Abstract
Subunit 2 of DNA damage-binding protein complex (DDB2) is an early sensor of nucleotide excision repair (NER) pathway for eliminating DNA damage induced by UV radiation (UVR) and cisplatin treatments of mammalian cells. DDB2 is modified by ubiquitin and poly(ADP-ribose) (PAR) in response to UVR, and these modifications play a crucial role in regulating NER. Here, using immuno-analysis of irradiated cell extracts, we have identified multiple post-irradiation modifications of DDB2 protein. Interestingly, although the DNA lesions induced by both UVR and cisplatin are corrected by NER, only the UV irradiation, but not the cisplatin treatment, induces any discernable DDB2 modifications. We, for the first time, show that the appearance of UVR-induced DDB2 modifications depend on the binding of DDB2 to the damaged chromatin and the participation of functionally active 26S proteasome. The in vitro and in vivo analysis revealed that SUMO-1 conjugations comprise a significant portion of these UVR-induced DDB2 modifications. Mapping of SUMO-modified sites demonstrated that UVR-induced SUMOylation occurs on Lys-309 residue of DDB2 protein. Mutation of Lys-309 to Arg-309 diminished the DDB2 SUMOylation observable both in vitro and in vivo. Moreover, K309R mutated DDB2 lost its function of recruiting XPC to the DNA damage sites, as well as the ability to repair cyclobutane pyrimidine dimers following cellular UV irradiation. Taken together, our results indicate that DDB2 is modified by SUMOylation upon UV irradiation, and this post-translational modification plays an important role in the initial recognition and processing of UVR-induced DNA damage occurring within the context of chromatin.
Introduction
Nucleotide excision repair (NER) is a versatile DNA repair pathway that removes a wide variety of DNA lesions, including cyclobutane pyrimidine dimers (CPDs) and 6–4 pyrimidine pyrimidone photoproducts (6–4PPs) induced upon UV irradiation, as well as cisplatin-induced intra-strand crosslinks and Benzo(a)pyrene diol-epoxide-induced bulky DNA adducts in living cells (1). The mammalian NER pathway utilizes more than 30 proteins to recognize DNA damage and its processing within the context of chromatin, which include the removal of a 24–32-nucleotide-long single-stranded DNA containing the lesion, synthesize a new fragment of DNA using the undamaged strand as a template, and finally ligate the nicked segment. Subunit 2 of DNA damage-binding protein complex (DDB2) is a key damage recognition sensor in the global genomic sub-pathway of NER to help initiate the entire damage removal squeal following cellular UV irradiation. Xeroderma pigmentosum complimentary group E cells lacking the functional DDB2 are deficient in repairing CPDs but are competent to repair 6-4PPs, albeit at a reduced rate (2,3). Notably, however, DDB2 was not found to be essential in the in vitro constituted NER system (4,5), suggesting that DDB2 is especially important for the repair of UV-damaged DNA occurring within the chromatin context.
Protein post-translational modifications via members of the ubiquitin family, e.g. ubiquitin itself and small ubiquitin-related modifier (SUMO), have been identified as key contributors for the maintenance of genome stability (6). Ubiquitin and SUMO can rapidly and reversibly change the properties, stability or localization of their target proteins without the need for de novo protein synthesis, making them ideal regulators for fine-tuning of DNA repair and damage response pathways (6,7). In fact, ever-increasing evidence demonstrates that protein ubiquitylation and SUMOylation play critical role in various DNA repair pathways (7,8).
DDB2 participates in the recognition of UV radiation (UVR)-induced CPD as a subunit of the E3 ubiquitin ligase complex CRL4–DDB2 (9,10). The CRL4-DDB2 complex ubiquitylates DDB2 itself at the damage site to facilitate its degradation (11–13), which promotes the subsequent recruitment of XPC for damage recognition and verification (13). The XPC protein accumulated at the damage sites is in turn also ubiquitylated by CRL4-DDB2 complex to enhance the binding of XPC to UV-damaged DNA (14). Moreover, this same E3 ligase also ubiquitylates histone H2A, H3 and H4 to affect nucleosome stability and chromatin remodeling (15,16), supporting the function of DDB2 in the removal of CPD in the context of chromatin (4,5,17).
In addition to UVR-induced ubiquitylation, DDB2 is reported to also get modified by PAR in response to UV irradiation (18,19). DDB2 associates with PAR polymerase-1 (PARP-1) in the vicinity of UV-damaged chromatin, stimulates its catalytic activity, and is modified by PAR (18). PARylation of DDB2 is suggested to regulate the stability and the chromatin retention time of DDB2 to facilitate the CPD repair (18,19).
In this study, we have systematically revealed the nature of DDB2 modifications as a very early response event, and their occurrence only upon UV irradiation, but not the cisplatin exposure. DDB2 must be physically recruited to UVR-damaged sites to enable its modification in a process that also requires the participation of functionally active 26S proteasome. The distinct multiple DDB2 modifications upon cellular UV irradiation include the DDB2 SUMOylation, at K309 residue, which plays a critical role in mediating the UVR-induced recruitment of XPC to damage site and the repair of UVR-induced CPD.
Materials and methods
Cell culture and treatment
HeLa cell line with over-expressed FLAG and HA-tagged DDB2 (HeLa-DDB2 cells) was a gift from Dr Yoshihiro Nakatani (Dana-Farber Cancer Institute, Boston, MA). Li-Fraumeni syndrome fibroblast 041 cell line was provided by Dr Michael Tainsky (MD Anderson Cancer Center, Houston, TX). All cell lines were grown in DMEM supplemented with 10% fetal calf serum and antibiotics at 37°C in a humidified atmosphere of 5% CO2. For overall UVR exposure, the cells were washed with PBS, irradiated with varying UVR doses and incubated in suitable medium for the desired time period. The irradiation was performed with a germicidal lamp at a dose rate of 1 J/m2/s as measured by a Kettering model 65 radiometer (Cole Palmer Instrument Co., Vernon Hill, IL). For cisplatin treatment, cells were maintained in medium with the desired doses of freshly prepared cisplatin (Sigma, St. Louis, MO) for 1 h, then washed with PBS and followed by incubation in fresh drug-free medium for varying times of post-treatment.
