Abstract
Introduction
In arthritic mice, a sympathetic influence is proinflammatory from the time point of immunization until the onset of disease (days 0–32), but reasons are unknown. Disruption of the major anti-inflammatory pathway through G<sub>αs</sub>-coupled receptors probably play a role. For example, noradrenaline cannot operate via anti-inflammatory β<sub>2</sub>-adrenoceptors but through proinflammatory α<sub>1/2</sub>-adrenoceptors. This might happen, first, through a loss of sympathetic nerve fibers in inflamed tissue with low neurotransmitter levels (noradrenaline only binds to high-affinity α-adrenoceptors) and, second, through an alteration in G-protein receptor coupling with a predominance of α-adrenergic signaling. We hypothesized that both mechanisms play a role in the course of collagen type II-induced arthritis (CIA) in the spleen in mice.
Methods
In CIA mice, nerve fiber density in the spleen was quantified by immunohistochemistry techniques. The functional impact of sympathetic nerve fibers in the spleen was studied by a microsuperfusion technique of spleen slices with a focus on the secretion of IFN-γ and IL-6 (proinflammatory) and TGF-β (anti-inflammatory).
Results
During CIA, sympathetic nerve fibers get increasingly lost from day14 until day 55 after immunization. The influence of electrically released noradrenaline diminishes in the course of arthritis. At all investigated time points (days 14, 32, and 55), only proinflammatory neuronal α-adrenergic effects on cytokine secretion were demonstrated (i.e., stimulation of IFN-γ and IL-6 and inhibition of TGF-β).
Conclusion
Sympathetic nerve fibers are rapidly lost in the spleen, and only proinflammatory α-adrenergic neuronal regulation of cytokine secretion takes place throughout the course of arthritis. These results support a predominance of a proinflammatory α-adrenergic sympathetic influence in arthritis.
Keywords: Sympathetic nervous system, Spleen, Cytokine regulation, Arthritis, Nerve fiber loss
Introduction
The influence of peripheral nerve fibers on joint inflammation is best demonstrated by the protection of the paretic body side in patients with hemiplegia (e.g., [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]). The role of the 2 types of peripheral nerve fibers that reach the joint is presently not fully understood, but studies on experimental arthritis show a proinflammatory influence of the sympathetic nervous system (SNS) and sensory afferent nerve fibers in the early phase of arthritis from experimental antigen immunization until the onset of the disease (summary: [11, 12, 13]).
In later phases of the disease, the proinflammatory impact of the SNS might switch to become anti-inflammatory, but sympathetic nerve fibers might not play a role because they are lost in inflamed tissue [14, 15]. In the later phases, cells positive for key enzymes of catecholamine secretion appear in the joints, spleen, lymph nodes, and bone marrow, and these cells exert anti-inflammatory effects in experimental arthritis [16, 17]. The role of the cholinergic system that does not directly innervate the joints is presently under investigation. It seems that cholinergic influences are anti-inflammatory [18, 19], but these findings have been challenged by some authors [20].
At the beginning of arthritis, the proinflammatory influence of sympathetic nerve fibers might be due to vascular changes in the synovial tissue and plasma extravasation [21], the propagation of pain and proinflammatory substance P secretion in the sense of neurogenic inflammation [22, 23], the regulation of lymphocyte migration [24, 25] and proinflammatory cytokines [26], the redistribution of energy-rich substrates to a highly activated immune system [11, 24], or a switch of anti-inflammatory Gαs signaling to proinflammatory Gαi signaling [13, 27], and other mechanisms.
While an entire generation of researchers thought that noradrenaline must be anti-inflammatory due to its activity via the β-adrenoceptor and the cAMP pathway, which is best exemplified by the inhibition of TNF, IFN-γ, and IL-12 [28, 29, 30], more recent data demonstrate that these anti-inflammatory effects are disrupted by the loss of sympathetic nerve fibers in inflamed tissue and the abovementioned proinflammatory Gαs-to-Gαi switch of receptor signaling [13, 14, 15, 27]. However, the functional influence of the sympathetic nerve fibers on cytokine secretion in a major target lymphoid organ like the spleen has not been studied for the entire course of arthritis.
The spleen offers an exceptional possibility to directly study the sympathetic nerve fiber/immune cell interaction, by examining spleen slices in a microsuperfusion machine developed by us [31], and used in the animal model of collagen type II-induced arthritis (CIA) [32, 33, 34]. This has the advantage that sympathetic nerve fibers and target immune cells remain in their compartments in a natural way. However, the full spectrum of adrenergic regulation of key cytokines in the spleen has never been demonstrated for the entire course of arthritis, i.e., from a time point shortly after immunization to disease onset and chronic late-stage disease. Such an analysis might find critical immune elements for the influence of the SNS on arthritis.
This study was initiated to investigate the sympathetic nerve fiber density in the spleen throughout CIA. In addition, sympathetic α1-, α2, and β-adrenergic regulation of cytokine secretion was investigated in the spleen slice model during the disease course. The effect of sympathetic noradrenaline was scrutinized using respective receptor antagonists. IFN-γ and IL-6 served as proinflammatory cytokines and TGF-β (a key cytokine of regulatory T cells) as an anti-inflammatory cytokine.
Materials and Methods
Animals
Female DBA/1 mice (aged 6–8 weeks) were purchased from Elevage Janvier (Le Genest-Saint-Isle, France). The mice were housed, 5–6 animals in a cage, and fed standard laboratory chow and water ad libitum under standard conditions. They were exposed to 12-h light/dark cycles. We used the spleens of control arthritic animals from other studies focusing on other issues over a longer period of time (2007–2015). These spleens would have been discarded but were used here for microsuperfusion experiments after being removed from the animals on day 14, 32, or 55 after immunization.
