TO THE EDITOR:
The B-cell antigen receptor (BCR) pathway seems to be critical in normal and malignant B cells such as chronic lymphocytic leukemia (CLL). Phosphatidylinositol 3-kinase (PI3K), specifically PI3Kδ/γ, constitute pivotal nodes in the BCR axis. The selective expression of PI3Kδ/γ in hematopoietic cells provided an impetus to create isoform-specific inhibitors to target this key kinase. Although several inhibitors have been created, duvelisib and idelalisib have moved far in the clinic1; in fact, the latter in combination with rituximab is an approved agent for treatment of relapsed/refractory CLL.2,3 Duvelisib targets the δ- and γ-isoforms, whereas idelalisib inhibits only the δ-isoform.1 Hence, we hypothesized that these inhibitors may have similar effects in downstream target proteins. Studies in the BCR pathway have demonstrated the impact of this nexus on CLL cell survival.4,5
In a recent study that used various platforms, we demonstrated significant changes in transcript and protein levels of Bcl-2 family members after treatment with duvelisib.6 Of the four antiapo-ptotic proteins, Bcl-2 protein was increased significantly in reverse phase protein array (RPPA; n = 16; P = .001) and immunoblot (median, 1.7-fold change; P = .034) assays. Among antiapoptotic proteins, Bcl-xL was not changed, whereas Bcl-2-A1 and Mcl-1 were slightly, but significantly decreased. Of BH3-only members, both Bim and Puma levels were increased. These observations were in circulating cells after duvelisib therapy (ie, after peripheral blood lymphocytosis).6 Idelalisib showed a similar phenomenon during phase I, single-agent investigations.7 To test our hypothesis, we evaluated these CLL lymphocytes from the peripheral blood before and after idelalisib treatment.
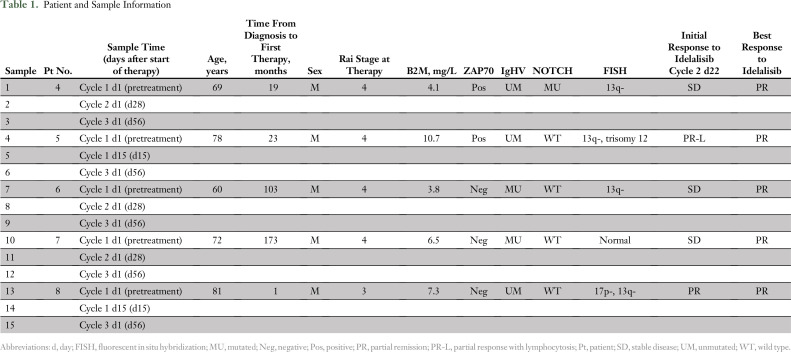
Fifteen samples from five patients were assessed (Table 1). All these patients with CLL were previously untreated and enrolled in a Dana-Farber Cancer Institute clinical trial of upfront idelalisib for 2 months followed by the addition of ofatumumab. All received idelalisib 150 mg twice per day as a single agent during the time these samples were collected.
Table 1.
Patient and Sample Information
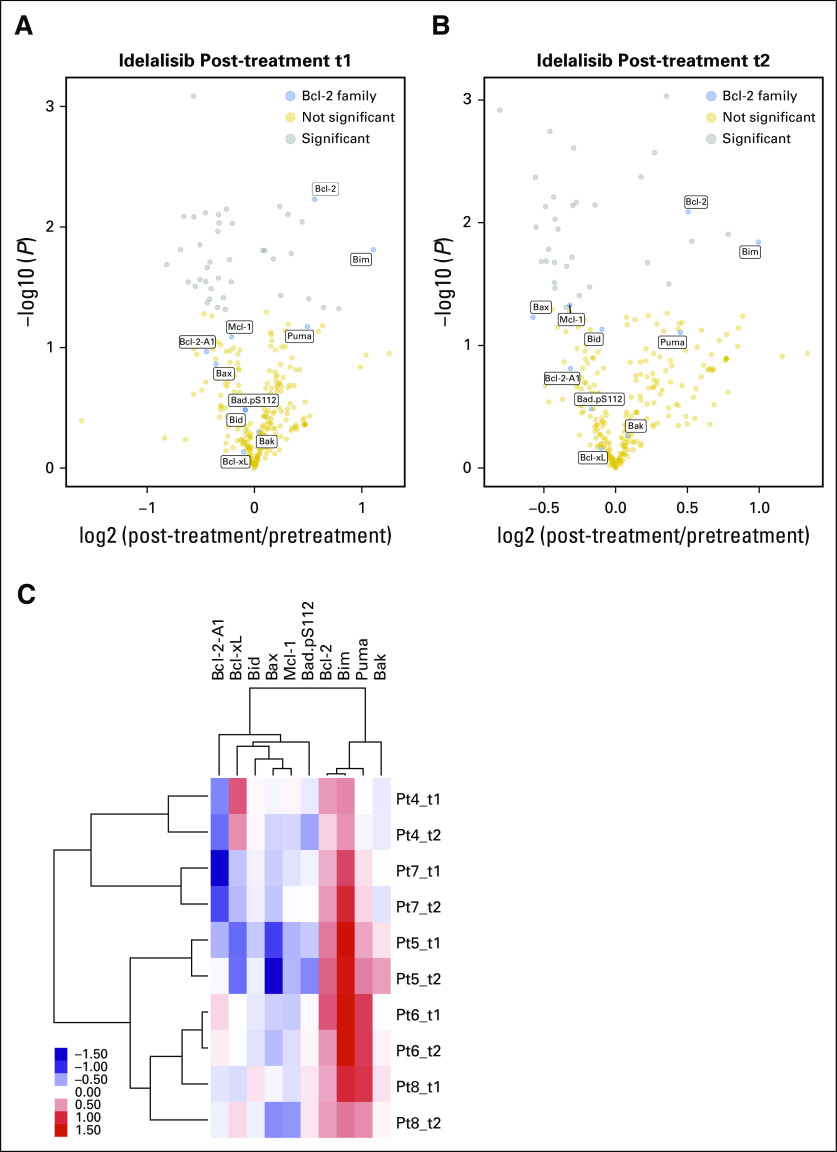
RPPA assays were performed from these samples to determine the protein expression of the Bcl-2 family. The Bcl-2 family proteins are Mcl-1, Bcl-xL, Bcl-2, and Bcl-2-A1 among the antiapoptotic members; Bax and Bak (multidomain proapoptotic members); and Bim, Bid, Puma, and Bad.pS155 among the BH3-only members. Close to 300 proteins were included in the RPPA assay, which included 10 members (blue symbols) of the Bcl-2 family (Figs 1A and 1B). The treatment duration at the time of measurement varied, starting as early as 15 days and as late as cycle 3 day 1 (ie, 56 days). A volcano plot demonstrates that 40 proteins after 15 or 28 days (post-treatment t1; Fig 1A) and 35 proteins after two cycles (56 days) of idelalisib (post-treatment t2; Fig 1B) were significantly modulated. Among the 10 Bcl-2 family proteins, Bcl-2 and Bim were modulated at a significant level (P = .006 and P = .01, respectively) after 15 to 28 days of therapy. After 56 days, Bcl-2, Bim, and Mcl-1 were the most significantly modulated proteins (P = .008, P = .01, and P = .05, respectively). Two-way hierarchical clustering and a heat map of fold changes in Bcl-2 family proteins demonstrated upregulation and downregulation of Bcl-2 family proteins. Among the four evaluated antiapoptotic proteins, Bcl-2 was consistently increased in all patient samples at every time point (Fig 1C). Bcl-2-A1 and Bcl-xL generally decreased but varied. Mcl-1 significantly decreased after two cycles of idelalisib (P = .04; Fig 1C). Among multidomain proapoptotic proteins, Bax was decreased, but Bak generally remained at a similar level. Among proapoptotic BH3-only domain proteins, Bid remained the same, but Bim was consistently increased at both evaluation times (P = .01; Fig 1C). Except for one patient sample, Puma levels also increased but not to a significant level (P = .07 and .08).
Fig 1.
Impact of idelalisib therapy on proteins with a focus on Bcl-2 family proteins in circulating chronic lymphocytic leukemia (CLL) cells. Primary CLL cells from five patients were isolated fresh from peripheral blood samples before treatment (baseline, designated as d0 [day 0]) and d15 (t1) or d28 (t1) and d56 (t2) after the start of oral idelalisib 150 mg twice per day. CLL cells were isolated by using standard Ficoll-hypaque technique from peripheral blood and were stored in liquid nitrogen at Dana-Farber Cancer Institute. Reverse phase protein array was done after lysis of cells at MD Anderson Cancer Center. The assay contained 300 proteins.8 Lysates were diluted and samples probed with antibodies and visualized by colorimetric reaction. Slides were scanned on a flatbed scanner to produce tag image file format images, and density was quantitated. Relative protein levels for each sample were determined by interpolation of each dilution curve from the standard curve of the slide. All data were normalized to protein loading and transformed to a linear value. (A) Volcano plot of the fold change of protein and statistical significance between pretreatment and post-treatment t1 (d15 and d28 after start of idelalisib therapy). Each symbol represents a protein. The proteins with P < .05 are shown in gray. The Bcl-2 family members are shown in blue. (B) Volcano plot of the fold change of protein and statistical significance between pretreatment and post-treatment t2 (ie, d56 after start of idelalisib treatment). (C) Two-way hierarchical clustering and heat map of fold changes between pretreatment and post-treatment samples for 10 Bcl-2 family member proteins. Pt, patient.