Plasmids, antibodies and reagents
Plasmids encoding N-terminal FLAG-tagged wild-type DDB2 (DDB2-WT) and mutant DDB2-K244E have been described previously (20). The DDB2-WT plasmid was also used to generate the point mutant of DDB2 by using QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The expression vector for HA-tagged ubiquitin was obtained from Dr Dirk Bohmann (European Molecular Biology Laboratory, Heidelberg, Germany). The plasmids were transfected into cells either by FuGene 6 (Roche, Indianapolis, IN) or by Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacture’s instruction. Proteasome proteolysis inhibitors, MG132 and Lactacystin, were purchased from EMD Millipore (La Jolla, CA. Final concentration, 10 μM). Anti-FLAG M2 Magnetic Beads were purchased from Sigma (St. Louis, MO). Goat anti-DDB2 (R&D Systems, Minneapolis, MN. 1:2000) was used for immunoblotting, mouse anti-DDB2 (Abcam, Cambridge, MA, 1:50) was used for immunofluorescence, mouse anti-Actin was purchased from Santa Cruz Biotechnology (Dallas, TX), rabbit anti-CPD (UV-2, generated in our laboratory; 1:1000) has been described previously (21,22), mouse anti-HA antibody was purchased from Roche Applied Science (Indianapolis, IN, 1:1000) and rabbit anti-SUMO-1 and rabbit anti-SUMO-2/3 were purchased from Cell Signaling Technology (Danvers, MA, 1:1000). In vitro SUMOylation was conducted using SUMOlink Kit (Active Motif, Carlsbad, CA).
Purification of DDB2 protein
Recombinant FLAG-tagged DDB2 was purified from Sf9 insect cells by one-step FLAG affinity purification as described earlier (23). Briefly, FLAG-tagged DDB2 baculovirus was generated using the Bac-to-Bac Baculovirus Expression System (Invitrogen), and used to infect Sf9 cells. After culturing for 2–3 days at 28°C, the cell pellet was lysed with buffer A (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, and 0.5% Triton X-100, protease inhibitor cocktail) for 10 min at 4°C. After centrifugation for 10 min at 900g at 4 °C, the pellet was lysed in buffer C (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.9, 1.5 mM MgCl2, 0.42 M NaCl, 25% v/v glycerol, 0.2 mM ethylenediaminetetraacetic acid, 0.5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail). The cleared lysate was incubated overnight with anti-FLAG M2 Magnetic Beads (Sigma) at 4°C. The beads were washed with buffer C four times, eluted with 3× FLAG peptide (Sigma) prepared in 50 mM Tris–HCl (pH 8.0), 150 mM NaCl. The eluate was concentrated and desalted with Amicon® Ultra 10K device (Millipore, Billerica, MA).
To purify DDB2 from cultured mammalian cells, FLAG-tagged DDB2-expressing plasmids were transfected into HeLa cells for 2 days. Cell lysates were prepared with RIPA buffer (50 mmol/l Tris–HCl, pH 7.4, 1% NP40, 0.25% sodium deoxycholate, 1 mmol/l ethylenediaminetetraacetic acid and 150 mmol/l NaCl), and incubated overnight with anti-FLAG M2 Magnetic Beads at 4°C. The beads were washed four times with RIPA buffer. DDB2 protein was eluted with 3× FLAG peptide.
Immunoprecipitation and immunoblotting
Immunoprecipitation was performed under denaturing conditions to detect DDB2 modifications. Whole cell lysates were prepared by direct boiling of cells in sodium dodecyl sulfate (SDS) lysis buffer (62 mM Tris–HCl pH 6.8, 2% SDS, 10% glycerol, 10 mM dithiothreitol and protease inhibitor cocktail). One milligram of total protein was further diluted 10-fold with RIPA buffer, incubated with anti-FLAG M2 Magnetic Beads overnight at 4°C. The beads were washed four times with RIPA buffer, and then boiled in 2× SDS sample buffer. The immunoprecipitates were then subjected to 8–16% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and the specific proteins were detected by immunoblotting with desired antibodies, as described above.
Fractionation of cellular proteins
Fractionation of cellular proteins was conducted as described previously (24). Briefly, the cells were trypsinized and resuspended in hypotonic buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.9), 10 mM KCl, 1.5 mM MgCl2, and protease inhibitor cocktail] and treated with 1% Triton X-100 to isolate nuclei. The nuclear pellet was then further extracted consecutively with increasing concentrations (0.3, 0.5 and 2.0 M) of NaCl to result in supernatant fractions, respectively, designated as 0.3, 0.5 and 2.0. Each protein fraction, corresponding to an equivalent cell number, was loaded for SDS–PAGE and analyzed by immunoblotting with indicated antibodies.
Local UV irradiation and immunofluorescence staining
Cells growing on glass coverslips were washed with PBS and UV irradiated as described previously (22). Briefly, an isopore polycarbonate filter (Millipore, Bedford, MA), containing pores of 5 μm in diameter, was placed on top of the cell monolayer. The filter-covered coverslips were irradiated from above with UV-C (254 nm) at 100 J/m2. The filter was then gently removed and the cells were incubated for 0.5 h followed by fixation and permeabilization with 2% paraformaldehyde and 0.5% Triton X-100 in PBS. The cells were then double stained with mouse anti-DDB2 and rabbit anti-CPD antibodies, or rabbit anti-XPC and mouse anti-CPD antibodies, as described previously (22). Fluorescence images were obtained with a Nikon Fluorescence Microscope 80i (Nikon, Tokyo, Japan) fitted with appropriate filters for FITC and Texas red. The digital images were then captured with a cooled charge-coupled device camera and processed with the help of its SPOT software (Diagnostic Instruments, Sterling Heights, MI).
In vitro SUMOylation
A 0.5 μg purified sample of FLAG-DDB2 was incubated with WT or mutant SUMO-1 for 3 h at 30°C in a 20 μl complete reaction system (SUMOlink SUMO-1 Kit). All the components were added in the order as instructed by the manual. The reaction was stopped by adding 20 μl of 2× SDS loading buffer, and boiled for 3 min. A 10 μl of this sample was subjected to SDS–PAGE and the membrane was probed with rabbit anti-SUMO-1 antibody (provided in the kit). The membrane was then stripped, and re-probed with goat anti-DDB2 antibody.
