Abstract
Background.
Von Hippel-Lindau (VHL) syndrome is a hereditary cancer syndrome characterized by a high risk of developing benign and malignant tumors, including central nervous system hemangioblastomas (CNS HB).. For an early diagnosis of VHL, before the occurrence of cancers (especially renal cancer), it is of huge importance to perform genetic testing in at risk patients. The aim of the study was to assess the psychological impact of VHL genetic testing in patients previously diagnosed with a CNS HB.
Methods.
From 1999 until 2015, 55 patients underwent surgery for CNS HB. Eleven patients were already screened for VHL mutations and 3 patients deceased before the start of the study. As for the remaining 42 patients, 24 accepted to be enrolled in the study. Assessment of psychological impact was performed by measuring anxiety levels, mood disorders, quality of life, and psychological consequences of screening.
Results.
Twenty-one of the enrolled 24 patients underwent genetic testing and only 12 patients came back for the communication of genetic results. The baseline psychological status did not differ between these 2 groups. Patients who attended visit 3 had similar anxiety levels compared to those who had not. Furthermore, they also experienced an improvement in two QoL dimension scores.
Conclusions.
There is no evidence of a negative psychosocial impact of VHL testing in patients with a previous history of CNS HB. We therefore recommend the recall of patients who have not been previously screened. Nonetheless, it is important to note that a substantial proportion of individuals did not respond to this screening invitation.
Keywords: VHL, hemangioblastoma, genetic screening, quality of life
Background
Von Hippel-Lindau (VHL) disorder is a rare autosomal dominant disease with a high penetrance (almost complete by the age of 60). It is caused by mutations in the VHL gene and is characterized by hemangioblastomas in the central nervous system (CNS) and retina, clear cell carcinoma of the kidney, pheochromocytomas or paragangliomas, renal and pancreatic cysts, cystadenomas (epididymal or pancreatic) or cystadenomas of probable mesonephric origin, pancreatic neuroendocrine tumors, and endolymphatic sac tumors. Bi-allelic inactivation of the VHL tumor suppressor gene, which is located on the small arm of chromosome 3, has been shown to be involved in the pathogenesis of these tumors [1–4]. The incidence of VHL disease is about 1 case per 36,000 live births. Genetic testing should include individuals with hemangioblastomas, multiple renal cysts and renal cell carcinoma (ccRCC), pheochromocytoma, and endolymphatic sac tumors. However, until present, the reported incidence of VHL seems to be significantly underestimated. In order to diagnose VHL in unrecognized patients, some countries have recalled patients for genetic testing if they have developed one or more tumors of the VHL spectrum during their lifetime. The screening of patients diagnosed with hemangioblastomas might be the most cost effective approach, since they are associated with VHL mutations in 20–50% of cases and generally occur before the development of ccRCC, which is becoming the main cause of death. VHL-mutation carriers and relatives may have higher levels of stress/distress. A recent study performed among 123 family members (including 66 mutation-carriers) from 48 VHL families found high levels of distress in about 50% of mutation-carriers and 36% of non-carriers [5]. Another study carried out by the same group, also showed distress in partners of individuals who were either diagnosed with, or were at high risk of developing tumors due to Li Fraumeni or VHL syndrome [6]. Genetic testing for hereditary cancer syndromes may also induce an accompanying anxiety. The assessment of baseline quality of life (QoL), depression, and anxiety prior to genetic testing might be interesting to evaluate, in order to adopt the best approach strategies and improve communication of medical information to patients in hopes of potentially improving their adherence to screening programs and outcomes. It has been shown that anxiety is associated with a premature drop out of surveillance programs [7]. With constant discovery of new genes implicated in cancers, we can presume that patient recalling for genetic screening of previously considered sporadic cancers will become more and more frequent. Therefore, this study could help serve in that regard. Until present, there has been no evaluation of the psychosocial impact of diagnostic VHL genetic testing in patients suspected of having the disease.
