Abstract
Background
Incidence of extraskeletal osteosarcoma (ESOS) is extremely low and the prognosis remains unclear. We conducted this study to explore prognostic factors and the role of chemotherapy in ESOS.
Material/Methods
We screened data from the Surveillance Epidemiology and End Results (SEER) database (1975–2016). Three hundred ten patients with ESOS were included and 49.4% (107/310) of them underwent chemotherapy. We performed logistic regression analysis to investigate potential factors determining selection of chemotherapy. An inverse probability of treatment weighting (IPTW) and propensity score matching (PSM)-adjusted Kaplan-Meier curve was created and log-rank test and Cox regression analysis were performed to compare overall survival (OS) and cancer-specific survival (CSS) in patients treated with and without chemotherapy. Subgroup analysis also was conducted based on age, tumor site, stage, size, and surgery.
Results
Chemotherapy in ESOS was not associated with improved OS in the unmatched cohort (HR, 0.764; 95% CI, 0.555–1.051; p=0.098). The insignificant treatment effect of chemotherapy was also noted in IPTW-adjusted (HR, 0.737; 95% CI, 0.533–1.021; p=0.066) and PSM-adjusted (HR, 0.804; 95% CI, 0.552–1.172; p=0.257) Cox regression analysis. The insignificant treatment effect was consistent across all subgroups and there was no significant heterogeneity of chemotherapy effect (all p for interaction >0.05).
Conclusions
The study suggested that chemotherapy has no significant benefit on prognosis of patients with ESOS. These findings should be considered when making treatment decisions about patients with ESOS.
MeSH Keywords: Chemotherapy, Adjuvant; Prognosis; Propensity Score SEER Program
Background
Extraskeletal osteosarcoma (ESOS), first defined in 1941 [1], is an extremely rare malignant mesenchymal tumor occurring in soft tissue without any skeletal attachment [2]. ESOS accounts for only 1% of soft tissue sarcomas and 2% to 4% of osteosarcomas [3,4]. In contrast to conventional osteosarcoma, ESOS occurs frequently among elderly patients, and tumors are widely dispersed throughout the body [4,5]. ESOS also has high rates of recurrence and distant metastasis, thus leading to poor prognosis [6].
Few studies of ESOS exist because of the low incidence of the disease, therefore, there is no consensus on a treatment protocol for it [6–8]. Surgical resection is the mainstay for treating ESOS [4]. Radiotherapy has been used in margin-positive resection [9]. Although chemotherapy is standard systematic treatment for osteosarcoma, its role in ESOS remains unclear [4,5,8]. Previous studies have reported that ESOS is insensitive to chemotherapy [7,10], while a small number of studies suggested that chemotherapy improved prognosis [4,8,9]. To our knowledge, the role of chemotherapy in ESOS has not been well explored in a study with comparatively large sample size.
To gain an overview of patients with ESOS, related data were retrieved from the SEER database. We further analyzed prognostic factors for this rare tumor and the role of chemotherapy on survival of patients with ESOS.
Material and Methods
Data sources and study population
The SEER database collects individual cancer data from 18 registries, covering about 30% of the US population [11]. The cancer data include no personal identifying information and data acquisition are permitted by the National Cancer Institute. We collected individual cancer data from this database via SEER*Stat software version 8.3.5.
We collected individual data on patients with ESOS from 1975 to 2016. Inclusion criteria were diagnosis of osteosarcoma according to ICD-O-3 histology code as the primary malignancy and limit of primary sites to extraskeletal sites according to the labeled primary site code. Data without positive histologic confirmation or exact follow-up time were excluded.
Covariates
We extracted data on sociodemographic, tumor-related, and treatment-related characteristics. Patients with ESOS were classified as those treated with and without chemotherapy based on SEER treatment code. Age and tumor size were divided based on the median of the groups respectively. Labeled primary site code did not refer to specific tumor location due to the properties of the database. For example, soft tissues of fingers, hands, forearms, and upper arms were all defined as soft tissues in the upper limb (labeled primary code=C49.1). Therefore, there were a total of four different groups of sites, including extremities (soft tissues of upper and lower limbs), trunk (soft tissues of thorax, abdomen and pelvis), viscera (liver, cecum, pancreas, lung, ovary, kidney and other visceral organs), and other sites. We divided tumor grades based on degree of differentiation. Based on codes in the SEER database [12], tumor stages were further categorized into localized, regional, and distant. Primary endpoints were overall survival (OS) and cancer-specific survival (CSS) rate. OS was considered as the period from the time from diagnosis to death due to any cause. CSS was considered as the time from diagnostic confirmation until death from ESOS.
Statistical analyses
Differences in sociodemographic, tumor-related, and treatment-related characteristics between patients treated with and without chemotherapy were assessed by Pearson’s chi-squared test. We introduced multiple imputation by chained equations (MICE) to deal with missing data on tumor size with relevant values [13,14]. Then we performed multivariable logistic regression to explore potential factors affecting selection of chemotherapy. Kaplan-Meier curve and log-rank test were calculated to compare OS and CSS rates in patients treated with and without chemotherapy. Comparison of each variable was analyzed by univariable Cox regression analysis. Variables closely approaching clinical significance (p<0.1) were noted. Multivariable Cox regression models were created by adjusting for variables selected from the univariate analysis and other potential variables.
Inverse probability of treatment weighting (IPTW) and propensity score matching (PSM) with a 1: 1 ratio and a caliper of 0.01 were used to balance bias of confounding factors that may affect chemotherapy allocation [15,16]. Multivariable logistic regression was performed to generate propensity scores (PS) for all variables, and then the weight was calculated and the matching was done based on the PS, respectively. We calculated standardized mean difference (SMD) to assess the balance of baseline characteristics after IPTW and PSM. IPTW-adjusted and PSM-adjusted Kaplan-Meier curves were drawn and comparisons of the treatments were analyzed with log-rank tests [17]. Landmark analysis was introduced to attenuate immortal time bias, if needed [18]. We then recreated IPTW-adjusted and PSM-adjusted multivariable Cox regression models and recalculated hazard ratios (HRs) [19]. Within the group of PSM, heterogeneity of chemotherapy treatment was evaluated via subgroup analysis according to variables including age, tumor site, stage, size, and surgery. We performed interaction tests to assess difference in the effect of chemotherapy between subgroups (Supplementary Figure 1).
Pearson’s chi-squared test, Logistic regression, and Cox regression analysis were conducted by SPSS (IBM, NY, United States). We created a Kaplan-Meier curve, performed a log-rank test, and created a forest plot presenting the results of subgroup analysis via GraphPad Prism 8 (GraphPad Software, Inc., CA, United States). MICE, IPTW, PSM, landmark analysis, bootstrap resampling, and interaction tests were conducted using R version 3.5.3 (http://www.r-project.org/). p<0.05 that was two-sided was defined as statistical significance.
Results
Characteristics associated with the use of chemotherapy
Between 1976 and 2016, we identified 310 patients with ESOS who were histologically confirmed as having the primary malignancy. Among them, 153 (49.4%) received chemotherapy and 157 (50.6%) did not receive that treatment (Supplementary Figure 1). Baseline characteristics of the original population with ESOS are presented in Table 1. The number of patients receiving chemotherapy increased over time while the proportion of different treatment groups was nearly equal (Supplementary Figure 2). Based on propensity score, IPTW and PSM achieved optimal balance between the two treatment groups (Table 1, Supplementary Table 1).
Table 1.
Sociodemographic and clinical characteristics of study patients.