Immuno-slot blot (ISB) analysis of DNA repair
The ISB assay was conducted as described previously (25). Briefly, genome DNA was isolated from UV irradiated cells and quantitated. Equal amounts of DNA were loaded onto nitrocellulose membranes, and the amounts of CPD were determined with monoclonal anti-CPD antibody (Cosmo Bio USA, Inc, Carlsbad, CA). Membranes were stripped and re-blotted with mouse anti-single strand DNA (ssDNA) antibody (Millipore) to serve as a loading control. The intensity of each band was quantified with ImageJ software.
Statistical analysis
Results are presented as the mean ± standard deviation. Statistical differences were determined by using two sample t-test for independent samples. P < 0.05 was considered statistically significant.
Results
DDB2 is modified upon UV irradiation
To visualize and characterize the modifications of DDB2 upon introduction of DNA damage, HeLa cells, with or without stable expression of FLAG-tagged DDB2, were subjected to UV irradiation and cultured further for different time periods. The ectopically expressed FLAG-tagged DDB2 was immunoprecipitated under denaturing conditions, and the immunoprecipitates were probed with anti-DDB2 antibody. As shown in the control lanes of Figure 1A, no DDB2 was recovered with anti-FLAG Magnetic Beads from extracts of HeLa cells. In contrast, DDB2 was prominently detected in the immuno-complexes recovered from HeLa-DDB2 cells. More importantly, several DDB2 antibody-reactive species, which migrated slower than the main DDB2 band, were also visualized in UV irradiated samples. The appearance of these modified DDB2 bands peaked at ~20 min and started to decrease at 60 min after UV irradiation. Moreover, modified DDB2 bands were formed very early and could be detected at <5 min post-UVR even with a dose as low as 10 J/m2 (Figure 1B). However, when cells were treated with cisplatin, such DDB2 modifications could not be detected (Figure 1C). It is obvious that while the DNA lesions induced by both UVR and cisplatin exposures are repaired by the NER pathway, only the UV irradiation, but not the cisplatin treatment, provokes the modifications of DDB2.
Figure 1.
DDB2 is modified upon UV irradiation but not cisplatin treatment. (A and B) Time- and dose-dependent modification of DDB2. HeLa cells and HeLa cells stably transfected with FLAG-HA-tagged DDB2 (HeLa-DDB2 cells) were UV irradiated and further cultured for the indicated time periods. Whole cell lysates were prepared by boiling in SDS lysis buffer for 10 min, and subjected to immunoprecipitation with anti-FLAG M2 Magnetic beads. The immunoprecipitates were resolved by SDS–PAGE and analyzed with anti-DDB2 antibody. *A non-specific band. (C) Failure of DDB2 modification by cisplatin treatment. HeLa-DDB2 cells were treated with UV at 100 J/m2, or cisplatin at 80 μM for 1 h and further cultured for 20 or 60 min. Immunoprecipitation and immunoblotting were conducted as above.
UVR-induced DDB2 modifications require the binding of DDB2 to damaged chromatin
We have previously established that DDB2 is not required for the repair of cisplatin-induced DNA damage (26), but it is critical to the repair of UVR-induced CPD by directly interacting with the lesions (24). Given that the UVR, but not the cisplatin treatment, induced the modifications of DDB2, we hypothesized that the interaction between DDB2 and DNA damage could be an essential prerequisite for provoking modifications of DDB2. By utilizing a sequential salt extraction technique, we have demonstrated that UV irradiation results in a tight association of DDB2 with damaged chromatin (24), also evidenced here by a pronounced decrease in the amount of DDB2 in the low-salt (0.3 M) fraction and a corresponding increase in the amount of DDB2 in the high-salt (2.0 M) fraction (Figure 2A, lanes 4–6 versus lanes 1–3). We also analyzed the redistribution of DDB2 within nuclei upon cisplatin treatment of cells. Contrary to UVR-initiated translocation, cisplatin treatment was unable to cause a change in the distribution of DDB2 in the nucleus (Figure 2A, lanes 7–9). This result indicates that DDB2 does not bind to cisplatin-damaged chromatin. To further confirm the importance of DNA damage-binding capacity of DDB2 in UVR-induced DDB2 modifications, we analyzed the UVR-induced modifications of a mutated DDB2 (K224E), which is known to lack the capacity for binding to UVR-damaged DNA (27,28). Thus, WT and K244E mutant DDB2 constructs were separately transfected into DDB2-deficient 041 cells. The transiently transfected cells were then UV irradiated and analyzed at 20 min post-treatment for the characteristic modified bands. Immunoprecipitation of denatured cell lysates revealed the typical DDB2 modifications in DDB2-WT transfected cells, but not in DDB2-K244E transfected cells (Figure 2B). The combined data indicate that UVR-induced modifications of DDB2 are dependent on the translocation and tighter binding of DDB2 to damaged DNA.
Figure 2.
UVR-induced modifications of DDB2 depend on its DNA-binding capacity. (A) Analysis of DDB2 translocation within chromatin following UVR and cisplatin treatments. HeLa cells were exposed to UVR (20 J/m2) or cisplatin (80 μM, 1 h) and further cultured for 30 min. The protein fractions were prepared on the basis of binding resistance to various concentrations of NaCl (0.3M, 0.5M and 2.0M). Individual protein fractions, corresponding to equivalent cell numbers, were subjected to immunoblotting with anti-DDB2 and anti-Actin antibodies. (B) Analysis of UVR-induced modifications of WT and mutated DDB2. DDB2-deficient 041 cells were stably transfected with FLAG-tagged DDB2-Wt or DDB2-K244E (which is unable to bind damaged DNA), UV irradiated and further cultured for 20 min. DDB2 was immunoprecipitated with anti-FLAG M2 Magnetic beads and detected with anti-DDB2 antibody.