The aim of the present study was to assess the psychosocial impact of VHL genetic testing in a sample of patients with previously diagnosed CNS hemangioblastomas.
Methods
Eligibility criteria
The eligibility criteria were: age >= 18 years, previous surgery for a CNS HB between 1999 and 2015 at the department of neurosurgery of La Timone University Hospital (Marseille, France), not previously screened for VHL disease, and alive at the time of the study. The eligibility of patients was assessed based on previous medical records.
Eligible patients were contacted by mail for a follow-up visit with the neurosurgical team in order to talk about new discoveries and developments regarding their disease. No information was given on potential genetic testing by mail.
The inclusion criteria were: agreement to attend the proposed follow-up visit, and to undergo genetic testing in the setting of this study.
The study was approved by the local ethics committee (Comité de Protection des Personnes Sud-Méditerranée II: 2014/03/04, NCT02120040).
Study design
This is a longitudinal study assessing participants during 3 evaluation times.
Study visit 1: Baseline evaluation with the neurosurgeon
The first visit was planned with a neurosurgeon. The neurosurgeon informed patients of the potential link between CNS HB and VHL disease and that the recommendation in this context was to perform genetic screening. A consent form was collected for each participant. The neurosurgeon performed a complete clinical evaluation (personal history, physical signs and symptoms). Patients had to fill out a booklet including self-reported questionnaires that assessed: anxiety, depression, and quality of life.
Study visit 2: Genetics consultation and testing
A second visit was planned after a maximum of 15 days following the first visit. During this visit, the patient met with an oncogeneticist and a psychologist. The geneticist provided detailed information about VHL disease and the mode of inheritance. A family pedigree tree was drawn. The psychologist performed an interview to assess physical suffering and fear of disease transmission to children. A blood sample was collected for DNA sequencing of the VHL gene to detect mutations (including large deletions).
Study visit 3: Communication of results
A third visit was planned with the oncogeneticist several months after the second visit to communicate results. Patients again filled out a booklet including self-reported questionnaires that assessed: anxiety, depression, quality of life, and psychological consequences of screening before the communication of results.
Patients had the possibility of benefiting from an intermediary consultation with the onco-geneticist between visit two and three if they felt the need to ask questions or clarify certain information.
Intruments to assess anxiety, depression and quality of life and psychological outcomes
Self-administered questionnaires were given to the patients at baseline (visit 1) and just before the feedback of the genetic testing (visit 3)
Level of anxiety was assessed with the Spielberger State-Trait Anxiety Inventory (STAI); with 20 items assessing trait anxiety (general anxiety level) and 20 items assessing state anxiety (anxiety level for a specific time). Scales range between 20–80, with higher scores corresponding to higher levels of anxiety [8, 9].
Depression was assessed using the Beck Depression Inventory (BDI). The score range is 0–39, with higher scores indicating worsening depression (score 0–<4: no depression, 4–<8: mild depression, 8–<30: moderate depression; score >30: severe depression) [10].
The QoL was assessed using the SF-36 questionnaire. SF-36 is a generic questionnaire that has eight subscales (Physical Function (PF), Social Functioning (SF), Role Physical (RP), Role Emotional (RE), Mental Health (MH), Vitality (Vi), Bodily Pain (BP), and General Health (GH)), with scores ranging from 0 (low) to 100 (high QoL level). Two component summary measures of SF-36, namely Physical and Mental Composite Scores (PCS and MCS, respectively) can be calculated. Higher scores indicate higher QoL levels [11].
The Psychological Consequences Questionnaire PCQ (part 1, negative consequences of screening) was used to evaluate the psychological consequences of the genetic screening. This specific instrument had been developed for a breast cancer screening study [12]. Part 1 of the questionnaire is composed of 12 questions. A total score is produced ranging from 0 to 10. The higher the score, the more negative the psychological consequences.
