Abstract
PURPOSE
Biliary tract cancers (BTCs), which include intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (EHC), and gallbladder cancer (GBC), have limited treatment options. We sought to comprehensively examine the clinical and molecular characteristics of BTCs with amplification or mutation of ERBB2.
METHODS
Demographic, outcome, and treatment response data were collected for patients with ERBB2-altered BTC identified by next-generation sequencing with Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets from 2014 to 2018.
RESULTS
A total of 517 patients with BTC underwent next-generation sequencing (ICC, n = 313; EHC, n = 93; GBC, n = 111). Twenty-eight patients (5.4%) had ERBB2 alterations, including 2.7% with ERBB2 gene amplification, 2.3% with ERBB2 mutation, and 0.4% with concurrent amplification and mutation. The prevalence of ERBB2 gene alterations was significantly higher in GBC (12.6%) than in ICC (2.2%) and EHC (7.5%; P < .001). In ERBB2-amplified tumors, the median fold change was 6.4 (range, 2.1 to 19.7), while in ERBB2-mutant tumors, the most frequent mutated domain was the extracellular domain (32%), with all mutations in this region involving the S310 codon. Frequent co-altered genes in this cohort were TP53 (54%), PIK3CA (21%), and CDKN2A (18%); KRAS amplification/mutation was found in 7% of patients. One patient with ERBB2-amplified EHC who enrolled in a basket trial (ClinicalTrials.gov identifier: NCT02675829) had a partial response to the human epidermal growth factor receptor 2–targeted antibody-drug conjugate ado-trastuzumab emtansine.
CONCLUSION
ERBB2 alterations are present in 5.4% of BTCs. When present, the degree of ERBB2 gene amplification is often high, and S310 codon mutations are the most common hotspot. These features, along with the presented case, support further development of human epidermal growth factor receptor 2–targeted therapy in ERBB2-mutant and/or -amplified BTC.
INTRODUCTION
Biliary tract cancer (BTC) comprises three clinically and molecularly distinct entities, intrahepatic cholangiocarcinoma (ICC), extrahepatic cholangiocarcinoma (EHC), and gallbladder cancer (GBC). The treatment options for these patients are limited and include chemotherapy with capecitabine in the adjuvant setting1 and the combination of gemcitabine with a platinum for patients with metastatic disease.2,3 In the second-line setting, the randomized phase III ABC-06 study showed that treatment with infusional fluorouracil, leucovorin, and oxaliplatin was associated with significant benefit compared with active symptom control, with a median survival of 6.2 and 5.3 months, respectively.4 Despite these treatments, overall survival (OS) in patients with metastatic disease remains < 1 year. Genomic profiling of BTCs with next-generation sequencing (NGS) platforms has shown that the three clinical subtypes have significant molecular differences.5 Whereas all three have a high prevalence of TP53 and CDKN2A mutations, genomic alterations in FGFR2 and IDH1 are mostly limited to ICC (approximately 20% each). KRAS mutations are observed more frequently in EHC than in ICC and GBC. In prior studies, the most frequently altered clinically actionable gene across all three BTC subtypes was ERBB2. ERBB2 encodes the human epidermal growth factor receptor 2 (HER2) receptor tyrosine kinase, which is most prevalent in GBC (12% to 19%).6,7 In a study of 195 patients with ICC and EHC who underwent targeted capture NGS, the prevalence of ERBB2 alterations was 4.6% (3.6% ERBB2 amplification and 1% ERBB2 mutation).8 Patients with EHC and ICC with ERBB2-amplified or -mutant tumors in this cohort had a shorter time to progression on first-line chemotherapy compared with patients with ERBB2 wild type.
CONTEXT
Key Objective
To describe the landscape of ERBB2 genomic alterations in biliary tract cancer and illustrate a case of a patient who responded to anti–human epidermal growth factor receptor 2 (HER2)–targeted therapy.
Knowledge Generated
ERBB2 amplification or mutations were identified in 28 (5.4%) of 517 patients with biliary tract cancer. ERBB2-driven biliary tract tumors had higher concurrent alterations of TP53 and PIK3CA, while KRAS alterations seemed to be less common. One patient with ERBB2-amplified extrahepatic cholangiocarcinoma had a partial response to the HER2-targeted antibody-drug conjugate ado-trastuzumab emtansine.
Relevance
Potentially targetable ERBB2 amplification and mutation are present in a significant group of biliary tract tumors, particularly in gallbladder cancer. This study supports prospective testing for ERBB2 alterations to develop novel anti-HER2 strategies in this population.
On the basis of demonstrated improved OS in pivotal phase III trials, drugs that target HER2 are standard treatment in HER2-overexpressed breast and gastric cancers.9,10 Given the low incidence of BTCs, the clinical characterization of relevant molecular subgroups, such as ERBB2-amplified or ERBB2-mutant BTCs, has been limited, and the clinical experience with anti-HER2 agents in this group has been largely anecdotal. A recent basket trial showed objective response in four (36%) of 11 patients with ERBB2-amplified or ERBB2-mutant BTC treated with the combination of the anti-HER2 antibodies trastuzumab and pertuzumab.11 The aim of the current study was to comprehensively describe the clinical and molecular characteristics of ERBB2-amplified or ERBB2-mutant BTCs identified within the context of a prospective tumor profiling initiative at Memorial Sloan Kettering (MSK) Cancer Center. We also report a durable response to the HER2-targeted antibody-drug conjugate ado-trastuzumab emtansine (T-DM1) in a patient with ERBB2-amplified BTC enrolled in a basket trial (ClinicalTrials.gov identifier: NCT02675829).
