Abstract
Purpose.
Nintedanib enhances the activity of chemotherapy in metastatic NSCLC. In this phase I/II study, we assessed safety and efficacy of nintedanib plus neoadjuvant chemotherapy, using major pathologic response (MPR) as primary endpoint.
Experimental Design:
Eligible patients had stage IB (≥4 cm)-IIIA resectable NSCLC. A safety run-in phase was followed by an expansion phase with nintedanib 200 mg PO bid (28 days), followed by 3 cycles of cisplatin (75 mg/m2), docetaxel (75 mg/m2) q21 days plus nintedanib, followed by surgery. With 33 planned patients, the study had 90% power to detect an MPR increase from 15% to 35%.
Results:
21 patients (stages I/II/III, N=1/8/12) were treated. One of 15 patients treated with nintedanib 200 mg achieved MPR (7%, 95% CI 0.2%−32%). Best ORR in 20 evaluable patients was 30% (6/20, 95% CI 12%−54%). 12-month RFS and OS were 66% (95% CI 47%−93%) and 91% (95% CI, 79%−100%), respectively. Most frequent treatment-related G3–4 toxicities were transaminitis and electrolyte abnormalities. Based on an interim analysis the study was discontinued for futility. Higher levels of CD3+ and cytotoxic CD3+CD8+ T cells were found in treated tumors of patients who were alive than in those who died (652.8 vs. 213.4 cells/mm2, P=0.048; 142.3 vs. 35.6 cells/mm2, P=0.018).
Conclusions:
Although tolerated, neoadjuvant nintedanib plus chemotherapy did not increase MPR rate compared to chemotherapy historical controls. Additional studies of the combination in this setting are not recommended. Post-treatment levels of tumor infiltrating T cells were associated with patient survival. Use of MPR facilitates the rapid evaluation of neoadjuvant therapies.
Keywords: Neoadjuvant chemotherapy, nintedanib, angiogenesis inhibitor, NSCLC, surrogate endpoint, major pathologic response
Introduction
In localized or loco-regional non-small cell lung cancer (NSCLC), surgical resection of the primary tumor and mediastinal lymph nodes is preferred to achieve prolonged survival. However, recurrences, often distal, occur in 30 to 70% of patients (1). The addition of adjuvant chemotherapy improves 5-year overall survival (OS) by 5.4%, with hazard ratio (HR) for death of 0.89 (P = 0.005) based on a meta-analysis of 4584 patients (2). Neoadjuvant chemotherapy offers very similar 5% survival benefit (HR 0.87, P = 0.007) (3), is better tolerated (4), and allows for evaluation of response both radiographically and pathologically (5), thus providing an opportunity to assess efficacy at an early time point (3).
Trials adopting OS and disease-free survival (DFS) endpoints require prolonged time to complete and can be resource consuming. Several studies have suggested that the degree of tumor regression after neoadjuvant chemotherapy, as determined by histopathologic findings in the resected tumor, correlates with and may serve as intermediate endpoint for long-term outcomes (6–9). From the analysis of 258 patients, our group has previously developed a scoring system that quantifies the percentage of viable tumor cells in at least one section per cm of tumor greatest diameter (10). After neoadjuvant chemotherapy, we observed a significant correlation between the residual larger percentage of viable tumor cells with a shorter DFS and OS. We defined a major pathologic response (MPR) to neoadjuvant chemotherapy as ≤ 10% viable tumor cells in the resected tumor, which occurred in 19% of patients and correlated with OS in two separate studies (10, 11). These results support the use of MPR as a suitable surrogate endpoint of efficacy in trials evaluating neoadjuvant therapies for resectable NSCLC (12).
Nintedanib is a potent orally available triple angiokinase inhibitor that targets vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) receptor signaling pathways. It has been approved in the European Union in combination with docetaxel for advanced, metastatic or locally recurrent NSCLC of adenocarcinoma histology after first-line chemotherapy based on PFS improvements over docetaxel alone, and OS improvements in the adenocarcinoma histology population (13). The role of preoperative nintedanib in NSCLC has not previously been explored. Nintedanib may prime the tumor vasculature for enhanced delivery of chemotherapy, by inhibiting VEGFR, FGFR and PDGFR in the endothelium, pericytes and smooth muscle cells (14), and possibly normalizing the distorted architecture of tumor microvessels (15, 16). Nintedanib monotherapy may also have a direct tumor anti-proliferative effect via blocking oncogenic receptor tyrosine kinases (17). In combination with chemotherapy, nintedanib is known to enhance the antitumor response to docetaxel and pemetrexed in NSCLC xenograft models (18).
We conducted a phase 1/2 clinical trial to test the primary hypothesis that neoadjuvant platinum doublet chemotherapy and nintedanib would be feasible and would increase MPR rate compared to previously published results of neoadjuvant chemotherapy alone in patients with resectable NSCLC stages IB-IIIA. Our secondary hypothesis was that a four-week course of priming nintedanib monotherapy given before chemotherapy would induce a response in a subset of patients, allowing exploratory analyses for predictive nintedanib biomarkers. Here, we report results of safety and efficacy of this regimen, as measured by MPR.
Materials and Methods
This was a single arm phase 1/2 study (NCT02225405) conducted at the University of Texas MD Anderson Cancer Center (MDACC), approved by the institutional review board (IRB) and conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Patient eligibility
Study inclusion criteria included: treatment naïve patients with NSCLC, histological subtypes adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, clinical stage IB (≥ 4 cm tumor size) to IIIA (only single N2 station positive for cancer) based on American Joint Committee on Cancer Staging Manual, 7th edition; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, age ≥ 18 years, and adequate bone marrow, renal, and liver organ function. All patients must have been surgical candidates, and invasive mediastinal staging was strongly recommended. Core exclusion criteria included: prior systemic or radiation therapy for current lung cancer, hypersensitivity to drugs, tumors centrally located or invading major blood vessels, any tumor cavitations, history of recent trauma or hemorrhagic or thromboembolic events, inherited propensity for bleeding or thrombosis, and prior malignancy requiring chemotherapy or radiation within 1 year.
Treatment Plan
All patients consented to the study. The study was designed as a 3+3 run-in phase to establish the safety of combining nintedanib with cisplatin and docetaxel in the neoadjuvant setting. During the run-in phase (Supplementary Figure 1A) nintedanib was given upfront with a fixed dose of cisplatin 75 mg/m2 and docetaxel 75 mg/m2 IV on day (D) 1 intravenously (IV), with repeated cycles every 21 days for a maximum of 3 cycles. Nintedanib was administered from cycle 1/day 2 until cycle 3/day 7 (nintedanib was held on the days of chemotherapy administration, i.e., day 1 of cycles 1, 2, and 3). The first three patients treated in the run-in phase received nintedanib at dose level −1 (150 mg PO bid), with plans to adjust the dose for the next three patients based on dose limiting toxicity (DLT).
The expansion phase (Supplementary Figure 1B) included a priming phase with nintedanib 200 mg PO bid monotherapy for 28 days. After priming therapy, patients received concomitant nintedanib 200 mg PO bid administered from cycle 1/day 2, until cycle 3/day 7 of chemotherapy with cisplatin 75 mg/m2 on and docetaxel 75 mg/m2 IV on D1 in cycles repeated every 21 days +7 days/−3 days for a maximum of 3 cycles. Nintedanib administration was held on the days of chemotherapy administration (i.e., day 1 of cycles 1, 2, and 3).
Surgical resection was performed 21 days after the last chemotherapy, and within 6 weeks after day 1 of the last chemotherapy cycle (range: 3–6 weeks after last cycle). The operative approach (thoracoscopy versus thoracotomy) and the extent of resection was based on surgeons’ judgment and included wedge, segmentectomy, lobectomy, or pneumonectomy with mediastinal lymph node dissection of at least 3 mediastinal nodal stations. The mediastinal N2 stations most routinely dissected included stations 4, 7 and 9 on the right and 4, 5, 7 and 9 on the left.