Statistical analysis
Patient characteristics were provided at each evaluation period. Scores of self-reported questionnaires were computed using the algorithms provided by the respective developers of the tools. Comparisons between groups (non-included vs. included patients, patients at visit 3 vs. patients lost to follow-up at visit 3) were performed using chi-2 tests or Fisher exact tests for qualitative variables, while Student t test or Mann Whitney tests were used for continuous variables. Relationships between baseline variables and variables collected at visit 3 were performed using paired non parametric Wilcoxon tests and non-parametric correlations.
Results
Study population
Fifty-five patients underwent a CNS HB operation between 1999 and 2015 in the neurosurgery department of La Timone University Hospital. Eleven patients had already been screened for VHL mutations (9 positive, 2 negative) and 3 patients had already deceased. All 9 patients with VHL mutations at least had a family history of VHL or other VHL-related tumors or multiple HBs at initial diagnosis (3 were operated on for spinal cord HB at initial surgery).
Among the 42 eligible patients invited for the baseline visit (with at least two reminder letters by mail), 24 attended and were included in the study. The sex ratio was 1:1, and the median age was 58 years (interquartile range 44–68 years). The sample characteristics are detailed in supplemental table 1. The 24 included and 18 non-included patients did not differ according to sex or age. The diagnosis of the CNS HB was based on neurological symptoms in 22 cases and was incidentally found (CNS mass) in the latter 2 cases. Out of the 24 enrolled patients, 21 attended the genetic testing visit (visit 2). The mean delay between CNS surgery and genetic testing was 6.8 years. Among the 21 patients who underwent genetic testing, only 12 attended visit 3. A flow chart is available in figure 1.
Figure 1. Quality of life scores of the study sample, and of French age-sex crossed norms.
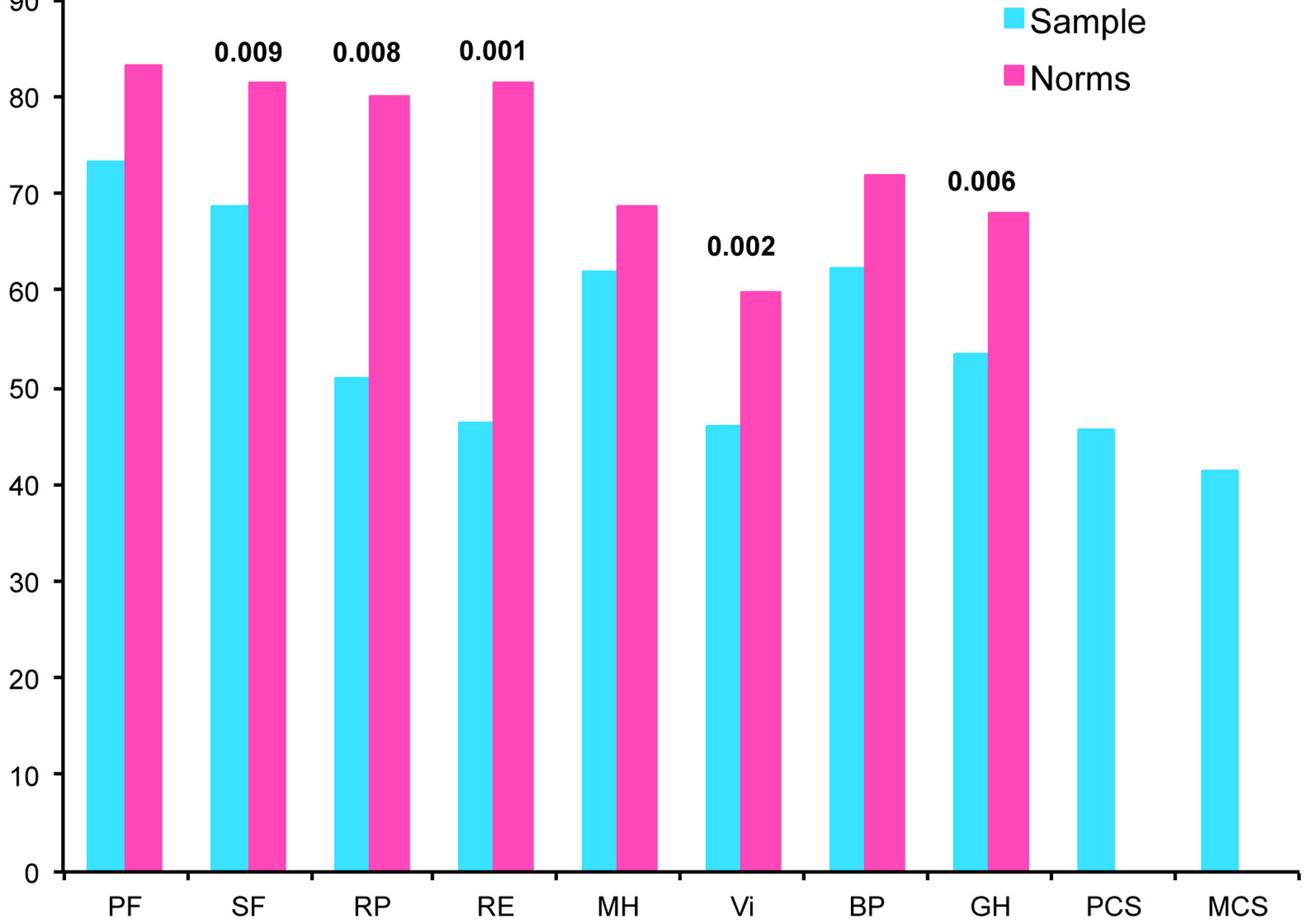
PF physical functioning. SF social functioning. RP role physical. RE role-emotional. MH mental health. Vi vitality. BP bodily pain. GH general health. PCS physical component summary. MCS
Psychological status at baseline
