Abstract
Purpose
Hotspot blood cell-free DNA (cfDNA) biomarker assays have limited utility in profiling tumor heterogeneity and burden and in capturing regional metastasis with low disease burden in patients with melanoma. We investigated the utility of a sensitive 54–cancer gene digital next-generation sequencing approach targeting blood cfDNA single nucleotide variants (SNVs) and copy number amplification for monitoring disease in patients with melanoma with regional or distant organ metastasis (DOM).
Patients and Methods
A total of 142 blood samples were evaluated by digital next-generation sequencing across two patient cohorts. Cohort 1 contained 44 patients with stage II, III, or IV disease with matched tumor DNA at the time of surgery or DOM. Cohort 2 consisted of 12 overlapping patients who were longitudinally monitored after complete lymph node dissection to DOM.
Results
In cohort 1, cfDNA SNVs were detected in 75% of patients. Tumor-cfDNA somatic SNV concordance was 85% at a variant allele fraction of ≥ 0.5%. An SNV load (number of unique SNVs detected) of greater than two SNVs and an SNV burden (total cumulative SNV VAF) of > 0.5% were significantly associated with worse overall survival (P < .05) in stage IV patients. In cohort 2, 98 longitudinal blood samples along with matched regional and distant metastases from 12 stage III patients were analyzed before complete lymph node dissection and throughout disease progression. cfDNA SNV levels correlated with tumor burden (P = .019), enabled earlier detection of recurrence compared with radiologic imaging (P < .01), captured tumor heterogeneity, and identified increasing SNVs levels before recurrence.
Conclusion
This study demonstrates significant utility for cfDNA profiling in patients with melanoma with regional and/or distant metastasis for earlier detection of recurrence and progression and in capturing tumor evolution and heterogeneity, thus impacting how patients with melanoma are monitored.
INTRODUCTION
After surgical resection of regional metastatic melanoma, longitudinal molecular profiling would greatly aid in monitoring for recurrence, progression, and therapeutic efficacy.1 Molecular profiling of melanoma biopsies or tumors becomes more challenging when patients experience progression to distant organ metastasis (DOM) as a result of procedure-related morbidity risks and limited sampling efficiency in capturing tumor heterogeneity.2
Blood cell-free DNA (cfDNA) biomarkers are minimally invasive and potentially allow routine monitoring of molecular changes in patients’ cancer over the course of therapy and follow-up.3 This would enable monitoring of treatment efficacy,4 recurrence, and/or subclonal mutation(s) tracking as tumors evolve or relapse.5 Unfortunately, no blood-based melanoma biomarker is available for early detection of recurrence, particularly in patients with stage III melanoma rendered clinically disease free upon surgery, except the problematic surrogate biomarker, serum lactate dehydrogenase (LDH).6 To address this need, we pioneered the investigation of cfDNA utility7-9 and explored different types of cfDNA biomarkers for monitoring patients with melanoma.4,9-12
A comprehensive profile of cfDNA mutations given the recent genomic classification of cutaneous melanoma (BRAF, NRAS, NF1, and triple wild-type)13,14 would provide real-time monitoring of postoperative residual disease to capture progression and enable earlier detection of recurrence compared with clinical or radiologic detection. Hotspot target blood assays (BRAF mutations) may not be suitable to comprehensively profile metastatic melanomas given the high intratumor heterogeneity.15-17 Blood cfDNA profiling using a cancer panel can address this heterogeneity issue if highly sensitive for metastatic disease. Overall, melanoma cfDNA profiling could provide a clinically informative approach for monitoring disease progression, heterogeneity, and earlier detection of DOM.
In this study, we systemically assessed the utility of profiling melanoma blood cfDNA using a sensitive digital next-generation sequencing (NGS) assay that includes a panel of 54 cancer genes in our clinically well-annotated patient cohorts with melanoma, with follow-up during a clinically disease-free period. Specifically, we focused on longitudinal cfDNA follow-up analysis in patients before curative surgery of American Joint Committee on Cancer (AJCC) stage III regional metastatic disease and throughout disease progression until the development of DOM. This approach can serve as a paradigm for future studies of tumor evolution and heterogeneity in blood during longitudinal patient follow-up.
PATIENTS AND METHODS
Patients and Specimens
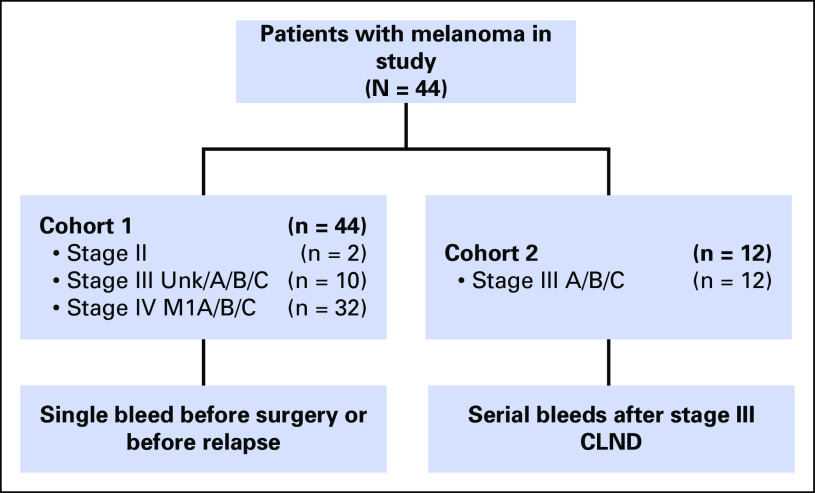
One hundred forty-two blood samples and available tumor tissue were prospectively collected from 44 patients with melanoma at Providence Saint John’s Health Center under approval of the Saint John’s Health Center/John Wayne Cancer Institute Joint Institutional Review Board and Western Institutional Review Board under standard operating procedures.18 The first patient cohort (cohort 1) included 44 patients with AJCC stage II, III, or IV melanoma, with blood samples collected before DOM relapse or elective surgery (Appendix Table A1); patients were treated with non–US Food and Drug Administration–approved immunotherapies and were confirmed to have no evidence of disease via computed tomography (CT) or magnetic resonance imaging scans after surgery. The second cohort (cohort 2) included patients overlapping with cohort 1 (Table 1) who were enrolled in a US Food and Drug Administration–registered phase III clinical trial for AJCC stage III melanoma (ClinicalTrials.gov identifier: NCT00052130; Appendix Fig A1).19 Briefly, after complete lymph node dissection (CLND) to render patients clinically disease free, patients were randomly assigned to one of the following two treatment arms: bacillus Calmette-Guérin plus Canvaxin melanoma vaccine (CancerVax, Carlsbad, CA) or bacillus Calmette-Guérin plus placebo, with all patient belonging to the treatment arm. No statistically significant clinical difference between the two randomized treatment arms was reported.20
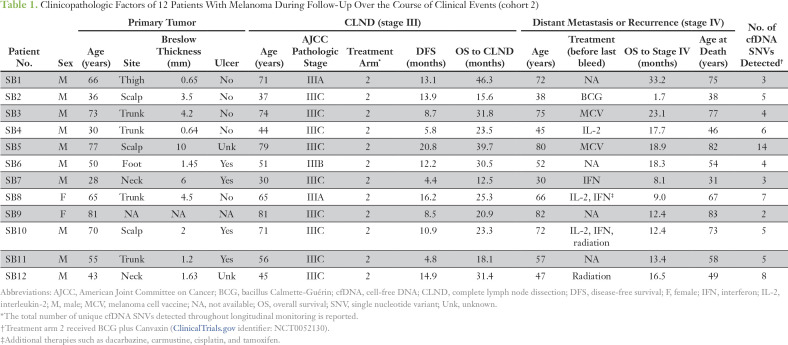
Table 1.
Clinicopathologic Factors of 12 Patients With Melanoma During Follow-Up Over the Course of Clinical Events (cohort 2)
Cohort 2 patients were selected on the basis of available blood samples during follow-up that were in accordance with the clinical trial’s protocol. Ninety-eight blood samples were collected at baseline (before CLND or before study) and during follow-up with available paired formalin-fixed paraffin-embedded (FFPE) tumors. Specifically, the blood was collected before CLND (AJCC stage III disease) and during follow-up every 2 to 4 months before DOM (AJCC stage IV disease). The follow-up time points were based on defined patient visits at 2, 4, and 12 months in year 1 and every 6 months in years 2 and 3. Standard clinical follow-up consisted of a patient visit with serum LDH blood testing, x-ray imaging every 3 months, and annual CT of the chest, abdomen, and pelvis along with brain CT or magnetic resonance imaging. Approximately six to nine serially collected blood samples per patient were available for assessment in the study. This study was performed in accordance with Reporting Recommendations for Tumor Marker Prognostic Studies.21
Sample Collection and DNA Purification
Peripheral blood was collected, and serum was isolated, centrifuged, and filtered before cryopreservation in aliquots at −80°C, as previously described7,11 under good laboratory practice conditions. Aliquots for the study were thawed only once before extraction. cfDNA was isolated from 2 mL of serum, and ≥ 5 ng of cfDNA was used for the assay, as previously described.22 DNA was extracted from surgical pathologist–confirmed melanoma tumor in FFPE specimens using the Zymo FFPE DNA kit (Zymo Research, Irvine, CA) and further purified by the OneStep PCR Inhibitor Removal Kit (Zymo Research) if contaminated with melanin, following the manufacturer’s instructions.