Post-operative radiation therapy (PORT) was administered based on final pathologic disease stage and the discretion of the treating physicians. Consideration for PORT was given to patients with clinically or pathologically positive N2 mediastinal disease, in cases of microscopically or grossly positive margins. Radiation used was either photon or proton based external beam radiation with doses of 50–66 Gy. Use of adjuvant chemotherapy was left to the discretion of the treating medical oncologist.
Multiplex immunofluorescence staining and scanning
Multiplex immunofluorescence (mIF) staining was performed using an automated staining system (BOND-RX; Leica Biosystems, Buffalo Grove, IL) in one 4-μm histologic tumor section obtained from representative FFPE tumor blocks using the Opal 7-Color fIHC Kit (Akoya Biosciences/PerkinElmer, Waltham, MA). The IF markers used were grouped into 1 6-antibody panel: pancytokeratin (epithelial cell marker; clone AE1/AE3, dilution 1:300, Dako, Santa Clara, CA), PD-L1 (clone E1L3N, dilution 1:3000, Cell Signaling Technology, Danvers, MA), CD68 (clone PG-M1, dilution 1:450, Dako), CD3 (cat#IS503, dilution 1:100, Dako), CD8 (clone C8/144B, dilution 1:300, Thermo Fisher Scientific, Waltham, MA), and PD-1 (clone EPR4877–2, dilution 1:250, Abcam, Cambridge, MA). The stained slides were scanned using the multispectral microscope Vectra 3.0.3 (Akoya Biosciences/PerkinElmer) (19). After slides were scanned in low magnification 10x, a pathology selected around five regions of interest (each ROI, 0.3345 mm2) to cover around 1.65 mm2 of tumor tissue using the phenochart 1.0.9 viewer (Akoya Biosciences/PerkinElmer PerkinElmer). ROIs were scanned at 20x magnification and were analyzed by a pathologist using InForm 2.4.4 image analysis software (Akoya Biosciences/PerkinElmer PerkinElmer).
Multispectral analysis
Tumor multispectral images containing immune markers were analyzed in the epithelial compartment, defined as malignant cell nests, and the stromal compartment, characterized by the fibrous tissue present between malignant cells (20). The individual cells defined by nuclei [DAPI] staining and identified by the cell segmentation tool were subjected to the phenotyping pattern recognition learning algorithm tool to characterize co-localization of the various cell populations using the markers in panel 1 as previously reported (21): malignant cells expressing PD-L1 (AE1/AE3+PD-L1+), T lymphocytes (CD3; pan T-cell marker), cytotoxic T cells (CD3+CD8+), tumor-associated macrophages (TAMs) (CD68+); and TAMs expressing PD-L1 (CD68+PD-L1+), T cells expressing PD-1 (antigen-experienced T cells, CD3+PD-1+), and cytotoxic T-cells expressing PD-1 (antigen-experienced cytotoxic T cells, CD3+CD8+PD-1+). Densities of each co-localized cell populations were quantified as average and the final data was expressed as number of cells/mm2 in the tumor and stroma compartments. Malignant cells and macrophages expressing PD-L1 were expressed also in percentages. All the data was consolidated using the R studio 3.5.3 (Phenopter 0.2.2 packet, Akoya Biosciences/PerkinElmer) and SAS 7.1 Enterprise.
Study Endpoints and Statistical Analysis
The primary objectives of the study were to determine safety of nintedanib in combination with cisplatin and docetaxel in all treated patients, and rate of MPR (≤ 10% viable tumor) in resected tumors in patients who received neoadjuvant therapy with nintedanib at maximum tolerated dose (i.e., dose level 0, including three patients accrued in the run-in phase and 12 patients accrued in the expansion phase).
Secondary endpoints were: objective response rates (ORR) to the entire neoadjuvant treatment, as well as to priming nintedanib, using Response Evaluation Criteria in Solid Tumors (RECIST v 1.1), recurrence-free survival (RFS), OS, correlation between MPR and RFS and OS, toxicity, perioperative morbidity and mortality, completeness of resection, correlation of imaging, blood and tissue biomarkers with efficacy and toxicity.
The primary hypothesis was that the combination of nintedanib and chemotherapy would increase the MPR rate from 15% to 35%. Based on the Simon’s two-stage design, with a 10% type I error rate and 90% power, we planned to enroll 19 patients in the first stage. If there were three or less responders, the proposed treatment would be considered inefficacious and the trial would be stopped. If ≥ 4 responders were seen, 14 more patients would be enrolled in the second stage to reach a total of 33 patients.
MPR was estimated with the exact 95% Clopper-Pearson confidence intervals (22). RFS and OS were estimated using the Kaplan-Meier (KM) method (23). RFS was defined as the time from the date of surgery to the first date of recurrence or death or last follow-up date (censor). For patients who received definitive radiation therapy, RFS was measured from the date radiation was completed and the patient achieved disease-free status to the first date of recurrence or death. Patients who did not receive surgery or definitive radiation, or who received definitive radiation but did not achieve DFS were not evaluated for RFS. OS was defined as the time from the date of surgery to death, or last follow-up (censor). Adverse events (AEs) were noted by grade and their relationship to the treatment within each dose cohort. Only those toxicities possibly or probably or definitely related to treatment were included in the toxicity analysis.
The levels of immune markers were summarized by descriptive statistics. Given the small number of events (8 recurrences [1 patient was not included in the analysis related to recurrence] and 3 deaths), we did not assess the associations of the markers with RFS and OS using time-to-event analysis approaches. Instead, we compared the marker levels between the patients who experienced recurrence and/or died before 17 months post-surgery (the maximum time to recurrence/death was 16.46 months) and those who were recurrence-free and alive beyond 17 months post-surgery, and between those who died before 17 months post-surgery (the maximum time-to-death was 15.67 months) and those who were still alive beyond 17 months post-surgery using Wilcoxon test and Kruskal-Wallis test (22). This approach was considered appropriate because all of patients without recurrence had been followed longer than the longest time-to-recurrence. Similarly, all of alive patients had been followed longer than the longest time-to-death. SAS version 9.4 and S-Plus version 8.04 were used to carry out the computations for all analyses.
Results
Patients
From July 2015 to May 2017, 24 patients were registered on trial: 3 patients were screen failures, due to comorbidities, stage IIIB (T4N2M0) at new baseline scans, and presence of central tumor with vascular invasion (Supplementary Figure 2, CONSORT Diagram). Twenty-one patients (15 female and 6 male), clinical stage IB (N = 1), stage IIA and IIB (N = 8), and stage IIIA (N = 12) were treated on the trial. Baseline patients’ characteristics are described in Table 1. Nine patients were treated in the run-in phase and 12 were treated in the expansion phase. In the run-in phase, the first three patients received nintedanib at dose level −1 (150 mg PO bid) concomitantly with chemotherapy, with plans to adjust the dose for the next three patients based on DLT. Because of one observed DLT with nintedanib dose level −1, three additional patients were treated at the same dose level. Since there were no additional DLTs, escalation to nintedanib dose level 0 (200 mg PO bid) ensued and the first three patients were treated at this dose. Since there were no DLTs in first three patients treated at this dose of nintedanib concomitantly with chemotherapy, the study entered the expansion phase. Twelve patients in the expansion phase received nintedanib monotherapy at dose level 0 for 28 days (priming phase) followed by nintedanib at dose level 0 concomitant with cisplatin and docetaxel for up to three cycles. Interim efficacy analysis was performed on all 15 patients treated at nintedanib dose level 0 (three in the run-in phase treated with concomitant nintedanib plus chemotherapy and 12 in the expansion phase treated with nintedanib priming monotherapy followed by concomitant nintedanib plus chemotherapy). All 21 patients underwent invasive mediastinal staging with endobronchial ultrasound (EBUS) and 14 (67%) were found to have node positive lung cancer, two in N1 hilar lymph nodes and 12 in N2 mediastinal nodes (single station). One patient had negative EBUS for N1 disease but baseline PET avidity at N1 station and was classified as N1.