At baseline, the mean state anxiety level was 42.9 (SD±10.1) and the mean trait anxiety level was 39.8 (SD±14.4). According to the BDI, 48%, 30%, and 22% of patients presented with no, mild, or moderate mood disorders, respectively. The QoL of the patients and French age-sex crossed norms are provided in Figure 2. For our sample, the lowest and highest scores were role-physical and physical functioning, respectively. Compared with French age-sex crossed norms, our sample reported significant lower scores for social functioning, role-physical, role-emotional, vitality, and general health dimensions. The mean (+/−SD) PCQ was 4.8 (±1.8).
Results from genetic testing
Among the 21 tested patients, genetic screening was positive in a single case, for a large deletion in the VHL gene. This patient had a family history of CNS HB and was operated on for 2 cerebellar HBs at initial presentation. Among the negative cases, none had a family history of CNS HB or other components of the VHL disease.
Psychological status of patients receiving the test results, and that of patients lost to follow-up
Only 12 patients (57%) who underwent genetic testing came back to visit 3. The mean (+/− SD) delay between the genetic testing (visit 2) and the communication of results (visit 3) was 7.3 (±2.9) months (median 7 months, minimum 2 months, and maximum 11 months). No patient needed an intermediary visit (between visits 2 end 3). The 9 individuals who were lost to follow-up at visit 3 did not differ in terms of sex, marital status, and educational level from the 12 patients who attended visit 3. However, they were significantly older and more likely to be non-working. Anxiety and mood disorder levels, QoL scores, and PCQ scores did not differ between the 2 groups. The details are provided in supplemental table 2.
Change of psychological status between visit 1 and visit 3
For the 12 patients who attended visit 3, the state anxiety level did not differ between the 2 evaluation times, but the trait anxiety level significantly decreased from a score of 41 at baseline, to 38 at visit 3. Two scores of QoL, vitality and general health dimensions, were significantly improved between baseline and visit 3. The mood disorder score and the PCQ score did not differ between the 2 evaluations. All results are presented in table 1.