| Characteristic | Unweighted study population* | Weighted study population** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n=310), n (%) | Chemotherapy (n=153), n (%) | No chemotherapy (n=157), n (%) | P value | Overall (%) | Chemotherapy (%) | No chemotherapy (%) | P value | ||
| Age(year) | <60 | 151 (48.7) | 89 (58.2) | 62 (39.5) | 0.001 | 48.5 | 48 | 49 | 0.878 |
| ≥60 | 159 (51.3) | 64 (41.8) | 95 (60.5) | 51.5 | 52 | 51 | |||
| Gender | Male | 145 (46.8) | 83 (54.2) | 62 (39.5) | 0.012 | 45.7 | 45.5 | 46 | 0.929 |
| Female | 165 (53.2) | 70 (45.8) | 95 (60.5) | 54.3 | 54.5 | 54 | |||
| Race | White | 249 (80.3) | 122 (79.7) | 127 (80.9) | 0.910 | 81.4 | 81.5 | 81.2 | 0.990 |
| Black | 38 (12.3) | 20 (13.1) | 18 (11.5) | 11.4 | 11.4 | 11.3 | |||
| Other | 23 (7.4) | 11 (7.2) | 12 (7.6) | 7.2 | 7.1 | 7.5 | |||
| Insurance type | Insured | 111 (35.8) | 54 (35.3) | 57 (36.3) | 0.379 | 36.9 | 36.3 | 37.6 | 0.978 |
| Any medicaid | 22 (7.1) | 14 (9.2) | 8 (5.1) | 6.9 | 7 | 6.8 | |||
| Unknown | 177 (57.1) | 85 (55.6) | 92 (58.6) | 56.2 | 56.7 | 55.6 | |||
| Marital status | Single | 55 (17.7) | 36 (23.5) | 19 (12.1) | 0.008 | 17.5 | 17.9 | 17.1 | 0.984 |
| Widowed/divorced | 76 (24.5) | 28 (18.3) | 48 (30.6) | 24.5 | 24.7 | 24.3 | |||
| Married | 170 (54.8) | 83 (54.2) | 87 (55.4) | 54.5 | 54.3 | 54.7 | |||
| Unknown | 9 (2.9) | 6 (3.9) | 3 (1.9) | 3.5 | 3.1 | 3.9 | |||
| Year of diagnosis | 1976 to 1985 | 20 (6.5) | 7 (4.6) | 13 (8.3) | 0.476 | 7 | 7.5 | 6.5 | 0.985 |
| 1986 to 1995 | 36 (11.6) | 18 (11.8) | 18 (11.5) | 11.3 | 11.2 | 11.5 | |||
| 1996 to 2005 | 102 (32.9) | 48 (31.4) | 54 (34.4) | 31.5 | 31.8 | 31.2 | |||
| 2006 to 2016 | 152 (49) | 80 (52.3) | 72 (45.9) | 50.2 | 49.5 | 50.8 | |||
| Primary site | Extremity | 135 (43.5) | 80 (52.3) | 55 (35) | 0.004 | 42.9 | 42.3 | 43.6 | 0.993 |
| Trunk | 84 (27.1) | 29 (19) | 55 (35) | 27.7 | 28.5 | 26.9 | |||
| Visceral | 51 (16.5) | 26 (17) | 25 (15.9) | 15.9 | 15.9 | 15.8 | |||
| Other | 40 (12.9) | 18 (11.8) | 22 (14) | 13.5 | 13.3 | 13.7 | |||
| Grade | Grade I/II | 22 (7.1) | 6 (3.9) | 16 (10.2) | 0.025 | 8.2 | 9.1 | 7.2 | 0.818 |
| Grade III/IV | 203 (65.5) | 110 (71.9) | 93 (59.2) | 63.6 | 63.9 | 63.2 | |||
| Unknown | 85 (27.4) | 37 (24.2) | 48 (30.6) | 28.2 | 27 | 29.6 | |||
| Stage | Localized | 146 (47.1) | 67 (43.8) | 79 (50.3) | 0.072 | 47.2 | 47.3 | 47.2 | 0.998 |
| Regional | 96 (31) | 43 (28.1) | 53 (33.8) | 30.7 | 30.6 | 30.9 | |||
| Distant | 52 (16.8) | 32 (20.9) | 20 (12.7) | 17.1 | 17 | 17.4 | |||
| Unstaged | 16 (5.2) | 11 (7.2) | 5 (3.2) | 5 | 5 | 4.5 | |||
| Tumor size (mm) | <73 | 155 (50) | 70 (45.8) | 85 (54.1) | 0.173 | 49.2 | 48.2 | 50.3 | 0.742 |
| ≥73 | 155 (50) | 83 (54.2) | 72 (45.9) | 50.8 | 51.8 | 49.7 | |||
| Radiation | Yes | 73 (23.5) | 40 (26.1) | 33 (21) | 0.349 | 24.6 | 24.7 | 24.4 | 0.956 |
| No | 237 (76.5) | 113 (73.9) | 124 (79) | 75.4 | 75.3 | 75.6 | |||
| Surgery | Yes | 272 (87.7) | 131 (85.6) | 141 (89.8) | 0.301 | 87.1 | 87.3 | 86.9 | 0.918 |
| No | 38 (12.3) | 22 (14.4) | 16 (10.2) | 12.9 | 12.7 | 13.1 | |||
Mm – millimeter.
Data are presented as number (percentage) of patients unless otherwise specified.
Data are presented as percentage of weighted study population unless otherwise specified.
After IPTW and PSM, the majority of SMDs for all covariables in IPTW cohorts and PSW cohorts were less than 10%, also indicating that bias of confounding factors was attenuated (Supplementary Figure 3). Multivariable logistic regression analysis identified characteristics significantly related to use of chemotherapy, including age ≥60 (odds ratio [OR], 0.57; 95% confidence interval [CI], 0.34–0.96; p=0.033), the trunk as primary tumor site (OR, 0.46; 95% CI, 0.25–0.85; p=0.012) and distant tumor stage (OR, 2.13; 95% CI, 1.02–4.44; p=0.044) (Table 2).
Table 2.
Multivariable logistic regression model of characteristics associated with chemotherapy.
| Characteristic | OR (95% CI) | P value | |
|---|---|---|---|
| Age (year) | <60 | Reference | NA |
| ≥60 | 0.57 (0.34–0.96) | 0.033 | |
| Gender | Male | Reference | NA |
| Female | 0.72 (0.42–1.21) | 0.209 | |
| Marital status | Single | Reference | NA |
| Widowed/divorced | 0.51 (0.23–1.16) | 0.110 | |
| Married | 0.63 (0.31–1.28) | 0.201 | |
| Unknown | 1.11 (0.23–5.31) | 0.895 | |
| Primary site | Extremity | Reference | NA |
| Trunk | 0.46 (0.25–0.85) | 0.012 | |
| Visceral | 0.68 (0.32–1.42) | 0.302 | |
| Other | 0.57 (0.26–1.23) | 0.151 | |
| Grade | Grade I/II | Reference | NA |
| Grade III/IV | 2.40 (0.84–6.86) | 0.101 | |
| Unknown | 1.55 (0.51–4.73) | 0.443 | |
| Stage | Localized | Reference | NA |
| Regional | 0.85 (0.48–1.51) | 0.575 | |
| Distant | 2.13 (1.02–4.44) | 0.044 | |
| Unstaged | 2.96 (0.88–9.94) | 0.080 |
NA – not applicable; OR – odds ratio.
Treatment effect of chemotherapy on survival in different cohorts
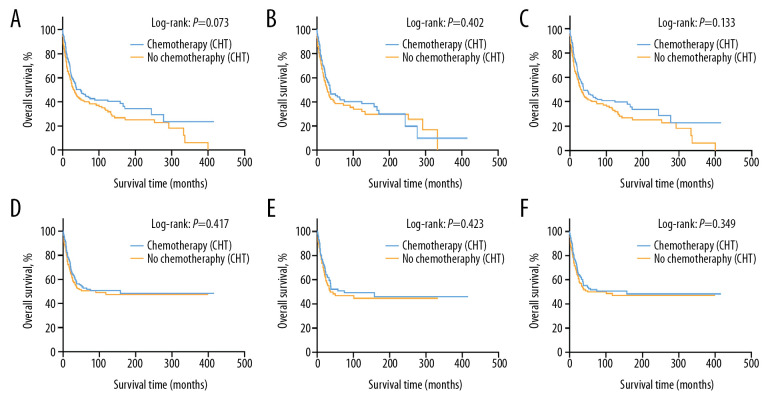
The 5-year OS rate was 45.4% (95% CI, 37.1–53.7%) in the group receiving chemotherapy and 40.0% (95% CI, 32.0–48.0%) in the group of patients treated without chemotherapy in the original unmatched cohort (p=0.073) (Figure 1A). Likewise, no statistical significance was noted in the PSM-adjusted and IPTW-adjusted cohorts, which balanced the bias of confounding variables and made the treatment effect comparable (Figure 1B, 1C). The 5-year OS was 42.8% (95% CI, 32.8–52.8%) for patients with chemotherapy and 38.5% (95% CI, 28.3–48.7%) for patients without chemotherapy in the PSM-adjusted cohort (P=0.402) and 45.5% (95% CI, 37.8–54.5%) for patients with chemotherapy and 40.5% (95% CI, 32.7–48.9%) for patients without chemotherapy in the IPTW-adjusted cohort (P=0.133). As landmark analysis in the PSM cohort illustrated that immortal time bias was controlled and no significant difference was noted in the effect of chemotherapy on prognosis of the two treatment groups at different time periods. (Supplementary Figure 4). No statistical significance in CSS rate was noted in the original unmatched cohort, with a 5-year CSS rate of 51.7% (95% CI, 43.3–60.1%) in the chemotherapy group and 50.0% (95% CI, 41.4–58.6%) in the non-chemotherapy group (P=0.417) (Figure 1D). There was still no significant statistical difference in CSS rate after adjustment for PSM- (P=0.423) and IPTW-adjustment (P=0.349) in these cohorts (Figure 1E, 1F).
Figure 1.
These graphs show Kaplan-Meier survival curves of overall survival and cancer-specific survival in (A, D) unmatched, (B, E) propensity score matched, and (C, F) inverse probability of treatment weighting cohorts.
Prognostic characteristics for survival of ESOS
In the univariable Cox regression model for OS rate of the original unmatched cohort (Table 3), treatment with chemotherapy failed to reach statistical significance (Hazard Ratio [HR], 0.774; 95% CI, 0.583–1.027; p=0.076). Similarly, in multivariable Cox regression models, treatment with chemotherapy still had nothing to do with OS after adjusting for all characteristics in model 2 (HR, 0.723; 95% CI, 0.589–1.011; p=0.055) and the characteristics selected in the univariable Cox regression model (p<0.1) including chemotherapy, age, race, marital status, primary sites, grade, stage, size, and surgery in model 1 (HR, 0.764; 95% CI, 0.555–1.051; p=0.098). The statistical significance of the characteristics in model 1 of the original unmatched group remained unchanged after 1000 bootstrap resamplings. In the IPTW cohort, use of chemotherapy did not improve OS in model 1 in multivariable Cox regression analysis adjusted for the characteristics mentioned above (HR, 0.737; 95% CI, 0.533–1.021; p=0.066) (Supplementary Table 2). There was also no significant effect of chemotherapy on OS in model 1 of the PSM cohort adjusted for related characteristics (HR, 0.804; 95% CI, 0.552–1.172; p=0.257) (Supplementary Table 3). For CSS rate between two groups, treatment with chemotherapy did not show significant therapeutic effect (Supplementary Table 4).
Table 3.