UVR-induced DDB2 modifications require the function of 26S proteasome
The most common modification of DDB2 upon UV irradiation is the ubiquitylation, and inhibition of the 26S proteasome is often used to enrich the cellular ubiquitylated proteins for their easy detection. Hence, we treated HeLa-DDB2 cells with proteasome inhibitor MG132 and analyzed the UVR-induced DDB2 modifications upon immunoprecipitation and immunoblotting. Surprisingly, MG132 failed to cause the typical enrichment of the modified DDB2. Instead, the UVR-induced modifications of DDB2 were actually inhibited in the presence of MG132 (Figure 3A). A similar result was obtained when cells were treated with another 26S proteasome-specific inhibitor, lactacystin (Figure 3B). These data suggest that the proteolytic function of 26S proteasome is required for UVR-induced DDB2 modifications. As indicated above, UVR-induced DDB2 modifications also need DDB2 to tightly bind to damaged DNA. Hence, it could be reasoned that inhibition of the 26S proteasome function prevents the tight binding of DDB2 to UV-damaged chromatin and, therefore, leads to a failure in modifying untargeted DDB2. To test this hypothesis, we first analyzed the redistribution of DDB2 to UVR-damaged chromatin in the presence and absence of MG132 by using the sequential salt extraction described above. As shown in Figure 3C, MG132 treatment did not affect the redistribution of DDB2 to high salt-resistant chromatin fraction upon UV irradiation. Next, we analyzed the effect of MG132 on the recruitment of DDB2 to UV-damaged sites by utilizing local UV irradiation and immunofluorescence, and found that MG132 treatment again did not affect the recruitment of DDB2 to CPD sites (Figure 3D and E). These results indicate that 26S proteasome is not necessary for the initial binding of DDB2 to CPD. Thus, 26S proteasome must play a role in UVR-induced DDB2 modifications only when DDB2 is fully recruited and physically resides at the damage sites.
Figure 3.
UVR-induced modifications of DDB2 require the function of 26S proteasome. (A and B), Effect of proteasome inhibitors on UVR-induced DDB2 modifications. HeLa-DDB2 cells were pre-treated with 26S proteasome inhibitors MG132 (A) or lactacystin (B) for 1 h, UV irradiated and further cultured for 20 min. DDB2 was immunoprecipitated with anti-FLAG M2 Magnetic beads and detected with anti-DDB2 antibody. (C–E) Effect of MG132 on translocation of DDB2 to UV-damaged chromatin. HeLa-DDB2 cells were pre-treated with MG132 for 1 h, UV irradiated and further cultured for 30 min. Cells were subjected to cellular fractionation with various concentrations of NaCl, and the distribution of DDB2 in nuclei was analyzed by immunoblotting with anti-DDB2 antibody (C). Cells growing on coverslips were UV irradiated through a membrane with micropores and further cultured for 30 min. Immunofluorescence staining was performed to determine the extent of co-localization of DDB2 and CPD (D). The ratio of DDB2 to CPD foci was determined upon scoring >100 individual nuclei in three visual fields for merged fluorescent signals of DDB2 and CPD foci (E).
Early UVR-induced modifications include the SUMOylation of DDB2
As 26S proteasome inhibition did not enrich UVR-induced rapid DDB2 modifications (Figure 3A), we speculated that these modifications are not in fact ubiquitylated forms. To test this prediction, we co-transfected FLAG-tagged DDB2 and HA-tagged ubiquitin into DDB2-deficient 041 cells, UV irradiated and cultured the cells further for various time periods in the absence or presence of MG132. The immunoprecipitates recovered by anti-FLAG antibody were probed with either anti-DDB2 antibody to view the modified DDB2 or anti-HA antibody to view the ubiquitylated DDB2. As shown in Figure 4A (left panel), in the absence of MG132 we failed to detect modified DDB2 analyzed up to 120 min Post-UVR. In contrast, hallmark smears of DDB2 species were readily detectable at 120 min after UV irradiation in the presence of MG132, and the pattern of this DDB2 modification was different from that at 20 min post-UVR. As expected, in the absence of MG132, DDB2 can be seen to promptly degrade following UV irradiation (Figure 4A, left lower panel). Next, we stripped this membrane and re-probed with anti-HA antibody to visualize the ubiquitylated DDB2. Consistent with previous report (11), ubiquitylated DDB2 was detected only in MG132-treated cells (Figure 4A, right panel). Thus, we concluded that the modifications of DDB2 observed at early times after UV irradiation do not represent the ubiquitylation.
Figure 4.
UVR-induced DDB2 modifications include SUMO-1 conjugation. (A) Identification of the modification of DDB2 following UV irradiation. DDB2-deficient 041 cells, with stably transfected FLAG-tagged DDB2, were transfected with HA-tagged ubiquitin for 48 h, treated with or without MG132 for 1 h, UV irradiated and further cultured for the indicated time periods. DDB2 was immunoprecipitated with anti-FLAG M2 Magnetic beads and detected with anti-DDB2 antibody (left panel). The membrane was stripped and re-blotted with anti-HA antibody to show ubiquitylated DDB2 (right panel). *A non-specific band. B, HeLa and HeLa-DDB2 cells were UV irradiated at 100 J/m2 and further cultured for 20 min, DDB2 was immunoprecipitated with anti-FLAG M2 Magnetic beads and detected with anti-DDB2 antibody. The membrane was stripped and re-blotted with anti-SUMO-1 or anti-SUMO-2/3 antibodies. Arrow marks SUMO-1 modified DDB2, *marks the immunoglobulin heavy chain. (C) Analysis of DDB2 SUMOylation in vitro. A 0.5 μg purified FLAG-DDB2 sample was incubated with WT or mutant SUMO-1 for 3 h at 30°C in a 20 μl in vitro SUMOylation reaction system. The reaction was stopped by adding 20 μl 2× SDS loading buffer, and boiled for 3 min. The samples were subjected to immunoblotting with anti-SUMO-1 antibody (left panel). The membrane was stripped and re-probed with anti-DDB2 antibody (right panel). Arrow marks SUMO-1 conjugated DDB2. *Marks free SUMO-1 protein.