Table 1.
Changes in psychological profile in patients who attended visit 3 (communication of genetic results)
| Baseline Visit 1 N=12 | communication of results Visit 3 N=12 | P-value* | ||
|---|---|---|---|---|
| Anxiety (Spielberger) | Trait (20–80)* | 33.0 [25.3;40.5] | 36 [23.8;40.0] | 0.722 |
| State (20–80)* | 42.5 [31.9;47.0] | 40.5 [27.5;45.8] | 0.028 | |
| Mood disorders (BDI) | T otal score (0–39)** | 4.5 [1.3;6.0] | 2.5 [1.3;5.0] | 0.572 |
| Quality of life(SF-36)*** | Physical functioning | 85 [72.5;95] | 90 [56.3;95.0] | 0.287 |
| Social functioning | 56.3 [50;97.0] | 81.3 [62.5; 100] | 0.135 | |
| Role physical | 25 [0;91.7] | 87.5 [6.0; 100] | 0.078 | |
| Role emotional | 41.7 [0;91.7] | 100 [8.3;100] | 0.206 | |
| Mental health | 64.0 [48.0;83.0] | 72.0 [57.0;88.0] | 0.133 | |
| Vitality | 45.0 [35.0;60.0] | 65.0 [36.3;75.0] | 0.005 | |
| Bodily pain | 61.5 [43.5;81.5] | 73.0 [46.0;81.5] | 0.760 | |
| General health | 57.0 [37.8;79.5] | 77.0 [52.0;82.0] | 0.026 | |
| Mental composite score | 43.6 [30.8;52.9] | 48.8 [39.9;52.1] | 0.060 | |
| Physical composite score | 47.8 [38.6;51.9] | 49.3 [39.0;58.5] | 0.754 | |
| Psychological consequences of testing (PCQ) | Total score (0–10)**** | 4.6 [3.6–5.4] | 4.0 [2.8–4.7] | 0.168 |
paired test between baseline visit (visit 1) and visit 3
BDI Beck Depression Inventory. SF-36 Short-Form 36, PCQ Psychological Consequences of Screening
higher score. higher anxiety level
higher score. higher mood disorder level
higher score. better quality of life
higher score, higher negative psychological consequence
The state anxiety level at visit 3 was significantly correlated to the anxiety levels and mood disorder levels at baseline, the mental health dimension, and the mental composite score of QoL at baseline, while the trait anxiety level at visit 3 was only significantly correlated to the ‘physical-like’ QoL dimension at visit 1 (bodily pain and physical composite score of SF-36). The baseline anxiety and mood disorders were correlated to 5 QoL dimensions and the mental composite score reported at visit 3. The score of negative psychological consequences of screening assessed at visit 3 was correlated to ‘physical-like’ QoL dimension at visit 1 (physical functioning and bodily pain). All the details are provided in supplementary table 3.
Discussion
To our best knowledge, this is the first study that specifically evaluates psychological consequences provoked by genetic screening for VHL disease. It also provides information regarding the impact of recalling patients after an initial diagnosis. The principle conclusions to be drawn from this study are:
Many patients with CNS HB were not screened for VHL mutations, especially those with apparently sporadic disease and unifocal lesions.
The recall procedure and waiting period for results of genetic testing did not seem to deteriorate the QoL nor increase anxiety, depressive symptoms, or worry of screened patients. Therefore, we recommend to recall those who were not previously screened.
A substantial proportion of individuals with previous history of CNS HB did not respond to our recall procedure. Among those who were screened, only half came for the communication of results. Therefore, everything must be done to screen at-risk patients for VHL at initial diagnosis.