Cox regression models for overall survival in patients with extraskeletal osteosarcoma in the unmatched cohort.
| Characteristic | Unadjusted* (unmatched cohort) | Model 1** (unmatched cohort) | Model 2*** (unmatched cohort) | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Chemotherapy | No | Reference | NA | Reference | NA | Reference | NA |
| Yes | 0.774 (0.583–1.027) | 0.076 | 0.764 (0.555–1.051) | 0.098 | 0.723 (0.589–1.011) | 0.055 | |
| Age(year) | <60 | Reference | NA | Reference | NA | Reference | NA |
| ≥60 | 1.848 (1.384–2.468) | <0.001 | 2.095 (1.503–2.922) | <0.001 | 2.165 (1.521–3.082) | <0.001 | |
| Gender | Male | Reference | NA | Reference | NA | ||
| Female | 0.953 (0.718–1.266) | 0.742 | 0.815 (0.576–1.155) | 0.251 | |||
| Race | White | Reference | NA | Reference | NA | Reference | NA |
| Black | 0.804 (0.505–1.281) | 0.359 | 0.941 (0.572–1.546) | 0.809 | 1.011 (0.612–1.668) | 0.968 | |
| Other | 0.578 (0.304–1.100) | 0.095 | 0.738 (0.374–1.456) | 0.381 | 0.678 (0.339–1.356) | 0.272 | |
| Insurance type | Insured | Reference | NA | Reference | NA | ||
| Any medicaid | 1.244 (0.682–2.269) | 0.477 | 1.166 (0.591–2.307) | 0.659 | |||
| Unknown | 0.786 (0.568–1.087) | 0.146 | 1.499 (0.737–3.045) | 0.264 | |||
| Marital status | Single | Reference | NA | Reference | NA | Reference | NA |
| Widowed/divorced | 1.945 (1.226–3.084) | 0.005 | 1.501 (0.902–2.496) | 0.118 | 1.631 (0.968–2.745) | 0.066 | |
| Married | 1.416 (0.926–2.164) | 0.108 | 1.196 (0.751–1.906) | 0.451 | 1.186 (0.733–1.918) | 0.487 | |
| Unknown | 1.705 (0.655–4.439) | 0.274 | 2.104 (0.789–5.612) | 0.137 | 1.657 (0.589–4.659) | 0.338 | |
| Year of diagnosis | 1976 to 1985 | Reference | NA | Reference | NA | ||
| 1986 to 1995 | 1.165 (0.621–2.188) | 0.634 | 1.476 (0.751–2.905) | 0.261 | |||
| 1996 to 2005 | 0.952 (0.535–1.692) | 0.867 | 1.032 (0.553–1.927) | 0.921 | |||
| 2006 to 2016 | 1.319 (0.745–2.338) | 0.342 | 2.195 (0.911–5.296) | 0.081 | |||
| Primary site | Extremity | Reference | NA | Reference | NA | Reference | NA |
| Trunk | 1.834 (1.287–2.611) | <0.001 | 2.181 (1.488–3.197) | <0.001 | 2.437 (1.632–3.638) | <0.001 | |
| Visceral | 3.642 (2.489–5.329) | <0.001 | 2.964 (1.951–4.505) | <0.001 | 3.337 (2.136–5.211) | <0.001 | |
| Other | 1.133 (0.703–1.826) | 0.609 | 1.382 (0.810–2.359) | 0.235 | 1.442 (0.833–2.495) | 0.191 | |
| Grade | Grade I/II | Reference | NA | Reference | NA | Reference | NA |
| Grade III/IV | 2.225 (1.088–4.553) | 0.029 | 2.102 (0.997–4.435) | 0.051 | 1.907 (0.894–4.068) | 0.095 | |
| Unknown | 2.652 (1.266–5.555) | 0.010 | 1.706 (0.793–3.669) | 0.172 | 1.768 (0.813–3.842) | 0.151 | |
| Stage | Localized | Reference | NA | Reference | NA | Reference | NA |
| Regional | 1.801 (1.284–2.525) | <0.001 | 1.718 (1.202–2.456) | 0.003 | 1.791 (1.234–2.601) | 0.002 | |
| Distant | 3.495 (2.366–5.163) | <0.001 | 2.456 (1.563–3.859) | <0.001 | 2.661 (1.644–4.308) | <0.001 | |
| Unstaged | 2.806 (1.550–5.080) | <0.001 | 1.523 (0.795–2.917) | 0.205 | 1.588 (0.801–3.151) | 0.186 | |
| Tumor size (mm) | <73 | Reference | NA | Reference | NA | Reference | NA |
| ≥73 | 2.684 (1.993–3.613) | <0.001 | 3.374 (2.420–4.704) | <0.001 | 3.399 (2.423–4.769) | <0.001 | |
| Radiation | Yes | Reference | NA | Reference | NA | ||
| No | 1.116 (0.799–1.560) | 0.519 | 0.664 (0.452–0.976) | 0.037 | |||
| Surgery | Yes | Reference | NA | Reference | NA | Reference | NA |
| No | 2.553 (1.743–3.740) | <0.001 | 2.302 (1.492–3.554) | <0.001 | 2.434 (1.561–3.796) | <0.001 | |
CI – confidence interval; NA – not applicable; HR – hazard ratio; mm – millimeter.
Univariable Cox regression analysis in the unmatched cohort.
Multivariable Cox regression adjusted for chemotherapy, age, race, marital status, primary site, grade, stage, tumor size and surgery in the unmatched cohort.
Multivariable Cox regression adjusted for all characteristics in the study in the unmatched cohort.
In the multivariable Cox regression analysis adjusted for related characteristics for OS rate in the original unmatched cohort (Table 3), age ≥60 (HR, 2.095; 95% CI, 1.503–2.922; p<0.001), the trunk (HR, 2.181; 95% CI, 1.488–3.197; p<0.001) and the visceral (HR, 2.964; 95% CI, 1.951–4.505; p<0.001) as the primary site of ESOS, the regional (HR, 1.718; 95% CI, 1.202–2.456; p=0.003) and the distant (HR, 2.456; 95% CI, 1.563–3.859; p<0.001) tumor stage, tumor size ≥73 mm (HR, 3.374; 95% CI, 2.420–4.704; p<0.001) and no surgical resection (HR, 2.302; 95% CI, 1.492–3.554; p<0.001) were all reported as independent prognostic factors for OS of ESOS. Independent prognostic factors reported in the multivariable Cox regression models in IPTW and PSM cohorts remained virtually the same after adjustment for related characteristics (Supplementary Tables 2, 3). Likewise, prognostic factors for OS rate remained statistically significant for CSS rate in patients with ESOS (Supplementary Table 4). CSS rate was also significantly associated with grade III/IV disease (HR, 4.344; 95% CI, 1.346–14.023; p=0.014).
Treatment effect of chemotherapy on survival in subgroups
Within subgroup analysis in the PSM-adjusted cohort, almost no significantly different treatment effects of chemotherapy were noted according to age, tumor sites, stage, size, or surgery (Figure 2). Specifically, there was a statistical difference in the effect of chemotherapy in the group that had regional ESOS (HR, 0.538; 95% CI, 0.295–0.979; p=0.042). However, use of chemotherapy did not reach statistical significance in patients with localized ESOS that was (HR, 1.189; 95% CI, 0.668–2.115; p=0.556) and ESOS <73 mm (HR, 1.011; 95% CI, 0.552–1.848; p=0.975). Of note, in patients with distant metastasis in the original unmatched cohort, chemotherapy failed to exert significant effect on OS and CSS rates (Supplementary Figure 5A, 5C). The insignificant treatment effects were consistent in the PSM-adjusted cohorts of patients with distant metastasis (Supplementary Figure 5B, 5D). We further performed interaction tests to analyze heterogeneity of the effect of chemotherapy in different subgroups. As illustrated in Figure 2, no statistically significant difference was noted in interaction tests.
Figure 2.
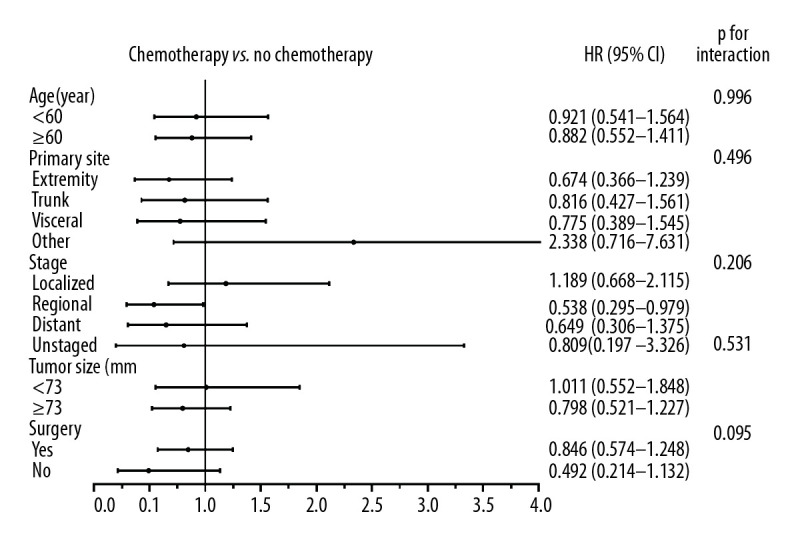
Forest plot representing the hazard ratio (HR) and 95% confidence interval (CI) of overall survival in patients treated with and without chemotherapy in subgroup analysis.
Discussion
In recent decades, uncertainty has existed about the optimal treatment strategy for ESOS because of the extremely low incidence and limited number of studies [6,20,21]. Surgery is regarded as the mainstay for ESOS, which has improved prognosis, as reported in many studies [4,9,10,21,22]. As one of predisposing factors for ESOS, radiation therapy was not universally included in conventional treatment and the effect of radiation therapy was limited [6,23,24]. Nevertheless, little was known about the effect of chemotherapy on prognosis of ESOS, and its role is still contentious [4–6,9,20–22,24]. Inconsistent results of treatment with chemotherapy may be explained by the relatively small sample size and bias of related studies [5,25,26]. To address the aforementioned limitations, we conducted IPTW- and PSM-adjusted analysis of a sample of 310 patients with ESOS who were assigned to groups with chemotherapy or without chemotherapy. No significant prognostic improvement was noted in the group receiving chemotherapy. In addition, subgroup analysis indicated that the insignificant treatment effect was consistent across all different subgroups. Several independent prognostic factors for ESOS were also identified through multivariable COX regression analysis. There are some noteworthy findings in the current study.