Prior investigations have demonstrated that agents that target Bcl-2 (navitoclax9 and venetoclax10) generally show effectiveness as a result of inherently high Bcl-2 protein levels in CLL cells. In contrast, levels of Mcl-1 protein were correlated with resistance to these agents. Among BH3-only proteins, Bim was positively correlated with Bcl-2 antagonist-induced apoptosis. Hence, we evaluated additional changes in these three important proteins. As shown in Figs 2A to 2C, Bcl-2, Mcl-1, and Bim levels were modulated in every sample, which were similar to those obtained with duvelisib.6
Fig 2.
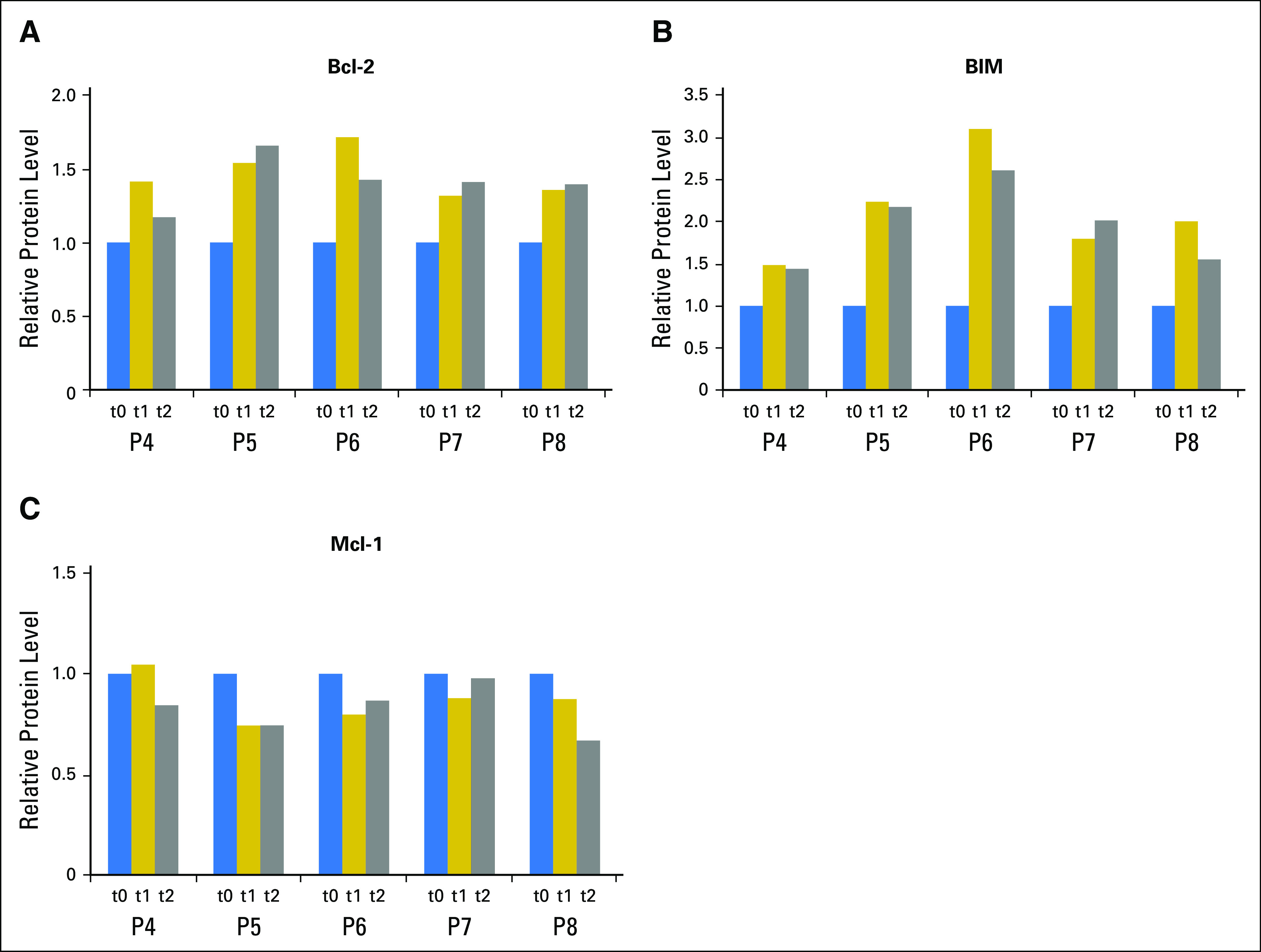
Idelalisib therapy results in an increase in Bcl-2 and Bim and a decrease in Mcl-1 protein. Bar graph of three proteins that show significant changes. Primary chronic lymphocytic leukemia cells from patients were isolated fresh from peripheral blood before and after therapy and subjected to reverse phase protein array analysis as described in Figure 1. All readouts are normalized with respect to pretreatment measurements. (A) Changes in the level of Bcl-2 protein after idelalisib therapy (t1 = 15 or 28 days and t2 = 56 days after treatment). P = .06 and .08 for the two evaluation times. (B) Changes in the expression level of Bim protein after idelalisib therapy. P = .01 and .02 for t1 and t2, respectively. (C) Changes in the expression level of Mcl-1 protein after idelalisib therapy. P = .08 and .05 for t1 and t2, respectively.