Table 1.
Patient and treatment characteristics.
| Variable | Category | Frequency Count | Percent of Total Frequency |
|---|---|---|---|
| Dose Cohort | −1: nintedanib 150 mg PO bid | 6 | 29% |
| 0: nintedanib 200 mg PO bid | 15 | 71% | |
| Sex | Female | 15 | 71% |
| Male | 6 | 29% | |
| Race | Asian | 2 | 10% |
| Hispanic | 2 | 10% | |
| Other | 1 | 5% | |
| White | 16 | 76% | |
| ECOG status | 0 | 12 | 57% |
| 1 | 9 | 43% | |
| Smoking | No | 9 | 43% |
| Yes | 12 | 57% | |
| HISTOLOGY | Adenocarcinoma | 15 | 71% |
| Large cell | 1 | 5% | |
| Squamous Cell Cancer | 5 | 24% | |
| Clinical Stage (AJCC 7th edition) | IB | 1 | 5% |
| IIA | 4 | 19% | |
| IIB | 4 | 19% | |
| IIIA | 12 | 57% | |
| Invasive Mediastinal Staging (EBUS) | Negative | 7 | 33% |
| Positive | 14 | 67% | |
| Neoadjuvant Therapy | Completion of 3 cycles of chemotherapy | 19 | 90% |
| Completion of nintedanib | 16 | 76% | |
| Surgery | Resection | 19 | 90% |
| Not resected | 2 | 10% | |
| Type of Surgery | Lobectomy | 16 | 76% |
| Lobectomy and wedge | 1 | 5% | |
| Pneumonectomy Left Right |
2 0 (0%) 2 (100%) |
10% |
|
| Postoperative Therapy | Adjuvant chemotherapy | 1 | 5% |
| Adjuvant radiation | 9 | 43% | |
| Definitive radiation | 2 | 10% |
Treatment
Treatment characteristics are in Table 1. Nineteen out of 21 treated patients (91%) completed all 3 cycles of chemotherapy. The third cycle of chemotherapy was omitted in two patients due to: treatment-related AEs (TRAEs) despite dose reduction in one, and lack of radiographic response following cycle 2 in both patients. In the 19 patients who received 3 cycles of chemotherapy, nintedanib was prematurely discontinued due to elevated liver enzymes (N = 1), non-compliance (N = 1), and grade 2 hemoptysis (N = 1). Nintedanib was also discontinued in the two patients who did not complete 3 cycles of chemotherapy.
Surgical resection was performed in 19 patients (19/21, 90%). A R0 resection was achieved in 18 (95%) patients and one patient underwent R1 resection (5%). Two patients were unresectable; one patient planned for pneumonectomy was no longer physically fit for the procedure after neoadjuvant treatment, and one was discovered to have pleural metastases during thoracotomy and resection was aborted. Lobectomy was performed in 16 (84%) of resected patients. Three of 19 resected patients (3/19, 16%) had mediastinal or hilar node down staging at surgery (1 N2 to ypN1, 1 N2 to ypN0, 1 N1 to ypN0), and one patient (1/19, 5%) was upstaged in the hilar nodes (N0 to ypN1). Only one patient received adjuvant chemotherapy and nine received PORT for N2 disease (43%, all stage IIIA; 8 baseline pN2 – found ypN2, 1 baseline pN2 - downstaged to ypN1 with focal pleural invasion). Two patients underwent definitive radiation due to unresectability (10%; 1 stage IIIA, 1 stage IIA). Two out of 12 patients with stage IIIA (N2) disease at baseline did not receive PORT due to postoperative disease progression (N = 1) and downstaging to ypN1.
Toxicity
The most common TRAEs by highest grade are presented in Table 2. In the run-in phase with nintedanib dose −1 administered concurrently with chemotherapy, the most common TRAEs were diarrhea (G1–2, N = 5, 83%), fatigue and nausea (G1–2, each N = 4, 67%), and alopecia (G1–2, N = 3, 50%). The only G3 toxicities seen were hyponatremia and alanine transaminitis, which occurred both in one patient (17%). No G4 or G5 AEs were noted. In the run-in phase with nintedanib level 0 combined with chemotherapy (N = 3) plus in the expansion phase with priming nintedanib level 0 followed by combination with chemotherapy (N = 12), most common TRAEs were G1–2 toxicities in all 15 patients. The overall most common TRAEs in this cohort included nausea (G1–2, N = 10, 67%; G3, N = 2; 13%), diarrhea (G1–2, N = 10, 67%; G3, N = 1, 7%), anemia (G1–2, N = 8, 53%; G3, N = 1, 7%), and alanine and aspartate transaminitis (G1–2, N = 6, 40%, G3, N = 2, 13%; and G1–2, N = 5, 34%, G3, N = 2, 13%, respectively). The only G4 toxicities in this cohort were hypokalemia and hypocalcemia, which both occurred in one patient (7%).
Table 2.
Number of patients with treatment-related adverse events (TRAEs) by highest grade receiving nintedanib 150 mg PO bid (dose level −1, N = 6) and nintedanib 200 mg PO bid (dose level 0, N = 15, including 3 in run-in phase and 12 in expansion phase).
| TRAE (Nintedanib 150 mg PO bid) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|---|
| DIARRHEA | 3 (50%) | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (83%) |
| FATIGUE | 2 (33%) | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (67%) |
| NAUSEA | 3 (50%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (67%) |
| ALOPECIA | 1 (17%) | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (50%) |
| HYPOMAGNESEMIA | 3 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (50%) |
| ANEMIA | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) |
| DEHYDRATION | 0 (0%) | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) |
| DYSGEUSIA | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) |
| HYPONATREMIA | 1 (17%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) | 2 (33%) |
| VOMITING | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) |
| WEIGHT LOSS | 1 (17%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33%) |
| ALT INCREASED | 0 (0%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) | 1 (17%) |
| ANOREXIA | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| AST INCREASED | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| HYPERBILIRUBINEMIA | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| CONSTIPATION | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| CREATININE INCREASED | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| DRY SKIN | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| ERYTHEMA MULTIFORME | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| GENERALIZED MUSCLE WEAKNESS | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| HYPERKALEMIA | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| HYPOKALEMIA | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| HYPOTENSION | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| MUCOSITIS ORAL | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| PAIN | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| PARESTHESIA | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| RASH ACNEIFORM | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| STOMACH PAIN | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (17%) |
| TRAE (Nintedanib 200 mg PO bid) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
| NAUSEA | 8 (53%) | 2 (13%) | 2 (13%) | 0 (0%) | 0 (0%) | 12 (80%) |
| DIARRHEA | 4 (27%) | 6 (40%) | 1 (7%) | 0 (0%) | 0 (0%) | 11 (74%) |
| ANEMIA | 6 (40%) | 2 (13%) | 1 (7%) | 0 (0%) | 0 (0%) | 9 (60%) |
| ALT INCREASED | 4 (27%) | 2 (13%) | 2 (13%) | 0 (0%) | 0 (0%) | 8 (53%) |
| AST INCREASED | 4 (27%) | 1 (7%) | 2 (13%) | 0 (0%) | 0 (0%) | 7 (47%) |
| LYMPHOPENIA | 0 (0%) | 6 (40%) | 1 (7%) | 0 (0%) | 0 (0%) | 7 (47%) |
| FATIGUE | 6 (40%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (40%) |
| HYPOKALEMIA | 3 (20%) | 2 (13%) | 0 (0%) | 1 (7%) | 0 (0%) | 6 (40%) |
| VOMITING | 3 (20%) | 1 (7%) | 2 (13%) | 0 (0%) | 0 (0%) | 6 (40%) |
| ALP INCREASED | 5 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (33%) |
| HYPERGLYCEMIA | 3 (20%) | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (33%) |