This study represents large cohorts of ESOS with 310 patients included based in the SEER database from 1976 to 2016. Because of the low incidence of ESOS, the majority of published studies concerning ESOS were case reports [27–29]. Median age was 60 years in the current study and most tumors were high-grade (grade III/IV), which was consistent with the common characteristics of ESOS [20,24]. The 5-year OS rate was 43.1% in current study, also comparable with those in previous studies with relatively large sample size of ESOS [6,20]. During the different time periods, the ratio of patients treated with and without chemotherapy remained relatively unchanged. It is of great significance to investigate several influential factors associated with use of chemotherapy. Age-related comorbidities and dysfunction may increase the risk of chemotherapy-related complications and morbidities [30,31]. The significantly negative effect of older age (≥60 years) on chemotherapy use was noted in the current study. However, the reason why chemotherapy was used less for ESOS in the trunk remains relatively unclear. The prognosis of osteosarcoma and soft tissue sarcomas with distant metastasis is poorer, and treatment options are more limited [32].
Chemotherapy, as one type of non-surgical treatment, is relatively favored in patients with distant metastases [33]. However, there was no significant benefit of chemotherapy on prognosis of ESOS in the current study. It has been reported that targeted agents can be used as treatment, but related information about that is still limited [34].
Based on the propensity scores calculated according to all the covariables that may mislead treatment allocation, allocation bias was largely attenuated [35]. The insignificant treatment effect of chemotherapy in the original cohort was also noted in the adjusted and matched groups on the basis of propensity scores. There are also several studies concerning treatment of ESOS in which chemotherapy had no effect on OS [6,7,9,10,22,24,25,36,37]. Although ESOS shares several similarities with conventional osteosarcoma in histology, the disease originates in various locations throughout the whole body and has very different clinicopathologic characteristics [10,21,25,33]. The name of extraskeletal osteosarcoma may lead to a misperception about ESOS, as it is actually more like soft tissue sarcoma and insensitive to chemotherapy [21,24]. It seemed that chemotherapy treatment impropved prognosis in one European study with 266 patients with ESOS [4]. However, the data in that study were collected from 16 centers throughout Europe where chemotherapy protocols were distinct, and ESOS was the secondary malignancy in 12 patients in that study. Univariable and further multivariable analysis without covariables matched or weighted may also lead to less reliable conclusions [15,38]. Another study that reported the potential for improved benefit with chemotherapy in patients with ESOS included only 17 patients, whose mean age was only 44 years [8]. Interestingly, chemotherapy protocols in studies of ESOS are divided into type of osteosarcoma and type of soft tissue sarcoma, while controversy about optimal protocols still exists [4,5,8,9,21,22,25,36,37]. Chemotherapy-induced complications remain a major cause of morbidities in patients with malignancy, and especially those who are elderly [39,40]. Considering the insignificant treatment effect on ESOS, chemotherapy may not be recommended as the conventional treatment in this disease.
In the current study, we also found that age ≥60 years, the trunk and viscera as the primary site of ESOS, regional and distant metastasis, size ≥73 mM, and no surgical resection were significantly related to poor prognosis of ESOS, which was also consistent in the IPTW and PSM cohorts. Soft tissue sarcomas in elderly patients tend to metastasize and relapse, but aggressive treatment is not suitable for those patients because of their age-related comorbidities and dysfunctions [30,31]. In one study that included 43 patients with ESOS, tumors located in the viscera were reported to be associated with poorer prognosis [9]. As several studies about ESOS indicated, tumor depth may be one significant predictor of patient survival [6,9,21,22,24]. Compared with the lower and upper extremities, the trunk and abdominal cavity have the anatomical space and may allow more extension of soft tissue sarcomas, leading to poor prognosis in those sites [41–43]. The SEER historic stage was introduced in the current study while there was a lack of complete information about the American Joint Committee on Cancer (AJCC) stage system within SEER data because of the database design [12]. Distant metastasis of tumor was related to poor prognosis and ESOS is no exception [44]. The poor prognosis for regional ESOS still needs further research. Median tumor size ranged from 5.9 cm to 10 cm in related studies of ESOS, and was indicated to be a prognostic factor for poorer prognosis [9,10,21,22,36,44]. Surgical resection may reduce tumor burden and improve survival and was regarded as optimal treatment for ESOS [4,9,10]. In the subgroup analysis, it is noteworthy that the insignificant treatment effect of chemotherapy was consistent across different subgroups. For patients with regional ESOS, chemotherapy seems to have a survival benefit. However, because analysis of five subgroups including 14 different characteristics suggested an insignificant treatment effect and all p for interaction > 0.05, the effect of chemotherapy on ESOS at the regional stage still needs further study.
This study also had limitations in design and data. First, detailed information about chemotherapy including chemotherapy regimens, doses, and the specific number of cycles was not recorded in the SEER database. Therefore, we could not make further conclusion about the specific treatment effects of chemotherapy stratified by regimens and other elements. Similar limitations can also be found in high-quality studies in which the specific protocol for chemotherapy was missing [45,46]. Second, although IPTW- and PSM-adjusted analysis are efficient for mitigating selection bias caused by observed cofounders, some unobserved cofounders, including specific surgical types, distant metastatic sites, and tumor necrosis rates, may have some effects on prognosis. Nevertheless, through systematic multiple analysis of 12 sociodemographic, tumor-related and treatment-related covariables, the insignificant treatment effect of chemotherapy was stable across all the cohorts. Third, we mainly concentrated on the treatment effect of chemotherapy on the OS and CSS in this study. Further study could include other aspects, such as treatment-related complications, treatment costs, and quality of life, to comprehensively assess patient status. Finally, this study is retrospective because of the properties of the SEER database [11]. However, it is not possible to conduct randomized controlled trials (RCT) of ESOS because of the epidemiological and clinical characteristics of the disease.
Conclusions
To summarize, this study suggests no significant benefit for chemotherapy on prognosis of ESOS. This study represents a systemic assessment of the comparative effectiveness of chemotherapy and no chemotherapy in a large cohort of patients with ESOS. Although there are several limitations, including the retrospective design and lack of some treatment information, we believe that these findings should be given serious consideration when making treatment decisions for patients with ESOS.
Supplementary Data
Study design. Data from patients with a histologically confirmed diagnosis of ESOS were extracted from the Surveillance Epidemiology and End Results (SEER) database (1975–2016), and then were analyzed according to the study design.
This graph illustrates use of chemotherapy for patients with ESOS over time in an original unmatched cohort from the SEER database, 1976 to 2016.
Supplementary Table 1.
Sociodemographic and clinical characteristics of study patients after propensity score matching.
| Characteristic | Overall (n=202) | Chemotherapy (n=101) | No chemotherapy (n=101) | P value | |
|---|---|---|---|---|---|
| Age (year) | <60 | 97 (48.0) | 49 (48.5) | 48 (47.5) | 1 |
| ≥60 | 105 (52.0) | 52 (51.5) | 53 (52.5) | ||
| Gender | Male | 91 (45.0) | 46 (45.5) | 45 (44.6) | 1 |
| Female | 111 (55.0) | 55 (54.5) | 56 (55.4) | ||
| Race | White | 163 (80.7) | 81 (80.2) | 82 (81.2) | 0.93 |
| Black | 25 (12.4) | 12 (11.9) | 13 (12.9) | ||
| Other | 14 (6.9) | 8 (7.9) | 6 (5.9) | ||
| Insurance type | Insured | 66 (32.7) | 32 (31.7) | 34 (33.7) | 0.761 |
| Any medicaid | 13 (6.4) | 8 (7.9) | 5 (5.0) | ||
| Unknown | 123 (60.9) | 61 (60.4) | 62 (61.4) | ||
| Marital status | Single | 35 (17.3) | 18 (17.8) | 17 (16.8) | 0.898 |
| Widowed/divorced | 47 (23.3) | 25 (24.8) | 22 (21.8) | ||
| Married | 113 (55.9) | 54 (53.5) | 59 (58.4) | ||
| Unknown | 7 (3.5) | 4 (4.0) | 3 (3.0) | ||
| Year of diagnosis | 1976 to 1985 | 16 (7.9) | 7 (6.9) | 9 (8.9) | 0.796 |
| 1986 to 1995 | 28 (13.9) | 12 (11.9) | 16 (15.8) | ||
| 1996 to 2005 | 63 (31.2) | 33 (32.7) | 30 (29.7) | ||
| 2006 to 2016 | 95 (47.0) | 49 (48.5) | 46 (45.5) | ||
| Primary site | Extremity | 84 (41.6) | 41 (40.6) | 43 (42.6) | 0.981 |
| Trunk | 53 (26.2) | 26 (25.7) | 27 (26.7) | ||
| Visceral | 37 (18.3) | 19 (18.8) | 18 (17.8) | ||
| Other | 28 (13.9) | 15 (14.9) | 13 (12.9) | ||
| Grade | Grade I/II | 12 (5.9) | 6 (5.9) | 6 (5.9) | 0.816 |
| Grade III/IV | 134 (66.3) | 65 (64.4) | 69 (68.3) | ||
| Unknown | 56 (27.7) | 30 (29.7) | 26 (25.7) | ||
| Stage | Localized | 91 (45) | 44 (43.6) | 47 (46.5) | 0.972 |
| Regional | 68 (33.7) | 35 (34.7) | 33 (32.7) | ||
| Distant | 34 (16.8) | 17 (16.8) | 17 (16.8) | ||
| Unstaged | 9 (4.5) | 5 (5.0) | 4 (4.0) | ||
| Tumor size (mm) | <73 | 99 (49.0) | 52 (51.5) | 47 (46.5) | 0.574 |
| ≥73 | 103 (51.0) | 49 (48.5) | 54 (53.5) | ||
| Radiation | Yes | 54 (26.7) | 25 (24.8) | 29 (28.7) | 0.634 |
| No | 148 (73.3) | 76 (75.2) | 72 (71.3) | ||
| Surgery | Yes | 175 (86.6) | 86 (85.1) | 89 (88.1) | 0.680 |
| No | 27 (13.4) | 15 (14.9) | 12 (11.9) |
mm – millimeter.
Standardized mean differences (SMDs) of different cohorts are presented in this graph.
As is shown in the landmark analysis in this graph, there was no significant difference in the effect of chemotherapy on survival of two treatment groups at different time periods.
Supplementary Table 2.