Immunohistochemistry of Sympathetic Nerve Fibers in the Spleen
A portion of the spleen not used for superfusion was fixed for 12–24 h in PBS containing 3.7% formaldehyde, and then incubated in PBS with 20% sucrose for another 12–24 h. Thereafter, the tissue was embedded in Tissue-Tek and quick-frozen. All tissue samples were stored at −80°C. A total of 6–8 cryosections (thickness 5–9 µm) were used for immunohistochemistry with a primary antibody against tyrosine hydroxylase (TH, the key enzyme for norepinephrine production in sympathetic nerve endings, cat. No. AB152, Millipore Germany, Schwalbach, Germany). An Alexa-546-conjugated secondary antibody (cat. No. A-11030 against mouse IgG, Molecular Probes, Leiden, The Netherlands) was used to achieve immunofluorescence staining of sympathetic nerve fibers. The number of splenic TH-positive sympathetic nerve fibers/mm2 was determined by averaging the number of stained nerve fibers (minimum length 50 µm, determined through a micrometer eyepiece) in 17 randomly selected high-power fields of view (×400). We controlled the positive nerve fiber staining by incubating the tissue with polyclonal control antibodies, which always yielded a negative result.
Induction of Arthritis and Removal of the Spleen
We performed these experiments as recently described [15]. Briefly, mice were immunized intradermally at the base of the tail with 100 µg of bovine collagen type II (Chondrex, Inc., via MD Biosciences, Zurich, Switzerland) emulsified in an equal volume of complete Freund's adjuvant on day 0 (Sigma, Taufkirchen, Germany).
The mice were sacrificed at 3 different time points during arthritis: the maximum immunization phase before the onset of the disease (day 14), shortly after the onset of the disease (day 32), and the late and chronic phase of the disease (day 55). At these time points, the release of splenic IFN-γ, IL-6, and TGF-β was detected from the superfusate of the spleen slices (technique below).
The spleen was removed after cervical dislocation at 11:00–12:00 a.m. and kept in ice-cold culture medium for 25 min (RPMI1640, 25 mM HEPES, pH 7.4, 5% FCS, 30 µM mercaptoethanol, 0.57 mM ascorbic acid, 1.3 mM calcium, 100 U/mL penicillin, and 100 µg/mL streptomycin [Sigma, Deisenhofen, Germany] and 8 µg/mL ciprofloxacin [Bayer, Leverkusen, Germany]). It was then cut into 0.35-mm-thick slices using a tissue chopper (Mickle Lab., Gomshall, UK) with the direction of cutting at a right angle to the longitudinal axis of the spleen, and these slices were washed carefully in the abovementioned medium to remove small particles and extravasated cells.
Superfusion Technique and Protocol and Standardization of Slices
The technique was recently demonstrated [32]. Briefly, spleen slices were transferred in the abovementioned medium to silicon superfusion microchambers with a volume of approximately 80 µL [32] (custom-made by F.I.T GmbH Fruth, 92331 Parsberg, Germany). The bottom and the top of the microchamber were equipped with platinum electrodes to apply electrical field stimulation to the tissue. The pulsating electrical field stimulates nerve fibers in the tissue, leading to the release of neurotransmitters from viable nerve terminals [32]. The electrical current was applied by a computer-driven, current-controlled, voltage-regulated stimulator (MR OEG, Vienna, Austria). The superfusion fluid was transported from sterile medium reservoirs (medium as above) using speed-controlled pumps (Ismatec, Wertheim-Mondfeld, Germany). Superfusion was performed for 6 h and a flow rate of 66 µL/min (1 slice/chamber, 32 chambers in parallel, i.e., 4 racks with 8 chambers each), a flow rate similar to that of interstitial fluid. The technique is demonstrated in detail in supplementary Figure 1.
Experiments with Antagonists and Electrical Stimulation
To indirectly study the effect of electrically released endogenous transmitters propranolol (β1/2-adrenergic antagonist), benoxathian (α1-adrenergic antagonist), and yohimbine (α2-adrenergic antagonist), all from Sigma, were used. We have used these antagonists previously [32, 33, 34], so we used only the most relevant concentration of 10−6 M in these experiments. The dilutions were prepared immediately before the experiments. In experiments to study noradrenaline effects, antagonists were added at 100 min until the end of superfusion. After a drug equilibration period of 20 min (at 100–120 min), the slices were electrically stimulated using a continuous train of 45,000 pulses of an action potential-like form (5 Hz, 2 ms, 43 mA). The technique is demonstrated in detail in online supplementary Figure 1 (for all online suppl. material, see www.karger.com/doi/10.1159/508109).
Determination of Cytokine Concentration in the Superfusate
Mouse IFN-γ in superfusate fractions was determined by ELISA (antibody pairs, BioLegend Inc., San Diego, CA, USA, via Biozol, Eching, Germany). The limit of detection was <8 pg/mL and the assay range 8–500 pg IFN-γ/mL. Similarly, mouse IL-6 in superfusate fractions was determined by a self-coated ELISA (antibody pairs, BioLegend Inc.). The limit of detection was <8 pg/mL and the assay range 8–500 pg/mL. Mouse TGF-β was similarly determined by ELISA (antibody pairs, eBioscience, Camarillo, CA, USA, via NatuTec, Frankfurt, Germany). The limit of detection was <60 pg/mL and the assay range 60–8,000 pg/mL. All Intra- and interassay coefficients of variation were <10%.
Presentation of the Data and Statistical Analysis
Using 32 chambers, we were able to investigate 32 slices in 1 experiment of 1 arthritic mouse on 1 day. Because of the technical effort, typically, 3–4 experiments were performed per week. Four different conditions were investigated: (A) 8 control slices without drug or electrical stimulation (ES), (B) 8 slices with an antagonist only, to study the nonneuronal effects of release neurotransmitters, (C) 8 slices with ES only, and (D) 8 slices with an antagonist and ES, to study the influence of the neuronally released noradrenaline. Since average phi (online suppl. Fig. 1) of one experiment varies from mouse to mouse the effects are demonstrated in percent of the control of each mouse (phi of the control is 100%). Mann-Whitney U test (SPSS, SPSS Inc., Chicago, IL, USA) was used to compare 2 groups and ANOVA on ranks was used to compare >2 groups (SPSS). p < 0.05 was the significance level.