| HYPERTENSION | 3 (20%) | 1 (7%) | 1 (7%) | 0 (0%) | 0 (0%) | 5 (33%) |
| HYPOCALCEMIA | 2 (13%) | 1 (7%) | 1 (7%) | 1 (7%) | 0 (0%) | 5 (33%) |
| HYPOMAGNESEMIA | 4 (27%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (33%) |
| ANOREXIA | 4 (27%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (27%) |
| COUGH | 3 (20%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (27%) |
| ABDOMINAL PAIN | 3 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) |
| HYPERBILIRUBINEMIA | 2 (13%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) |
| CONSTIPATION | 3 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) |
| CREATININE INCREASED | 2 (13%) | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 3 (20%) |
| DEHYDRATION | 0 (0%) | 3 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) |
| DYSGEUSIA | 2 (13%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) |
| DYSPEPSIA | 2 (13%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) |
| FLUSHING | 2 (13%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) |
| HYPONATREMIA | 2 (13%) | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 3 (20%) |
| METABOLISM AND NUTRITION DISORDERS | 3 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (20%) |
| CHILLS | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13%) |
| DYSPNEA | 1 (7%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13%) |
| GERD | 1 (7%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13%) |
| GASTROINTESTINAL DISORDERS | 1 (7%) | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 2 (13%) |
| HICCUPS | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13%) |
| NEUTROPENIA | 1 (7%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13%) |
| THROMBOCYTOPENIA | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13%) |
| RENAL AND URINARY DISORDERS | 1 (7%) | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 2 (13%) |
| SYNCOPE | 0 (0%) | 0 (0%) | 2 (13%) | 0 (0%) | 0 (0%) | 2 (13%) |
| LEUKOPENIA | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13%) |
| ABDOMINAL DISTENSION | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| ALOPECIA | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| BLOATING | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| BLOOD AND LYMPHATIC SYSTEM DISORDERS | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| BLURRED VISION | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| BONE PAIN | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| BRONCHOPULMONARY HEMORRHAGE | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| DIZZINESS | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| EPISTAXIS | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| GENERALIZED MUSCLE WEAKNESS | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| HEADACHE | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| HEARING IMPAIRED | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| HYPERKALEMIA | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| HYPERMAGNESEMIA | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| HYPOALBUMINEMIA | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| HYPOPHOSPHATEMIA | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| INFECTIONS AND INFESTATIONS | 0 (0%) | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 1 (7%) |
| MALAISE | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| MYALGIA | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| NERVOUS SYSTEM DISORDERS | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| PAIN IN EXTREMITY | 0 (0%) | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| SINUS TACHYCARDIA | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| STOMACH PAIN | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| VTE | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
| WEIGHT LOSS | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) |
Perioperative outcomes for all 19 resected patients were notable for median length of hospital stay of 4 days (range 2–20). Pulmonary complications (N = 4, 21%) included prolonged air leak in three patients and respiratory failure/acute respiratory distress syndrome (ARDS) in one patient. Atrial fibrillation occurred in two patients (10%). Three patients required one unit of postoperative blood transfusion (16%). One patient died within 30-days from ARDS, which was not attributed to the study drugs, rather to baseline pulmonary fibrosis despite adequate preoperative pulmonary function.
Efficacy
Among 15 patients treated with nintedanib dose level 0, three with concomitant nintedanib plus chemotherapy and 12 with priming nintedanib followed by chemotherapy plus nintedanib, only one achieved MPR [6.7% (95% CI 0.17%, 31.95%)]. In the whole study population (N = 21), the MPR rate was 9.5% (2/21, 95% CI 1.17%, 30.38%), as the second MPR was observed after neoadjuvant chemotherapy plus nintedanib at dose level −1 during the run-in phase (Figure 1). Two patients (10%, 2/21) were unresectable after neoadjuvant therapy and failed to achieve the primary endpoint.
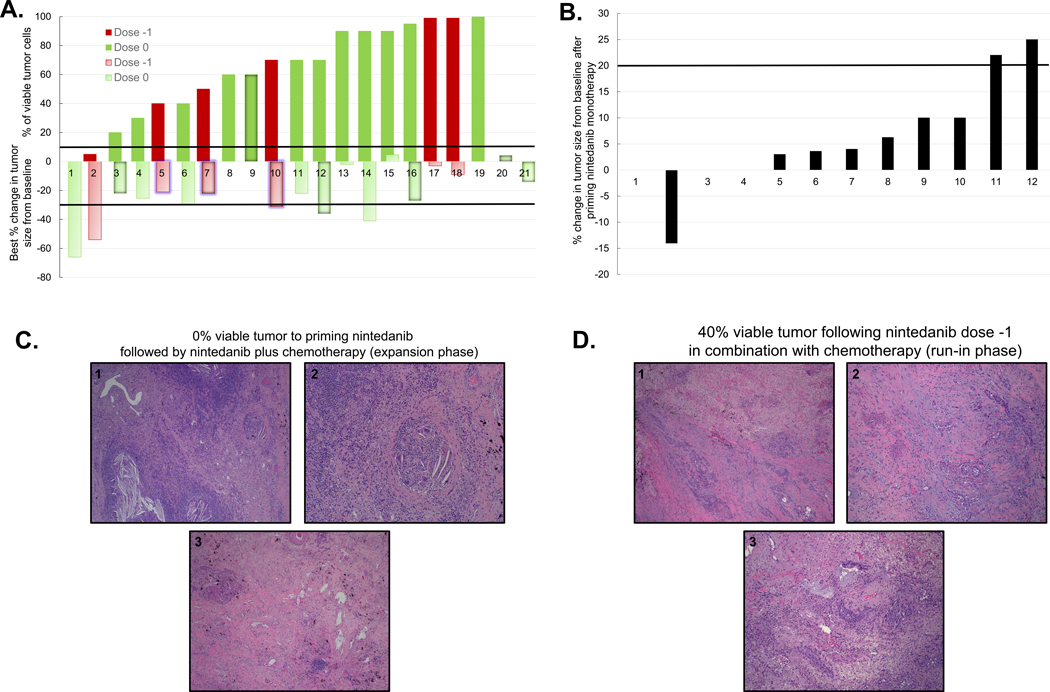
Figure 1. Pathologic and radiographic responses in evaluable patients.
A. Bar graph depicting the percentage of viable tumor and best percentage change in tumor size from baseline in evaluable patients. The solid red and green bars depict the percentage of viable tumor in 19 patients who underwent surgery following neoadjuvant therapy, according to nintedanib dose level. The light red and green bars depict the best percentage change in tumor size from baseline in 20 evaluable patients by RECIST, according to dose level. One patient (#19) was not radiographically evaluable due to post-obstructive pneumonia. For two unresectable patients (included in MPR analysis as failures to achieve MPR), the percentage of viable tumor is not available (# 20 and 21). The upper solid black line depicts the 10% cutoff for the definition of MPR; the lower solid black line depicts the −30% cutoff for definition of radiographic partial response (PR) by RECIST. The bars representing patients whose tumor progressed are labeled with a centered shadow, patients who died are labeled with blue glow and those who had progression of disease and then died are labeled with both shadow and glow. Dose −1: nintedanib 150 mg PO bid; dose 0: nintedanib 200 mg PO bid. B. Bar graph depicts the radiographic percentage change in tumor size compared to baseline in 12 patients treated with priming nintedanib 200 mg PO bid monotherapy in the expansion phase. The solid black line depicts the 20% cutoff for definition of radiographic PD by RECIST. C. Representative microscopic images of resected tumor with pathologic complete response (pCR) to nintedanib priming followed by combination with chemotherapy. 1: Low magnification image of tumor bed demonstrates no residual viable tumor, dense inflammatory infiltrate, cholesterol cleft granulomas and fibrosis. 2: High magnification microscopic image of tumor bed demonstrates cholesterol cleft giant cell granuloma and a dense lymphocytic infiltrate. 3: Low magnification image of tumor bed shows extensive fibrosis with focal inflammatory infiltrate. D. Representative microscopic images of resected tumor with 40% viable tumor to nintedanib plus chemotherapy.1: low magnification image shows poorly differentiated adenocarcinoma in a background of marked fibrosis/scar. 2 and 3: high magnification image demonstrates residual viable tumor with focal necrosis, mixed inflammatory infiltrate and marked fibrosis.