Cox regression models for OS in patients with ESOS in the IPTW cohort.
| Characteristic | Model 1* (weighted cohort) | Model 2** (weighted cohort) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Chemotherapy | No | Reference | NA | Reference | NA |
| Yes | 0.737 (0.533–1.021) | 0.066 | 0.735 (0.529–1.019) | 0.065 | |
| Age(year) | <60 | Reference | NA | Reference | NA |
| ≥60 | 2.066 (1.472–2.901) | <0.001 | 2.239 (1.575–3.183) | <0.001 | |
| Gender | Male | Reference | NA | ||
| Female | 0.865 (0.578–1.294) | 0.481 | |||
| Race | White | Reference | NA | Reference | NA |
| Black | 0,817 (0.448–1.491) | 0.509 | 0.897 (0.488–1.648) | 0.727 | |
| Other | 0.679 (0.346–1.331) | 0.259 | 0.649 (0.325–1.297) | 0.221 | |
| Insurance type | Insured | Reference | NA | ||
| Any medicaid | 1.339 (0.639–2.803) | 0.439 | |||
| Unknown | 1.787 (0.869–3.673) | 0.114 | |||
| Marital status | Single | Reference | NA | Reference | NA |
| Widowed/divorced | 1.238 (0.705–2.175) | 0.458 | 1.361 (0.774–2.393) | 0.285 | |
| Married | 1.074 (0.624–1.847) | 0.798 | 1.116 (0.645–1.929) | 0.695 | |
| Unknown | 2.824 (0.992–8.039) | 0.052 | 2.222 (0.801–6.955) | 0.171 | |
| Year of diagnosis | 1976 to 1985 | Reference | NA | ||
| 1986 to 1995 | 1.405 (0.754–2.621) | 0.284 | |||
| 1996 to 2005 | 0.903 (0.511–1.599) | 0.727 | |||
| 2006 to 2016 | 2.078(0.917–4.707) | 0.079 | |||
| Primary site | Extremity | Reference | NA | Reference | NA |
| Trunk | 2.347 (1.605–3.433) | <0.001 | 2.559 (1.709–3.833) | <0.001 | |
| Visceral | 3.071 (1.918–4.919) | <0.001 | 3.384 (2.078–5.509) | <0.001 | |
| Other | 1.097 (0.593–2.029) | 0.769 | 1.184 (0.637–2.202) | 0.593 | |
| Grade | Grade I/II | Reference | NA | Reference | NA |
| Grade III/IV | 1.383 (0.747–2.562) | 0.302 | 1.235 (0.598–2.551) | 0.568 | |
| Unknown | 1.222 (0.643–2.323) | 0.541 | 1.162 (0.579–2.334) | 0.672 | |
| Stage | Localized | Reference | NA | Reference | NA |
| Regional | 1.632 (1.139–2.37) | <0.001 | 1.629 (1.119–2.371) | 0.010 | |
| Distant | 2.204 (1.376–3.532) | <0.001 | 2.311 (1.364–3.916) | 0.002 | |
| Unstaged | 1.114 (0.521–2.377) | 0.781 | 1.093 (0.487–2.455) | 0.829 | |
| Tumor size (mm) | <73 | Reference | NA | Reference | NA |
| ≥73 | 3.969 (2.805–5.616) | <0.001 | 4.095 (2.840–5.904) | <0.001 | |
| Radiation | Yes | Reference | NA | ||
| No | 0.757 (0.509–1.126) | 0.169 | |||
| Surgery | Yes | Reference | NA | Reference | NA |
| No | 2.841 (1.716–4.701) | <0.001 | 3.049 (1.811–5.133) | <0.001 | |
CI – confidence interval; EOSS – extraskeletal osteosarcoma; IPTW – inverse probability of treatment weighting; NA – not applicable; HR – hazard ratio; mm – millimeter; OSM – osteosarcoma.
Multivariable Cox regression adjusted for chemotherapy, age, race, marital status, primary site, grade, stage, tumor size and surgery in the weighted cohort.
Multivariable Cox regression adjusted for all characteristics in the study of weighted cohort.
Supplementary Table 3.
Cox regression models for OS in patients with ESOS in the PSM cohort.
| Characteristic | Model 1* (Matched cohort) | Model 2** (Matched cohort) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Chemotherapy | No | Reference | NA | Reference | NA |
| Yes | 0.804 (0.552–1.172) | 0.257 | 0.814 (0.557–1.189) | 0.287 | |
| Age (year) | <60 | Reference | NA | Reference | NA |
| ≥60 | 1.782 (1.187–2.676) | 0.005 | 2.062 (1.304–3.259) | 0.002 | |
| Gender | Male | Reference | NA | ||
| Female | 0.905 (0.583–1.403) | 0.654 | |||
| Race | White | Reference | NA | Reference | NA |
| Black | 0.899 (0.486–1.663) | 0.735 | 0.962 (0.513–1.805) | 0.905 | |
| Other | 0.746 (0.286–1.946) | 0.551 | 0.773 (0.288–2.079) | 0.611 | |
| Insurance type | Insured | Reference | NA | ||
| Any medicaid | 1.661 (0.726–3.798) | 0.229 | |||
| Unknown | 1.915 (0.811–4.525) | 0.138 | |||
| Marital status | Single | Reference | NA | Reference | NA |
| Widowed/divorced | 0.927 (0.506–1.698) | 0.806 | 0.954 (0.507–1.795) | 0.884 | |
| Married | 0.834 (0.482–1.442) | 0.516 | 0.862 (0.484–1.534) | 0.612 | |
| Unknown | 2.004(0.698–5.753) | 0.197 | 2.079 (0.655–6.601) | 0.214 | |
| Year of diagnosis | 1976 to 1985 | Reference | NA | ||
| 1986 to 1995 | 1.357 (0.644–2.863) | 0.422 | |||
| 1996 to 2005 | 0.763 (0.376–1.546) | 0.453 | |||
| 2006 to 2016 | 1.535 (0.588–4.005) | 0.382 | |||
| Primary site | Extremity | Reference | NA | Reference | NA |
| Trunk | 2.148 (1.319–3.495) | 0.002 | 2.329 (1.389–3.904) | 0.001 | |
| Visceral | 2.521 (1.481–4.292) | 0.001 | 2.767 (1.577–4.853) | <0.001 | |
| Other | 0.881 (0.452–1.715) | 0.709 | 0.951 (0.477–1.889) | 0.883 | |
| Grade | Grade I/II | Reference | NA | Reference | NA |
| Grade III/IV | 1.015 (0.445–2.319) | 0.971 | 1.061 (0.452–2.486) | 0.893 | |
| Unknown | 0.886 (0.366–2.144) | 0.788 | 0.903 (0.365–2.237) | 0.826 | |
| Stage | Localized | Reference | NA | Reference | NA |
| Regional | 1.757 (1.105–2.793) | 0.017 | 1.762 (1.092–2.842) | 0.021 | |
| Distant | 2.626 (1.512–4.561) | 0.001 | 3.221 (1.773–5.849) | <0.001 | |
| Unstaged | 1.585 (0.702–3.581) | 0.268 | 1.582 (0.652–3.838) | 0.311 | |
| Tumor size (mm) | <73 | Reference | NA | Reference | NA |
| ≥73 | 4.571 (2.952–7.705) | <0.001 | 4.967 (3.132–7.878) | <0.001 | |
| Radiation | Yes | Reference | NA | ||
| No | 0.641 (0.401–1.025) | 0.063 | |||
| Surgery | Yes | Reference | NA | Reference | NA |
| No | 3.957 (2.269–6.902) | <0.001 | 4.345 (2.432–7.763) | <0.001 | |
CI – confidence interval; ESOS – extraskeletal osteosarcoma; NA – not applicable; HR – hazard ratio; mm – millimeter; OSM – osteosarcoma; PSM – propensity score matched.
Multivariable Cox regression adjusted for chemotherapy, age, race, marital status, primary site, grade, stage, tumor size and surgery in the matched cohort.
Multivariable Cox regression adjusted for all characteristics in the study in the matched cohort.
Supplementary Table 4.
Cox regression models for cancer-specific survival in patients with extraskeletal osteosarcoma in the unmatched cohort.