METHODS
Patients
Demographic data and treatment histories were collected for all patients with ERBB2-altered BTC who consented for prospective tumor genomic profiling using the US Food and Drug Administration–authorized MSK-IMPACT assay between February 2014 and June 2018. Informed consent for tumor profiling and clinical data collection was obtained under the Genomic Profiling in Cancer Patients protocol (ClinicalTrials.gov identifier: NCT01775072), which was approved by the MSK institutional review board.
Data Collection
Clinical data were extracted from electronic medical records. Variables collected were demographic characteristics (age, sex, race), Eastern Cooperative Oncology Group performance status, tumor type (ICC, EHC, GBC), metastatic sites, systemic treatments received, and clinical outcomes. All tumors were prospectively reviewed to confirm histology and to estimate tumor purity.
Molecular Profiling and Genomic Analysis
Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) was performed as previously described in a Clinical Laboratory Improvement Amendments–certified laboratory.12,13 MSK-IMPACT sequences at high coverage all exons and selected intronic and noncoding regions of up to 468 cancer-associated genes. MSK-IMPACT is capable of detecting mutations, small insertions and deletions, copy number alterations, and selected structural rearrangements (Data Supplement). Genes were classified as amplified if they had a fold change ≥ 2. Additional ERBB2 copy number alterations with a fold change < 2 and ≥ 1.5 were classified as copy number gains. Concordance of 98.4% for ERBB2 amplification with immunohistochemistry and fluorescence in situ hybridization was established in a validation set of 252 patients.14 ERBB2 amplification testing by MSK-IMPACT is clinically validated and approved by New York State.
Statistical Analysis
Descriptive statistics were used to summarize the characteristics of this cohort. For the analysis of the prevalence of molecular alterations, a point estimate of the percentage of patients along with an exact 95% CI was reported. The χ2 or Fisher’s exact test was used to identify significant associations between specific ERBB2 alterations and clinical features of interest. P < .05 was considered statistically significant (two sided). Median progression-free survival (PFS) and OS were calculated using the Kaplan-Meier method. Both OS and PFS were calculated from first-line chemotherapy start date. SAS 9.3 software (SAS Institute, Cary, NC) was used for statistical analyses.
Illustrative Case
One patient with metastatic ERBB2-amplified EHC was treated with T-DM1 in a basket trial at MSK for patients with HER2-amplified or HER2-mutant cancers in the cohort of other solid tumors. Methods and partial results of this trial have been presented previously,15,16 but to our knowledge, this would be the first publication about the patient with BTC from the basket trial. This study was approved by the MSK institutional review board, and all patients signed informed consent in accordance with the precepts of the Declaration of Helsinki.
RESULTS
Patients and Molecular Profile
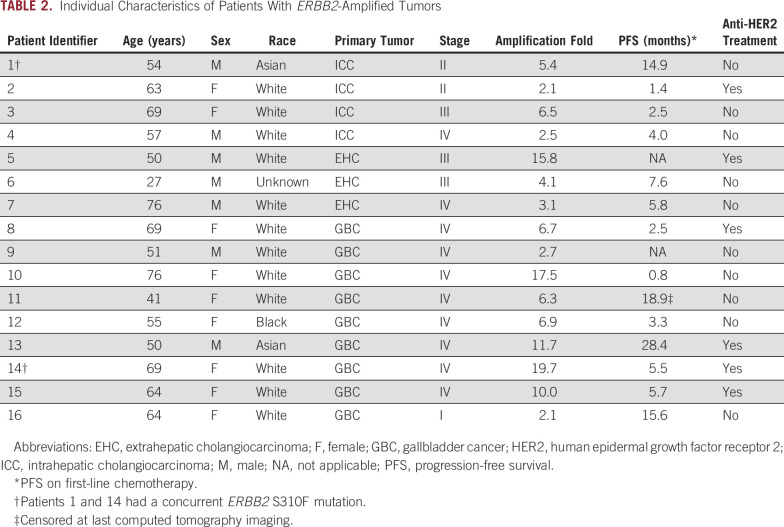
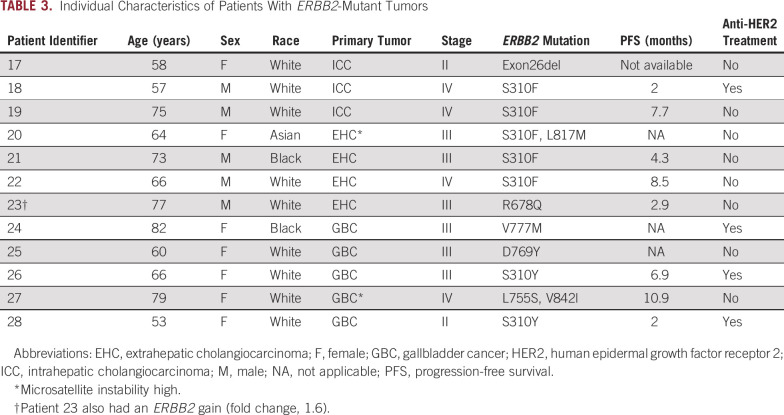
During the period considered in this study, 517 patients with BTC underwent MSK-IMPACT testing. The primary tumor site was ICC, EHC, and GBC in 313, 93, and 111 patients, respectively. Twenty-eight patients (5.4%; 95% CI, 3.6% to 7.7%) had ERBB2 gene alterations, including 2.7% with gene amplification (95% CI, 1.5% to 4.5%), 2.3% with ERBB2 gene mutation (95% CI, 1.2% to 4%), and 0.4% with concurrent amplification and mutation (95% CI, 0.05% to 1.4%). The prevalence of ERBB2 alterations was highest in GBC (12.6%) compared with EHC (7.5%) and ICC (2.2%; P < .001; Data Supplement). In the subset of patients with BTC with ERBB2-amplified or ERBB2-mutant tumors, the median age at diagnosis was 64 years (range, 27-82 years), and the most frequent stage of presentation was stage IV (50%). Baseline characteristics of this cohort are listed in Table 1; individual characteristics of the patients are listed in Tables 2 and 3.