Results
Loss of Sympathetic Nerve Fibers in the Spleen
One argument for a missing anti-inflammatory influence via β-adrenergic signaling was the loss of sympathetic nerve fibers from inflamed tissue. Loss of sympathetic nerve fibers would result in lower noradrenaline concentrations at target cells, thus, according to the model, only binding to high-affinity α-adrenergic receptors is expected [35].
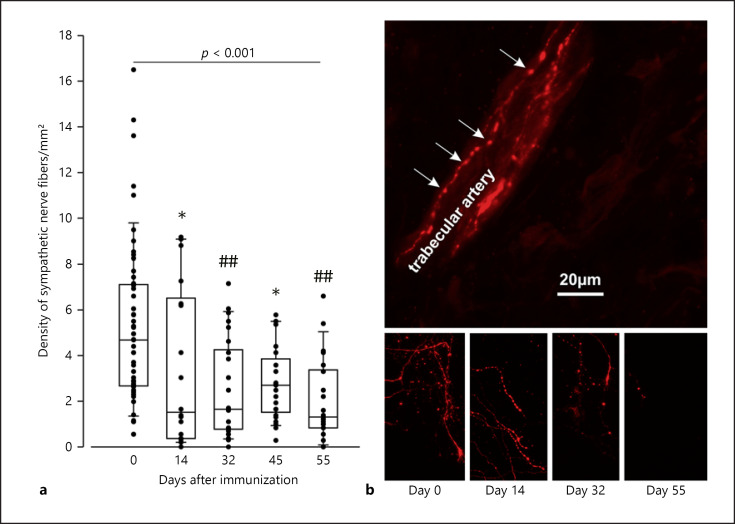
We observed a rapid decrease in sympathetic nerve fiber density in the key lymphoid target organ, the spleen (Fig. 1). The reduction was already visible at day 14 but became more pronounced in the course of arthritis. On day 55, only 20% of the original sympathetic nerve fiber density was detected, which is nearly similar to effects obtained through chemical sympathectomy with 6-hydroxydopamine (6-OHDA) demonstrated previously [36].
Fig. 1.
Density of sympathetic nerve fibers in the spleen of control and arthritic rats. a Density of sympathetic tyrosine hydroxylase-positive nerve fibers. Each dot represents the result for 1 mouse (mean from 10–17 high-power fields). * p < 0.05, ## p < 0.005 versus control 8 (day 0). The pvalue above the boxes gives the result from the ANOVA on ranks test for all groups. Data are given as box-plots with the 10th (whisker), 25th, 50th (median), 75th, and 90th (whisker) percentiles. b Immunohistochemical staining of sympathetic nerve fibers. In the top panel, a typical bead chain staining of a splenic artery is given. The arrows point to varicosities along the nerve fiber.
Electrically Induced Inhibition of IFN-γ and IL-6 and Stimulation of TGF-β.
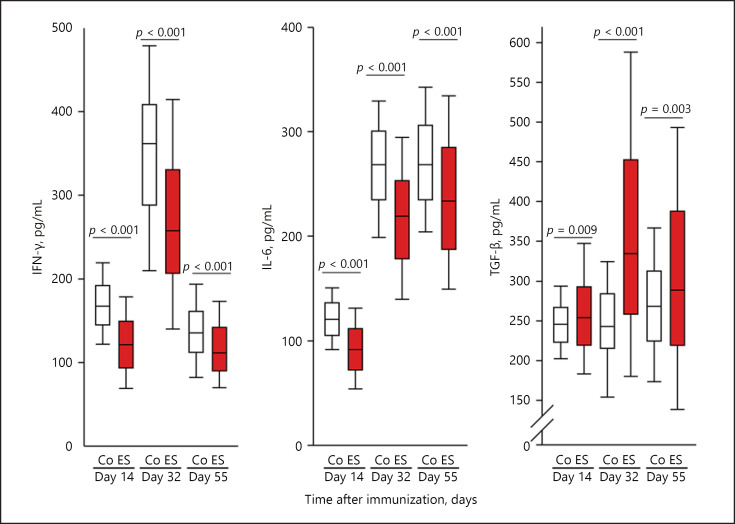
In arthritis research, IFN-γ and IL-6 constitute immunostimulatory and proinflammatory cytokines, while TGF-β is a cytokine of regulatory immune phenomena with anti-inflammatory potential. IFN-γ and IL-6 were inhibited by ES in the course of the arthritis, while TGF-β was stimulated by the same electrically released neurotransmitters (Fig. 2). This effect of electrically released neurotransmitters on cytokine secretion was stable during the entire course (Fig. 2). The individual cytokines showed different peaks and lows, i.e., IFN-γ was high around day 32, IL-6 increased from day 32 onwards, and TGF-β remained relatively stable throughout the course and reached somewhat higher levels around day 55 (Fig. 2).
Fig. 2.
Effects of electrically released neurotransmitters on cytokine secretion (IFN-γ, IL-6, and TGF-β). The individual panels show control conditions (Co, white boxes) and conditions under electrical stimulation (ES, red boxes) on days 14, 32, and 55 during arthritis. p values compare groups using Mann-Whitney U test statistics. Data are given as box-plots with the 10th (whisker), 25th, 50th (median), 75th, and 90th (whisker) percentiles. For each condition, at least 25 mice (200 spleen slices) were used.
Adrenergic Regulation of Cytokine Secretion
To study the influence of neuronal adrenergic signaling pathways, ES was combined with adrenergic antagonists to neutralize the electrically released neurotransmitter noradrenaline.