Digital NGS
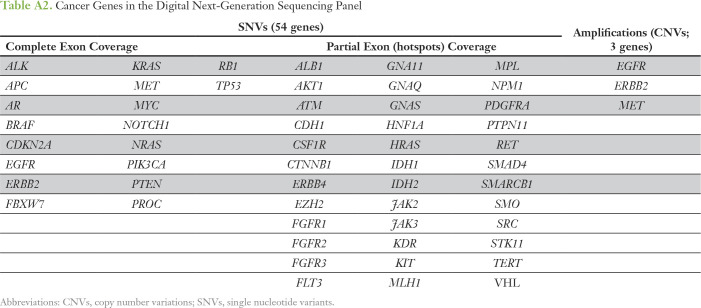
The characteristics and methodology of the digital NGS assay containing a panel of 54 cancer genes have been previously described.22 NGS was performed at Guardant Health (Redwood City, CA), a Clinical Laboratory Improvement Amendments–certified, College of American Pathologists–accredited laboratory. The panel covers all known frequent melanoma driver mutations14 and includes full exon coverage of 18 genes, critical exons for the 36 remaining genes (ie, having somatic mutations reported in the Catalogue of Somatic Mutations in Cancer23), and three copy number variations (CNVs). The variant allele fraction (VAF) was calculated as the number of cfDNA molecules with variants at a given nucleotide position divided by the total number of unique cfDNA molecules at that position. The panel has a cfDNA single nucleotide variant (SNV) limit of detection of 0.1%. cfDNA CNV analysis for three genes, EGFR, ERBB2, and MET, has been previously described22 with limits of detection of 0.2, 0.5, and 0.2 extra copies, respectively. cfDNA SNVs were categorized as somatic variants through referencing the Catalogue of Somatic Mutations in Cancer database23 or as variants of uncertain significance upon additional reference to the Database of Short Genetic Variation. SNV load was calculated on the basis of the number of unique SNVs per patient excluding CNVs.
Validation of Digital NGS
To validate cfDNA SNVs identified by digital NGS, DNA from the resected matched tumor (regional or distant metastasis) was subjected to custom targeted sequencing using a TruSeq Custom Amplicon panel (Illumina, San Diego, CA) as performed by the John Wayne Cancer Institute Sequencing Center. Illumina’s nondigital TruSeq Amplicon panel NGS was determined to have a 1% VAF cutoff suitable for FFPE tumor DNA. The 150-base pair amplicon panel design, specific for the cfDNA SNVs identified, was generated by the Illumina DesignStudio software. Libraries were prepared with the TruSeq Custom Amplicon Low Input Library Prep Kit (Illumina), following the manufacturer’s protocol, and sequenced on the Illumina MiSeq with 150-base pair single-end reads. An average sequencing depth of 8,000× across the targeted region was achieved. Raw sequencing reads were trimmed using Trimmomatic (version 0.33),24 mapped to the human genome (1000 Genomes (b37) build) using BWA (version 0.7.12)25 at default settings, and processed using GATK (version 3.4)26 base quality score recalibration and indel realignment in accordance with the GATK best practices recommendations. The number of reads mapping to each locus of interest was counted using the mpileup function in SAMtools.27
Concordance Analysis
SNV concordance in paired tumor tissue and blood samples was determined as positive when the mutation was found in both tumor and cfDNA or negative when cfDNA SNVs were not detected in the paired tumor. The percent agreement was calculated for individual SNVs across the cohort. The overall concordance rate is the average of the percent agreement for all SNVs analyzed.
Biostatistical Analysis
SNV burden or total cumulative SNV VAF before and after recurrence was compared using the Wilcoxon signed rank test. Comparison of cfDNA analysis versus radiologic imaging or LDH for detection of DOM was assessed using Fisher’s exact test, where an LDH cutoff value of 190 U/L was used.6 Fisher’s exact and F tests were performed for categorical and continuous variables, respectively. The Kaplan-Meier method was used for survival analysis groupings with cfDNA status and analyzed using the log-rank test. cfDNA status cutoff values were evaluated using the cutp() function in statistical R package, survMisc.28 The Gompertz survival regression29-31 was used to evaluate the disease-free survival (DFS) differences between cfDNA SNV groups. Cox proportional hazards regression was used for adjusting clinical factors in multivariable analyses. All statistical analyses were performed with R Studio (R Studio, Boston, MA).
RESULTS
cfDNA SNVs and Association With Outcomes
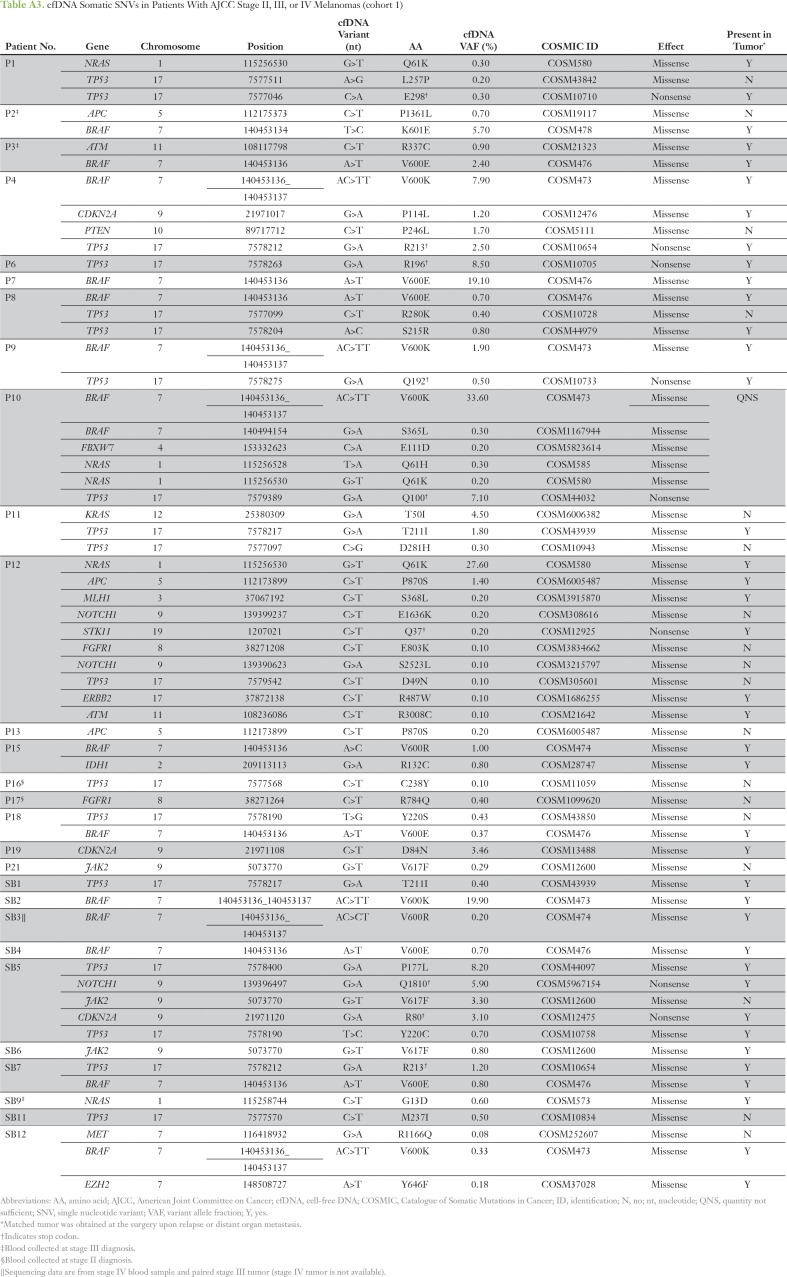
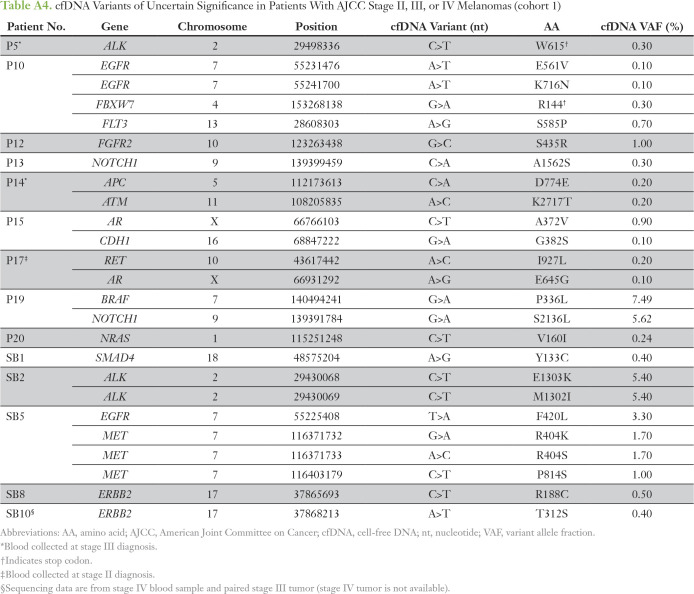
The digital NGS assay was used to analyze 54 clinically relevant cancer genes covering all major melanoma driver genes (Appendix Table A2) in blood cfDNA of patients with AJCC stage II, III, or IV melanoma (cohort 1; Appendix Table A1) collected before DOM relapse or elective surgery. cfDNA SNVs were detected in 75% of patients (33 of 44 patients) at VAFs ranging from 0.1% to 33.6% (Appendix Tables A3 and A4). Eleven patients negative for cfDNA SNVs had stage IV (M1B, n = 1; M1C, n = 7) or stage III disease (A, B, or C, n = 1 each). The most frequently mutated genes, BRAF, TP53, and NRAS (Appendix Fig A2), align with those previously reported in our studies in the melanoma tissue mutational landscape.13,14 To confirm that cfDNA somatic SNVs (Appendix Table A3) were tumor derived, custom targeted amplicon sequencing was performed in matched tumor DNA (cohort 1). Tumor-cfDNA somatic SNV concordance was detected at 68% (n = 57), 85% (n = 33), and 100% (n = 23) for somatic SNVs at VAFs of > 0%, ≥ 0.5%, and ≥ 1%, respectively. Interestingly, 100% concordance of the hotspot driver mutations BRAFV600 (n = 12) and NRASQ61K (n = 2) in paired tumor-cfDNA samples was not a result of high SNV burden because the individual VAFs ranged from 0.2% to 28%.