TABLE 1.
Baseline Characteristics
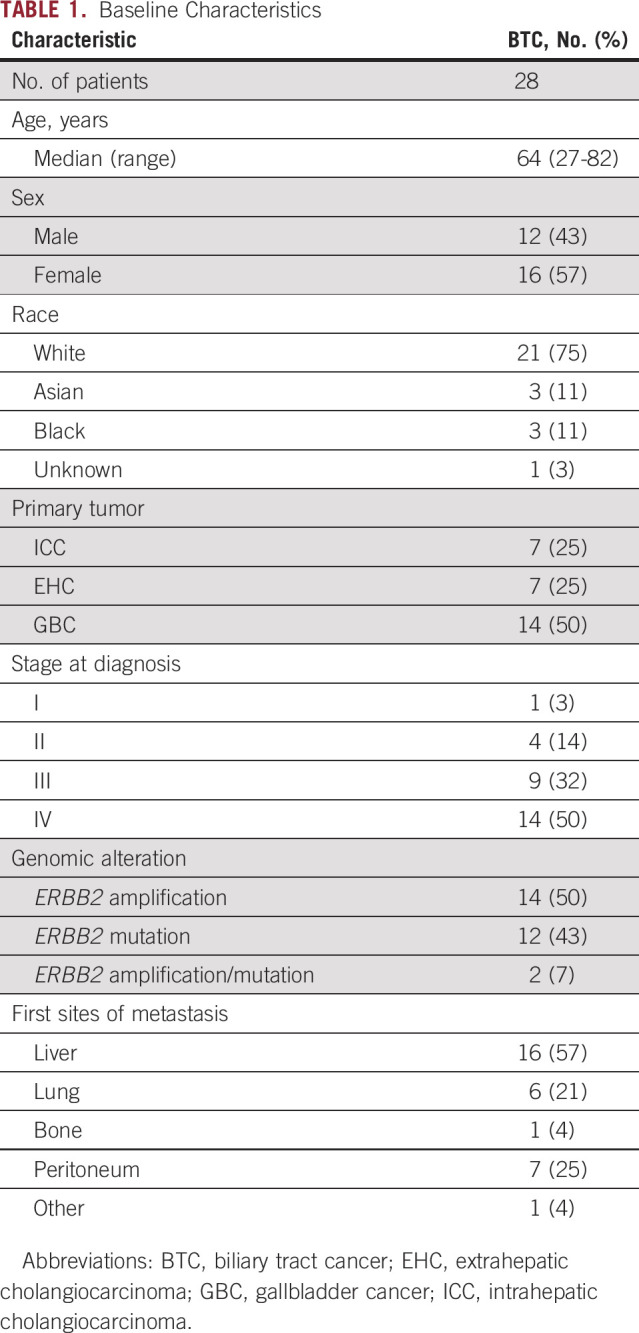
TABLE 2.
Individual Characteristics of Patients With ERBB2-Amplified Tumors
TABLE 3.
Individual Characteristics of Patients With ERBB2-Mutant Tumors
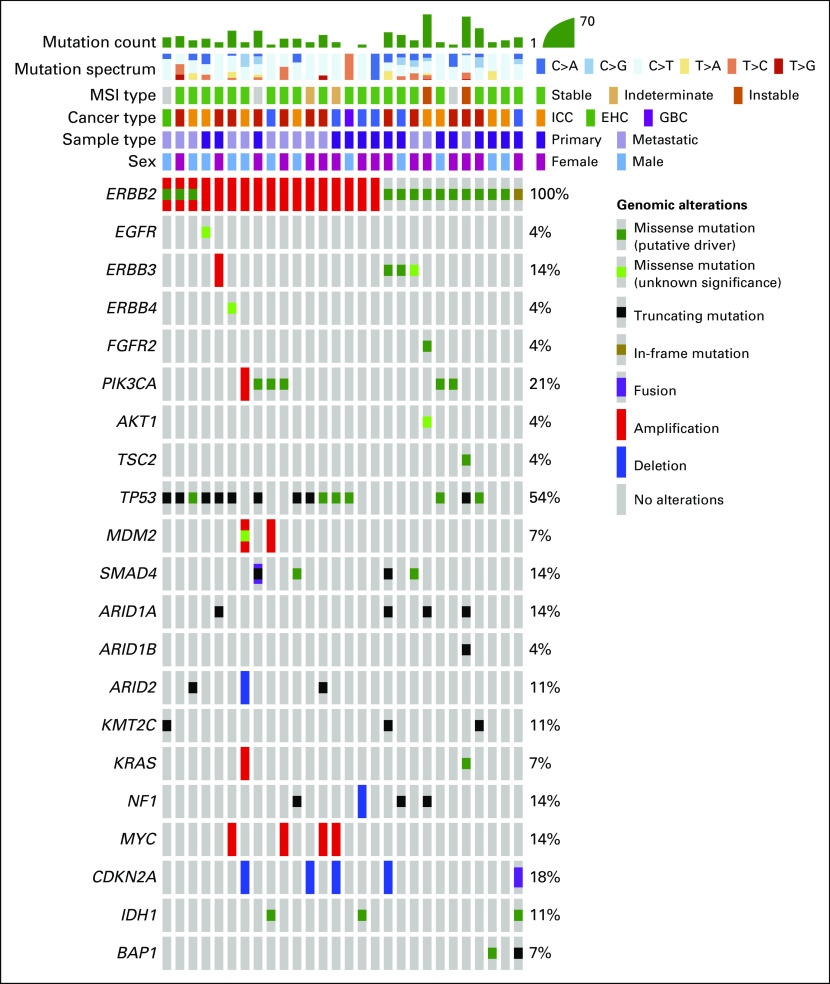
Median sample sequencing coverage was 681×. In the subgroup with ERBB2-amplified BTC, the median fold change of the ERBB2 gene was 6.4 (range, 2.1-9.7; interquartile range, 2.8-11.3). The most frequently mutated domain of HER2 was the extracellular domain (32%), and all mutations in this region arose at the S310 codon (Fig 1). Missense mutations represented the most common alteration in the mutated group (94%). Two patients with ERBB2 mutation had concurrent amplification, and one patient with an ERBB2 mutation had concurrent ERBB2 copy number gain (Fig 2). Two patients (7%) in the ERBB2-altered cohort were classified as microsatellite instability (MSI) high, whereas in the ERBB2-negative BTC cohort, the prevalence of MSI-high tumors was only 1% (P = .051). Both patients with MSI-high tumors had two concurrent ERBB2 mutations (Table 3). In the ERBB2-altered cohort, there were also more frequent TP53 mutations (54% v 28%; P = .0089), ERBB3 alterations (14% v 6%; P = .1), and PIK3CA alterations (21% v 5%; P = .0042), while KRAS amplification/mutation, a potential mechanism of resistance to HER2-targeted therapy, was less frequent (7% v 16%, P = .2866). Genomic alterations of patients with ERBB2-negative BTC are shown in the Data Supplement. In the 22 patients with BTC with metastatic ERBB2-amplified or ERBB2-mutant tumors who received a variety of first-line chemotherapy regimens at our center, the median PFS and OS was 5.7 months and 15.3 months, respectively, with a median follow-up of 39 months. In an exploratory analysis, patients with GBC (n = 91) who harbored ERBB2 alterations had a longer OS than those with ERBB2 wild type (24.9 v 13.4 months; P = .031).
FIG 1.
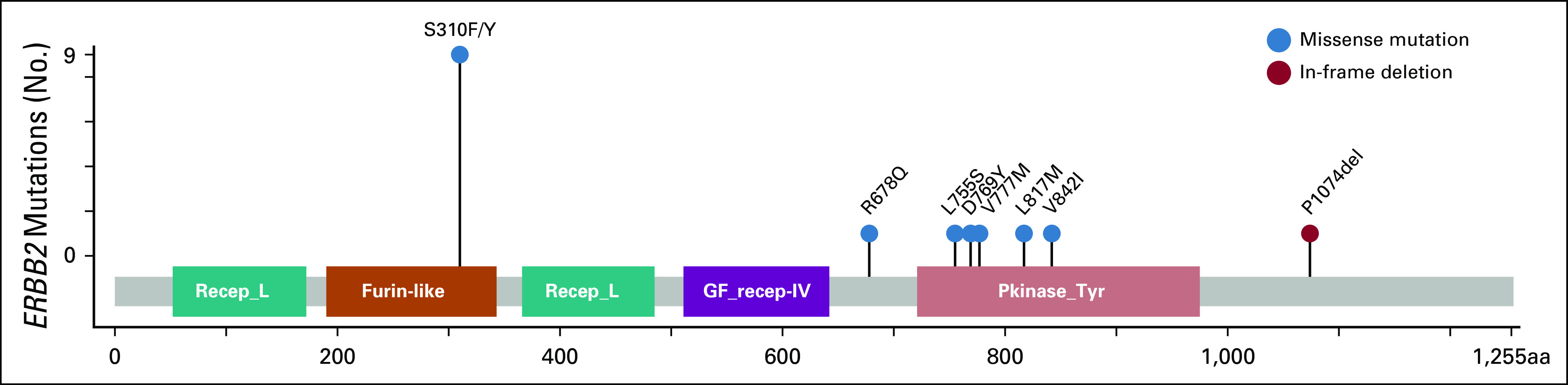
Lollipop plot of ERBB2 coding variants detected by MSK-IMPACT. aa, amino acid; Furin-like, furin-like cysteine-rich region; GF_recept-IV, growth factor receptor domain IV; Pkinase_Tyr, protein tyrosine kinase domain; Recep_L, receptor L domain.
FIG 2.
Oncoprint of biliary tract cancers with ERBB2 amplification or mutation. EHC, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; ICC, intrahepatic cholangiocarcinoma; MSI, microsatellite instability.