In general, these data suggest that BCR pathway inhibition leads to high Bcl-2 and Bim protein levels and a decline in Mcl-1 protein levels in CLL cells, which was also previously reported with ibrutinib therapy.11 Given that the BCR axis inhibitors do not induce overt apoptosis of CLL cells, strategies to combine venetoclax with idelalisib, duvelisib, or ibrutinib should result in apoptosis and removal of CLL cells from peripheral blood. Such trials currently are being pursued.
ACKNOWLEDGMENT
Supported in part by an MD Anderson Cancer Center Support Grant, a CLL Global Research Foundation Alliance grant, and Sponsored Research Agreement from Gilead to V.G. The samples are from an investigator-initiated clinical trial (J.R.B.) that was supported by Gilead. J.R.B and V.G. are members of the Chronic Lymphocytic Leukemia Consortium. A.K. is supported by MD Anderson Cancer Center Support Grant P30 CA016672 (Bioinformatics Shared Resource). Q.Y. and P.M. contributed equally to this work. J.R.B. and V.G. are co-senior authors.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Changes in Bcl-2 Family Protein Profile During Idelalisib Therapy Mimic Those During Duvelisib Therapy in Chronic Lymphocytic Leukemia Lymphocytes
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or po.ascopubs.org/site/ifc.
Qingshan Yang
No relationship to disclose
Prexy Modi
No relationship to disclose
Anil Korkut
Stock and Other Ownership Interests: Vivoz Biolabs (I)
Patents, Royalties, Other Intellectual Property: drug combinations for treatment of melanoma and other cancers, C. Sander and A. Korkut US Patent App. 14/722,768 (Inst); systems and methods for identifying drug combinations for reduced drug resistance in cancer treatment, C. Sander and A. Korkut US Patent App. 14/722,733 (Inst)
Stacey M. Fernandes
No relationship to disclose
John Hanna
No relationship to disclose
Jennifer R. Brown
Honoraria: Janssen Pharmaceuticals, AbbVie
Consulting or Advisory Role: Gilead Sciences, Pharmacyclics, Janssen Pharmaceuticals, Roche, Genentech, Celgene, Sun Pharma, Infinity Pharmaceuticals, AstraZeneca, Redx Pharma, Astellas Pharma, AbbVie
Research Funding: Gilead Sciences
Varsha Gandhi
Research Funding: Gilead Sciences
REFERENCES
- 1.Yang Q, Modi P, Newcomb T, et al. Idelalisib: First-in-class PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia, small lymphocytic leukemia, and follicular lymphoma. Clin Cancer Res. 2015;21:1537–1542. doi: 10.1158/1078-0432.CCR-14-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller BW, Przepiorka D, de Claro RA, et al. FDA approval: Idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res. 2015;21:1525–1529. doi: 10.1158/1078-0432.CCR-14-2522. [DOI] [PubMed] [Google Scholar]
- 4.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel VM, Balakrishnan K, Douglas M, et al. Duvelisib treatment is associated with altered expression of apoptotic regulators that helps in sensitization of chronic lymphocytic leukemia cells to venetoclax (ABT-199) Leukemia. doi: 10.1038/leu.2016.382. [epub ahead of print on February 3, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MD Anderson Cancer Center: Reverse phase protein array. https://www.mdanderson.org/research/research-resources/core-facilities/functional-proteomics-rppa-core.html.
- 9.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2015;21:3705–3715. doi: 10.1158/1078-0432.CCR-14-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]