It has been reported that DDB2 can be modified by SUMO-1 in response to UV irradiation (29). To ascertain whether the modified bands are SUMOylated forms, we immunoprecipitated FLAG-DDB2 from UV-irradiated HeLa-DDB2 cells under denaturing conditions, and analyzed the putative SUMOylation of DDB2 with either anti-SUMO-1 or anti-SUMO-2/3 antibodies. As shown in Figure 4B, an anti-SUMO-1 antibody-reactive band was identified in the sample of UV-irradiated HeLa-DDB2 cells (middle panel), and the band was located in the same position as the modified DDB2 (left panel). In contrast, no specific band was revealed by using anti-SUMO2/3 antibody (right panel). This result indicates that at least one band among the multiple modified DDB2 bands is SUMO-1-conjugated DDB2. We further analyzed the ability of DDB2 to be conjugated by SUMO-1 in vitro. For this, purified DDB2 protein was incubated with purified WT or mutated SUMO-1 protein in the in vitro SUMOylation system, and the subsequent immunoblot analysis of the reactants revealed a clear SUMO-1 conjugated-DDB2 (Figure 4C). Taken together, our data indicate that UVR-induced multiple modifications of DDB2 include SUMO-1 conjugation.
UVR-induced SUMOylation occurs at K309 of DDB2
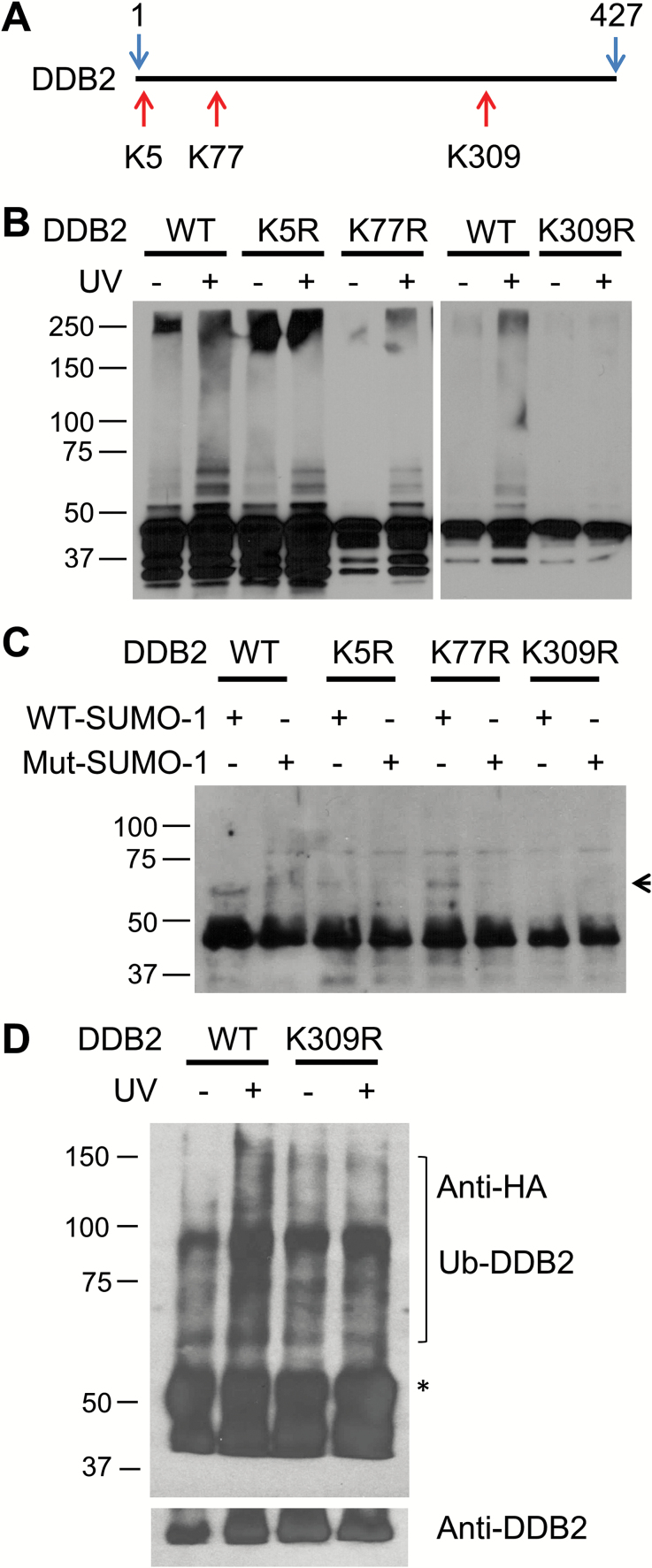
To identify the SUMOylation site in DDB2, we first screened the full-length human DDB2 sequence in SUMOplot (http://www.abgent.com/doc/sumoplot). Three potential SUMOylation sites identified were K5, K77 and K309 residues of DDB2 (Figure 5A). To validate the culprit SUMOylation site(s) in DDB2, we individually mutated these three lysines to arginine (K5R, K77R and K309R), and transfected the constructs into HeLa cells. UVR-induced modifications of DDB2 were once again detected by using standardized immunoprecipitation and immunoblotting. As shown in Figure 5B, K5R and K77R mutation did not affect the UVR-induced DDB2 modifications. However, K309R mutation completely abolished the DDB2 modification upon UV irradiation, indicating that K309 is the valid SUMOylation site in DDB2. To further confirm this finding, we expressed WT, and K5R, K77R, K309R mutated DDB2 in HeLa cells, purified these WT and mutated DDB2 forms with anti-FLAG magnetic beads, and subjected the proteins to in vitro SUMOylation. As shown in Figure 5C, WT, K5R and K77R DDB2 proteins were able to conjugate with WT-SUMO-1. In contrast, K309R DDB2 protein was unable to conjugate with WT-SUMO-1. These data indicate that UVR-induced SUMOylation occurs specifically at K309 residue of DDB2.
Figure 5.