Case Report From the T-DM1 Basket Trial
A 52-year-old man was diagnosed with localized EHC in the context of acute pancreatitis. Initial surgical management included a left-sided hepatectomy, cholecystectomy, and regional lymphadenectomy. Pathology showed moderately differentiated adenocarcinoma, with a multifocal tumor (the largest mass was 7 cm in size) with invasion of the left-side hepatic duct and the liver hilar soft tissue. Four of 12 lymph nodes were involved, with tumor and perineural invasion also present (pT3N1Mx, stage IIIB). Tumor genomic profiling showed ERBB2 amplification (fold change, 15.8) and a TP53 mutation (NM_000546) p.E271* (c.811G>T). The patient then received adjuvant gemcitabine, but within 3 months of initiation of systemic chemotherapy, imaging showed progression of disease in the posterior mediastinum, liver, and peritoneum. A biopsy specimen of the mediastinal metastasis confirmed recurrence; immunohistochemistry revealed 3+ HER2 protein overexpression. The patient was then treated with T-DM1 3.6 mg/kg once every 21 days in a basket trial and achieved a confirmed partial response after 12 weeks of treatment (Fig 3). Despite ongoing treatment with T-DM1, the patient’s disease progressed after 8.6 months with the development of a new 2.2-cm (short-axis) retrocaval lymph node. Cancer antigen 19-9 was 10,700 U/mL before starting T-DM1 and decreased to 73 U/mL after achieving a confirmed partial response, with cancer antigen 19-9 remaining stable at the time of disease progression (62 U/mL). However, with the development of a new site of disease, T-DM1 was discontinued.
FIG 3.
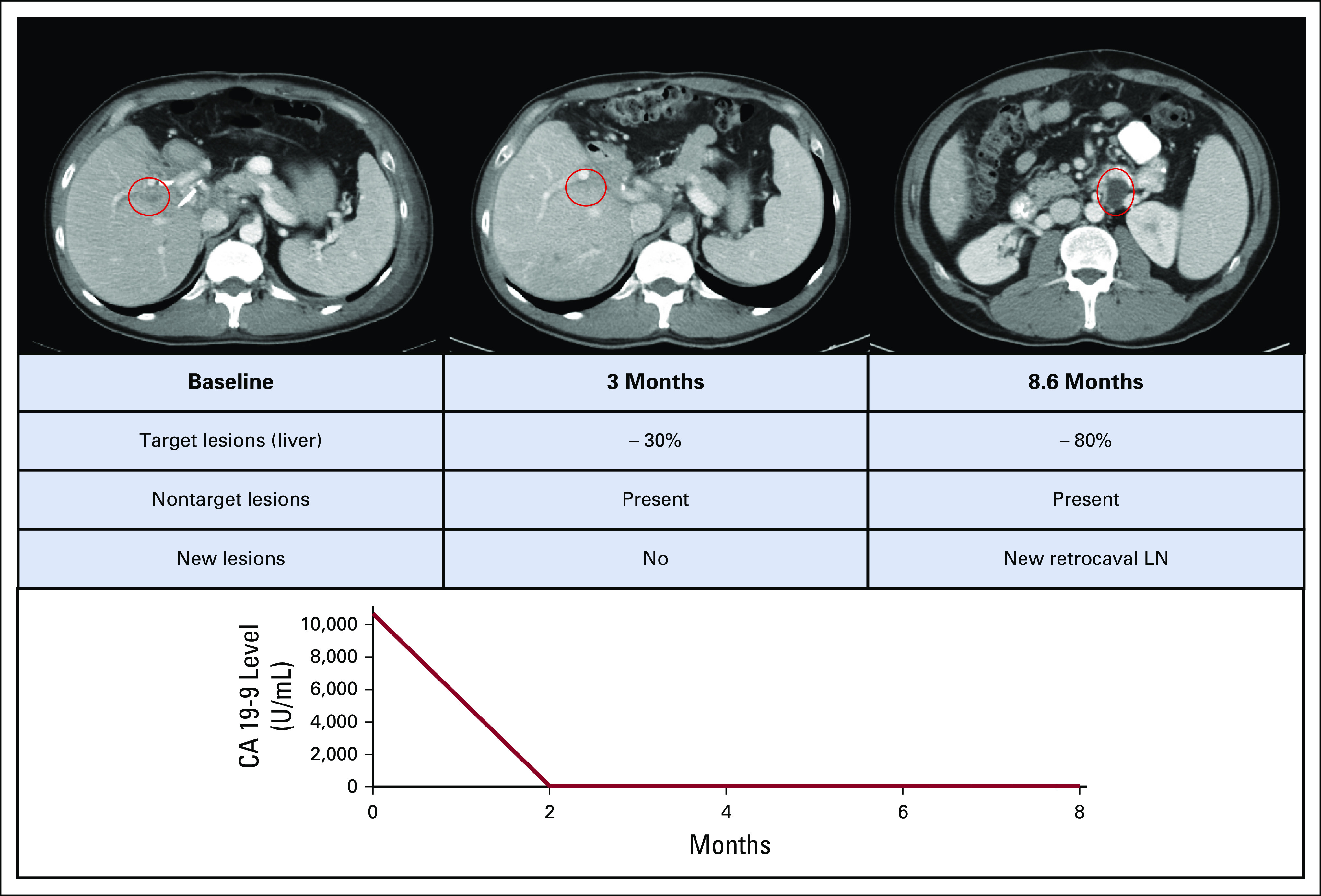
Clinical course of a patient with ERBB2-amplified biliary tract cancer treated with ado-trastuzumab emtansine. CA, cancer antigen; LN, lymph node.