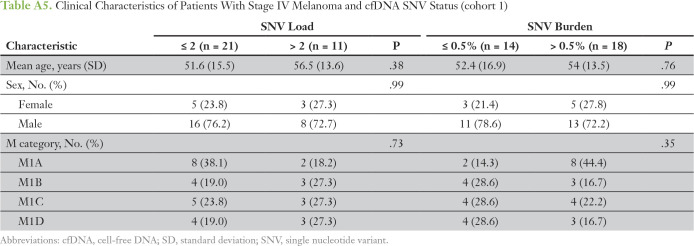
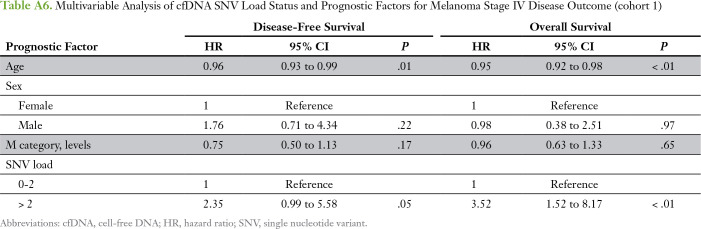
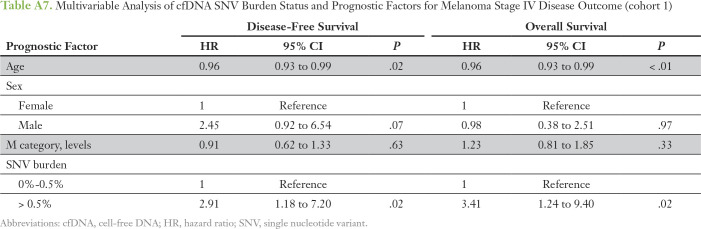
The number of different cfDNA SNVs (SNV load), ranging from zero to four, and the total cumulative SNV VAF (SNV burden), ranging from 0.1% to 1%, as cutoffs were analyzed for association with overall survival (OS) and DFS in stage IV patients only (n = 32; Appendix Table A5). Patients with more than two cfDNA SNVs had a significantly worse OS compared with patients with two or fewer SNVs (median OS, 8.6 v 17.5 months, respectively; P = .026; Fig 1A). An SNV burden of > 0.5% was significantly associated with worse OS compared with an SNV burden ≤ 0.5% (median OS, 9.2 v 16.4 months, respectively; P = .049; Fig 1B). The total increase in mean lifetime DFS was 5.8 and 8.7 months for patients with lower cfDNA SNV load or burden, respectively (Appendix Fig A3). One patient lost to follow-up was omitted from the survival analysis. Multivariable analysis showed that higher SNV load and burden were independent prognostic factors for worse OS and DFS after adjusting for age, sex, and M category (Appendix Tables A6 and A7). Altogether, this suggests that cfDNA status may be a prognostic indicator in cutaneous melanoma.
Fig 1.
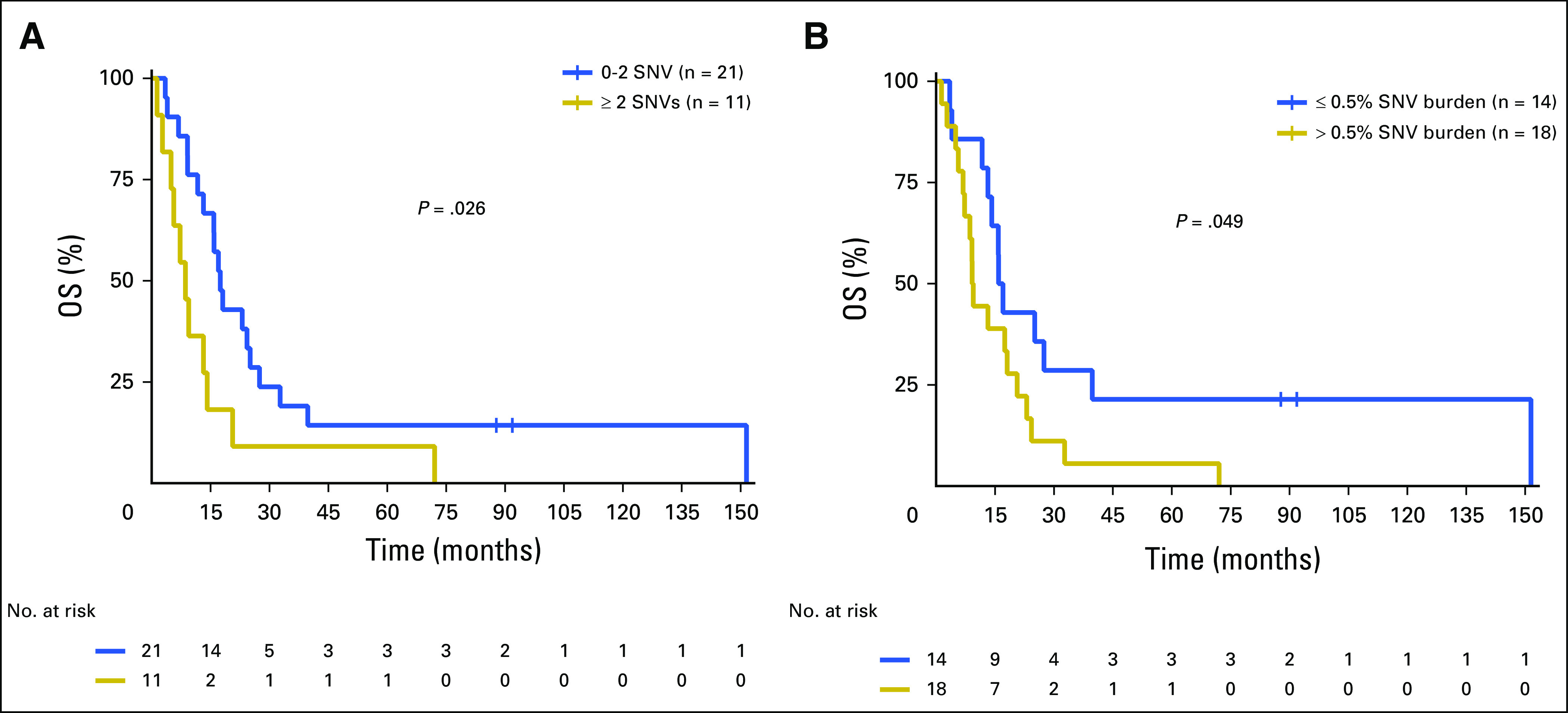
Association of baseline cell-free DNA (cfDNA) single nucleotide variant (SNV) load and burden with overall survival (OS) in patients with melanoma. A single blood sample obtained from patients in cohort 1 was analyzed for known OS outcome based on (A) SNV load and (B) SNV burden.
cfDNA Profiling After CLND
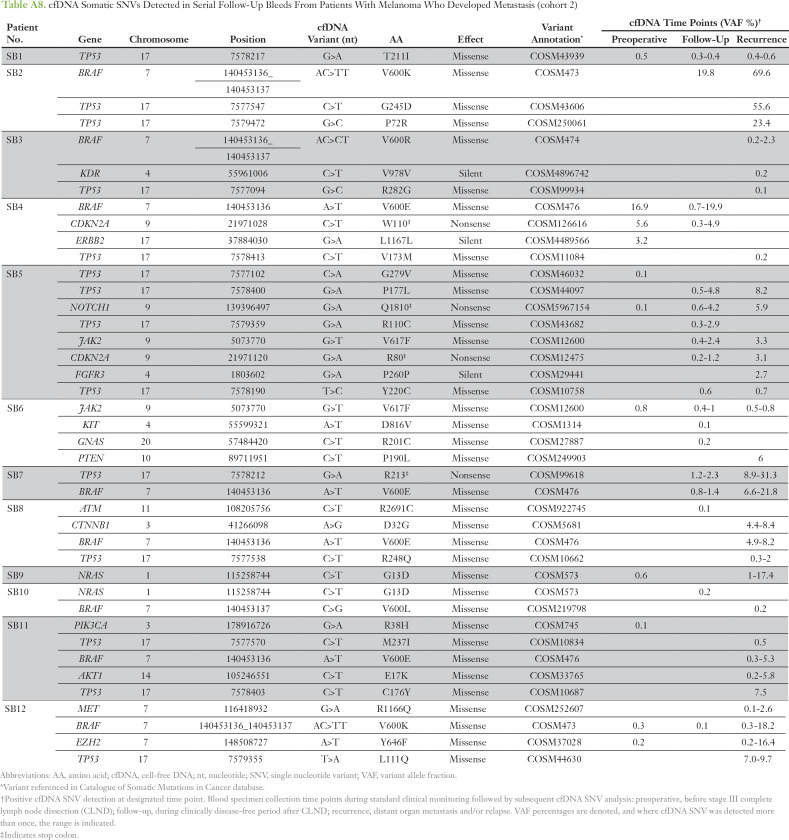
Digital NGS was performed to longitudinally profile cfDNA SNVs in 98 blood samples collected from 12 patients with melanoma (cohort 2). Serial blood sampling occurred at three major clinical time points, as detailed in Figure 2A. The cfDNA analysis of all serially collected blood samples is summarized per patient over longitudinal follow-up for somatic SNVs (Appendix Table A8) and variants of uncertain significance (Appendix Table A9). The most frequent SNVs detected were in TP53 (75%) and BRAF (58%; Fig 3A), reflecting frequently reported metastatic melanoma DNA mutations.14 Lack of pre-CLND cfDNA SNV detection in three patients was unlikely to be a result of low tumor burden, because all three patients had stage IIIC disease with positive lymph nodes (SB3, n = 9; SB7, n = 1; and SB10, n = 1). Representative cfDNA SNV profiling during disease progression is shown in Figure 2B for all SNVs detected. Before CLND surgery, the cfDNA VAF was detected at high levels, whereas these levels decreased after curative surgery, reflecting the tumor burden reduction. After disease recurrence, VAF levels increased up to 500-fold. Increasing VAF (P = .019) and SNV burden (P = .039) after relapse was strongly associated with disease progression in this patient cohort (cohort 2; Fig 3B).
Fig 2.