DDB2 can be conjugated by SUMO-1 at K309. (A) SUMOylation sites on the DDB2 protein from SUMOplot prediction analysis. (B) Identification of SUMOylation sites on the DDB2 protein. WT or mutated DDB2 was transfected into HeLa cells, UV irradiated and immunoprecipitated with anti-FLAG Magnetic beads, detected with anti-DDB2 antibody. (C) Validation of the SUMOylation site on the DDB2 protein in the in vitro SUMOylation system. Purified WT or mutated FLAG-DDB2 protein from mammalian cells was subjected to the in vitro SUMOylation reaction, and detected with anti-DDB2 antibody. Arrow marks SUMO-1 conjugated DDB2. D, DDB2-deficient 041 cells were transfected with FLAG-tagged DDB2-WT or DDB2-K309R, along with HA-tagged ubiquitin for 48 h, treated with MG132 for 1 h, UV irradiated and further cultured for 2 h. DDB2 was immunoprecipitated with anti-FLAG M2 Magnetic beads and detected with anti-HA antibody to show ubiquitylated DDB2. The membrane was stripped and re-blotted with anti-DDB2 antibody. *A non-specific band.
Given that MG132 treatment prevents the sumoylation of DDB2 (Figure 3A and B), but does not prevent the ubiquitylation of DDB2 (Figure 4A), it appears that DDB2 sumoylation is not a prerequisite for DDB2 ubiquitylation. To determine whether K309R mutation does also affect UV-induced DDB2 ubiquitylation, we co-transfected FLAG-tagged DDB2-WT or DDB2-K309R along with HA-tagged ubiquitin into 041 cells, treated with UV radiation and further cultured for 2 h in the presence of MG132. The immunoprecipitates recovered by anti-FLAG antibody were probed with anti-HA antibody to view the ubiquitylated DDB2. As shown in Figure 5D, ubiquitylated DDB2 was detected only in MG132-treated 041 cells that were transfected with DDB2-WT, but not with DDB2-K309R, indicating that K309 residue is also critical to UV-induced DDB2 ubiquitylation.
DDB2 K309 modifications are required for the repair of UVR induced CPD by facilitating the recruitment of XPC to the damage site
To define the functional role of DDB2 modifications at the K309 residue in the repair of UVR-induced CPD, we expressed the WT and K309R DDB2 into DDB2-deficient 041 cells, by respective transfection of the constructs (Figure 6A), and UV irradiated the cells followed by their culture up to 24 h post-UVR. The ISB analysis of initial and remaining CPD was carried out to determine the NER efficiency for CPD in these engineered cells. As shown in Figure 6B, DDB2-WT overexpression significantly enhanced the repair of CPD. However, a similar overexpression of K309R mutated DDB2, which is unable to get modified with SUMO-1 and ubiquitin, could not alter the efficiency of CPD removal. These data indicate that DDB2 modifications at K309, including SUMOylation, play an important functional role in the repair of UVR-induced CPD.
Figure 6.
DDB2 modifications at K309 are essential to repair CPD through facilitating the recruitment of XPC to damage sites. (A) DDB2 expression in 041 cells transfected with Wt or mutated DDB2-expressing plasmids. 041 cells were transiently transfected with Wt or K309R DDB2 for 48 h and immunoblotting of protein extracts was conducted to determine the expression of DDB2. (B) DNA repair efficiency of cells possessing WT or mutated DDB2; 041 cells transfected with WT or K309R DDB2 were UV irradiated and further cultured for different time periods. ISB analysis was performed with anti-CPD antibody to detect the amount of CPD remaining at different times. N = 3, Bar: SD, *P < 0.05; **P < 0.01. (C–F) Effect of non-modified DDB2 on the recruitment of DDB2 and XPC to UV damage sites; 041 cells transfected with WT or K309R DDB2 were UV irradiated through micropore membrane filters, further cultured for 30 min, and subjected to immunofluorescence to detect the co-localization of CPD and DDB2 (C), as well as CPD and XPC (E). The ratio of DDB2 to CPD and XPC to CPD foci was determined by analyzing more than 100 individual nuclei for merged fluorescent signals of DDB2 and CPD, as well as XPC and CPD foci in three visual fields (D and F). Bar: SD, **P < 0.01.
As an initial DNA damage recognition factor, DDB2 is critical for the recruitment of XPC to UVR-induced CPD sites (24). Given that K309R mutated DDB2 compromised the NER of CPD, we reasoned that DDB2 modifications at K309 could be an important determinant of the subsequent lesion handover and recruitment of XPC. To test this hypothesis, we transfected DDB2-WT and DDB2-K309R into 041 cells, respectively, and determined the extent of XPC recruitment to CPD sites via local micropore UV irradiation and immunofluorescent visualization. We first confirmed that K309R mutation does not affect the recruitment of DDB2 to UV-damaged DNA, reflected by similar co-localizing DDB2 and CPD foci in 041 cells transfected with DDB2-WT and DDB2-K309R (Figure 6C and D). As shown in Figure 6E and F, very few DNA damage (red) co-localizing XPC foci (green) could be observed in 041 cells transfected with empty vector. In contrast, clear XPC foci specifically targeting DNA damage (green, red and merged yellow) were found in 041 cells transfected with DDB2-WT. More importantly, these same damage co-localizing XPC foci were rarely found (only red) in cells transfected with DDB2-K309R, indicating that the recruitment of XPC at CPD sites does, in fact, require DDB2 modifications including SUMOylation. Taken together, these data demonstrate that the DDB2 modifications at K309 are essential post-irradiation response elements that promote the recruitment of XPC to damage sites for orchestrating an effective CPD repair.
Discussion
Some of the important post-translational protein modifications, e.g. phosphorylation, acetylation, ubiquitylation and SUMOylation, are known to help orchestrate different steps of NER (30) and other DNA repair pathways. In this study, we have shown the induction of a heterogeneous set of DDB2 modifications following cellular UV irradiation. A systematic characterization of these modifications indicates that DDB2 is specifically modified through SUMO-1, and that these modifications require the participation of functional 26S proteasome.