DISCUSSION
This study reports that potentially targetable ERBB2 amplification or mutation was present in 5.4% of a cohort of 517 patients with BTC who underwent prospective tumor molecular characterization at our institution. The prevalence of ERBB2 alterations was lowest in ICC (2.2%) and highest in GBC (12.6%). A similar gradient was described in a previous study that reported 16%, 11%, and 3% of ERBB2 amplification in GBC, EHC, and ICC, respectively.5 We also found that MSI-high tumors were more frequent in the ERBB2-altered cohort compared with tumors without these alterations. Similar findings have been described in ERBB2-mutant colon cancer17; however, it is not clear whether this represents a biologically meaningful association or passenger ERBB2 mutations are more frequent in tumors with an increased tumor mutational burden. In our cohort, patients with ERBB2 amplification and ERBB2 mutation were treated with anti-HER2 therapies. ERBB2-activating mutations have been previously described to be present in 1% to 8% of BTCs; the potential therapeutic significance of these mutations is an active area of research.5 As an example, 20 patients with HER2-mutant BTC were enrolled in a basket trial of the pan-HER tyrosine kinase inhibitor neratinib (ClinicalTrials.gov identifier: NCT01953926), with two (10%) achieving a partial response.18 This trial found that the clinical activity of neratinib varied as a function of tumor site, with the most promising clinical activity observed in HER2-mutant breast cancer (32%) but no RECIST responses in several other cancer types, including bladder and colon cancers.19 The likelihood of clinical response to neratinib also varied as a function of mutation type. Historically, patients whose tumors had an ERBB2 mutation in the absence of gene amplification or protein overexpression were not considered to be optimal for monoclonal antibody–based therapies. However, studies have suggested that patients with HER2-mutant lung cancer can respond to antibody-drug conjugates. In another basket study, the response rate of patients with HER2-mutant non–small-cell lung cancer to T-DM1 was 44%.15 It remains unknown whether HER2-targeted antibody drug conjugates would have similar activity in HER2-mutant BTC.
We report here a patient with ERBB2-amplified BTC who achieved a partial response to the HER2-targeted antibody-drug conjugate T-DM1. Of note, this patient had the third highest level of ERBB2 gene amplification by NGS in this cohort (fold change, 15.8). This high degree of gene amplification may be relevant because in a previous study of patients with metastatic ERBB2-amplified gastric cancer treated with trastuzumab-based therapy, those in the top quartile of ERBB2 amplification levels by NGS had the longest median PFS at 24.3 months.20 Some novel anti-HER2 agents have shown promising activity in nongastric, nonbreast, ERBB2-altered tumors. The antibody-drug conjugate trastuzumab deruxtecan was found to have a response rate of 36% in a variety of HER2-expressing solid tumors, including cancers with ERBB2 mutations.21,22 In a phase I study, the safety and preliminary activity of the bispecific HER2-targeted antibody ZW25 was explored. This drug simultaneously binds the trastuzumab- and pertuzumab-binding domains of HER2.23 Dose-limiting toxicities were not observed, and in a heavily pretreated population of 33 ERBB2-amplified tumors, a response rate of 36% was reported. In this cohort, six patients had nongastric, nonbreast tumors, including one patient with an ERBB2-amplified GBC who had a partial response.24 Although novel HER2-targeted drugs are being tested in basket trials, conduct of dedicated trials that include an ERBB2-altered BTC cohort has been more challenging. Currently, a phase II trial in China is evaluating the combination of gemcitabine plus oxaliplatin and trastuzumab in ERBB2-amplified BTC (ClinicalTrials.gov identifier: NCT02836847). Similarly, the development of a potential companion diagnostic for anti-HER2 drugs in this population is still controversial. HER2 overexpression by immunohistochemistry and fluorescence in situ hybridization has been pivotal for the approval of HER2-targeted therapies in breast and gastric cancers.9,10 However, tissue-based NGS analyses can identify both mutation and amplification along with other potential actionable targets. Given the good correlation for amplification among these methods14 and the increasing clinical use of NGS, we believe that this approach represents an appropriate inclusion criterion in clinical trials. A strong correlative research program in these trials is key to refining the optimal biomarker strategy in this population.
This study has clear limitations. First, the cohort represents a selected population treated at a single academic cancer center that favors clinical trial enrollment; hence, the patients with BTC studied may have had different characteristics compared with those being treated in a community setting. Second, the sample size precluded adequately powered comparisons among subgroups, such as different types of genomic alterations and different tumor sites. It also has been suggested that reports of exceptional responders may be enriched for patients with favorable tumor biology.25 The Von Hoff criteria propose that if the PFS on the reported treatment is greater than 1.3 times the PFS on the prior line of therapy, the reported treatment can be considered beneficial.26 In the case presented here, the patient progressed within 3 months after starting adjuvant chemotherapy and had a PFS of 8.6 months on first-line T-DM1, which supports a clinically meaningful benefit. So far, this patient has been the only confirmed partial response to T-DM1 in six patients with ERBB2-amplified BTC enrolled in the basket trial (median fold change in nonresponders, 8.4).
To conclude, ERBB2 amplification and mutation are present in a subset of patients with BTC, most commonly GBC. In these tumors, the degree of amplification is often high, and the most prevalent hotspot involves the S310 codon. These findings, along with a durable response to T-DM1 in a patient with ERBB2-amplified BTC, support prospective testing for ERBB2 alterations in BTC and provide a foundation for the development novel anti-HER2 therapies for patients with locally advanced and metastatic BTC.