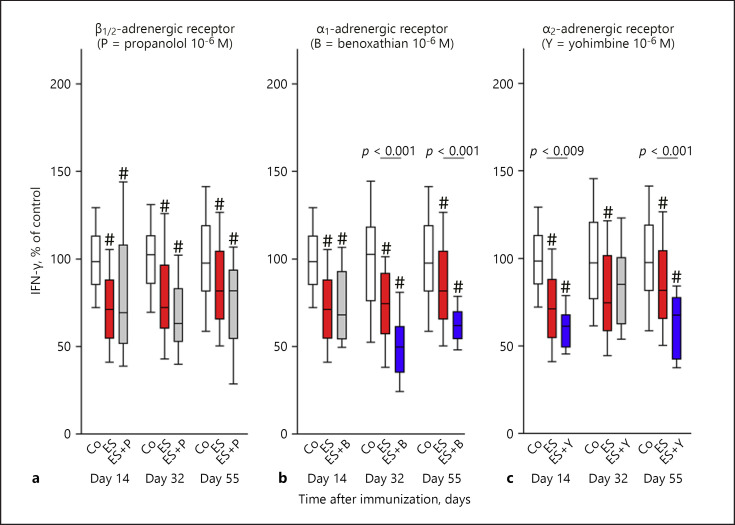
In the case of IFN-γ, β-adrenergic signaling did not play a role throughout the entire course of the arthritis (Fig. 3a). However, neuronal α1-adrenergic signaling stimulated IFN-γ secretion on days 32 and 55, because the antagonist had a decreasing influence on cytokine secretion (Fig. 3b). A very similar result was observed for neuronal α2-adrenergic signaling on days 14 and 55 (Fig. 3c).
Fig. 3.
Neuronal adrenergic regulation of IFN-γ during arthritis. The graph shows β-adrenergic (a), α1-adrenergic (b), and α2-adrenergic (c) effects. The individual panels show control conditions (Co, white boxes), conditions under electrical stimulation (ES, red boxes), and conditions under ES + antagonist (grey boxes) on days 14, 32, and 55 during arthritis. The blue boxes demonstrate the significant effects of the respective antagonist in relation to ES alone (p values are given). # p < 0.005 compared to control conditions (white box). Data are given as box-plots with the 10th (whisker), 25th, 50th (median), 75th, and 90th (whisker) percentiles. For each condition with ES + antagonist, at least 3–5 mice (24–40 spleen slices) were used. Control conditions and conditions with ES only were done with at least 13 mice (104 spleen slices).
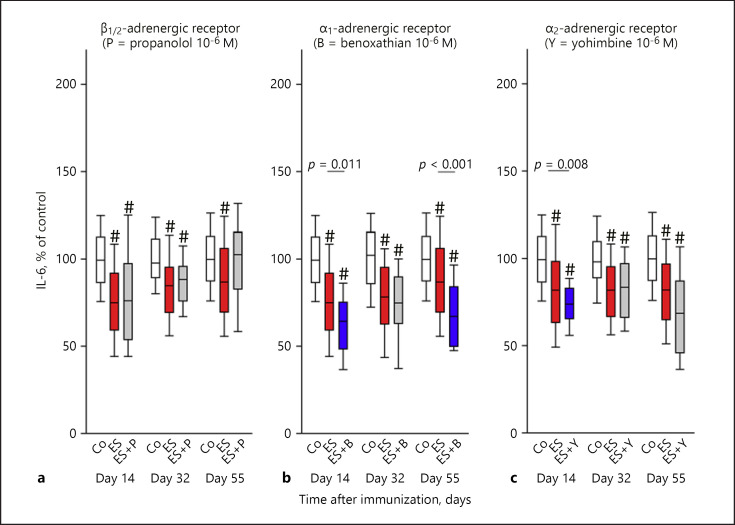
Similarly to IFN-γ, β-adrenergic signaling did not affect IL-6 secretion during the course of the arthritis (Fig. 4a). However, IL-6 was neuronally stimulated by the α1-adrenergic influence on days 14 and 55 (Fig. 4b), and the α2-adrenergic influence on day 14 only (Fig. 4c).
Fig. 4.
Neuronal adrenergic regulation of IL-6 during arthritis. The graph shows β-adrenergic (a), α1-adrenergic (b), and α2-adrenergic (c) effects. The individual panels show control conditions (Co, white boxes), conditions under electrical stimulation (ES, red boxes), and conditions under ES + antagonist (grey boxes) on days 14, 32, and 55 in the course of arthritis. The blue boxes demonstrate the significant effects of the respective antagonist in relation to ES alone (p values are given). #p < 0.005 compared to control conditions (white box). Data are given as box-plots with the 10th (whisker), 25th, 50th (median), 75th, and 90th (whisker) percentiles. For each condition with ES + antagonist, at least 3–5 mice (24–40 spleen slices) were used. Control conditions and conditions with ES only were done with at least 12 mice (96 spleen slices).
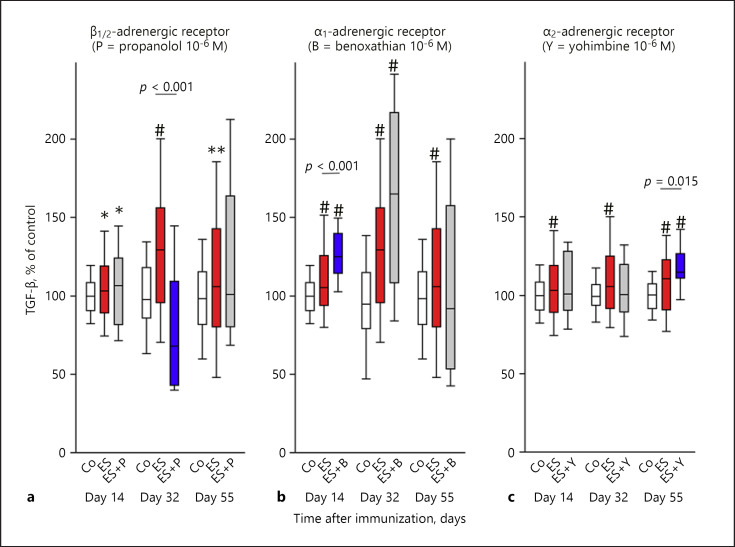
Adrenergic regulation of TGF-β is illustrated in Figure 5. On day 32, TGF-β was β-adrenergically stimulated because the β-adrenoceptor antagonist inhibited TGF-β secretion compared to ES only (Fig. 5a). However, no β-adrenergic regulation of TGF-β was observed on days 14 and 55. In addition, neuronal α1-adrenergic effects were observed on day 14 and as a trend on day 32 (0.05 < p <0.1) (Fig. 5b), and neuronal α2-adrenergic effects were present on day 55 (Fig. 5c). These α-adrenergic effects were of a TGF-β inhibitory nature because the antagonist increased cytokine secretion (Fig. 5b, c).