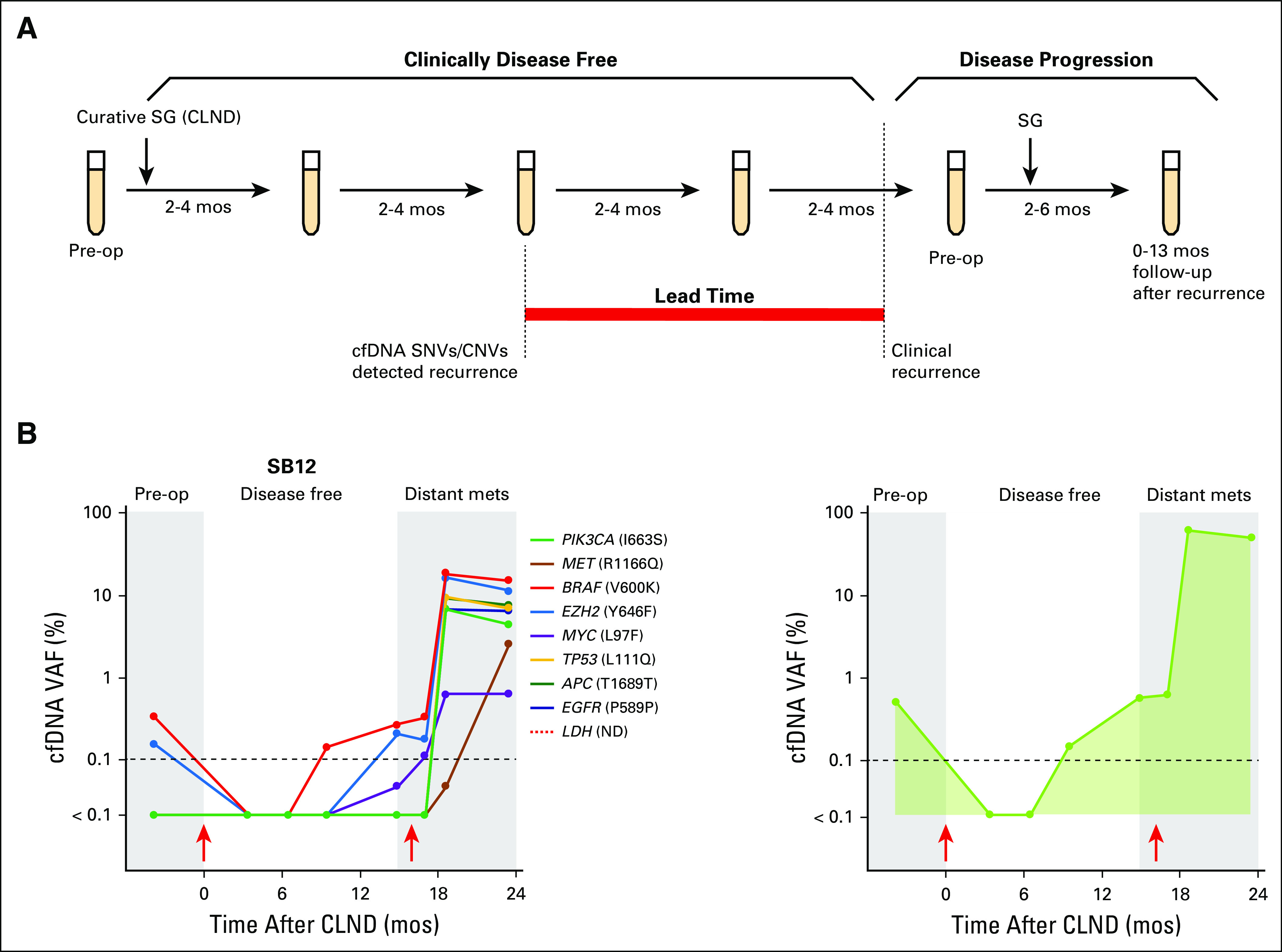
Cell-free DNA (cfDNA) monitoring in patients with melanoma. (A) Serial blood collection schematic for digital next-generation sequencing. Red bar indicates lead time over standard follow-up. (B) cfDNA dynamics in patient SB12 (left: log; right: stacked). Lung distant metastasis (15 months), subsequent surgical resection (17 months), and brain metastasis (23 months) occurred. Red arrows indicate surgery; dashed line indicates limit of detection. CLND, complete lymph node dissection; CNV, copy number variation; mets, metastases; mos, months; LDH (ND), lactate dehydrogenase not done longitudinally; Pre-op, preoperative; SG, surgery; SNV, single nucleotide variant; VAF, variant allele fraction.
Fig 3.
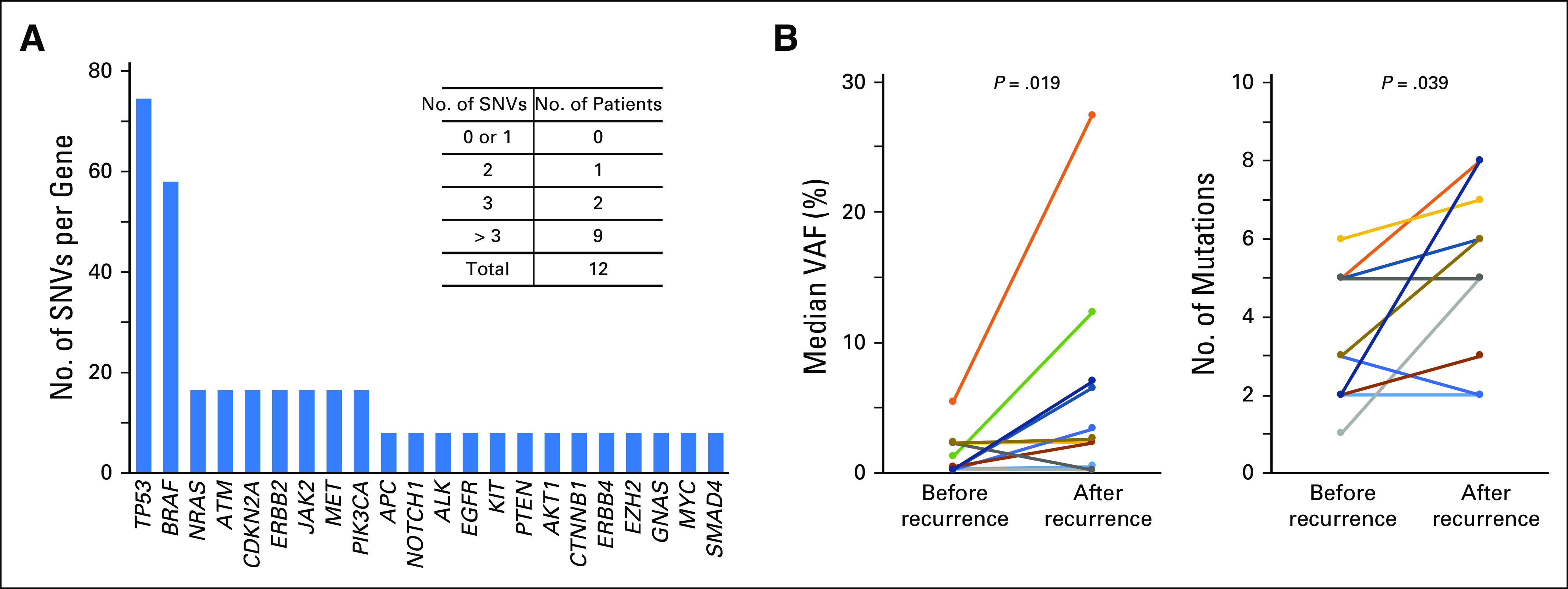
Cell-free DNA (cfDNA) single nucleotide variant (SNV) profiling reflects tumor burden. (A) Frequency of SNVs identified per gene across the cohort containing 98 serial bleeds from 12 patients (cohort 2). (B) Significant correlation of increasing variant allele fraction (VAF; left) and total number of cfDNA variants (right) with tumor burden in 11 patients.
Earlier Detection of DOM by Longitudinal cfDNA Analysis
We evaluated whether longitudinal cfDNA profiles can detect residual or progressive disease after CLND to provide earlier detection of DOM compared with clinical or radiologic imaging. Given their utility in AJCC staging32 and emerging prognostic utility in immunotherapy, LDH levels were also evaluated for recurrence monitoring.33,34 LDH values were longitudinally assessed in eight patients (Fig 4). In the four remaining serially profiled patients, LDH values were only available at baseline and were within normal levels (≤ 190 U/L). Only 25% of patients (two of patients) had elevated LDH levels at the point of DOM, whereas 100% of patients had detectable cfDNA SNVs. cfDNA SNV and CNV monitoring was able to detect DOM significantly earlier than clinical or radiologic detection by a median of 7.5 months (95% CI, 3.17 to 12.0 months; P < .01) and earlier than LDH (P = .01). There was no significant correlation between preoperative cfDNA SNV burden and recurrence-free survival (P = .3). In patient SB11, the presence of new somatic SNVs (BRAFV600E and AKT1E17K) during follow-up and upon DOM suggests the value of cfDNA monitoring for tumor heterogeneity after surgery. This pattern of new SNVs upon recurrence was similarly seen in five additional patients (Fig 4 and Appendix Fig A4). Altogether, the longitudinal cfDNA SNV profiles suggest the possibility of monitoring disease through detecting the dynamic cfDNA SNV levels during disease-free follow-up.
Fig 4.