Footnotes
Supported by National Institutes of Health Memorial Sloan Kettering Cancer Center Core Grant No. P30 CA 008748, the Conquer Cancer Foundation, and Cycle for Survival. The Memorial Sloan Kettering–sponsored clinical trial (ClinicalTrials.gov identifier: NCT02675829) received funding from the Conquer Cancer Foundation and Genentech.
AUTHOR CONTRIBUTIONS
Conception and design: Sebastian Mondaca, Bob T. Li, Ghassan K. Abou-Alfa
Financial support: David B. Solit, Ghassan K. Abou-Alfa
Administrative support: Sebastian Mondaca, David B. Solit, Bob T. Li, Ghassan K. Abou-Alfa
Provision of study material or patients: Eileen M. O’Reilly, David B. Solit, Bob T. Li, Ghassan K. Abou-Alfa
Collection and assembly of data: Sebastian Mondaca, Pedram Razavi, Chongrui Xu, Michael Offin, Mackenzie Myers, Mikaela Bradley, Eileen M. O’Reilly, David B. Solit, Ghassan K. Abou-Alfa
Data analysis and interpretation: Sebastian Mondaca, Pedram Razavi, Chongrui Xu, Michael Offin, Mackenzie Myers, Maurizio Scaltriti, Jaclyn F. Hechtman, Eileen M. O’Reilly, Michael F. Berger, David B. Solit, Bob T. Li, Ghassan K. Abou-Alfa
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sebastian Mondaca
Consulting or Advisory Role: Foundation Medicine
Pedram Razavi
Consulting or Advisory Role: Novartis
Research Funding: GRAIL (Inst), Illumina (Inst)
Chongrui Xu
Honoraria: AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Pfizer, Roche
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer
Travel, Accommodations, Expenses: AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, Pfizer, Roche, Bristol-Myers Squibb, MSD
Michael Offin
Consulting or Advisory Role: PharmaMar, Novartis, Targeted Oncology
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme
Maurizio Scaltriti
Stock and Other Ownership Interests: Loxo Oncology
Honoraria: Menarini, ADC Therapeutics
Consulting or Advisory Role: Menarini
Research Funding: Menarini (Inst), Immunomedics (Inst), TargImmune Therapeutics (Inst), Puma Biotechnology (Inst), Daiichi Sankyo (Inst)
Patents, Royalties, Other Intellectual Property: “PI3K Inhibitors and Uses Thereof,” US Provisional Application No. 62/742,163, inventors: Maurizio Scaltriti, Dan Heller, José Baselga, Yosef Shamay, Carles Monterrubio, Amaia Arruabarrena (Inst); “Methods for Detecting HER2 Dimerization in Patients With HER2 Mutant Lung Cancers and Predicting Responsiveness to Ado-Trastuzumab Emtansine Therapy,” SK2018-038-01, inventors: Maurizio Scaltriti, Bob T. Li (Inst); “Methods for Predicting Responsiveness of Lung Cancer Patients to HER2-Targeting Therapies,” US Provisional Application No. 62/685,057, inventors: Maurizio Scaltriti, Bob T. Li (Inst)
Travel, Accommodations, Expenses: Menarini
Jaclyn F. Hechtman
Honoraria: WebMD
Consulting or Advisory Role: COR2ED, Axiom Healthcare Strategies
Research Funding: Bayer AG, Eli Lilly, Boehringer Ingelheim
Eileen M. O’Reilly
Consulting or Advisory Role: Ipsen, Merck
Research Funding: AstraZeneca (Inst), MedImmune (Inst)
Michael F. Berger
Consulting or Advisory Role: Roche
Research Funding: GRAIL
David B. Solit
Stock and Other Ownership Interests: Loxo Oncology
Consulting or Advisory Role: Pfizer, Loxo Oncology, Illumina, Intezyne Technologies, Vivideon Therapeutics, Eli Lilly
Travel, Accommodations, Expenses: Merck KGaA
Bob T. Li
Consulting or Advisory Role: Roche, Biosceptre International, Thermo Fisher Scientific, Mersana, Guardant Health, Hengrui Therapeutics
Research Funding: Roche (Inst), Genentech (Inst), Illumina (Inst), BioMed Valley Discoveries (Inst), AstraZeneca (Inst), GRAIL (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Guardant Health (Inst), Amgen (Inst)
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Astellas Pharma, Celsion, Celgene, Sanofi, Silenseed (I), Sillajen, Boston Scientific, CASI Pharmaceuticals, Onxeo, Roche, Bristol-Myers Squibb, EMD Serono (I), Gilead Sciences (I), Vicus Therapeutics (I), Servier, Agios, Aslan Pharmaceuticals, Bayer AG, Delcath Systems, Eisai, Halozyme, Ipsen, Merck Serono, Sirtex Medical, AstraZeneca, MedImmune, Amgen, Antengene, Aptus Clinical, Carsgen Therapeutics, CytomX Therapeutics (I), Daiichi Sankyo, Debiopharm Group, Exelixis, Inovio Pharmaceuticals, PCI Biotech, Yakult, 3DMedcare, Alignmed, BeiGene, BiolineRx, BridgeBioPharma, Cipla, Genoscience Pharma, Hengrui Pharmaceutical, Jazz Pharmaceuticals, Kyowa Hakko Kirin, Janssen Pharmaceuticals (I), LAM Therapeutics, Eli Lilly, Loxo Oncology (I), Mina, Newlink Genetics (I), Novella Clinical, Pfizer (I), Pharmacyte Biotech (I), Pharmacyclics (I), Pieris Pharmaceuticals (I), QED, RedHill Biopharma, SOBI (I), Targovax (I), Tekmira, twoXAR, Yiviva
Research Funding: Bayer AG (Inst), Exelixis (Inst), Genentech (Inst), Roche (Inst), CASI Pharmaceuticals (Inst), MedImmune (Inst), AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Incyte (Inst), MabVax (Inst), Momenta Pharmaceuticals (Inst), OncoMed (Inst), Agios (Inst), Array BioPharma (Inst), Celgene (Inst), Eli Lilly (Inst), Novartis (Inst), Acta Biologica (Inst), BeiGene (Inst), Halozyme (Inst), Polaris (Inst), OncoQuest (Inst), Puma Biotechnology (Inst), QED (Inst)
Travel, Accommodations, Expenses: Polaris
No other potential conflicts of interest were reported.