| Characteristic | Unadjusted* (Unmatched cohort) | Model 1** (Unmatched cohort) | Model 2*** (Unmatched cohort) | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Chemotherapy | No | Reference | NA | Reference | NA | Reference | NA |
| Yes | 0.887 (0.637–1.236) | 0.479 | 0.765 (0.533–1.099) | 0.147 | 0.727 (0.503–1.049) | 0.088 | |
| Age (year) | <60 | Reference | NA | Reference | NA | Reference | NA |
| ≥60 | 1.547 (1.107–2.163) | 0.011 | 1.672 (1.149–2.432) | 0.007 | 1.680 (1.123–2.513) | 0.012 | |
| Gender | Male | Reference | NA | Reference | NA | ||
| Female | 0.933 (0.670–1.299) | 0.681 | 0.922 (0.619–1.375) | 0.692 | |||
| Race | White | Reference | NA | Reference | NA | ||
| Black | 0.944 (0.567–1.571) | 0.826 | 1.219 (0.702–2.116) | 0.482 | |||
| Other | 0.650 (0.303–1.395) | 0.269 | 0.825 (0.358–1.904) | 0.652 | |||
| Insurance type | Insured | Reference | NA | Reference | NA | ||
| Any medicaid | 1.201 (0.606–2.381) | 0.600 | 0.940 (0.427–2.070) | 0.878 | |||
| Unknown | 0.853 (0.593–1.228) | 0.392 | 1.529 (0.697–3.357) | 0.290 | |||
| Marital status | Single | Reference | NA | Reference | NA | Reference | NA |
| Widowed/divorced | 1.864 (1.074–3.236) | 0.027 | 1.573 (0.866–2.856) | 0.137 | 1.682 (0.910–3.109) | 0.097 | |
| Married | 1.525 (0.924–2.517) | 0.099 | 1.440 (0.835–2.482) | 0.190 | 1.410 (0.797–2.495) | 0.238 | |
| Unknown | 1.717 (0.584–5.053) | 0.326 | 2.123 (0.703–6.412) | 0.182 | 1.739 (0.541–5.591) | 0.353 | |
| Year of diagnosis | 1976 to 1985 | Reference | NA | Reference | NA | ||
| 1986 to 1995 | 1.269 (0.578–2.787) | 0.553 | 1.422 (0.610–3.313) | 0.415 | |||
| 1996 to 2005 | 0.950 (0.465–1.942) | 0.888 | 0.849 (0.391–1.843) | 0.680 | |||
| 2006 to 2016 | 1.266 (0.629–2.549) | 0.509 | 1.809 (0.655–4.994) | 0.253 | |||
| Primary site | Extremity | Reference | NA | Reference | NA | Reference | NA |
| Trunk | 1.608 (1.051–2.460) | 0.028 | 1.953 (1.250–3.053) | 0.003 | 2.138 (1.338–3.415) | 0.001 | |
| Visceral | 3.716 (2.416–5.717) | <0.001 | 3.079 (1.914–4.952) | <0.001 | 3.462 (2.069–5.791) | <0.001 | |
| Other | 1.108 (0.628–1.954) | 0.724 | 1.377 (0.731–2.593) | 0.322 | 1.441 (0.748–2.778) | 0.275 | |
| Grade | Grade I/II | Reference | NA | Reference | NA | Reference | NA |
| Grade III/IV | 4.738 (1.502–14.952) | 0.008 | 4.344 (1.346–14.023) | 0.014 | 4.076 (1.250–13.297) | 0.020 | |
| Unknown | 4.923 (1.521–15.936) | 0.008 | 2.779 (0.838–9.220) | 0.095 | 2.850 (0.850–9.558) | 0.090 | |
| Stage | Localized | Reference | NA | Reference | NA | Reference | NA |
| Regional | 1.830 (1.218–2.751) | 0.004 | 1.543 (1.009–2.359) | 0.045 | 1.718 (1.099–2.686) | 0.018 | |
| Distant | 4.142 (2.675–6.413) | <0.001 | 2.717 (1.642–4.495) | <0.001 | 3.255 (1.878–5.644) | <0.001 | |
| Unstaged | 3.274 (1.698–6.315) | <0.001 | 1.673 (0.817–3.423) | 0.159 | 1.957 (0.906–4.227) | 0.088 | |
| Tumor size (mm) | <73 | Reference | NA | Reference | NA | Reference | NA |
| ≥73 | 2.766 (1.947–3.931) | <0.001 | 3.026 (2.066–4.432) | <0.001 | 3.184 (2.153–4.710) | <0.001 | |
| Radiation | Yes | Reference | NA | Reference | NA | ||
| No | 0.977 (0.669–1.428) | 0.906 | 0.575 (0.367–0.901) | 0.016 | |||
| Surgery | Yes | Reference | NA | Reference | NA | Reference | NA |
| No | 2.894 (1.887–4.438) | <0.001 | 2.533 (1.568–4.092) | <0.001 | 2.729 (1.652–4.506) | <0.001 | |
CI – confidence interval; NA – not applicable; HR – hazard ratio; mm – millimeter.
Univariable Cox regression analysis in the unmatched cohort.
Multivariable Cox regression adjusted for chemotherapy, age, marital status, primary site, grade, stage, tumor size and surgery in the unmatched cohort.
Multivariable Cox regression adjusted for all characteristics in the study in the unmatched cohort.
These graphs show Kaplan-Meier survival curves of overall survival and cancer-specific survival in (A, C) unmatched and (B, D) propensity score-matched cohorts of patients with distant metastasis.
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (NSFC; No. 81902745), Hunan Provincial Research and Development Program in Key Areas (2019WK2071), Natural Science Foundation of Hunan Province (No. 2018JJ3716), Key Research and Development Program of Hunan Province Science and Technology Department (No. 2017DK2013), Scholar Support Funding from the Hunan Association for Science and Technology (No. 2017TJ-Q19) and the Hunan Provincial financial department program (No. [2018]95)
References
- 1.Wilson H. Extraskeletal ossifying tumors. Ann Surg. 1941;113(1):95–112. doi: 10.1097/00000658-194101000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campanacci M. Bone and soft tissue tumors: Clinical features, imaging, pathology and treatment. Springer Science & Business Media; 2013. [Google Scholar]
- 3.Rosenberg A, Nielsen G, Fletcher J, et al. WHO classification of tumors: Pathology and genetics of tumors of soft tissue and bone. 2005 [Google Scholar]
- 4.Longhi A, Bielack SS, Grimer R, et al. Extraskeletal osteosarcoma: A European Musculoskeletal Oncology Society study on 266 patients. Eur J Cancer. 2017;74:9–16. doi: 10.1016/j.ejca.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Roller LA, Chebib I, Bredella MA, Chang CY. Clinical, radiological, and pathological features of extraskeletal osteosarcoma. Skelet Radiol. 2018;47(9):1213–20. doi: 10.1007/s00256-018-2908-6. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: Response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521–27. doi: 10.1200/JCO.2002.20.2.521. [DOI] [PubMed] [Google Scholar]
- 7.McCarter MD, Lewis JJ, Antonescu CR, Brennan MF. Extraskeletal osteosarcoma: Analysis of outcome of a rare neoplasm. Sarcoma. 2000;4(3):119–23. doi: 10.1080/13577140020008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein-Jackson SY, Gosheger G, Delling G, et al. Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol. 2005;131(8):520–26. doi: 10.1007/s00432-005-0687-7. [DOI] [PubMed] [Google Scholar]
- 9.Paludo J, Fritchie K, Haddox CL, et al. Extraskeletal osteosarcoma: Outcomes and the role of chemotherapy. Am J Clin Oncol. 2018;41(9):832–37. doi: 10.1097/COC.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma. Am J Clin Oncol. 2016;39(1):32–36. doi: 10.1097/COC.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub 1975–2016 varying) – Linked To County Attributes – Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019 based on the November 2018 submission
- 12.Ruhl JL, Hurlbut A, Ries LAG, et al. Codes and coding instructions. National Cancer Institute; Bethesda, MD: 2018. [Google Scholar]
- 13.Stuart EA, Azur M, Frangakis C, Leaf P. Multiple imputation with large data sets: A case study of the Children’s Mental Health Initiative. Am J Epidemiol. 2009;169(9):1133–39. doi: 10.1093/aje/kwp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DB. Multiple imputation for nonresponse in surveys. Vol. 81. John Wiley & Sons; 2004. [Google Scholar]
- 15.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–71. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33(7):1242–58. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabbri N, Tiwari A, Umer M, Vanel D. Extraskeletal osteosarcoma: Clinico-pathologic features and results of multimodal management. J Clin Oncol. 2010;28(15 Suppl):Supp_XIV. [Google Scholar]
- 21.Fan Z, Patel S, Lewis VO, et al. Should high-grade extraosseous osteosarcoma be treated with multimodality therapy like other soft tissue sarcomas? Clin Orthopaedics Related Res. 2015;473(11):3604–11. doi: 10.1007/s11999-015-4463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao Z, Qiu M, Yang J, et al. Outcomes of surgery and/or combination chemotherapy for extraskeletal osteosarcoma: A single-center retrospective study from China. Sci Rep. 2019;9(1):4816. doi: 10.1038/s41598-019-41089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiklund TA, Blomqvist CP, Räty J, et al. Postirradiation sarcoma. Analysis of a nationwide cancer registry material. Cancer. 1991;68(3):524–31. doi: 10.1002/1097-0142(19910801)68:3<524::aid-cncr2820680313>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: A review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2. doi: 10.2106/JBJS.M.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nystrom LM, Reimer NB, Reith JD, et al. The treatment and outcomes of extraskeletal osteosarcoma: Institutional experience and review of the literature. Iowa Orthopaedic J. 2016;36:98–103. [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Lee MR, Lee SJ, et al. Extraosseous osteosarcoma: Single institutional experience in Korea. Asia Pac J Clin Oncol. 2010;6(2):126–29. doi: 10.1111/j.1743-7563.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu L, Yang SJ. Primary osteosarcoma of the liver: Case report and literature review. Path Oncology Res. 2018;(26):115–20. doi: 10.1007/s12253-018-0483-8. [DOI] [PubMed] [Google Scholar]
- 28.Al-Janabi Y, Al-Janabi K, Tzafetta K, Petkar M. Primary cutaneous osteosarcoma of the scalp. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-222641. bcr-2017-222641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian J, Zhang XY, Gu P, et al. Primary thoracic extraskeletal osteosarcoma: A case report and literature review. J Thoracic Dis. 2017;9(12):E1088–95. doi: 10.21037/jtd.2017.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30(15):1829–34. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 31.Biau DJ, Ferguson PC, Turcotte RE, et al. Adverse Effect of older age on the recurrence of soft tissue sarcoma of the extremities and trunk. J Clin Oncology. 2011;29(30):4029–35. doi: 10.1200/JCO.2010.34.0711. [DOI] [PubMed] [Google Scholar]
- 32.Trovik C, Bauer H, Alvegård T, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36(6):710–16. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 33.Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: Spectrum of imaging findings. Am J Roentgenol. 2012;198(1):W31–37. doi: 10.2214/AJR.11.6927. [DOI] [PubMed] [Google Scholar]
- 34.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries [Internet] Bethesda (MD): National Cancer Institute (US); 2020. Mar 25, Osteosarcoma and Malignant Fibrous Histiocytoma of Bone Treatment (PDQ): Health Professional Version. 2002. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65736/ [Google Scholar]
- 35.Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: Analysis of the National Cancer Database. J Clin Oncol. 2018;36(6):600–8. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 36.Berner K, Bjerkehagen B, Bruland OS, Berner A. Extraskeletal osteosarcoma in Norway, between 1975 and 2009, and a brief review of the literature. Anticancer Res. 2015;35(4):2129–40. [PubMed] [Google Scholar]
- 37.Patel SR, Benjamin RS. Primary extraskeletal osteosarcoma? Experience with chemotherapy. J Natl Cancer Inst. 1995;87(17):1331-a. doi: 10.1093/jnci/87.17.1331-a. [DOI] [PubMed] [Google Scholar]
- 38.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56(4):1117–27. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 40.Meric F, Milas M, Hunt KK, et al. Impact of neoadjuvant chemotherapy on postoperative morbidity in soft tissue sarcomas. J Clin Oncol. 2000;18(19):3378–83. doi: 10.1200/JCO.2000.18.19.3378. [DOI] [PubMed] [Google Scholar]
- 41.Zhao R, Yu X, Feng Y, et al. The influence of anatomic location on outcomes in patients with localized primary soft tissue sarcoma. Jpn J Clin Oncol. 2018;48(9):799–805. doi: 10.1093/jjco/hyy105. [DOI] [PubMed] [Google Scholar]
- 42.Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, et al. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall. Acta Orthopaedica. 2014;85(3):323–32. doi: 10.3109/17453674.2014.908341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmoud O, Tunceroglu A, Chokshi R, et al. Overall survival advantage of chemotherapy and radiotherapy in the perioperative management of large extremity and trunk soft tissue sarcoma; A large database analysis. Radiother Oncol. 2017;124(2):277–84. doi: 10.1016/j.radonc.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 44.Thampi S, Matthay KK, Boscardin WJ, et al. Clinical features and outcomes differ between skeletal and extraskeletal osteosarcoma. Sarcoma. 2014;2014 doi: 10.1155/2014/902620. 902620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seisen T, Krasnow RE, Bellmunt J, et al. Effectiveness of adjuvant chemotherapy after radical nephroureterectomy for locally advanced and/or positive regional lymph node upper tract urothelial carcinoma. J Clin Oncol. 2017;35(8):852–60. doi: 10.1200/JCO.2016.69.4141. [DOI] [PubMed] [Google Scholar]
- 46.Seisen T, Jamzadeh A, Leow JJ, et al. Adjuvant chemotherapy vs. observation for patients with adverse pathologic features at radical cystectomy previously treated with neoadjuvant chemotherapy. JAMA Oncol. 2018;4(2):225–29. doi: 10.1001/jamaoncol.2017.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study design. Data from patients with a histologically confirmed diagnosis of ESOS were extracted from the Surveillance Epidemiology and End Results (SEER) database (1975–2016), and then were analyzed according to the study design.