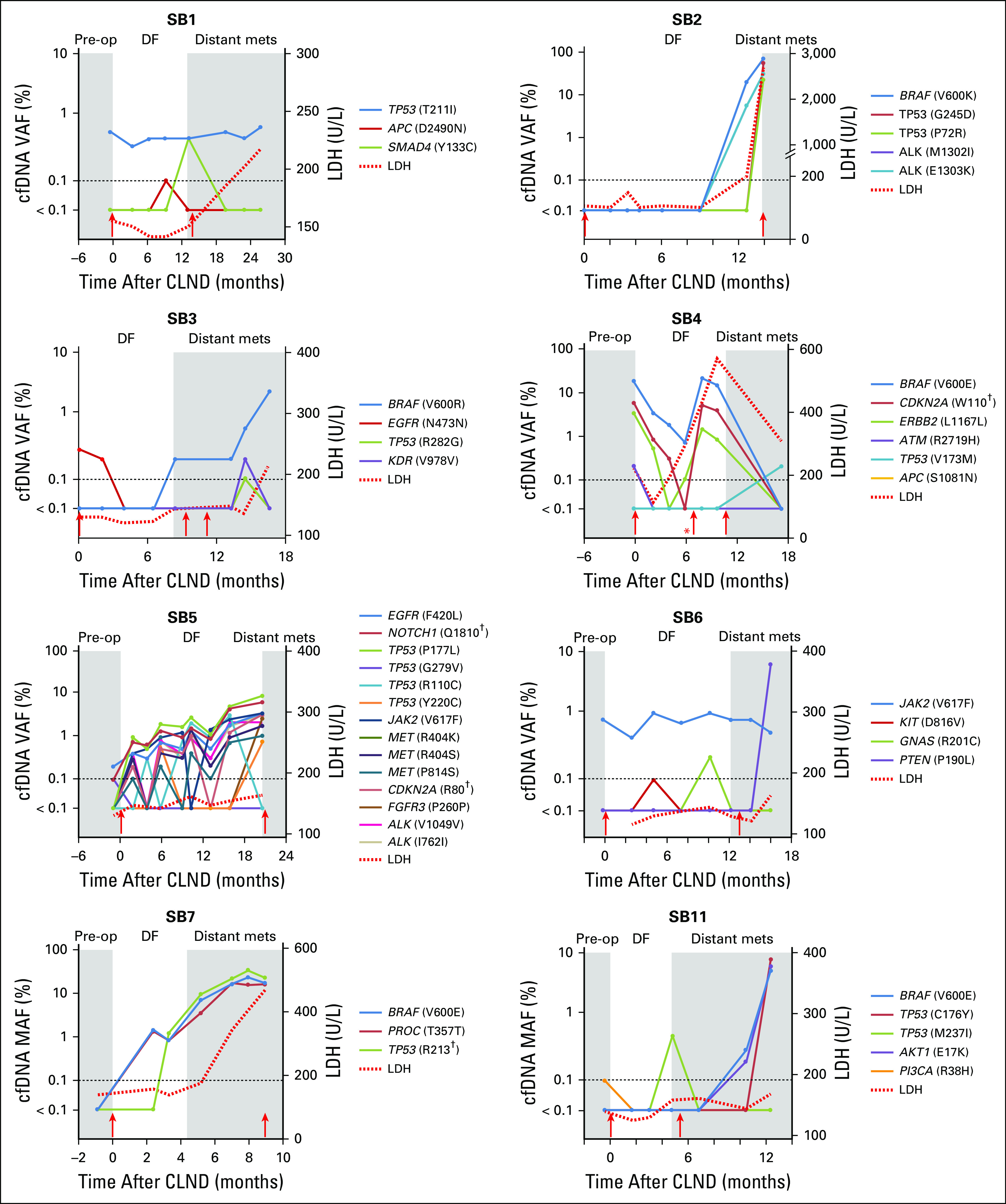
Cell-free DNA monitoring enables earlier detection of distant metastasis recurrence. For eight patients (cohort 2), clinical events are denoted in gray and white. Dashed line indicates normal lactate dehydrogenase (LDH) level (≤ 190 U/L) and digital next-generation sequencing limit of detection (≥ 0.1%). Red arrows indicate surgical resection. (*) Local recurrence before distant metastasis or relapse. (†) Stop codon. DF, disease-free period of follow-up every 2 to 4 months; Distant mets: distant metastasis or relapse; Pre-op, American Joint Committee on Cancer stage III diagnosis before complete lymph node dissection; VAF, variant allele fraction.
MET and EGFR cfDNA amplification
The recent association of CNV detection in melanoma tumors with clinical outcome and treatment response35,36 has yet to be clearly demonstrated in blood. In cohort 2, we identified cfDNA amplification during longitudinal follow-up in EGFR, ERBB2, and MET (Appendix Table A10). Patients SB2 and SB9 contained detectable EGFR and MET amplification that could aid in cfDNA monitoring given undetectable or low cfDNA SNV burden during follow-up. Patients SB4, SB7, SB10, and SB11 contained cfDNA EGFR/MET amplification during follow-up, reflecting cfDNA SNV dynamics (data not shown). Furthermore, two of four patients with preoperative EGFR/MET cfDNA amplification had detectable EGFR/MET cfDNA amplification postoperatively, reflecting residual disease presence.
cfDNA Monitoring Captures Tumor Evolution
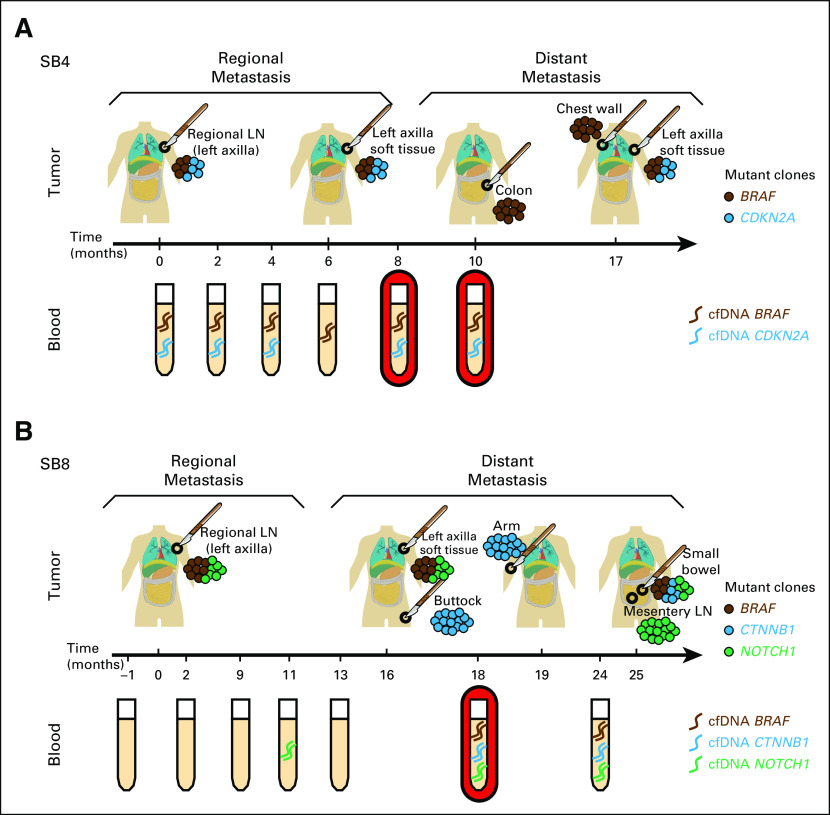
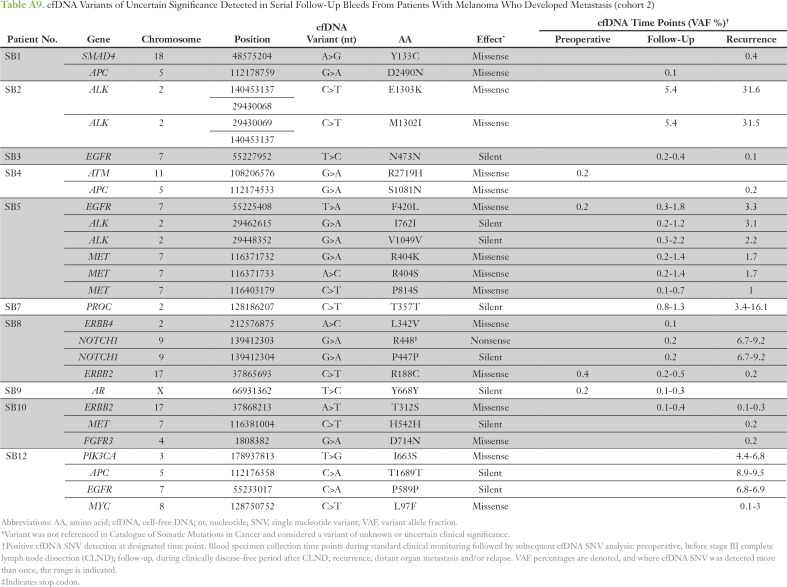
Given high tumor heterogeneity, cfDNA monitoring was evaluated to determine whether dynamic or evolving tumor SNV profiles can be captured, precluding the need for repetitive invasive tissue biopsies. To this end, amplicon sequencing was performed in matched regional and distant metastases. The tumor-cfDNA concordance, defined as the presence of cfDNA SNVs in any serially collected blood sample to any matched tumors sequenced, ranged from 66% to 100%, with an average concordance of 81.5% (Appendix Table A11). Interestingly, intertumoral heterogeneity among the metastatic sites was captured in the cfDNA profile as highlighted by representative patients in Figure 5. cfDNA profiling in patient SB4 (Fig 5A) captured heterogeneous tumor clones 2 to 7 months before distant metastasis biopsy, as revealed by detection of CDKN2A and BRAF somatic SNVs in blood. In patient SB8 (Fig 5B), cfDNA profiling captured all heterogeneous tumor clones 1 month before distant metastasis biopsy by monitoring the detection of BRAF, NOTCH1, and CTNNB1 cfDNA SNVs in blood.
Fig 5.
Cell-free DNA (cfDNA) single nucleotide variant (SNV) analysis reveals clonal tumor heterogeneity during disease progression. Time course of cfDNA SNV serial profiling in blood and matched metastatic tumors. (A) Patient SB4 (top: tumor; bottom: blood). CDKN2A and BRAF SNVs were detected in blood at 8 and 10 months before subsequent relapse. (B) Patient SB8 (top: tumor; bottom: blood). All SNVs were detected at 18 and 24 months before subsequent relapse. Scalpel indicates surgical resection; red-shaded serial bleed indicates lead time of cfDNA detection over tissue biopsy; dash along timeline indicates blood and tumor biopsy time points. LN, lymph node.
DISCUSSION
Given the highly aggressive nature of melanoma, monitoring patients for recurrence after curative surgery is particularly valuable.37 This study investigates the utility of cfDNA profiling using a sensitive 54–cancer gene panel digital NGS assay in patients with regional metastasis after CLND. We focused on cfDNA profiling during longitudinal follow-up at clinically relevant times, namely before curative elective surgery, during disease-free follow-up, and at relapse. Coupled with repeated analysis of tumor sites during disease progression, this strategy allowed monitoring of disease progression and evolution from the regional metastasis. This is critical for understanding how to use long-term longitudinal cfDNA analysis to best guide management of patients with melanoma.
The study explores the application of cfDNA analysis in melanoma for earlier recurrence detection compared with standard radiologic imaging after surgery, and the results support previous oncologic blood cfDNA studies.38-41 However, this study provides a novel view of cfDNA SNV dynamics. The assay containing a cancer panel that includes known melanoma driver genes minimizes the need for tumor DNA sequencing to identify baseline SNVs and proved advantageous in profiling dynamic cfDNA SNV levels, particularly for SNVs not detectable at the time of surgery. Furthermore, cfDNA profiling from the regional metastasis compared with advanced stages enabled a significantly earlier detection of DOM compared with imaging or serum LDH when monitoring patients after CLND when all imaging and testing were performed every 2 to 4 months, highlighting the sensitivity of cfDNA SNV detection during a clinically disease-free period.