We observed different DDB2 modifications upon cellular UV irradiation by applying clearly distinguishing immunoprecipitation and immunoblotting schemes. Thus, for the first time, we showed that cisplatin is unable to induce these DDB2 modifications, although both UVR-induced DNA lesions and cisplatin-induced intra-strand crosslinks are prime substrates of NER (1). UVR-induced CPDs are characterized by small deformations of the DNA double helix, the DNA is unwound and kinked by ~7–9° relative to B-form DNA (31,32), whereas 6–4PPs produce even more significant structural distortions in the DNA double helix than CPDs because the pyrimidine planes within the 6–4PP are almost perpendicular, and a bending angle of 44° has been observed (32). The chemotherapeutic agent, cisplatin, forms primarily 1, 2-intrastrand crosslinks between adjacent purines in DNA, e.g. cis-Pt(NH3)2d(GpG) (Pt-GG), with Pt bound to two adjacent guanines, and cis-Pt(NH3)2d(ApG) (Pt-AG), in which the Pt is bound to adenine and an adjacent guanine. Similar to UVR-induced 6–4PP, these 1, 2-intrastrand crosslinks also unwind the DNA duplex in the vicinity of the site of platination, bending it 50–78° toward the major groove (33,34). Given that DDB2 is only required for the repair of UVR-induced CPD, but not UVR-induced 6–4PP (3,24,35) and cisplatin-induced intrastrand crosslinks (26), it could be argued that DDB2 and its modifications are only required for facilitating the recognition of minor helix-distorting lesions.
We have demonstrated that the ability of DDB2 to bind the damaged DNA within chromatin is essential for UVR to provoke DDB2 modifications. Moreover, in order for DDB2 to get modified, it must intimately reside at the damage site within chromatin. In essence, the unmodified DDB2 is first recruited to the UVR-induced CPD sites, and then DNA damage-anchored DDB2 is promptly modified to help execute its key NER-specific role in local chromatin remodeling as well as the recruitment of the immediate downstream factor, XPC. This is further confirmed by our data indicating that DDB2 does not tightly bind to cisplatin-induced chromatin and, therefore, cannot be modified upon the treatment of cells with cisplatin.
Another interesting finding of this study is related to the function of 26S proteasome that was seen as essential for UVR-induced DDB2 modifications, and 26S proteasome was shown to play its role in protein modification only after DDB2 is recruited to the damage sites. Inhibition of 26S proteasome failed to affect the DDB2 ubiquitylation upon UV irradiation, but blocked the other DDB2 modifications occurring rapidly after UV treatment. The 26S proteasome has been implicated in the optimal execution of NER by promoting the recruitment of XPC to the UVR-induced DNA damage sites (36). Given that XPC recruitment to UV-damaged sites requires DDB2 PARylation (18) and SUMOylation, one can reason that 26S proteasome could promote XPC recruitment by initially facilitating DDB2 modification that eventually leads to the efficient NER.
DDB2 has been reported to get modified by PAR upon UV irradiation. However, we were unable to observe PARylated DDB2 (Figure S1) in the obviously competent immunoprecipitates pulled down by anti-FLAG antibody and the detection with the same anti-PAR antibodies that have been described in the literature (19). Nevertheless, the use of PARP-1 inhibitor PJ-34, showed that PARP inhibition partially reduced the UVR-induced DDB2 modifications, suggesting a PARP-1-mediated modification, possibly DDB2 PARylation, constitute the UVR-induced rapid modifications of DDB2 (Figure S1). Recently, DDB2 was found to be modified by SUMO-1 upon UV irradiation (29). Our in vivo immunoprecipitation and in vitro SUMOylation analyses confirmed this important UVR-induced DDB2 SUMOylation. In addition, we have now identified the K309 residue of DDB2 as the site where SUMO-1 is conjugated. From a mechanistic standpoint, upon cellular UV irradiation, modifications of DDB2 at K309, including SUMOylation, promote the subsequent recruitment of XPC to the damage site, and functionally contributes to the repair of UVR-induced CPD.
To summarize, our data demonstrate that modifications of DDB2 at K309, including SUMOylation, are indispensable as they facilitate the recruitment of XPC to enable the repair of UVR-induced CPD. In addition, the multiple modifications of DDB2 induced upon UV irradiation, including the SUMOylation, require the 26S proteasome function and are formed only after DDB2 is recruited to the DNA damage sites within chromatin.
Supplementary material
Supplementary date are available at Carcinogenesis online.
Supplementary Material
Acknowledgements
We greatly acknowledge Drs Yoshihiro Nakatani (Dana-Farber Cancer Institute, Boston, MA) and Michael Tainsky (MD Anderson Cancer center, Houston, TX) for the HeLa-DDB2 and 041 cell lines. We also thank the members of the Wani laboratory for their valuable input.