This graph illustrates use of chemotherapy for patients with ESOS over time in an original unmatched cohort from the SEER database, 1976 to 2016.
Supplementary Table 1.
Sociodemographic and clinical characteristics of study patients after propensity score matching.
| Characteristic | Overall (n=202) | Chemotherapy (n=101) | No chemotherapy (n=101) | P value | |
|---|---|---|---|---|---|
| Age (year) | <60 | 97 (48.0) | 49 (48.5) | 48 (47.5) | 1 |
| ≥60 | 105 (52.0) | 52 (51.5) | 53 (52.5) | ||
| Gender | Male | 91 (45.0) | 46 (45.5) | 45 (44.6) | 1 |
| Female | 111 (55.0) | 55 (54.5) | 56 (55.4) | ||
| Race | White | 163 (80.7) | 81 (80.2) | 82 (81.2) | 0.93 |
| Black | 25 (12.4) | 12 (11.9) | 13 (12.9) | ||
| Other | 14 (6.9) | 8 (7.9) | 6 (5.9) | ||
| Insurance type | Insured | 66 (32.7) | 32 (31.7) | 34 (33.7) | 0.761 |
| Any medicaid | 13 (6.4) | 8 (7.9) | 5 (5.0) | ||
| Unknown | 123 (60.9) | 61 (60.4) | 62 (61.4) | ||
| Marital status | Single | 35 (17.3) | 18 (17.8) | 17 (16.8) | 0.898 |
| Widowed/divorced | 47 (23.3) | 25 (24.8) | 22 (21.8) | ||
| Married | 113 (55.9) | 54 (53.5) | 59 (58.4) | ||
| Unknown | 7 (3.5) | 4 (4.0) | 3 (3.0) | ||
| Year of diagnosis | 1976 to 1985 | 16 (7.9) | 7 (6.9) | 9 (8.9) | 0.796 |
| 1986 to 1995 | 28 (13.9) | 12 (11.9) | 16 (15.8) | ||
| 1996 to 2005 | 63 (31.2) | 33 (32.7) | 30 (29.7) | ||
| 2006 to 2016 | 95 (47.0) | 49 (48.5) | 46 (45.5) | ||
| Primary site | Extremity | 84 (41.6) | 41 (40.6) | 43 (42.6) | 0.981 |
| Trunk | 53 (26.2) | 26 (25.7) | 27 (26.7) | ||
| Visceral | 37 (18.3) | 19 (18.8) | 18 (17.8) | ||
| Other | 28 (13.9) | 15 (14.9) | 13 (12.9) | ||
| Grade | Grade I/II | 12 (5.9) | 6 (5.9) | 6 (5.9) | 0.816 |
| Grade III/IV | 134 (66.3) | 65 (64.4) | 69 (68.3) | ||
| Unknown | 56 (27.7) | 30 (29.7) | 26 (25.7) | ||
| Stage | Localized | 91 (45) | 44 (43.6) | 47 (46.5) | 0.972 |
| Regional | 68 (33.7) | 35 (34.7) | 33 (32.7) | ||
| Distant | 34 (16.8) | 17 (16.8) | 17 (16.8) | ||
| Unstaged | 9 (4.5) | 5 (5.0) | 4 (4.0) | ||
| Tumor size (mm) | <73 | 99 (49.0) | 52 (51.5) | 47 (46.5) | 0.574 |
| ≥73 | 103 (51.0) | 49 (48.5) | 54 (53.5) | ||
| Radiation | Yes | 54 (26.7) | 25 (24.8) | 29 (28.7) | 0.634 |
| No | 148 (73.3) | 76 (75.2) | 72 (71.3) | ||
| Surgery | Yes | 175 (86.6) | 86 (85.1) | 89 (88.1) | 0.680 |
| No | 27 (13.4) | 15 (14.9) | 12 (11.9) |
mm – millimeter.
Standardized mean differences (SMDs) of different cohorts are presented in this graph.
As is shown in the landmark analysis in this graph, there was no significant difference in the effect of chemotherapy on survival of two treatment groups at different time periods.
Supplementary Table 2.
Cox regression models for OS in patients with ESOS in the IPTW cohort.
| Characteristic | Model 1* (weighted cohort) | Model 2** (weighted cohort) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Chemotherapy | No | Reference | NA | Reference | NA |
| Yes | 0.737 (0.533–1.021) | 0.066 | 0.735 (0.529–1.019) | 0.065 | |
| Age(year) | <60 | Reference | NA | Reference | NA |
| ≥60 | 2.066 (1.472–2.901) | <0.001 | 2.239 (1.575–3.183) | <0.001 | |
| Gender | Male | Reference | NA | ||
| Female | 0.865 (0.578–1.294) | 0.481 | |||
| Race | White | Reference | NA | Reference | NA |
| Black | 0,817 (0.448–1.491) | 0.509 | 0.897 (0.488–1.648) | 0.727 | |
| Other | 0.679 (0.346–1.331) | 0.259 | 0.649 (0.325–1.297) | 0.221 | |
| Insurance type | Insured | Reference | NA | ||
| Any medicaid | 1.339 (0.639–2.803) | 0.439 | |||
| Unknown | 1.787 (0.869–3.673) | 0.114 | |||
| Marital status | Single | Reference | NA | Reference | NA |
| Widowed/divorced | 1.238 (0.705–2.175) | 0.458 | 1.361 (0.774–2.393) | 0.285 | |
| Married | 1.074 (0.624–1.847) | 0.798 | 1.116 (0.645–1.929) | 0.695 | |
| Unknown | 2.824 (0.992–8.039) | 0.052 | 2.222 (0.801–6.955) | 0.171 | |
| Year of diagnosis | 1976 to 1985 | Reference | NA | ||
| 1986 to 1995 | 1.405 (0.754–2.621) | 0.284 | |||
| 1996 to 2005 | 0.903 (0.511–1.599) | 0.727 | |||
| 2006 to 2016 | 2.078(0.917–4.707) | 0.079 | |||
| Primary site | Extremity | Reference | NA | Reference | NA |
| Trunk | 2.347 (1.605–3.433) | <0.001 | 2.559 (1.709–3.833) | <0.001 | |
| Visceral | 3.071 (1.918–4.919) | <0.001 | 3.384 (2.078–5.509) | <0.001 | |
| Other | 1.097 (0.593–2.029) | 0.769 | 1.184 (0.637–2.202) | 0.593 | |
| Grade | Grade I/II | Reference | NA | Reference | NA |
| Grade III/IV | 1.383 (0.747–2.562) | 0.302 | 1.235 (0.598–2.551) | 0.568 | |
| Unknown | 1.222 (0.643–2.323) | 0.541 | 1.162 (0.579–2.334) | 0.672 | |
| Stage | Localized | Reference | NA | Reference | NA |
| Regional | 1.632 (1.139–2.37) | <0.001 | 1.629 (1.119–2.371) | 0.010 | |
| Distant | 2.204 (1.376–3.532) | <0.001 | 2.311 (1.364–3.916) | 0.002 | |
| Unstaged | 1.114 (0.521–2.377) | 0.781 | 1.093 (0.487–2.455) | 0.829 | |
| Tumor size (mm) | <73 | Reference | NA | Reference | NA |
| ≥73 | 3.969 (2.805–5.616) | <0.001 | 4.095 (2.840–5.904) | <0.001 | |
| Radiation | Yes | Reference | NA | ||
| No | 0.757 (0.509–1.126) | 0.169 | |||
| Surgery | Yes | Reference | NA | Reference | NA |
| No | 2.841 (1.716–4.701) | <0.001 | 3.049 (1.811–5.133) | <0.001 | |
CI – confidence interval; EOSS – extraskeletal osteosarcoma; IPTW – inverse probability of treatment weighting; NA – not applicable; HR – hazard ratio; mm – millimeter; OSM – osteosarcoma.