REFERENCES
- 1.Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 2.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 3.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: A comparative multicentre study in Japan. Br J Cancer. 2010;103:469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamarca A, Palmer D, Wasan H, et al: ABC-06 | A randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol 37, 2019 (suppl; abstr 4003) [Google Scholar]
- 5.Javle M, Bekaii-Saab T, Jain A, et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer. 2016;122:3838–3847. doi: 10.1002/cncr.30254. [DOI] [PubMed] [Google Scholar]
- 6.Roa I, de Toro G, Schalper K, et al. Overexpression of the HER2/neu gene: A new therapeutic possibility for patients with advanced gallbladder cancer. Gastrointest Cancer Res. 2014;7:42–48. [PMC free article] [PubMed] [Google Scholar]
- 7.Galdy S, Lamarca A, McNamara MG, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017;36:141–157. doi: 10.1007/s10555-016-9645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin Cancer Res. 2018;24:4154–4161. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 11. Javle MM, Hainsworth JD, Swanton C, et al: Pertuzumab + trastuzumab for HER2-positive metastatic biliary cancer: Preliminary data from MyPathway. J Clin Oncol 35, 2017 (suppl; abstr 402) [Google Scholar]
- 12.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1038/nm.4333. Zehir A, Benayed R, Shah RH, et al: Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [Erratum: Nat Med 23:1004, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1016/j.jmoldx.2016.09.010. Ross DS, Zehir A, Cheng DT, et al: Next-generation assessment of human epidermal growth factor receptor 2 (ERBB2) amplification status: Clinical validation in the context of a hybrid capture-based, comprehensive solid tumor genomic profiling assay. J Mol Diagn 19:244-254, 2017 [Erratum J Mol Diagn 19:485, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li BT, Shen R, Buonocore D, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: Results from a phase II basket trial. J Clin Oncol. 2018;36:2532–2537. doi: 10.1200/JCO.2018.77.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. doi: 10.1200/JCO.2018.77.9777. Li BT, Makker V, Buonocore DJ, et al: A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol 36, 2018 (suppl; abstr 2502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross JS, Fakih M, Ali SM, et al. Targeting HER2 in colorectal cancer: The landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer. 2018;124:1358–1373. doi: 10.1002/cncr.31125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harding J, Cleary J, Shapiro G, et al: O-005: Treating HER2-mutant advanced biliary tract cancer with neratinib: Benefits of HER2-directed targeted therapy in the phase 2 SUMMIT ‘basket’ trial. Ann Oncol 30:mdz154.004, 2019. [Google Scholar]
- 19. doi: 10.1038/s41586-019-0974-0. Hyman DM, Piha-Paul SA, Won H, et al: HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554:189-194, 2018 [Erratum: Nature 566:E11-E12, 2019] [DOI] [PubMed] [Google Scholar]
- 20.Janjigian YY, Sanchez-Vega F, Jonsson P, et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018;8:49–58. doi: 10.1158/2159-8290.CD-17-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwata H, Tamura K, Doi T, et al: Trastuzumab deruxtecan (DS-8201a) in subjects with HER2-expressing solid tumors: Long-term results of a large phase 1 study with multiple expansion cohorts. J Clin Oncol 36, 2018 (suppl; abstr 2501) [Google Scholar]
- 22. Park H, Doi T, Modi S, et al: OA02.07: Updated results of phase 1 study of DS-8201a in HER2-expressing or –mutated advanced non-small-cell lung cancer. J Thorac Oncol 13:S324, 2018. [Google Scholar]
- 23. Weisser N, Wickman G, Davies R, et al: Preclinical development of a novel biparatopic HER2 antibody with activity in low to high HER2 expressing cancers. Cancer Res 77, 2017 (suppl; abstr 31) [Google Scholar]
- 24. Meric-Bernstam F, Beeram M, Mayordomo JI, et al: Single agent activity of ZW25, a HER2-targeted bispecific antibody, in heavily pretreated HER2-expressing cancers. J Clin Oncol 36, 2018 (suppl; abstr 2500) [Google Scholar]
- 25.Nishikawa G, Luo J, Prasad V. A comprehensive review of exceptional responders to anticancer drugs in the biomedical literature. Eur J Cancer. 2018;101:143–151. doi: 10.1016/j.ejca.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Von Hoff DD, Stephenson JJ, Jr, Rosen P, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol. 2010;28:4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]