The MPR rates observed in the current study were lower than expected; therefore, we performed an interim analysis after enrollment of 15 patients treated with nintedanib dose level 0 to estimate the probability of continuing the expansion phase of the trial from the first stage to the second stage. By design, ≥ 4 responders among the first 19 patients treated were needed during the first stage of the trial. With only one MPR (7%) in the cohort treated with nintedanib 200 mg PO bid, the probability of 3 additional responses to continue the trial beyond the initial 19 patients was only 5.4%, even when an optimistic prior p beta (0.35, 0.65) was assumed (mean of the MPR rate of 0.35). Therefore, it was recommended that the trial be closed due to lack of expected treatment efficacy.
In the overall population, best ORR as assessed by RECIST was 30% (6/20 evaluable patients, 95% CI 11.89%, 54.28%), with six confirmed partial responses (PRs), and 14 patients achieving stable disease (SD) (Figure 1A). Disease control rate (DCR), defined as SDs plus PRs (best responses) was 100% (20/20 patients). As shown in Figure 1B, no radiographic responses were observed in 12 patients following nintedanib priming monotherapy at 200 mg PO bid; nine experienced SD, one was not evaluable due post-obstructive pneumonia (patient 1), and two patients had PD. The two patients with radiographic PD after priming experienced SD at restaging scans following combined neoadjuvant nintedanib plus chemotherapy treatment, prior to surgery. Representative hematoxylin and eosin (H&E) images reveal pathologic complete response (pCR) to nintedanib 200 mg PO bid priming followed by nintedanib combined with chemotherapy in a patient treated in the expansion phase (Figure 1C), and 40% residual viable tumor cells in a patient treated with nintedanib 150 mg PO bid combined with chemotherapy in the run-in phase (Figure 1D). There were no significant associations between histology and MPR and/or best radiographic response to therapy. One of 15 (6.7%) patients with adenocarcinoma and one of six patients with other histologies (16.7%) had MPR (P = 0.5, Supplementary Table 1); five of 14 (35.7%) patients with adenocarcinoma had PR, and one of six patients with other histologies (16.7%) had PR (P = 0.61, Supplementary Table 2).
Two patients with MPR also had a radiographic response to neoadjuvant treatment and are alive and without disease (Figure 1A). Among four patients who achieved radiographic PR but not MPR, two patients recurred (50%). Representative CT images depicting the association between radiographic and pathologic responses are shown in Figure 2. CT images reveal radiographic PR in patients with pCR after nintedanib priming followed by nintedanib plus chemotherapy (Figure 2A, top panel), and MPR after completion of nintedanib dose −1 plus chemotherapy (Figure 2A, bottom panel), respectively. Representative CT images of two patients with radiographic response but no MPR after nintedanib priming followed by nintedanib plus chemotherapy and completion of nintedanib dose −1 plus chemotherapy are in Figure 2B (top and bottom panel, respectively).
Figure 2. Representative images of partial radiographic responses and pathologic tumor regression following neoadjuvant nintedanib and chemotherapy.
A. Representative CT images at baseline, after 28 days of priming nintedanib dose 0 and post 3 cycles of nintedanib and chemotherapy (expansion phase; upper panels); representative CT images at baseline, and post 3 cycles of combined nintedanib dose −1 plus chemotherapy (run-in phase; lower panels) in patients with PR and pCR or MPR. B. Representative CT images at baseline, after 28 days of priming nintedanib and post 3 cycles of combined nintedanib and chemotherapy (upper panels); representative CT images at baseline, and post 3 cycles of nintedanib dose −1 plus chemotherapy (lower panels) in patients with PR and no MPR. Black arrows indicate primary lung cancer. Dose −1: nintedanib 150 mg PO bid; Dose 0: nintedanib 200 mg PO bid. PR, partial response; pCR, pathologic complete response; MPR, major pathologic response.
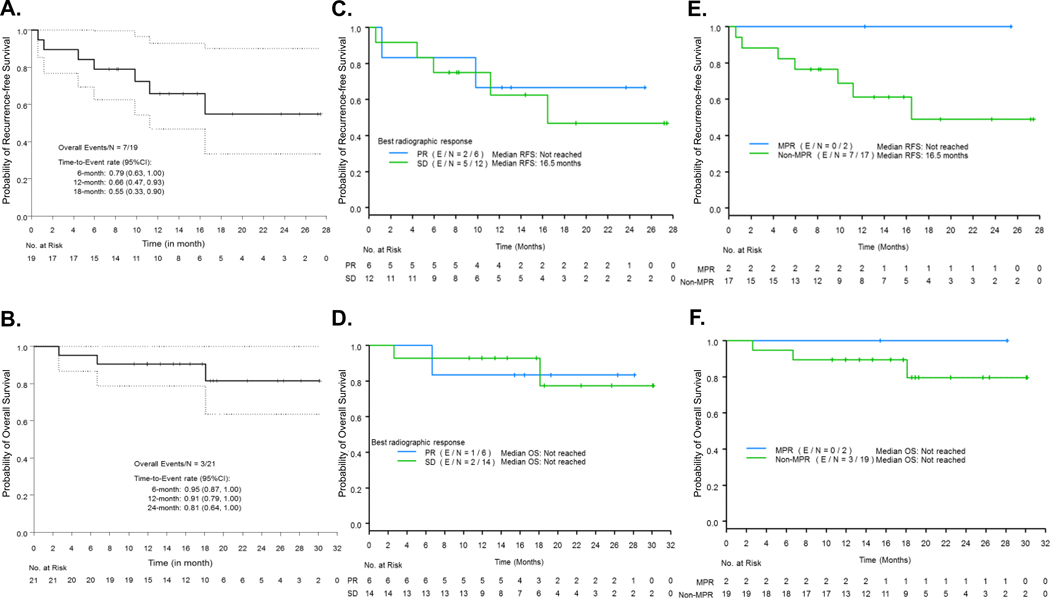
At the median follow up duration of 18.6 months from registration, the probability of 12-month RFS was 66% (CI 47%−93%), with seven recurrences at the time of analysis (7/19, 37%). Two patients were not evaluated for RFS, as they did not receive surgery or definitive radiation, or did not achieve disease-free status (Figure 3A). The 12-months OS was 91% (CI 79%−100%), and three deaths occurred at the time of follow up (3/21, 14%), (Figure 3B). One patient experienced postoperative ARDS and two patients died from distant recurrence.
Figure 3. Kaplan-Meier (KM) curves for probability of RFS and OS in the whole study population and as determined by radiographic response and MPR.
A. KM curves for RFS. Two patients (2/21) were not evaluable for RFS and were not included in the analysis, as they did not receive surgery or definitive radiation therapy, or who received definitive radiation therapy but did not achieve disease-free status. B. KM curves for OS. N: number of patients; CI: confidence interval, RFS: recurrence-free survival, OS: overall survival. C, D. KM curves for RFS (C) and OS (D) according to PR vs. SD as measured by RECIST. Two patients (2/21) were not evaluable for RFS, and were not included in the analysis. E, F. KM curves for RFS (E) and OS (F) according to MPR vs. no MPR. N: number of total patients; E: number of events. PR: partial response, SD: stable disease; MPR: major pathologic response.