An important limitation regarding the results of the psychosocial impact of genetic testing is related to the high proportion of patients (50%) that did not respond to our invitation letters. We can explain this low response rate with many reasons. Firstly, the average age was relatively high in our cohort. This reflects a population that generally has more co-morbidity and is thus, less inclined to show up for supplementary consultations in addition to their own. Furthermore, the time interval between surgery and invitation letters was very long and it is probable that some patients preferred not to re-live their previous experience. Finally, the department of neurosurgery is a regional and national referral center that recruits patients from distant areas, which might explain why some patients did not participate. We cannot rule out the possibility that the most anxious patients did not take part in this study because of excess fear and stress of re-entering the context of their previous disease. This is probably the case for the patient with the VHL mutation. Surprisingly, about half of the patients didn’t come back for the communication of results. These patients were significantly older and most often non-working compared to those who came back, and therefore, possibly less concerned about their health.
Many studies have shed light on the amount of stress provoked by screening for other genetic pathologies predisposing to cancer such as Lynch Syndrome [13], Li Fraumeni Syndrome [14], and BRCA1/BRCA2 mutations in breast and ovarian cancers [15, 16]. However, unlike in our study, these have been conducted after the diagnosis of cancer. One study showed that 6% to 30% of patients benefiting from genetic screening for colorectal cancer had significantly higher levels of psychological distress and worry related to cancer before and after the genetic test [17]. The principle risk factors included: female sex, level of education, a higher baseline level of psychological distress prior to genetic counselling, a previous history of minor or major depression, and lack of social support. Another study was able to identify adoptable measures in order to better prepare patients for genetic testing, especially involving the support of close friends and relatives and improving self-confidence [18]. Early detection and better management of psychosocial difficulties of patients seems primordial, as it allows, according to a recent study, for improvement in patient adherence to screening programs for pathologies associated with VHL [7].
In conclusion, there is no evidence of a negative psychological impact related to the recall procedure and waiting period for genetic results in apparently sporadic cases of operated CNS HB. As a consequence, we recommend the recall of all those who were not previously screened. With constant discovery of new genes implicated in cancers, we anticipate that patient recalling for genetic testing will become more and more frequent and it is important to measure, prevent, and manage psychosocial risks associated with these screening programs.
Supplementary Material
Acknowledgements
The study was initiated and designed by the investigators and sponsored by Assistance Publique des Hôpitaux de Marseille (APHM). It has been financially supported by the « Fondation maladies rares ».
Footnotes
Competing interests. the authors declare The authors declare that they have no competing interests.
Consent statement
Written informed consent was obtained from the patients for this publication. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- 1.Glasker S, Bender BU, Apel TW, van Velthoven V, Mulligan LM, Zentner J, Neumann HP: Reconsideration of biallelic inactivation of the VHL tumour suppressor gene in hemangioblastomas of the central nervous system. Journal of neurology, neurosurgery, and psychiatry 2001, 70:644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JY, Dong SM, Park WS, Yoo NJ, Kim CS, Jang JJ, Chi JG, Zbar B, Lubensky IA, Linehan WM, et al. : Loss of heterozygosity and somatic mutations of the VHL tumor suppressor gene in sporadic cerebellar hemangioblastomas. Cancer research 1998, 58:504–508. [PubMed] [Google Scholar]