Abbreviations
- 6-4PP
6-4 pyrimidine pyrimidone photoproducts
- CPD
cyclobutane pyrimidine dimers
- DDB2
subunit 2 of DNA damage-binding protein complex
- ISB
immuno-slot blot
- NER
nucleotide excision repair
- SUMO
small ubiquitin-related modifier
- UVR
ultraviolet radiation
- WT
wild-type
References
- 1. de Laat W.L., et al. (1999) Molecular mechanism of nucleotide excision repair. Genes Dev., 13, 768–785. [DOI] [PubMed] [Google Scholar]
- 2. Hwang B.J., et al. (1999) Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair. Proc. Natl. Acad. Sci. U. S. A., 96, 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moser J., et al. (2005) The UV-damaged DNA binding protein mediates efficient targeting of the nucleotide excision repair complex to UV-induced photo lesions. DNA Repair (Amst)., 4, 571–582. [DOI] [PubMed] [Google Scholar]
- 4. Aboussekhra A., et al. (1995) Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell, 80, 859–868. [DOI] [PubMed] [Google Scholar]
- 5. Mu D., et al. (1995) Reconstitution of human DNA repair excision nuclease in a highly defined system. J. Biol. Chem., 270, 2415–2418. [DOI] [PubMed] [Google Scholar]
- 6. Zhu Q., et al. (2017) Nucleotide excision repair: finely tuned molecular orchestra of early pre-incision events. Photochem. Photobiol., 93, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ulrich H.D. (2012) Ubiquitin and SUMO in DNA repair at a glance. J. Cell Sci., 125(Pt 2), 249–254. [DOI] [PubMed] [Google Scholar]
- 8. Ulrich H.D. (2014) Two-way communications between ubiquitin-like modifiers and DNA. Nat. Struct. Mol. Biol., 21, 317–324. [DOI] [PubMed] [Google Scholar]
- 9. Shiyanov P., et al. (1999) Cullin 4A associates with the UV-damaged DNA-binding protein DDB. J. Biol. Chem., 274, 35309–35312. [DOI] [PubMed] [Google Scholar]
- 10. Groisman R., et al. (2003) The ubiquitin ligase activity in the DDB2 and CSA complexes is differentially regulated by the COP9 signalosome in response to DNA damage. Cell, 113, 357–367. [DOI] [PubMed] [Google Scholar]
- 11. Nag A., et al. (2001) The xeroderma pigmentosum group E gene product DDB2 is a specific target of cullin 4A in mammalian cells. Mol. Cell. Biol., 21, 6738–6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsuda N., et al. (2005) DDB2, the xeroderma pigmentosum group E gene product, is directly ubiquitylated by Cullin 4A-based ubiquitin ligase complex. DNA Repair (Amst)., 4, 537–545. [DOI] [PubMed] [Google Scholar]
- 13. El-Mahdy M.A., et al. (2006) Cullin 4A-mediated proteolysis of DDB2 protein at DNA damage sites regulates in vivo lesion recognition by XPC. J. Biol. Chem., 281, 13404–13411. [DOI] [PubMed] [Google Scholar]
- 14. Sugasawa K., et al. (2005) UV-induced ubiquitylation of XPC protein mediated by UV-DDB-ubiquitin ligase complex. Cell, 121, 387–400. [DOI] [PubMed] [Google Scholar]
- 15. Kapetanaki M.G., et al. (2006) The DDB1-CUL4ADDB2 ubiquitin ligase is deficient in xeroderma pigmentosum group E and targets histone H2A at UV-damaged DNA sites. Proc. Natl. Acad. Sci. U.S.A., 103, 2588–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H., et al. (2006) Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol. Cell, 22, 383–394. [DOI] [PubMed] [Google Scholar]
- 17. Otrin V.R., et al. (1998) Relationship of the xeroderma pigmentosum group E DNA repair defect to the chromatin and DNA binding proteins UV-DDB and replication protein A. Mol. Cell. Biol., 18, 3182–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Robu M., et al. (2013) Role of poly(ADP-ribose) polymerase-1 in the removal of UV-induced DNA lesions by nucleotide excision repair. Proc. Natl. Acad. Sci. U.S.A., 110, 1658–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pines A., et al. (2012) PARP1 promotes nucleotide excision repair through DDB2 stabilization and recruitment of ALC1. J. Cell Biol., 199, 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Q.E., et al. (2007) Ubiquitylation-independent degradation of Xeroderma pigmentosum group C protein is required for efficient nucleotide excision repair. Nucleic Acids Res., 35, 5338–5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wani A.A., et al. (1984) Antibodies to UV irradiated DNA: the monitoring of DNA damage by ELISA and indirect immunofluorescence. Photochem. Photobiol., 40, 465–471. [DOI] [PubMed] [Google Scholar]
- 22. Wang Q.E., et al. (2003) Tumor suppressor p53 dependent recruitment of nucleotide excision repair factors XPC and TFIIH to DNA damage. DNA Repair (Amst)., 2, 483–499. [DOI] [PubMed] [Google Scholar]
- 23. Zhao R., et al. (2014) DNA damage-binding complex recruits HDAC1 to repress Bcl-2 transcription in human ovarian cancer cells. Mol. Cancer Res., 12, 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Q.E., et al. (2004) UV radiation-induced XPC translocation within chromatin is mediated by damaged-DNA binding protein, DDB2. Carcinogenesis, 25, 1033–1043. [DOI] [PubMed] [Google Scholar]
- 25. Han C., et al. (2015) Cdt2-mediated XPG degradation promotes gap-filling DNA synthesis in nucleotide excision repair. Cell Cycle, 14, 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barakat B.M., et al. (2010) Overexpression of DDB2 enhances the sensitivity of human ovarian cancer cells to cisplatin by augmenting cellular apoptosis. Int. J. Cancer, 127, 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nichols A.F., et al. (1996) Mutations specific to the xeroderma pigmentosum group E Ddb- phenotype. J. Biol. Chem., 271, 24317–24320. [DOI] [PubMed] [Google Scholar]
- 28. Scrima A., et al. (2008) Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell, 135, 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsuge M., et al. (2013) SUMOylation of damaged DNA-binding protein DDB2. Biochem. Biophys. Res. Commun., 438, 26–31. [DOI] [PubMed] [Google Scholar]
- 30. Ciccia A., et al. (2010) The DNA damage response: making it safe to play with knives. Mol. Cell, 40, 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang C.I., et al. (1991) Site-specific effect of thymine dimer formation on dAn.dTn tract bending and its biological implications. Proc. Natl. Acad. Sci. U.S.A., 88, 9072–9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim J.K., et al. (1995) Contrasting structural impacts induced by cis-syn cyclobutane dimer and (6-4) adduct in DNA duplex decamers: implication in mutagenesis and repair activity. Photochem. Photobiol., 62, 44–50. [DOI] [PubMed] [Google Scholar]
- 33. Takahara P.M., et al. (1995) Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature, 377, 649–652. [DOI] [PubMed] [Google Scholar]
- 34. Gelasco A., et al. (1998) NMR solution structure of a DNA dodecamer duplex containing a cis-diammineplatinum(II) d(GpG) intrastrand cross-link, the major adduct of the anticancer drug cisplatin. Biochemistry, 37, 9230–9239. [DOI] [PubMed] [Google Scholar]
- 35. Fitch M.E., et al. (2003) In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. J. Biol. Chem., 278, 46906–46910. [DOI] [PubMed] [Google Scholar]
- 36. Wang Q.E., et al. (2005) Cellular ubiquitination and proteasomal functions positively modulate mammalian nucleotide excision repair. Mol. Carcinog., 42, 53–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.