The necessity of monitoring tumor evolution is highlighted by the different mutations found between matched primary and metastatic tumors.14,42 Melanoma cfDNA analysis focusing only on BRAF/NRAS hotspot mutations43-48 not only excludes wild-type patients (> 25%), but also limits the ability to assess dynamic levels of subclonal mutations and tumor heterogeneity found in early-stage metastatic melanoma tumors16 that may be indicative of tumor progression and therapy resistance.45-47 In this study, capture of clinically relevant cfDNA SNVs and CNVs that can impact treatment stratification was seen. Longitudinal follow-up captured dynamic cfDNA SNV levels, reflecting tumor heterogeneity, and the potential increase of subclonal cfDNA mutation levels upon relapse, potentially indicative for alternative treatment regimens.49,50
The 54–cancer gene panel also permitted evaluation of CNVs and SNV load and burden in melanoma blood cfDNA. Recently, the association of melanoma tumor CNVs with therapeutic outcomes has suggested their potential use for monitoring disease.36,37 cfDNA CNV utility was evident because EGFR, ERBB2, and MET cfDNA amplifications were detected during follow-up, suggesting that cfDNA amplification has the potential to monitor for occult micrometastatic residual disease after surgery. A significant association between preoperative EGFR and/or MET cfDNA amplification (cohort 2) and OS was seen but requires further verification. This study demonstrates the feasibility of detecting cfDNA amplification of metastasis driver genes in melanoma, supporting the reported associations of MET/EGFR amplification with tumor progression and poor outcome.51,52 cfDNA SNV load and burden were independent prognostic factors for OS in stage IV patients when age, sex, and M category were not significantly different between the dichotomized groups. cfDNA SNV load and burden factors were also analyzed as continuous variables (data not shown) and demonstrated the same finding. The association of cfDNA SNV load or burden status with worse OS supports recent findings of a high cfDNA SNV load (> three SNVs) correlating with immunotherapy response.53 Altogether, this highlights a cfDNA SNV load and burden trend that needs further validation in a larger patient cohort in a multicenter study. In addition, future studies are needed to determine the impact of longitudinal follow-up cfDNA monitoring in patients with early-stage regional melanoma.
To our knowledge, this is the first study to report blood cfDNA multiplex gene analysis in patients with regional AJCC stage III melanoma metastasis over longitudinal follow-up through DOM. Overall, our cfDNA analysis reveals informative SNV and CNV profiles, potentially precluding the need for invasive tumor biopsies. This approach allows for real-time monitoring of tumor progression and evolution, earlier recurrence detection, and discovery of new SNVs and CNVs indicative of therapy resistance. These capabilities are all critical to developing personalized care to effectively manage metastatic melanomas.
ACKNOWLEDGMENT
We acknowledge Dr Ian Hutchinson, PhD, Editorial Services, for his support in proofreading and editing of this article, and Dr Yuuki Iida, MD, for technical assistance in preparation of the article. We also acknowledge Kimberly Banks for her assistance in specimen logistics and data analysis coordination at Guardant Health. We thank Stacey Stern, Melanoma Database Coordinator, for assistance in providing clinical trial information. In memoriam, we thank Donald L. Morton for supporting and encouraging this study and providing advice.
Appendix
Fig A1.
Schematic of patient cohorts in the study. Melanoma stage and blood specimen collection time points are shown. CLND, complete lymph node dissection; Unk, unknown.
Fig A2.
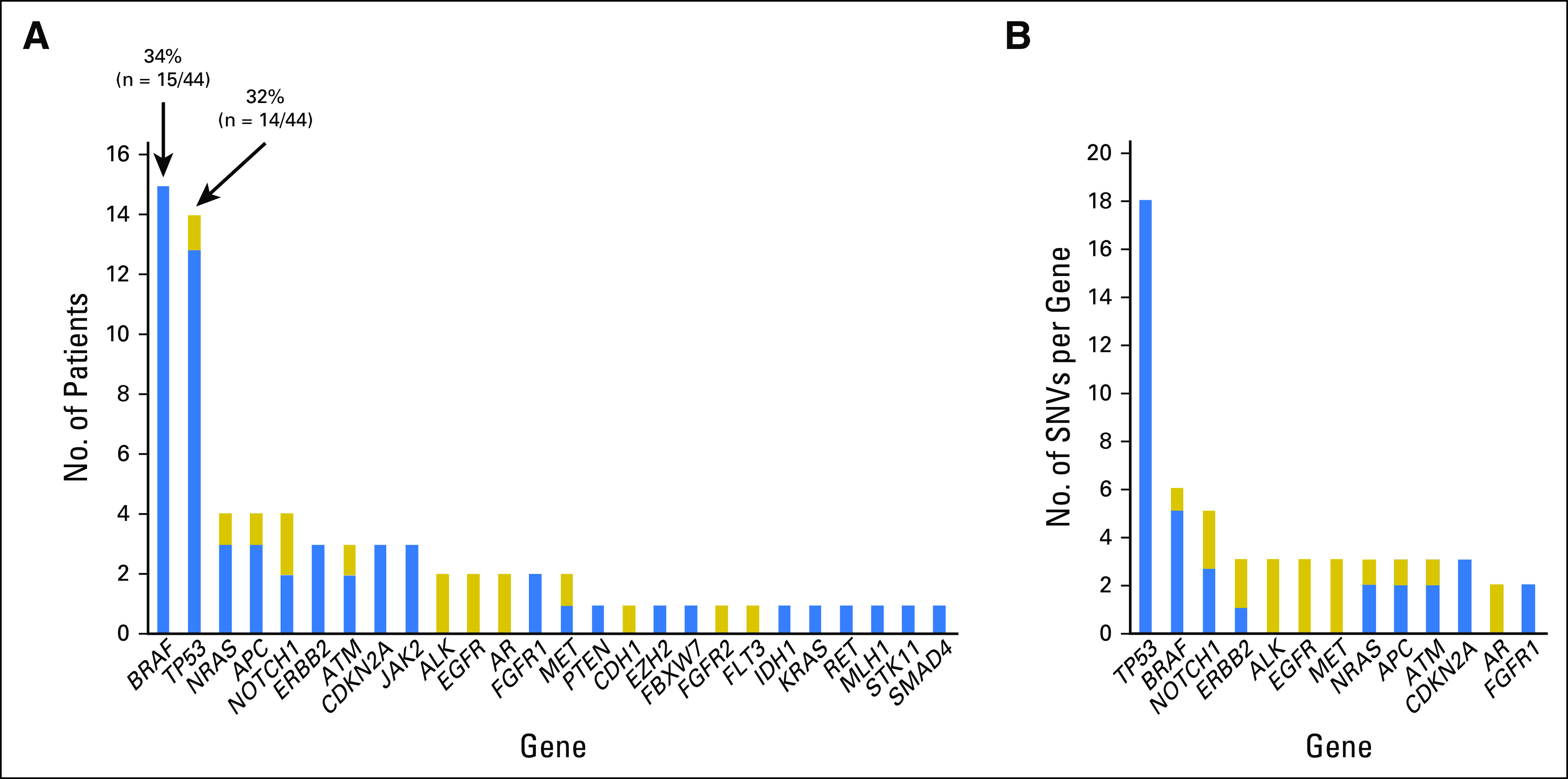
Measurement of cell-free DNA single nucleotide variants (SNVs) in 44 patients with melanoma (cohort 1) at a single presurgical/prerelapse time point. (A) Frequency of SNVs identified per gene across the cohort. (B) Number of SNVs (≥ 2) detected per gene across the cohort. Blue represents somatic SNVs; gold represents variants of unknown significance.
Fig A3.
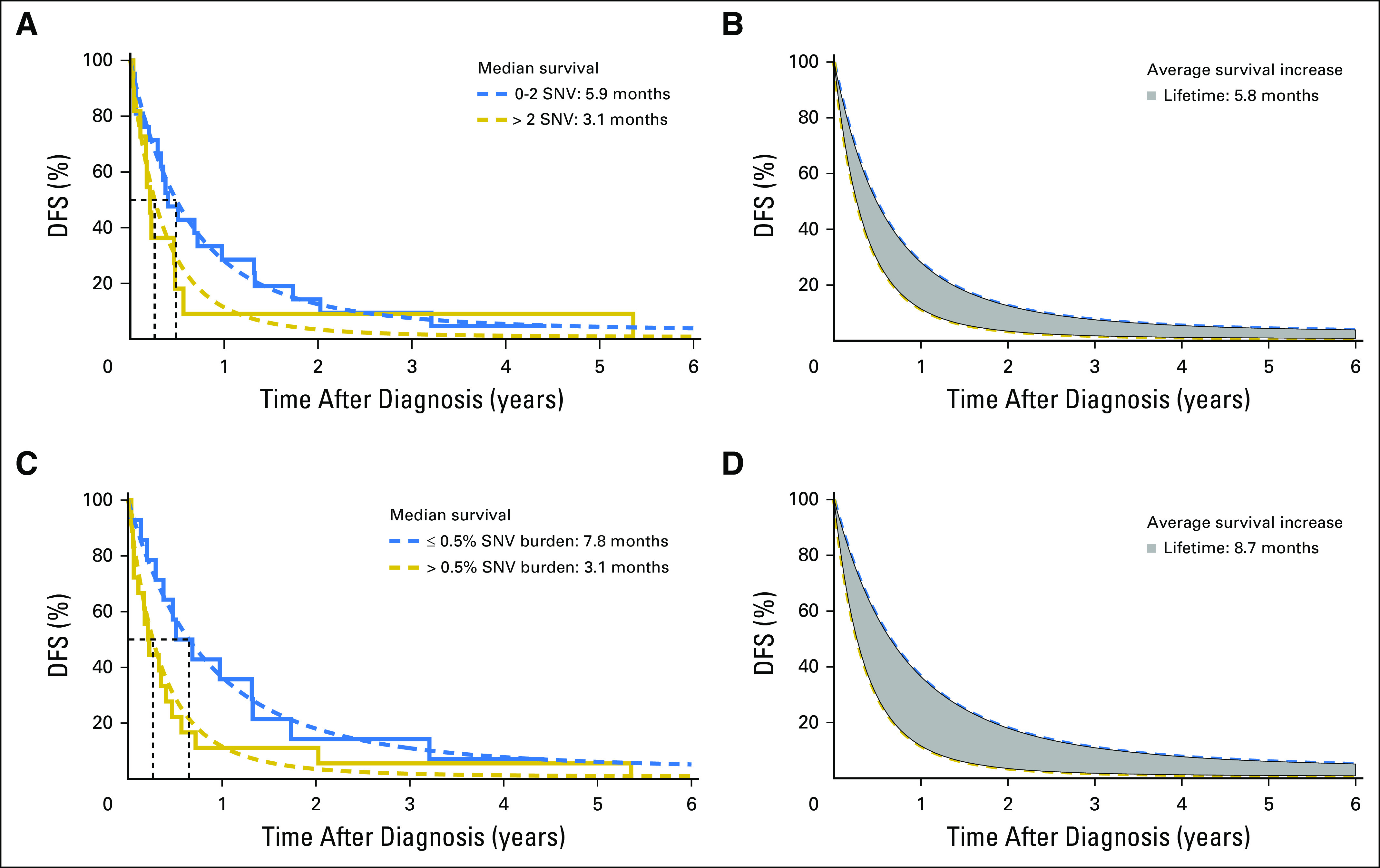
Disease-free survival (DFS) for cell-free DNA (cfDNA) single nucleotide variant (SNV) load and burden in patients with stage IV melanoma (cohort 1). The Kaplan-Meier curves for (A) two cfDNA SNV load groups and for (C) two cfDNA SNV burden groups with the fitted (dashed lines) Gompertz curves. (B and D) The difference in mean DFS for the two patient groups (gray area) is shown. The end point for DFS is the time to event related to the disease itself or death from a disease-specific event (ie, noncancer deaths are censored).