Exploratory analyses and immune marker studies
We performed exploratory analyses between the overall best radiographic response evaluated by RECIST (SD versus PR), MPR, and probability of survival outcomes (RFS and OS) without formal statistical comparisons. While radiographic tumor response was not associated with differences in RFS (Figure 3C) nor OS (Figure 3D), MPR was associated with distinct separation of KM curves in RFS (Figure 3E) and OS (Figure 3F). Among 18 patients evaluable for RFS, two of six patients with PR progressed compared to five of 12 patients with SD. Among 20 patients evaluable for OS, one of six patients with PR died as compared to two of 14 with SD. Noteworthy, no PDs nor deaths were observed in the two patients who achieved MPR, whereas, among those individuals who failed to achieve MPR, seven of 17 evaluable patients for RFS progressed or died, and three of 19 patients evaluable for OS progressed or died (Figure 3E and 3F).
The results of correlative analyses comparing immune phenotypes in resected tumors following neoadjuvant chemotherapy and nintedanib in alive patients as compared to those who died and in patients who were alive without recurrence as compared to those who recurred/died (P value cutoff < 0.1) are reported. These exploratory analyses revealed significantly lower levels of CD3+ T cells infiltrating the tumor compartment (median, 61.3 vs. 229 cells/mm2, P = 0.031, Figure 4A), as well as lower levels of total CD3+ T cells infiltrating tumor and stroma compartments (median, 213.4 vs. 652.8 cells/mm2, P = 0.048, Figure 4B) in patients who died as compared to levels in tumors of patients who were still alive at time of follow up. Similarly, significantly lower level of cytotoxic CD3+CD8+ T cells in the stroma compartment (median, 72.11 vs. 348.8 cells/mm2, P = 0.031, Figure 4C) and of total cytotoxic CD3+CD8+ T cells were noted in patients who died (median, 35.6 vs. 142.3 cells/mm2, P = 0.018, Figure 4D) as compared to levels in tumors of patients who were still alive. We observed numerically increased densities of CD3+ T cells, cytotoxic CD3+CD8+ T cells and macrophages (CD68+) in the stroma compartment of tumors resected from patients who were alive without recurrence as compared to that of patients who recurred/died of their disease (CD3+ T cells, median 1702.97 vs. 548.86 cells/mm2, P = 0.097; cytotoxic CD3+CD8+ T cells, median 452.16 vs. 139.96 cells/mm2, P = 0.073; macrophages, median 344.03 cells/mm2 vs. 135.76 cells/mm2, P = 0.097, respectively) (Figure 4E-G). Interestingly, we also noted that the amounts of total CD3+PD-1+ antigen-experienced T cells were lower within the tumor and stroma compartments of tumor tissues resected from patients who had not had disease recurrence as compared to that of patients who recurred/died (median, 0.00 vs. 6.52 mm2 cells/mm2, P = 0.09) (Figure 4H). Taken together, the results of these analyses suggested that higher densities of immune cells, including T cells, cytotoxic T cells and macrophages, and numerically lower levels of antigen-experienced T cells in resected tumor tissues following neoadjuvant nintedanib and chemotherapy are associated with recurrence-free and alive status in this cohort.
Figure 4. Levels of T cell infiltration in tumors of patients treated with neoadjuvant chemotherapy and nintedanib according to survival status.
A. Higher levels of T cells (CD3+) infiltrating the tumor compartment of resected tumors are shown in patients still alive (N = 12) compared to patients who died (N = 3). B. Higher levels of T cells (CD3+) infiltrating tumor and stroma compartments of resected tumors are shown in patients still alive (N = 12) compared to patients who died (N = 3). C. Higher levels of cytotoxic T cells (CD3+CD8+) infiltrating the stroma compartment of resected tumors are shown in patients still alive (N = 12) compared to patients who died (N = 3). D. Higher levels of cytotoxic T cells (CD3+CD8+) infiltrating the tumor and stroma compartments of resected tumors are shown in alive patients (N = 12) compared to patients who died (N = 3). E. Higher levels of T cells (CD3+) infiltrating the stroma compartment of resected tumors are shown in patients still alive without recurrence (N = 7) compared to patients who recurred/died (N = 7). F. Higher levels of cytotoxic T cells (CD3+CD8+) infiltrating the stroma compartment of resected tumors are shown in patients still alive with no recurrence (N = 7) compared to patients who had recurred/died (N = 7). G. Higher levels of macrophages (CD68+) infiltrating the stroma compartment of resected tumors are shown in patients still alive with no recurrence (N = 7) compared to patients who had recurred/died (N = 7). H. Lower levels of antigen-experienced T cells (CD3+PD-1+) infiltrating the tumor and stroma compartments of resected tumors are shown in patients still alive with no recurrence (N = 7) compared to patients who had recurred/died (N = 7). In all panels, data is shown as median, interquartiles, and minimum and maximum values.
Discussion
In this phase 1/2 trial for patients with previously untreated, resectable NSCLC, we demonstrated that the combination regimen of neoadjuvant cisplatin, docetaxel, and nintedanib is safe and feasible. However, we did not observe an improved MPR rate with the addition of nintedanib to the neoadjuvant chemotherapy backbone of cisplatin and docetaxel, and, therefore, we do not recommend additional studies using this agent alone or in combination with chemotherapy in the neoadjuvant setting.
To expedite the investigation of novel compounds in the neoadjuvant setting, we constructed a platform of signal-finding studies testing the addition of novel agents to the existing platinum-docetaxel base paradigm using MPR as primary efficacy endpoint. We have reported that 19% of patients treated with neoadjuvant platinum doublet chemotherapy achieved MPR (10, 11). In this study, nintedanib with platinum doublet chemotherapy was overall safe and feasible, with a historically similar resectability rate of 90% (24), and no delays in surgery. The best response rate by RECIST to neoadjuvant chemotherapy and nintedanib (30%) was slightly lower than the historical controls of neoadjuvant chemotherapy (at least 40%). Previous studies have shown that anti-angiogenic agents can normalize and remodel the tumor vasculature for improved delivery of chemotherapy (16), and that nintedanib may augment the efficacy of chemotherapy in preclinical models of NSCLC (18). The priming phase of neoadjuvant nintedanib alone tested whether tumors would decrease in size via inhibition of receptor tyrosine kinases, degradation of tumor vessels and subsequent tumor necrosis (14), or via direct antitumor activity (17). No responses were noted with nintedanib monotherapy, and two patients with radiographic evidence of PD post nintedanib alone experienced SD after combined nintedanib-chemotherapy regimen as compared to pre-neoadjuvant treatment baseline scans. Similar findings were observed with bevacizumab monotherapy, suggesting ineffectiveness of these drugs as single agent neoadjuvant therapy (25).
Our results highly suggest that MPR may be a better surrogate of outcome as compared to RECIST. Patients with MPR also demonstrated a radiographic PR, however, 50% of patients with radiographic response failed to achieve MPR, and all recurred. This finding agrees with an analysis conducted by our group to determine the relationship between CT-measured response, histopathologic response (≤ 10% viable tumor) and survival outcomes in 160 NSCLC patients treated with neoadjuvant platinum doublet chemotherapy. In that study, we found that histopathologic response was a stronger predictor of OS (P = 0.002), as compared to CT response (P = 0.03), and noted a 41% overall discordance rate between CT response and histopathologic response, indicating that radiographic CT response is not a reliable predictor of survival in NSCLC patients undergoing surgical resection after neoadjuvant chemotherapy (26).