Multivariable Cox regression adjusted for chemotherapy, age, race, marital status, primary site, grade, stage, tumor size and surgery in the weighted cohort.
Multivariable Cox regression adjusted for all characteristics in the study of weighted cohort.
Supplementary Table 3.
Cox regression models for OS in patients with ESOS in the PSM cohort.
| Characteristic | Model 1* (Matched cohort) | Model 2** (Matched cohort) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Chemotherapy | No | Reference | NA | Reference | NA |
| Yes | 0.804 (0.552–1.172) | 0.257 | 0.814 (0.557–1.189) | 0.287 | |
| Age (year) | <60 | Reference | NA | Reference | NA |
| ≥60 | 1.782 (1.187–2.676) | 0.005 | 2.062 (1.304–3.259) | 0.002 | |
| Gender | Male | Reference | NA | ||
| Female | 0.905 (0.583–1.403) | 0.654 | |||
| Race | White | Reference | NA | Reference | NA |
| Black | 0.899 (0.486–1.663) | 0.735 | 0.962 (0.513–1.805) | 0.905 | |
| Other | 0.746 (0.286–1.946) | 0.551 | 0.773 (0.288–2.079) | 0.611 | |
| Insurance type | Insured | Reference | NA | ||
| Any medicaid | 1.661 (0.726–3.798) | 0.229 | |||
| Unknown | 1.915 (0.811–4.525) | 0.138 | |||
| Marital status | Single | Reference | NA | Reference | NA |
| Widowed/divorced | 0.927 (0.506–1.698) | 0.806 | 0.954 (0.507–1.795) | 0.884 | |
| Married | 0.834 (0.482–1.442) | 0.516 | 0.862 (0.484–1.534) | 0.612 | |
| Unknown | 2.004(0.698–5.753) | 0.197 | 2.079 (0.655–6.601) | 0.214 | |
| Year of diagnosis | 1976 to 1985 | Reference | NA | ||
| 1986 to 1995 | 1.357 (0.644–2.863) | 0.422 | |||
| 1996 to 2005 | 0.763 (0.376–1.546) | 0.453 | |||
| 2006 to 2016 | 1.535 (0.588–4.005) | 0.382 | |||
| Primary site | Extremity | Reference | NA | Reference | NA |
| Trunk | 2.148 (1.319–3.495) | 0.002 | 2.329 (1.389–3.904) | 0.001 | |
| Visceral | 2.521 (1.481–4.292) | 0.001 | 2.767 (1.577–4.853) | <0.001 | |
| Other | 0.881 (0.452–1.715) | 0.709 | 0.951 (0.477–1.889) | 0.883 | |
| Grade | Grade I/II | Reference | NA | Reference | NA |
| Grade III/IV | 1.015 (0.445–2.319) | 0.971 | 1.061 (0.452–2.486) | 0.893 | |
| Unknown | 0.886 (0.366–2.144) | 0.788 | 0.903 (0.365–2.237) | 0.826 | |
| Stage | Localized | Reference | NA | Reference | NA |
| Regional | 1.757 (1.105–2.793) | 0.017 | 1.762 (1.092–2.842) | 0.021 | |
| Distant | 2.626 (1.512–4.561) | 0.001 | 3.221 (1.773–5.849) | <0.001 | |
| Unstaged | 1.585 (0.702–3.581) | 0.268 | 1.582 (0.652–3.838) | 0.311 | |
| Tumor size (mm) | <73 | Reference | NA | Reference | NA |
| ≥73 | 4.571 (2.952–7.705) | <0.001 | 4.967 (3.132–7.878) | <0.001 | |
| Radiation | Yes | Reference | NA | ||
| No | 0.641 (0.401–1.025) | 0.063 | |||
| Surgery | Yes | Reference | NA | Reference | NA |
| No | 3.957 (2.269–6.902) | <0.001 | 4.345 (2.432–7.763) | <0.001 | |
CI – confidence interval; ESOS – extraskeletal osteosarcoma; NA – not applicable; HR – hazard ratio; mm – millimeter; OSM – osteosarcoma; PSM – propensity score matched.
Multivariable Cox regression adjusted for chemotherapy, age, race, marital status, primary site, grade, stage, tumor size and surgery in the matched cohort.
Multivariable Cox regression adjusted for all characteristics in the study in the matched cohort.
Supplementary Table 4.
Cox regression models for cancer-specific survival in patients with extraskeletal osteosarcoma in the unmatched cohort.
| Characteristic | Unadjusted* (Unmatched cohort) | Model 1** (Unmatched cohort) | Model 2*** (Unmatched cohort) | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Chemotherapy | No | Reference | NA | Reference | NA | Reference | NA |
| Yes | 0.887 (0.637–1.236) | 0.479 | 0.765 (0.533–1.099) | 0.147 | 0.727 (0.503–1.049) | 0.088 | |
| Age (year) | <60 | Reference | NA | Reference | NA | Reference | NA |
| ≥60 | 1.547 (1.107–2.163) | 0.011 | 1.672 (1.149–2.432) | 0.007 | 1.680 (1.123–2.513) | 0.012 | |
| Gender | Male | Reference | NA | Reference | NA | ||
| Female | 0.933 (0.670–1.299) | 0.681 | 0.922 (0.619–1.375) | 0.692 | |||
| Race | White | Reference | NA | Reference | NA | ||
| Black | 0.944 (0.567–1.571) | 0.826 | 1.219 (0.702–2.116) | 0.482 | |||
| Other | 0.650 (0.303–1.395) | 0.269 | 0.825 (0.358–1.904) | 0.652 | |||
| Insurance type | Insured | Reference | NA | Reference | NA | ||
| Any medicaid | 1.201 (0.606–2.381) | 0.600 | 0.940 (0.427–2.070) | 0.878 | |||
| Unknown | 0.853 (0.593–1.228) | 0.392 | 1.529 (0.697–3.357) | 0.290 | |||
| Marital status | Single | Reference | NA | Reference | NA | Reference | NA |
| Widowed/divorced | 1.864 (1.074–3.236) | 0.027 | 1.573 (0.866–2.856) | 0.137 | 1.682 (0.910–3.109) | 0.097 | |
| Married | 1.525 (0.924–2.517) | 0.099 | 1.440 (0.835–2.482) | 0.190 | 1.410 (0.797–2.495) | 0.238 | |
| Unknown | 1.717 (0.584–5.053) | 0.326 | 2.123 (0.703–6.412) | 0.182 | 1.739 (0.541–5.591) | 0.353 | |
| Year of diagnosis | 1976 to 1985 | Reference | NA | Reference | NA | ||
| 1986 to 1995 | 1.269 (0.578–2.787) | 0.553 | 1.422 (0.610–3.313) | 0.415 | |||
| 1996 to 2005 | 0.950 (0.465–1.942) | 0.888 | 0.849 (0.391–1.843) | 0.680 | |||
| 2006 to 2016 | 1.266 (0.629–2.549) | 0.509 | 1.809 (0.655–4.994) | 0.253 | |||
| Primary site | Extremity | Reference | NA | Reference | NA | Reference | NA |
| Trunk | 1.608 (1.051–2.460) | 0.028 | 1.953 (1.250–3.053) | 0.003 | 2.138 (1.338–3.415) | 0.001 | |
| Visceral | 3.716 (2.416–5.717) | <0.001 | 3.079 (1.914–4.952) | <0.001 | 3.462 (2.069–5.791) | <0.001 | |
| Other | 1.108 (0.628–1.954) | 0.724 | 1.377 (0.731–2.593) | 0.322 | 1.441 (0.748–2.778) | 0.275 | |
| Grade | Grade I/II | Reference | NA | Reference | NA | Reference | NA |
| Grade III/IV | 4.738 (1.502–14.952) | 0.008 | 4.344 (1.346–14.023) | 0.014 | 4.076 (1.250–13.297) | 0.020 | |
| Unknown | 4.923 (1.521–15.936) | 0.008 | 2.779 (0.838–9.220) | 0.095 | 2.850 (0.850–9.558) | 0.090 | |
| Stage | Localized | Reference | NA | Reference | NA | Reference | NA |
| Regional | 1.830 (1.218–2.751) | 0.004 | 1.543 (1.009–2.359) | 0.045 | 1.718 (1.099–2.686) | 0.018 | |
| Distant | 4.142 (2.675–6.413) | <0.001 | 2.717 (1.642–4.495) | <0.001 | 3.255 (1.878–5.644) | <0.001 | |
| Unstaged | 3.274 (1.698–6.315) | <0.001 | 1.673 (0.817–3.423) | 0.159 | 1.957 (0.906–4.227) | 0.088 | |
| Tumor size (mm) | <73 | Reference | NA | Reference | NA | Reference | NA |
| ≥73 | 2.766 (1.947–3.931) | <0.001 | 3.026 (2.066–4.432) | <0.001 | 3.184 (2.153–4.710) | <0.001 | |
| Radiation | Yes | Reference | NA | Reference | NA | ||
| No | 0.977 (0.669–1.428) | 0.906 | 0.575 (0.367–0.901) | 0.016 | |||
| Surgery | Yes | Reference | NA | Reference | NA | Reference | NA |
| No | 2.894 (1.887–4.438) | <0.001 | 2.533 (1.568–4.092) | <0.001 | 2.729 (1.652–4.506) | <0.001 | |
CI – confidence interval; NA – not applicable; HR – hazard ratio; mm – millimeter.
Univariable Cox regression analysis in the unmatched cohort.
Multivariable Cox regression adjusted for chemotherapy, age, marital status, primary site, grade, stage, tumor size and surgery in the unmatched cohort.
Multivariable Cox regression adjusted for all characteristics in the study in the unmatched cohort.
These graphs show Kaplan-Meier survival curves of overall survival and cancer-specific survival in (A, C) unmatched and (B, D) propensity score-matched cohorts of patients with distant metastasis.