Fig A4.
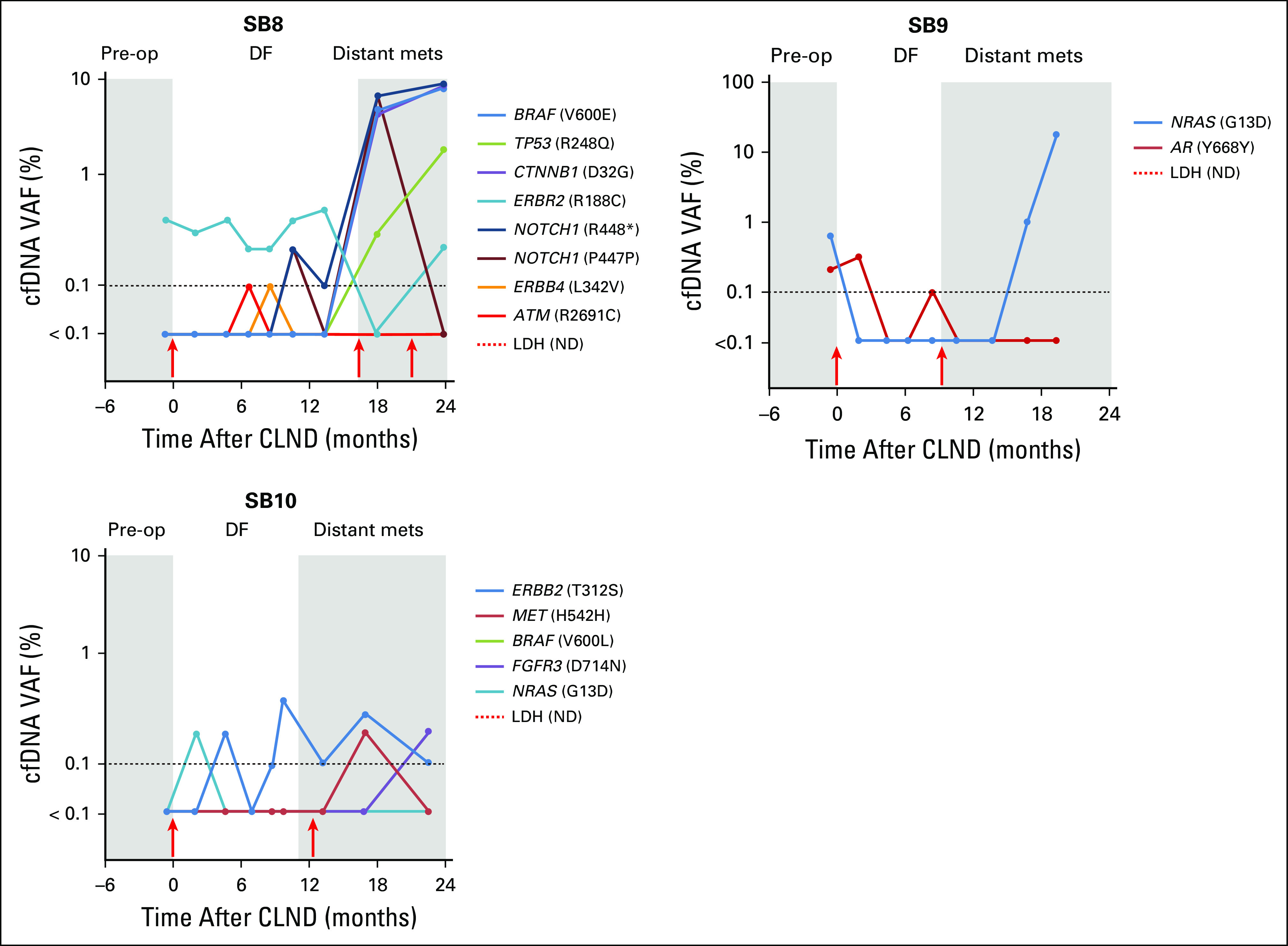
Cell-free DNA (cfDNA) single nucleotide variant (SNV) monitoring of patients with melanoma over disease progression (cohort 2). cfDNA SNV profiling during disease progression in patients with melanoma is displayed (cohort 2). For each time course, progressive clinical events are highlighted in gray or white. Dashed line indicates digital next-generation sequencing limit of detection (≥ 0.1%); red arrows indicate surgical resection. DF, disease-free period of follow-up every 2 to 4 months; Distant mets: distant metastasis or relapse; LDH (ND), lactate dehydrogenase not done longitudinally; Pre-op, American Joint Committee on Cancer stage III diagnosis before complete lymph node dissection; VAF, variant allele fraction.
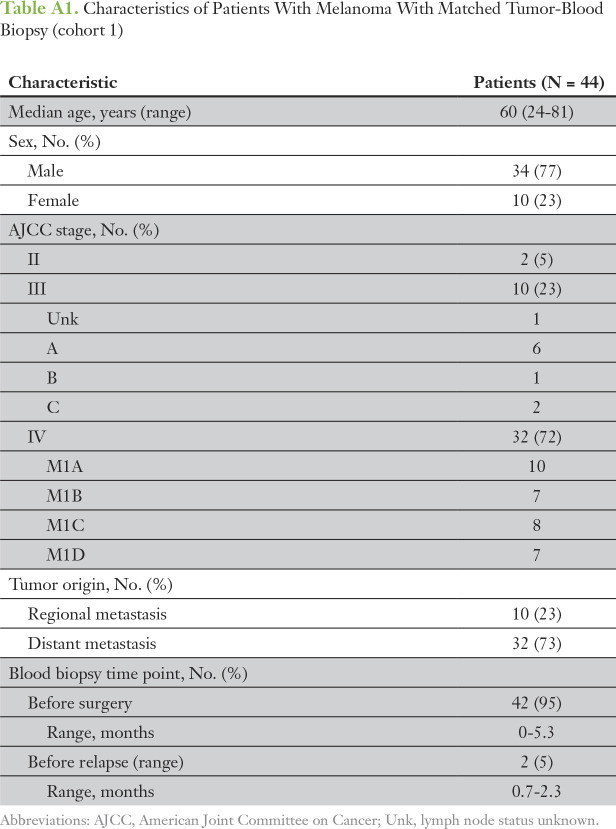
Table A1.
Characteristics of Patients With Melanoma With Matched Tumor-Blood Biopsy (cohort 1)
Table A2.
Cancer Genes in the Digital Next-Generation Sequencing Panel
Table A3.
cfDNA Somatic SNVs in Patients With AJCC Stage II, III, or IV Melanomas (cohort 1)
Table A4.
cfDNA Variants of Uncertain Significance in Patients With AJCC Stage II, III, or IV Melanomas (cohort 1)
Table A5.
Clinical Characteristics of Patients With Stage IV Melanoma and cfDNA SNV Status (cohort 1)
Table A6.
Multivariable Analysis of cfDNA SNV Load Status and Prognostic Factors for Melanoma Stage IV Disease Outcome (cohort 1)
Table A7.
Multivariable Analysis of cfDNA SNV Burden Status and Prognostic Factors for Melanoma Stage IV Disease Outcome (cohort 1)
Table A8.
cfDNA Somatic SNVs Detected in Serial Follow-Up Bleeds From Patients With Melanoma Who Developed Metastasis (cohort 2)
Table A9.
cfDNA Variants of Uncertain Significance Detected in Serial Follow-Up Bleeds From Patients With Melanoma Who Developed Metastasis (cohort 2)
Table A10.
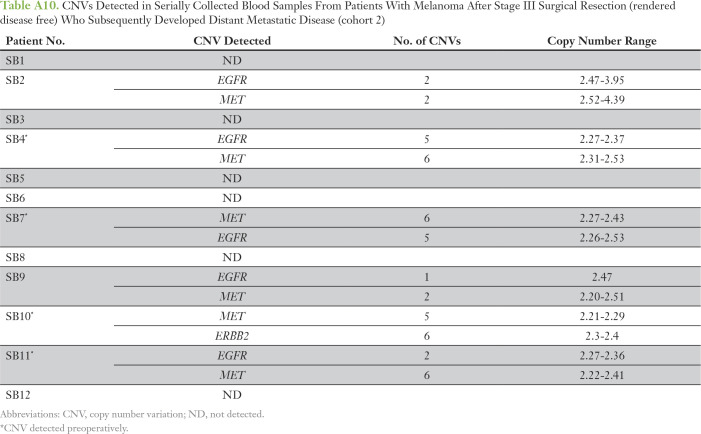
CNVs Detected in Serially Collected Blood Samples From Patients With Melanoma After Stage III Surgical Resection (rendered disease free) Who Subsequently Developed Distant Metastatic Disease (cohort 2)
Table A11.