Fig. 5.
Neuronal adrenergic regulation of TGF-β during arthritis. The graph shows β-adrenergic (a), α1-adrenergic (b), and α2-adrenergic (c) effects. The individual panels show control conditions (Co, white boxes), conditions under electrical stimulation (ES, red boxes), and conditions under ES + antagonist (grey boxes) on days 14, 32, and 55 in the course of arthritis. The blue boxes demonstrate the significant effects of the respective antagonist in relation to ES alone (p values are given). * p < 0.05, ** p < 0.01, # p < 0.005 compared to control conditions (white box). Data are given as box-plots with the 10th (whisker), 25th, 50th (median), 75th, and 90th (whisker) percentiles. For each condition with ES + antagonist, at least 3–5 mice (24–40 spleen slices) were used. Control conditions and conditions with ES only were done with at least 8 mice (64 spleen slices).
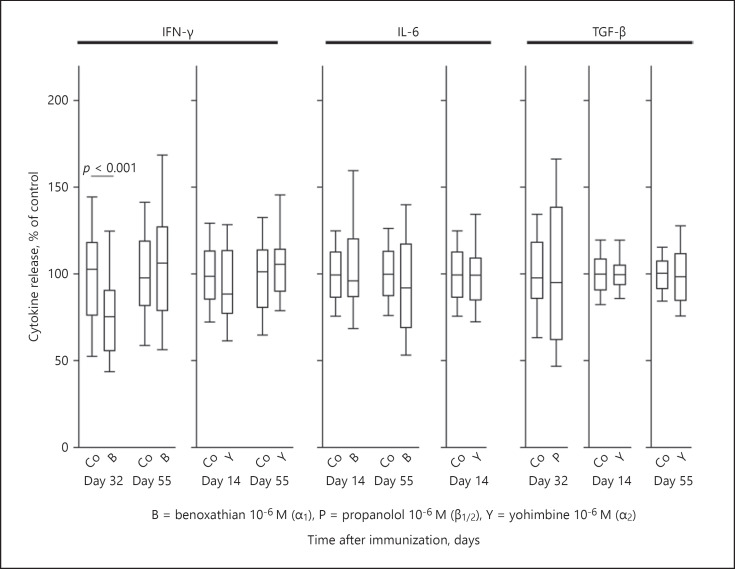
To understand the role of the neuronal aspect of the inhibition or stimulation of cytokine secretion in the spleen, one needs to investigate the pure effect of the respective antagonist on cytokine secretion in the absence of ES. If an antagonist has a significant effect in the absence of ES, the effect is likely independent of electrically released neurotransmitters. Thus, all experiments with a significant effect of an antagonist (Fig. 3, 4, 5, blue boxes) were repeated with the same antagonist concentration in the absence of ES.
Generally, the antagonist had no effect on spontaneous cytokine secretion (Fig. 6), indicating no effects outside of the ES. There was 1 exception to this rule, i.e., the α1-adrenergic antagonist benoxathian inhibited IFN-γ secretion on day 32 (Fig. 6). As the α1-adrenergic antagonist inhibited IFN-γ secretion with and without ES (Fig. 3, 6), this particular effect might be independent of neuronal pathways.
Fig. 6.
Effect of adrenergic antagonists on cytokine secretion independent of electrical stimulation. The individual panels show control conditions (Co) and conditions with an adrenergic antagonist. This graph only demonstrates those conditions where an adrenergic antagonist had a significant influence on electrically modulated cytokine secretion (Fig. 3, 4, 5, blue boxes). Data are given as box-plots with the 10th (whisker), 25th, 50th (median), 75th, and 90th (whisker) percentiles. For each condition with an antagonist, 3–5 mice (24–40 spleen slices) were used. Control conditions were carried out with at least 10 mice (80 spleen slices).
Discussion
Sympathetic nerve fibers in the spleen get lost during experimental arthritis. Proinflammatory cytokines like IFN-γ and IL-6 are stimulated by α1/2-adrenergic pathways but not changed by β-adrenergic signaling. The anti-inflammatory regulatory cytokine TGF-β was stimulated β-adrenergically only on day 32, but at other time points, only α1/2-adrenergic inhibitory effects were observed (days 14 and 55). The study detected a strong α-adrenergic neuronal influence on splenic cytokine secretion but almost no β-adrenergic neuronal regulation.
In the early days of investigation of sympathetic effects on inflammation, between 1940 and 1980, the SNS was thought to play a proinflammatory role [37, 38, 39]. Indeed, the SNS is a critical proinflammatory component of neurogenic inflammation, which is particularly evident during the first hours of induction of inflammation. This is most probably due to the supportive effects of neurotransmitters on plasma extravasation, and the α2-adrenergic effects on pain transmission and directed migration of immune cells to sites of inflammation. The latter aspect also plays a critical role in experimental arthritis because the SNS stimulates lymphocyte egress from lymphoid organs [24]. Another positive effect of the SNS on leukocytes was shown recently in the form of leukocyte progenitor proliferation and differentiation [25]. All these effects fit nicely into the overall concept of the fight and flight response where wounding, infection, and adequate immune response would be relevant aspects of survival [40].