Our trial illustrates the utility of using MPR as the primary endpoint in signal-finding studies for resectable, locally advanced NSCLC. With only 21 treated patients, we are able to recommend against further evaluation of nintedanib in the neo-adjuvant setting. By contrast, the phase 3 E1505 trial assessing the role of adjuvant bevacizumab required 1501 patients, approximately 10 years from activation to reporting, and similarly failed to show any benefit from the VEGF antibody in this setting (27). At the current pace of development of new drugs for advanced NSCLC, it will be challenging to test most promising agents using strategies that require large trials with long-term follow up. The neoadjuvant platform developed by our group may provide an alternative framework to quickly screen drugs that should or should not proceed to testing in later-phase trials. Interestingly, a 44-patient study using chemotherapy plus bevacizumab in the neoadjuvant setting (25) reported an MPR rate of 27% (11/41 resected, 3 unresectable), and might have been able to predict lack of activity of adjuvant bevacizumab in the E1505 study. Although appealing, the neoadjuvant, MPR-based study platform poses additional challenges. The phase III NATCH trial recently reported MPR to be associated with an improved 5-year OS (P = 0.026) in the squamous but not in the non-squamous population (P = 0.586), suggesting that this endpoint might not be applicable to all situations, histologies, or drugs (28).
The landscape of lung cancer therapy has been evolving towards immunotherapy and the success of immune checkpoint blockade expanded from metastatic to locally advanced/unresectable and, most recently, to the neoadjuvant setting. In the first reported study of 21 patients (stage IB-IIIA) treated with two doses of nivolumab followed by surgery, authors observed a MPR of 45% (9/20) with a radiographic response rate of only 10% (2/21) (29). In the randomized phase 2 trial NEOSTAR (NCT03158129), which evaluated neoadjuvant nivolumab and nivolumab plus ipilimumab in resectable NSCLC patients using MPR as primary endpoint, the MPR rate in the intention-to-treat population was 17% and 33%, respectively (30). Based on the study design, these results suggested the combination regimen to be promising for further evaluation, facilitating the rapid assessment of novel combination therapies in the neoadjuvant setting. Highly encouraging results have been recently reported on the role of neoadjuvant chemo-immunotherapy for patients with resectable NSCLC. Shu and colleagues reported a MPR rate of 50%, including three patients with pCR (21%) in 14 patients with resected NSCLC following neoadjuvant atezolizumab combined with chemotherapy (NCT02716038) (31). In the NADIM study (NCT03081689), the authors (32) evaluated neoadjuvant combination platinum-based chemotherapy plus nivolumab given in three cycles to patients with stage IIIA NSCLC followed by surgical resection. In 89% (41/46) of resected patients, authors reported 83% (34/41) MPR rate, of which 59% (24/41) achieved pCR. In our cohort of patients treated with nintedanib plus platinum doublet chemotherapy, the probability of 12-month RFS (66%) and 12-months OS (91%) were overall similar to the survival rates observed and/or calculated following neoadjuvant platinum chemotherapy historical controls in our institution (DFS 79.7%; OS 85.3% (10) and DFS 67.8%; OS 81.6% (33)), as well as in larger cohorts of patients treated with neoadjuvant chemotherapy (PFS 68%; OS 82% SWOG9900 Study) (24). As a comparison, the initial clinical experiences with neoadjuvant immunotherapy reported slightly higher short-term survival rates (calculated 12-month RFS 83%, and 24-month RFS 69% following neoadjuvant monotherapy (34); 12-month PFS 98%, and 12-month OS 96% following neoadjuvant nivolumab plus chemotherapy (32)), suggesting, perhaps, a potentially improved benefit from neoadjuvant immune-based therapies over chemotherapy on survival outcomes. It remains to be seen whether these results will be validated by other studies and whether MPR to immunotherapy-based neoadjuvant strategies will correlate with favorable long-term outcomes.
The results of our correlative analyses comparing the levels of immune markers expressed in tumors resected following neoadjuvant chemotherapy and nintedanib revealed that T cells, including cytotoxic T cells, are less abundant in tumors of patients who died, as well as in tumors of patients who experienced disease recurrence compared to those of patients who were still alive, and those who had not recurred, respectively. These findings are in agreement with existing reports suggesting that neoadjuvant chemotherapy is associated with increased levels of CD3+ T cells in resected NSCLCs, and that patients who received neoadjuvant chemotherapy and had higher density of helper T cells in their resected tumors had improved OS (19). It remains to be determined the specific modulatory role of nintedanib in reducing the immunosuppressive tumor microenvironment as compared to neoadjuvant chemotherapy in resected NSCLC samples, and this is the focus of future studies. These results confirm previously reported findings, however, given the early closure of the study for futility, have limited prognostic value. While the power of our exploratory analyses is limited and could change as clinical outcome data mature, we view the results of these exploratory studies as hypothesis-generating and not the primary conclusion of our study. The primary conclusion of the study regarding the impact of neoadjuvant chemotherapy plus nintedanib on MPR is mature.
The main limitation of this study is its small sample size. Given the lower than expected pathologic response rates, the study was terminated due to the lack of treatment efficacy. Whether the lower than expected MPR rate of 6.7% with nintedanib as compared to 15–19% MPR in historical controls of chemotherapy alone or 22% MPR with chemotherapy plus an anti-angiogenic agents is due to chance alone or other reasons is presently unknown. Although pre-treatment testing of driver mutations in surgically resectable tumors is not considered the standard of care at the moment, it is possible that the responses to our neoadjuvant combination therapy were influenced by unique tumor mutations or microenvironments with unfavorable, yet untested, features for anti-angiogenic therapy. We did not observe a significant association between histology and MPR or radiographic response, and the small sample size of our study did not allow for further investigation of the associations between MPR and other baseline patient characteristics. Also, the limited number of responding patients (with only two MPRs overall, and no patients responding to priming nintedanib monotherapy by RECIST) precluded initially planned biomarker discovery and larger exploratory analyses. Testing of additional exploratory biomarkers (e.g. circulating tumor DNA or fecal microbiome) could have shed light on the reasons why the responses to this regimen are lower than expected; however, at the initiation of this study these tests were not as readily available as they are today.
In conclusion, nintedanib combined with platinum doublet chemotherapy did not increase MPR compared to historical controls of neoadjuvant chemotherapy alone, and, therefore, further studies with this regimen are not recommended. This finding allows us to move on to testing alternative classes of agents either alone or in combination with chemotherapy, corroborating a major advantage of the neoadjuvant platform study design. By utilizing MPR as a surrogate endpoint of therapeutic efficacy, we continue to support this framework to rapidly test novel compounds that should be investigated further as novel neoadjuvant regimens in resectable NSCLC, which we have now expanded to include immunotherapy combinations.
Supplementary Material
Statement of Translational Significance.
In this study, we assessed the safety and efficacy of nintedanib, a multitargeted angiokinase inhibitor, plus neoadjuvant chemotherapy in patients with resectable non-small cell lung cancer (NSCLC) using major pathologic response (MPR) as surrogate of clinical efficacy. The goal of the study was to demonstrate that neoadjuvant nintedanib and chemotherapy induce higher rates of MPR compared to historical controls of neoadjuvant chemotherapy alone. Although overall well tolerated, neoadjuvant nintedanib plus chemotherapy did not increase the MPR rate compared to historical controls of chemotherapy alone, leading to discontinuation of the study for futility. The use of MPR as a surrogate marker for efficacy after neoadjuvant therapy allowed the rapid investigation of nintedanib in the neoadjuvant setting and halted further studies evaluating this agent in the preoperative setting.
Acknowledgments
Funding/Support: We thank the patients and their families who participated in the reported research; all members of our clinical research and regulatory teams for their assistance. Support for the study was partially provided by Boehringer Ingelheim, the Lung SPORE grant 5 P50 CA070907, the NIH/NCI P30 CA016672 Cancer Center Support Grant (CCSG New Faculty Award), the Conquer Cancer Foundation of the American Society of Clinical Oncology (ASCO) Career Development Award 2018, and the Bruton Endowed Chair in Tumor Biology. The study was also partially supported by the generous philanthropic contributions to the University of Texas MD Anderson Cancer Center Lung Cancer Moon Shot Program, the University of Texas MD Anderson Cancer Center CG Johnson Foundation Advanced Scholar Program Funds and Khalifa Scholars Program (from Khalifa Bin Zayed Al Nahyan Foundation), the University of Texas MD Anderson Cancer Center Physician Scientist Program (T.J. Martell Foundation), and the Bob Mayberry Foundation.