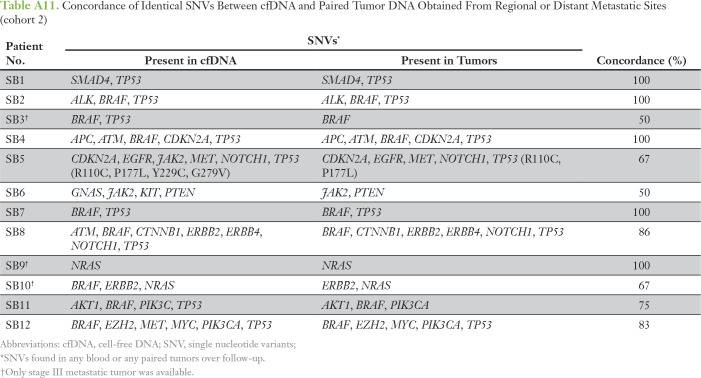
Concordance of Identical SNVs Between cfDNA and Paired Tumor DNA Obtained From Regional or Distant Metastatic Sites (cohort 2)
Footnotes
Supported by the Dr Miriam and Sheldon G. Adelson Medical Research Foundation (D.S.B.H.) and National Institutes of Health, National Cancer Institute Grant No. 1R01CA167967-01A1 (D.S.B.H.).
AUTHOR CONTRIBUTIONS
Conception and design: Selena Y. Lin, Sharon K. Huang, AmirAli Talasaz, Dave S.B. Hoon
Financial support: Dave S.B. Hoon
Administrative support: Dave S.B. Hoon
Provision of study material or patients: Dave S.B. Hoon
Collection and assembly of data: Selena Y. Lin, Sharon K. Huang, Kelly T. Huynh, Diego M. Marzese, Richard B. Lanman, Dave S.B. Hoon
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Selena Y. Lin
No relationship to disclose
Sharon K. Huang
No relationship to disclose
Kelly T. Huynh
No relationship to disclose
Matthew P. Salomon
No relationship to disclose
Shu-Ching Chang
No relationship to disclose
Diego M. Marzese
No relationship to disclose
Richard B. Lanman
Employment: Guardant Health, Veracyte
Leadership: Guardant Health, Biolase
Stock and Other Ownership Interests: Guardant Health, Biolase
Research Funding: Guardant Health
AmirAli Talasaz
Employment: Guardant Health
Leadership: Guardant Health
Stock and Other Ownership Interests: Guardant Health
Research Funding: Guardant Health
Patents, Royalties, Other Intellectual Property: Guardant Health
Travel, Accommodations, Expenses: Guardant Health
Dave S.B. Hoon
Consulting or Advisory Role: Guardant Health
REFERENCES
- 1.Johnson DB, Pollack MH, Sosman JA: Emerging targeted therapies for melanoma. Expert Opin Emerg Drugs 21:195-207, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Nicholl MB, Elashoff D, Takeuchi H, et al. : Molecular upstaging based on paraffin-embedded sentinel lymph nodes: Ten-year follow-up confirms prognostic utility in melanoma patients. Ann Surg 253:116-122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wittner C, Park S, Rifai N, et al. : Circulating tumor cells and circulating tumor DNA as a real time liquid biopsy approach, in Rifai N. (ed): Tietz Textbook Clinical Chemistry and Molecular Diagnostics (ed 6). New York, NY, Saunders, 2016, pp 1111-1144 [Google Scholar]
- 4.Shinozaki M, O’Day SJ, Kitago M, et al. : Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res 13:2068-2074, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girotti MR, Gremel G, Lee R, et al. : Application of sequencing, liquid biopsies and patient-derived xenografts for personalized medicine in melanoma. Cancer Discov 6:286-299, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Yao YH, Li BG, et al. : Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: A systematic review and meta-analysis. Sci Rep 5:9800, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujiwara Y, Chi DD, Wang H, et al. : Plasma DNA microsatellites as tumor-specific markers and indicators of tumor progression in melanoma patients. Cancer Res 59:1567-1571, 1999 [PubMed] [Google Scholar]
- 8.Taback B, O’Day SJ, Boasberg PD, et al. : Circulating DNA microsatellites: Molecular determinants of response to biochemotherapy in patients with metastatic melanoma. J Natl Cancer Inst 96:152-156, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto A, O’Day SJ, Taback B, et al. : Allelic imbalance on 12q22-23 in serum circulating DNA of melanoma patients predicts disease outcome. Cancer Res 64:4085-4088, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Marzese DM, Hirose H, Hoon DS: Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients. Expert Rev Mol Diagn 13:827-844, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Hoshimoto S, Kuo CT, Chong KK, et al. : AIM1 and LINE-1 epigenetic aberrations in tumor and serum relate to melanoma progression and disease outcome. J Invest Dermatol 132:1689-1697, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoon DS, Spugnardi M, Kuo C, et al. : Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene 23:4014-4022, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodis E, Watson IR, Kryukov GV, et al. : A landscape of driver mutations in melanoma. Cell 150:251-263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Network : Genomic classification of cutaneous melanoma. Cell 161:1681-1696, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SK, Hoon DS: Liquid biopsy utility for the surveillance of cutaneous malignant melanoma patients. Mol Oncol 10:450-463, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harbst K, Lauss M, Cirenajwis H, et al. : Multiregion whole-exome sequencing uncovers the genetic evolution and mutational heterogeneity of early-stage metastatic melanoma. Cancer Res 76:4765-4774, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi H, Morton DL, Kuo C, et al. : Prognostic significance of molecular upstaging of paraffin-embedded sentinel lymph nodes in melanoma patients. J Clin Oncol 22:2671-2680, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faries MB, Thompson JF, Cochran AJ, et al. : Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med 376:2211-2222, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshimoto S, Shingai T, Morton DL, et al. : Association between circulating tumor cells and prognosis in patients with stage III melanoma with sentinel lymph node metastasis in a phase III international multicenter trial. J Clin Oncol 30:3819-3826, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morton D, Mozzillo N, Thompson J, et al. : An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. J Clin Oncol 25:8508-8508, 2007 (suppl; abstr 8508) [Google Scholar]
- 21.McShane LM, Altman DG, Sauerbrei W, et al. : Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 97:1180-1184, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lanman RB, Mortimer SA, Zill OA, et al. : Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS One 10:e0140712, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes SA, Beare D, Boutselakis H, et al. : COSMIC: Somatic cancer genetics at high-resolution. Nucleic Acids Res 45:D777-D783, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolger AM, Lohse M, Usadel B: Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30:2114-2120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Durbin R: Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754-1760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cibulskis K, McKenna A, Fennell T, et al. : ContEst: Estimating cross-contamination of human samples in next-generation sequencing data. Bioinformatics 27:2601-2602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Handsaker B, Wysoker A, et al. : The sequence alignment/map format and SAMtools. Bioinformatics 25:2078-2079, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dardis C: survMisc: Miscellaneous functions for survival data. https://cran.r-project.org/web/packages/survMisc/index.html.
- 29.Kiche J, Aduda J, Anthiany K, et al. : Comparison of survival models and estimation of their parameters with respect to mortality in a given population. Math Theory Modeling 4:164-176, 2014 [Google Scholar]
- 30.Chang S-C, Wang M, Beckerman JG, et al. : A method to estimate the mean lifetime survival increase of statin therapy. Future Cardiol 12:539-544, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Nardi A, Schemper M: Comparing Cox and parametric models in clinical studies. Stat Med 22:3597-3610, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Balch CM, Gershenwald JE, Soong SJ, et al. : Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199-6206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martens A, Wistuba-Hamprecht K, Geukes Foppen M, et al. : Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res 22:2908-2918, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345-1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch D, Kemmerling R, Davis S, et al. : Chromothripsis and focal copy number alterations determine poor outcome in malignant melanoma. Cancer Res 73:1454-1460, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson MA, Zhao F, Khare S, et al. : Copy number changes are associated with response to treatment with carboplatin, paclitaxel, and sorafenib in melanoma. Clin Cancer Res 22:374-382, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong SQ, Raleigh JM, Callahan J, et al. : Circulating tumor DNA analysis and functional imaging provide complementary approaches for comprehensive disease monitoring in metastatic melanoma. JCO Precis Oncol 10.1200/PO.16.00009 [epub on April 27, 2017] [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Murillas I, Schiavon G, Weigelt B, et al. : Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med 7:302ra133-302ra133, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Reinert T, Schøler LV, Thomsen R, et al. : Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut 65:625-634, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Birkenkamp-Demtröder K, Christensen E, Nordentoft I, et al. : Monitoring treatment response and metastatic relapse in advanced bladder cancer by liquid biopsy analysis. Eur Urol 73:535-540, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Almodovar K, Iams WT, Meador CB, et al. : Longitudinal cell-free DNA analysis in patients with small cell lung cancer reveals dynamic insights into treatment efficacy and disease relapse. J Thorac Oncol 13:112-123, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cohen O, Kim D, Oh C, et al: Whole exome and transcriptome sequencing of resistant ER+ metastatic breast cancer. San Antonio Breast Cancer Symposium, San Antonio, TX, December 6-10, 2016 (abstr S1-01) [Google Scholar]
- 43.Chang GA, Tadepalli JS, Shao Y, et al. : Sensitivity of plasma BRAFmutant and NRASmutant cell-free DNA assays to detect metastatic melanoma in patients with low RECIST scores and non-RECIST disease progression. Mol Oncol 10:157-165, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gray ES, Rizos H, Reid AL, et al. : Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget 6:42008-42018, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanmamed MF, Fernández-Landázuri S, Rodríguez C, et al. : Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem 61:297-304, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Tsao SC-H, Weiss J, Hudson C, et al. : Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations. Sci Rep 5:11198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JH, Long GV, Boyd S, et al. : Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol 28:1130-1136, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Lee R, Gremel G, Marshall A, et al. : Circulating tumor DNA predicts survival in patients with resected high risk stage II/III melanoma. Ann Oncol 29:490-496, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson SC: Translational medicine: A target for pharmacological intervention in an untreatable human disease. Science 346:1192, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Kreso A, O’Brien CA, van Galen P, et al. : Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 339:543-548, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rákosy Z, Vízkeleti L, Ecsedi S, et al. : EGFR gene copy number alterations in primary cutaneous malignant melanomas are associated with poor prognosis. Int J Cancer 121:1729-1737, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Engelman JA, Zejnullahu K, Mitsudomi T, et al. : MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316:1039-1043, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Khagi Y, Goodman AM, Daniels GA, et al. : Hypermutated circulating tumor DNA: Correlation with response to checkpoint inhibitor-based immunotherapy. Clin Cancer Res 23:5729-5736, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]