With immune cell culture experiments and cytokine determination starting in the early 1980s, the picture completely changed. Now, using control T lymphocytes, B lymphocytes, and macrophages that are briefly stimulated with a cell-specific stimulus, noradrenaline inhibits many proinflammatory cytokines such as TNF [28, 41], IFN-γ [29], and IL-12 [42], which are often dependent on Gαs-induced increase of cAMP [43, 44, 45]. The first author was caught up in this thinking for 2 decades (1990–2010) when, in parallel, the concept of alternative activation of Gαs-coupled receptors appeared [13, 46, 47]. In addition, the timing of the SNS influence on immune function during the course of experimental arthritis became an important new target [15, 26]. We and other groups demonstrated the unequivocal proinflammatory role of the SNS in the immunization phase until the onset of arthritis ([11, 12, 13, 48], summary). Necessarily, the concept of sympathetic anti-inflammatory influences needed some revision.
Nowadays, a strong argument for a proinflammatory influence of the SNS on inflammation and immunity came along with the loss of sympathetic nerve fibers in inflamed tissue [14, 15]. Loss of sympathetic nerve fibers happens in many different diseases, and it is accompanied by a gradual shift from a β-adrenergic to an α-adrenergic influence in signaling because of the expected noradrenaline concentration in the tissue [35]. If noradrenaline levels are low, signaling goes through the α-adrenoceptors because of their higher affinity to noradrenaline than the β-adrenoceptors. In our study, the loss of sympathetic nerve fibers was not only observable in inflamed tissue but also in the spleen. Thus, noradrenaline concentrations in the spleen might be largely changed, similar to chemical sympathectomy with 6-hydroxy-dopamine. In summary, the arthritic disease induces a state of sympathectomy in the spleen.
Other groups have shown that the loss of sympathetic nerve fibers happens distant to the entry of nerve fibers into the spleen [36]. In earlier studies, we featured the loss of these nerve fibers on specific nerve repellent factors secreted from macrophages ([11, 35], review). Repulsion of nerve fibers does not mean destruction of these nerve fibers, which can be seen in sympathetic neurite outgrowth assays when using sympathetic chain ganglia and sympathetic repulsion factors like semaphorin-3F [49]. Thus, the increased density of sympathetic nerve fibers in the hilus region of the spleen observed in other studies [36] could simply be the expression of repelled and pushed nerve fibers that look like a collapsed concertina at their entry into the spleen.
A second major argument for a proinflammatory influence came with the idea of alternative Gαs-coupled receptor signaling of activated immune cells like in rheumatoid arthritis [13, 27]. This signaling induces proinflammatory effects via the mitogen-activated protein kinase pathway (MAPK). It has been described that, in such a situation, the classical anti-inflammatory Gαs/cAMP/protein kinase A pathway is eliminated and a proinflammatory pathway with MAPK is installed [13, 27, 46]. Under the new conditions, α2-adrenergic signaling, in particular, becomes proinflammatory [27] (supported in other studies [50]). Our study demonstrates exactly this kind of α-adrenergic dominance, which started as early as day 14, long before disease onset, and remained constant throughout the chronic phase of the disease.
This raises the question of whether neuroimmunomodulation in the spleen can change peripheral joint inflammation, and whether this happens in humans, too. The spleen is a central, secondary lymphoid organ, which can be involved in systemic autoimmune phenomena such as autoimmune thrombocytopenia, or autoimmune hemolytic anemia associated with systemic lupus erythematosus [51]. Its role in rheumatoid arthritis is not well known but, in severe cases of Felty syndrome, splenectomy might be an important therapeutic option. In experimental arthritis, the spleen can have importance because splenectomy attenuates experimental arthritis [52]. In earlier studies on the DBA/1 mouse, we also observed that splenectomy postpones arthritis onset (R.H.S., unpubl. data). In the animal model of experimental arthritis in DBA/1 mice, one assumed that several immune reactions, like the clonal expansion of T and B cells and systemic cytokine secretion in/from the spleen, added to peripheral joint inflammation.
In conclusion, this study describes a strong loss of sympathetic nerve fibers in a key lymphoid organ, the spleen. It further demonstrates a strong α-adrenergic signaling in the natural compartment of a spleen slice that contributed to proinflammatory effects. While researchers talked for decades of a β-adrenergically effective agonist therapy for arthritis (or similar, i.e., via other Gαs-coupled receptors), this study and other current studies clearly point to an antagonistic therapy towards the α-adrenoceptor or other Gαi-coupled receptors.
Statement of Ethics
Experiments were conducted according to institutional and governmental regulations for animal use (Government of the Oberpfalz AZ 621–2531.1–17/01, AZ 54–2531.1–24/06, AZ 54–2531.1–07/08, AZ 54–2532.1–43/12, AZ 54–2532.1–04/13, and AZ 54–2532.1–04/13).
Disclosure Statement
The authors declare there are no conflicts of interest.
Funding Source
This study was partly supported by a grant from the Deutsche Forschungsgemeinschaft to R.H.S. (grant No. DFG STR 511/36–1).
Author Contributions
R.H.S.: development of the concept, drafting the paper, generating the figures, final approval. B.D.: generation of data, drafting parts of the paper, final approval. L.R.: generation of data, drafting parts of the paper, final approval.