- 3.Kanno H, Kondo K, Ito S, Yamamoto I, Fujii S, Torigoe S, Sakai N, Hosaka M, Shuin T, Yao M: Somatic mutations of the von Hippel-Lindau tumor suppressor gene in sporadic central nervous system hemangioblastomas. Cancer research 1994, 54:4845–4847. [PubMed] [Google Scholar]
- 4.Maher ER, Yates JR, Ferguson-Smith MA: Statistical analysis of the two stage mutation model in von Hippel-Lindau disease, and in sporadic cerebellar haemangioblastoma and renal cell carcinoma. Journal of medical genetics 1990, 27:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammens CR, Bleiker EM, Verhoef S, Hes FJ, Ausems MG, Majoor-Krakauer D, Sijmons RH, van der Luijt RB, van den Ouweland AM, Van Os TA, et al. : Psychosocial impact of Von Hippel-Lindau disease: levels and sources of distress. Clinical genetics 2010, 77:483–491. [DOI] [PubMed] [Google Scholar]
- 6.Lammens CR, Bleiker EM, Verhoef S, Ausems MG, Majoor-Krakauer D, Sijmons RH, Hes FJ, Gomez-Garcia EB, Van Os TA, Spruijt L, et al. : Distress in partners of individuals diagnosed with or at high risk of developing tumors due to rare hereditary cancer syndromes. Psycho-oncology 2011, 20:631–638. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen A, Alonso E, Ochoa A, De Biase I, Familiar I, Yescas P, Sosa AL, Rodriguez Y, Chavez M, Lopez-Lopez M, Bidichandani SI: Uptake of genetic testing and long-term tumor surveillance in von Hippel-Lindau disease. BMC medical genetics 2010, 11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spielberger C: Manual for the state-trait anxiety inventory (form Y). Palo Alto, CA: consulting psychologists; 1983. [Google Scholar]
- 9.Spielberger C,RLG, Lushene R: Manual for the state-trait anxiety inventory (Self-evaluation questionnaire). Palo Alto, CA: consulting psychologists; 1970. [Google Scholar]
- 10.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961, 4:561–571. [DOI] [PubMed] [Google Scholar]
- 11.Leplege A, Ecosse E, Verdier A, Perneger TV: The French SF-36 Health Survey: translation, cultural adaptation and preliminary psychometric evaluation. Journal of clinical epidemiology 1998, 51:1013–1023. [DOI] [PubMed] [Google Scholar]
- 12.Cockburn J, De Luise T, Hurley S, Clover K: Development and validation of the PCQ: a questionnaire to measure the psychological consequences of screening mammography. Social science & medicine 1992, 34:1129–1134. [DOI] [PubMed] [Google Scholar]
- 13.Palmquist AE, Koehly LM, Peterson SK, Shegog M, Vernon SW, Gritz ER: “The cancer bond”: exploring the formation of cancer risk perception in families with Lynch syndrome. Journal of genetic counseling 2010, 19:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lammens CR, Aaronson NK, Wagner A, Sijmons RH, Ausems MG, Vriends AH, Ruijs MW, van Os TA, Spruijt L, Gomez Garcia EB, et al. : Genetic testing in Li-Fraumeni syndrome: uptake and psychosocial consequences. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010, 28:3008–3014. [DOI] [PubMed] [Google Scholar]
- 15.Bosch N, Junyent N, Gadea N, Brunet J, Ramon y Cajal T, Torres A, Grana B, Velasco A, Darder E, Mensa I, Balmana J: What factors may influence psychological well being at three months and one year post BRCA genetic result disclosure? Breast 2012, 21:755–760. [DOI] [PubMed] [Google Scholar]
- 16.Vos J, van Asperen CJ, Oosterwijk JC, Menko FH, Collee MJ, Gomez Garcia E, Tibben A: The counselees’ self-reported request for psychological help in genetic counseling for hereditary breast/ovarian cancer: not only psychopathology matters. Psycho-oncology 2013, 22:902–910. [DOI] [PubMed] [Google Scholar]
- 17.Bleiker EM, Esplen MJ, Meiser B, Petersen HV, Patenaude AF: 100 years Lynch syndrome: what have we learned about psychosocial issues? Familial cancer 2013, 12:325–339. [DOI] [PubMed] [Google Scholar]
- 18.Read CY, Perry DJ, Duffy ME: Design and psychometric evaluation of the Psychological Adaptation to Genetic Information Scale. Journal of nursing scholarship : an official publication of Sigma Theta Tau International Honor Society of Nursing / Sigma Theta Tau 2005, 37:203–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