Footnotes
Conflicts of Interests: T.C. reports speaker’s fees from Society for Immunotherapy of Cancer (SITC) and Bristol-Myers Squibb, consulting fees from MedImmune/AstraZeneca and Bristol-Myers Squibb, and advisory role fees from EMD Serono and Bristol-Myers Squibb. B.S. reports consulting fees from Bristol-Myers Squibb. M.C.B.G. has received research funding from Siemens Healthcare. A.T. reports research funding from and advisory role for Boehringer Ingelheim. D.G. reports honoraria for scientific advisory boards from AstraZeneca, Sanofi, Alethia Biotherapeutics and Janssen, research support from Janssen, Takeda, Ribon Therapeutics and AstraZeneca. W.N.W. reports consulting or advisory role fees from Clovis Oncology and AstraZaneca, speaker’s fees from Boehringer Ingelheim, honoraria from Roche/Genentech, AstraZaneca, Boehringer Ingelheim, Bristol-Myers Squibb, Merck; research funding from OSI Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, and Merck. J.V.H. reports consulting fees and advisory role fees from Bristol-Myers Squibb, AstraZeneca, Merck, Genentech, EMD Serono, Boehringer Ingelheim, Spectrum, Lilly, Novartis, GSK, and Pfizer. The remaining authors have no conflicts of interest to report.
References
- 1.Goldstraw P, Crowley J, Chanksy K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: Proposals for the revision of the TNM stage groupings in the forthcoming (Seventh) edition of the TNM classification of malignant tumors (vol 8, pg 706–714, 2007). Journal of Thoracic Oncology. 2007;2(10):985-. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(21):3552–9. [DOI] [PubMed] [Google Scholar]
- 3.Group NM-aC. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383(9928):1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felip E, Rosell R, Maestre JA, Rodriguez-Paniagua JM, Moran T, Astudillo J, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(19):3138–45. [DOI] [PubMed] [Google Scholar]
- 5.McElnay P, Lim E. Adjuvant or neoadjuvant chemotherapy for NSCLC. J Thorac Dis. 2014;6 Suppl 2:S224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junker K, Thomas M, Schulmann K, Klinke F, Bosse U, Muller KM. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol. 1997;123(9):469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Junker K, Langner K, Klinke F, Bosse U, Thomas M. Grading of tumor regression in non-small cell lung cancer : morphology and prognosis. Chest. 2001;120(5):1584–91. [DOI] [PubMed] [Google Scholar]
- 8.Liu-Jarin X, Stoopler MB, Raftopoulos H, Ginsburg M, Gorenstein L, Borczuk AC. Histologic assessment of non-small cell lung carcinoma after neoadjuvant therapy. Mod Pathol. 2003;16(11):1102–8. [DOI] [PubMed] [Google Scholar]
- 9.Yamane Y, Ishii G, Goto K, Kojima M, Nakao M, Shimada Y, et al. A novel histopathological evaluation method predicting the outcome of non-small cell lung cancer treated by neoadjuvant therapy: the prognostic importance of the area of residual tumor. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2010;5(1):49–55. [DOI] [PubMed] [Google Scholar]
- 10.Pataer A, Kalhor N, Correa AM, Raso MG, Erasmus JJ, Kim ES, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2012;7(5):825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cascone T, Gold KA, Swisher SG, Liu DD, Fossella FV, Sepesi B, et al. Induction Cisplatin Docetaxel Followed by Surgery and Erlotinib in Non-Small Cell Lung Cancer. Ann Thorac Surg. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellmann MD, Chaft JE, William WN, Jr., Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–55. [DOI] [PubMed] [Google Scholar]
- 14.Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer research. 2008;68(12):4774–82. [DOI] [PubMed] [Google Scholar]
- 15.Ackermann M, Kim YO, Wagner WL, Schuppan D, Valenzuela CD, Mentzer SJ, et al. Effects of nintedanib on the microvascular architecture in a lung fibrosis model. Angiogenesis. 2017;20(3):359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–27. [DOI] [PubMed] [Google Scholar]
- 17.Hilberg F, Tontsch-Grunt U, Baum A, Le AT, Doebele RC, Lieb S, et al. Triple Angiokinase Inhibitor Nintedanib Directly Inhibits Tumor Cell Growth and Induces Tumor Shrinkage via Blocking Oncogenic Receptor Tyrosine Kinases. J Pharmacol Exp Ther. 2018;364(3):494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilberg F, Brandstetter I. C7–03: Efficacy of BIBF 1120, a potent triple angiokinase inhibitor, in models of human non-small cell lung cancer is augmented by chemotherapy. Journal of Thoracic Oncology. 2007;2(8):S380. [Google Scholar]
- 19.Parra ER, Villalobos P, Behrens C, Jiang M, Pataer A, Swisher SG, et al. Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer. 2018;6(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakabayashi O, Yamazaki K, Oizumi S, Hommura F, Kinoshita I, Ogura S, et al. CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Sci. 2003;94(11):1003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stack EC, Foukas PG, Lee PP. Multiplexed tissue biomarker imaging. J Immunother Cancer. 2016;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woolson RaC WR. . Statistical Methods for the Analysis of Biomedical Data. . 2nd Edition, John Wiley & Sons, New York.; 2002. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 24.Pisters KM, Vallieres E, Crowley JJ, Franklin WA, Bunn PA Jr., Ginsberg RJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(11):1843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaft JE, Rusch V, Ginsberg MS, Paik PK, Finley DJ, Kris MG, et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous non-small-cell lung cancers. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8(8):1084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.William WN Jr., Pataer A, Kalhor N, Correa AM, Rice DC, Wistuba II, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8(2):222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakelee HA, Dahlberg SE, Keller SM, Tester WJ, Gandara DR, Graziano SL, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2017;18(12):1610–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remon J, Martinez-Marti A, Carcereny Costa E, Zeron-Medina Cuairan J, Sansano I, Mate JL, et al. 1278PDMajor pathological response after preoperative chemotherapy as a surrogate marker of survival in early-stage non-small cell lung cancer: cohort of NATCH phase III trial. Annals of Oncology. 2017;28(suppl_5):mdx381.004-mdx381.004. [Google Scholar]
- 29.Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. The New England journal of medicine. 2018;378(21):1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cascone T, William WN, Weissferdt A, Lin HY, Leung CH, Carter BW, et al. Neoadjuvant nivolumab (N) or nivolumab plus ipilimumab (NI) for resectable non-small cell lung cancer (NSCLC): Clinical and correlative results from the NEOSTAR study. Journal of Clinical Oncology. 2019;37(15_suppl):8504-. [Google Scholar]
- 31.Shu CA, Grigg C, Chiuzan C, Garofano RF, Patel V, Hernandez S, et al. Neoadjuvant atezolizumab + chemotherapy in resectable non-small cell lung cancer (NSCLC). Journal of Clinical Oncology. 2018;36(15_suppl):8532-. [Google Scholar]
- 32.Provencio M, Nadal E, Insa A, Campelo RG, Casal J, Domine M, et al. OA13.05 NADIM Study: Updated Clinical Research and Outcomes. Journal of Thoracic Oncology. 2019;14(10):S241. [Google Scholar]
- 33.Cascone T, Gold KA, Swisher SG, Liu DD, Fossella FV, Sepesi B, et al. Induction Cisplatin Docetaxel Followed by Surgery and Erlotinib in Non-Small Cell Lung Cancer. Ann Thorac Surg. 2018;105(2):418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuss JE, Smith KN, Anagnostou V, Zhang J, Zahurak M, Caushi J, et al. Neoadjuvant nivolumab in resectable non-small cell lung cancer: Extended follow-up and molecular markers of response. Journal of Clinical Oncology. 2019;37(15_suppl):8524-. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.