Supplementary Material
Supplementary data
Supplementary data
References
- 1.Thompson M, Bywaters EG. Unilateral rheumatoid arthritis following hemiplegia. Ann Rheum Dis. 1962 Dec;21((4)):370–7. doi: 10.1136/ard.21.4.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glick EN. Asymmetrical rheumatoid arthritis after poliomyelitis. BMJ. 1967 Jul;3((5556)):26–8. doi: 10.1136/bmj.3.5556.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velayos EE, Cohen BS. The effect of stroke on well-established rheumatoid arthritis. Md State Med J. 1972 Mar;21((3)):38–42. [PubMed] [Google Scholar]
- 4.Smith RD. Effect of hemiparesis on rheumatoid arthritis. Arthritis Rheum. 1979 Dec;22((12)):1419–20. doi: 10.1002/art.1780221225. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton S. Unilateral rheumatoid arthritis in hemiplegia. J Can Assoc Radiol. 1983 Mar;34((1)):49–50. [PubMed] [Google Scholar]
- 6.Nakamura K, Akizuki M, Kimura A, Chino N. [A case of polyarthritis developed on the non-paralytic side in a hemiplegic patient] Ryumachi. 1994 Jun;34((3)):656–61. [PubMed] [Google Scholar]
- 7.Glynn JJ, Clayton ML. Sparing effect of hemiplegia on tophaceous gout. Ann Rheum Dis. 1976 Dec;35((6)):534–5. doi: 10.1136/ard.35.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veale D, Farrell M, Fitzgerald O. Mechanism of joint sparing in a patient with unilateral psoriatic arthritis and a longstanding hemiplegia. Br J Rheumatol. 1993 May;32((5)):413–6. doi: 10.1093/rheumatology/32.5.413. [DOI] [PubMed] [Google Scholar]
- 9.Lee JC, Salonen DC, Inman RD. Unilateral hemochromatosis arthropathy on a neurogenic basis. J Rheumatol. 1997 Dec;24((12)):2476–8. [PubMed] [Google Scholar]
- 10.Keyszer G, Langer T, Kornhuber M, Taute B, Horneff G. Neurovascular mechanisms as a possible cause of remission of rheumatoid arthritis in hemiparetic limbs. Ann Rheum Dis. 2004 Oct;63((10)):1349–51. doi: 10.1136/ard.2003.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pongratz G, Straub RH. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat Rev Rheumatol. 2013 Feb;9((2)):117–26. doi: 10.1038/nrrheum.2012.181. [DOI] [PubMed] [Google Scholar]
- 12.Schaible HG, Straub RH. Function of the sympathetic supply in acute and chronic experimental joint inflammation. Auton Neurosci. 2014 May;182:55–64. doi: 10.1016/j.autneu.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Lorton D, Bellinger DL. Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int J Mol Sci. 2015 Mar;16((3)):5635–65. doi: 10.3390/ijms16035635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weidler C, Holzer C, Harbuz M, Hofbauer R, Angele P, Schölmerich J, et al. Low density of sympathetic nerve fibres and increased density of brain derived neurotrophic factor positive cells in RA synovium. Ann Rheum Dis. 2005 Jan;64((1)):13–20. doi: 10.1136/ard.2003.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Härle P, Möbius D, Carr DJ, Schölmerich J, Straub RH. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 2005 Apr;52((4)):1305–13. doi: 10.1002/art.20987. [DOI] [PubMed] [Google Scholar]
- 16.Jenei-Lanzl Z, Capellino S, Kees F, Fleck M, Lowin T, Straub RH. Anti-inflammatory effects of cell-based therapy with tyrosine hydroxylase-positive catecholaminergic cells in experimental arthritis. Ann Rheum Dis. 2015 Feb;74((2)):444–51. doi: 10.1136/annrheumdis-2013-203925. [DOI] [PubMed] [Google Scholar]
- 17.Capellino S, Weber K, Gelder M, Härle P, Straub RH. First appearance and location of catecholaminergic cells during experimental arthritis and elimination by chemical sympathectomy. Arthritis Rheum. 2012 Apr;64((4)):1110–8. doi: 10.1002/art.33431. [DOI] [PubMed] [Google Scholar]
- 18.Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. 2016 Jul;113((29)):8284–9. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis. 2010 Sep;69((9)):1717–23. doi: 10.1136/ard.2009.118554. [DOI] [PubMed] [Google Scholar]
- 20.Bassi GS, Dias DP, Franchin M, Talbot J, Reis DG, Menezes GB, et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav Immun. 2017 Aug;64:330–43. doi: 10.1016/j.bbi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green PG, Luo J, Heller PH, Levine JD. Neurogenic and non-neurogenic mechanisms of plasma extravasation in the rat. Neuroscience. 1993 Feb;52((3)):735–43. doi: 10.1016/0306-4522(93)90422-c. [DOI] [PubMed] [Google Scholar]
- 22.Jänig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog Brain Res. 1996;113:161–84. doi: 10.1016/s0079-6123(08)61087-0. [DOI] [PubMed] [Google Scholar]
- 23.Levine JD, Clark R, Devor M, Helms C, Moskowitz MA, Basbaum AI. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984 Nov;226((4674)):547–9. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- 24.Klatt S, Stangl H, Kunath J, Lowin T, Pongratz G, Straub RH. Peripheral elimination of the sympathetic nervous system stimulates immunocyte retention in lymph nodes and ameliorates collagen type II arthritis. Brain Behav Immun. 2016 May;54:201–10. doi: 10.1016/j.bbi.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Vasamsetti SB, Florentin J, Coppin E, Stiekema LC, Zheng KH, Nisar MU, et al. Sympathetic Neuronal Activation Triggers Myeloid Progenitor Proliferation and Differentiation. Immunity. 2018 Jul;49((1)):93–106.e7. doi: 10.1016/j.immuni.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lubahn CL, Schaller JA, Bellinger DL, Sweeney S, Lorton D. The importance of timing of adrenergic drug delivery in relation to the induction and onset of adjuvant-induced arthritis. Brain Behav Immun. 2004 Nov;18((6)):563–71. doi: 10.1016/j.bbi.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Jenei-Lanzl Z, Zwingenberg J, Lowin T, Anders S, Straub RH. Proinflammatory receptor switch from Gαs to Gαi signaling by β-arrestin-mediated PDE4 recruitment in mixed RA synovial cells. Brain Behav Immun. 2015 Nov;50:266–74. doi: 10.1016/j.bbi.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Spengler RN, Chensue SW, Giacherio DA, Blenk N, Kunkel SL. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J Immunol. 1994 Mar;152((6)):3024–31. [PubMed] [Google Scholar]
- 29.Sanders VM, Baker RA, Ramer-Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2-adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997 May;158((9)):4200–10. [PubMed] [Google Scholar]
- 30.Agarwal SK, Marshall GD., Jr Beta-adrenergic modulation of human type-1/type-2 cytokine balance. J Allergy Clin Immunol. 2000 Jan;105((1 Pt 1)):91–8. doi: 10.1016/s0091-6749(00)90183-0. [DOI] [PubMed] [Google Scholar]
- 31.Straub RH, Lang B, Falk W, Schölmerich J, Singer EA. In vitro superfusion method for the investigation of nerve-immune cell interaction in murine spleen. J Neuroimmunol. 1995 Aug;61((1)):53–60. doi: 10.1016/0165-5728(95)00073-b. [DOI] [PubMed] [Google Scholar]
- 32.Straub RH, Rauch L, Fassold A, Lowin T, Pongratz G. Neuronally released sympathetic neurotransmitters stimulate splenic interferon-gamma secretion from T cells in early type II collagen-induced arthritis. Arthritis Rheum. 2008 Nov;58((11)):3450–60. doi: 10.1002/art.24030. [DOI] [PubMed] [Google Scholar]
- 33.Straub RH, Rauch L, Rauh L, Pongratz G. Sympathetic inhibition of IL-6, IFN-γ, and KC/CXCL1 and sympathetic stimulation of TGF-β in spleen of early arthritic mice. Brain Behav Immun. 2011 Nov;25((8)):1708–15. doi: 10.1016/j.bbi.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Meinel T, Pongratz G, Rauch L, Straub RH. Neuronal α1/2-adrenergic stimulation of IFN-γ, IL-6, and CXCL-1 in murine spleen in late experimental arthritis. Brain Behav Immun. 2013 Oct;33:80–9. doi: 10.1016/j.bbi.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther. 2014;16((6)):504. doi: 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorton D, Lubahn C, Sweeney S, Major A, Lindquist CA, Schaller J, et al. Differences in the injury/sprouting response of splenic noradrenergic nerves in Lewis rats with adjuvant-induced arthritis compared with rats treated with 6-hydroxydopamine. Brain Behav Immun. 2009 Feb;23((2)):276–85. doi: 10.1016/j.bbi.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Selye H, Fortier C. Adaptive reactions to stress. Res Publ Assoc Res Nerv Ment Dis. 1949 Dec;29:3–18. [PubMed] [Google Scholar]
- 38.Levine JD, Goetzl EJ, Basbaum AI. Contribution of the nervous system to the pathophysiology of rheumatoid arthritis and other polyarthritides. Rheum Dis Clin North Am. 1987 Aug;13((2)):369–83. [PubMed] [Google Scholar]
- 39.Levine JD, Coderre TJ, Helms C, Basbaum AI. Beta 2-adrenergic mechanisms in experimental arthritis. Proc Natl Acad Sci USA. 1988 Jun;85((12)):4553–6. doi: 10.1073/pnas.85.12.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014 May;58((2-3)):193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- 41.van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994 May;62((5)):2046–50. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996 Sep;108((5)):374–81. [PubMed] [Google Scholar]
- 43.Renz H, Gong JH, Schmidt A, Nain M, Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988 Oct;141((7)):2388–93. [PubMed] [Google Scholar]
- 44.Verghese MW, McConnell RT, Strickland AB, Gooding RC, Stimpson SA, Yarnall DP, et al. Differential regulation of human monocyte-derived TNF alpha and IL-1 beta by type IV cAMP-phosphodiesterase (cAMP-PDE) inhibitors. J Pharmacol Exp Ther. 1995 Mar;272((3)):1313–20. [PubMed] [Google Scholar]
- 45.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995 Feb;181((2)):775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, et al. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc Natl Acad Sci USA. 2003 Feb;100((3)):940–5. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007 Apr;100((7)):950–66. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 48.Bellinger DL, Lorton D. Sympathetic Nerve Hyperactivity in the Spleen: Causal for Nonpathogenic-Driven Chronic Immune-Mediated Inflammatory Diseases (IMIDs)? Int J Mol Sci. 2018 Apr;19((4)):19. doi: 10.3390/ijms19041188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kunath J, Delaroque N, Szardenings M, Neundorf I, Straub RH. Sympathetic nerve repulsion inhibited by designer molecules in vitro and role in experimental arthritis. Life Sci. 2017 Jan;168:47–53. doi: 10.1016/j.lfs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang QH, Hao JW, Li GL, Ji XJ, Yao XD, Dong N, et al. Proinflammatory switch from Gαs to Gαi signaling by Glucagon-like peptide-1 receptor in murine splenic monocyte following burn injury. Inflamm Res. 2018 Feb;67((2)):157–68. doi: 10.1007/s00011-017-1104-9. [DOI] [PubMed] [Google Scholar]
- 51.Barron N, Arenas-Osuna J, Medina G, Cruz-Dominguez MP, Gonzalez-Romero F, Velasques-Garcia JA, et al. Splenectomy in systemic lupus erythematosus and autoimmune hematologic disease: a comparative analysis. Clin Rheumatol. 2018;37:943–8. doi: 10.1007/s10067-018-3979-4. [DOI] [PubMed] [Google Scholar]
- 52.Kimpel D, Dayton T, Fuseler J, Gray L, Kannan K, Wolf RE, et al. Splenectomy attenuates streptococcal cell wall-induced arthritis and alters leukocyte activation. Arthritis Rheum. 2003 Dec;48((12)):3557–67. doi: 10.1002/art.11424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data