Abstract
PURPOSE
Increases in androgen receptor (AR) copy number (CN) can be detected in plasma DNA when patients develop metastatic castration-resistant prostate cancer. We aim to evaluate the association between AR CN as a continuous variable and clinical outcome.
PATIENTS AND METHODS
PCR2023 was an international, multi-institution, open-label, phase II study of abiraterone acetate plus prednisolone (AAP) or abiraterone acetate plus dexamethasone that included plasma AR assessment as a predefined exploratory secondary end point. Plasma AR CN data (ClinicalTrials.gov identifier: NCT01867710) from this study (n = 133) were pooled with data from the following three other cohorts: cohort A, which was treated with either AAP or enzalutamide (n = 73); the PREMIERE trial (ClinicalTrials.gov identifier: NCT02288936) of biomarkers for enzalutamide (n = 94); and a phase II trial from British Columbia (ClinicalTrials.gov identifier: NCT02125357) that randomly assigned men to either AAP or enzalutamide (n = 201). The primary outcome measures for the biomarker analysis were overall survival and progression-free survival.
RESULTS
Using multivariable fractional polynomials analysis using Cox regression models, a nonlinear relationship between plasma AR CN and outcome was identified for overall survival, where initially for small incremental gains in CN there was a large added hazard ratio that plateaued at higher CN. The CN cut point associated with the highest local hazard ratio was 1.92. A similar nonlinear association was observed with progression-free survival. In an exploratory analysis of PCR2023, the time from start of long-term androgen-deprivation therapy to start of AAP or abiraterone acetate plus dexamethasone was significantly shorter in patients with plasma AR CN of 1.92 or greater than patients with plasma AR CN of less than 1.92 (43 v 130 weeks, respectively; P = .005). This was confirmed in cohort A (P = .003), the PREMIERE cohort (P = .03), and the British Colombia cohort (P = .003).
CONCLUSION
Patients with metastatic castration-resistant prostate cancer can be dichotomized by a plasma AR CN cut point of 1.92. Plasma AR CN value of 1.92 or greater identifies aggressive disease that is poorly responsive to AR targeting and is associated with a prior short response to primary androgen-deprivation therapy.
INTRODUCTION
Standard of care treatment at development of metastatic castration-resistant prostate cancer (mCRPC) is inhibition of androgen receptor (AR) signaling with either abiraterone acetate administered with prednisone (AAP; 5 mg twice a day) or enzalutamide. The duration of benefit is variable, with some patients experiencing rapid progression and some responding for several years.1,2 There is a clinical need to develop biomarker strategies to stratify patients at emergence of mCRPC on the basis of their predicted benefit from AR targeting.
Obtaining metastatic tumor biopsies is challenging in clinical practice because most mCRPC is restricted to bone, especially in men with lower volume disease. Studies across multiple tumor types have demonstrated potential clinical utility of plasma tumor DNA for identifying somatic genomic aberrations (rearrangements, mutations, or copy number [CN]) relating to resistance in the metastatic setting, especially at key clinical decision points.3-6
AR gene aberrations are rare before treatment with primary androgen-deprivation therapy (ADT) but progressively increase in prevalence in patients with mCRPC.7 AR CN amplification in prostate cancer cells is associated with increased AR expression8 and has a wide variable continuous range, with some mCRPC tumors harboring an average of 25 copies.9 It has been proposed that plasma AR gain or mutations are associated with worse outcomes with second-generation AR-targeting agents.10-12 However, it is unclear how this biomarker performs because the presence or absence of AR CN gain is likely insufficient to robustly predict benefit from AR-targeting agents. We aimed to evaluate the association between AR CN as a continuous variable and clinical outcome. For this evaluation, we performed a pooled analysis of AR CN in chemotherapy-naïve patients with mCRPC receiving abiraterone acetate or enzalutamide in four independent clinical studies that included biomarker evaluation as a secondary objective.
CONTEXT
Key Objective
Several studies have assessed plasma DNA, including the androgen receptor (AR), as a biomarker for patient stratification. Variable plasma AR copy number (CN) parameters have been used, making it challenging to interpret differences in associations with outcome observed across multiple analyses. We aimed to define the most appropriate framework for outcome-based stratification using plasma AR CN in patients with metastatic castration-resistant prostate cancer starting therapy with abiraterone acetate or enzalutamide.
Knowledge Generated
This pooled analysis demonstrates a nonlinear relationship of plasma AR CN with outcome, where an AR CN cut point of 1.92 or greater has the strongest association with shorter progression-free survival and overall survival on AR-targeting therapies as well as a shorter duration of benefit from prior primary androgen-deprivation therapy.
Relevance
A fixed cut point for plasma AR CN of 1.92 is a pragmatic way to clinically implement AR assessment as a liquid biomarker for metastatic castration-resistant prostate cancer.
PATIENTS AND METHODS
Participants and Study Design
This was a pooled analysis of four cohorts. The PCR2023 cohort (ClinicalTrials.gov identifier: NCT01867710) was an international, multi-institutional, open-label, parallel-arm, phase II study of abiraterone acetate in asymptomatic or minimally symptomatic, chemotherapy-naïve patients with mCRPC.13 Patients were randomly assigned 1:1:1:1 to abiraterone acetate and one of four different glucocorticoid regimens (prednisone 5 mg twice a day or once a day or 2.5 mg twice a day or dexamethasone 0.5 mg once a day) to evaluate tolerability. The trial was not designed to detect differences in clinical outcome between corticosteroid doses, so for the purposes of biomarker analysis, all patients were grouped together. Clinical outcome data were obtained after completion of the main study and extension protocols, with a closure date of June 5, 2018.
The other three cohorts have been described previously (ClinicalTrials.gov identifier: NCT02288936).10,12 Cohort A is a subset of patients that included patients treated with either abiraterone acetate (in combination with prednisone 5 mg twice a day) or enzalutamide and recruited to biomarker protocols in the Royal Marsden (London, United Kingdom; Protocol No. REC 04/Q0801/6) or the Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (Meldola, Italy; Protocol No. REC 2192/2013).10 The PREMIERE trial (European Union Clinical Trial Register identifier: 2014-003192-28; ClinicalTrials.gov identifier: NCT02288936) was sponsored and conducted by the Spanish Oncology Genitourinary Group and was a biomarker-driven study in men treated with enzalutamide. The final cohort, the British Columbia (BC) cohort, was from a randomized phase II trial of AAP versus enzalutamide (ClinicalTrials.gov identifier: NCT02125357) that was sponsored and conducted by the BC Cancer Agency.12
For the biomarker analyses from all four studies, only patients with histologically confirmed prostate adenocarcinoma and no neuroendocrine differentiation; progressive disease despite castrate levels of serum testosterone (< 50 ng/dL); ongoing medical or prior surgical castration; and no prior treatment with chemotherapy, AAP, or enzalutamide were included. Specific selection criteria by cohort are specified in the Data Supplement. All four studies obtained institutional review board and ethics committee approval and were conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. All patients provided written informed consent for the biomarker research.
Procedures
Plasma was collected 30 days before starting treatment (Data Supplement). DNA from PCR2023, cohort A, and PREMIERE was analyzed by droplet digital polymerase chain reaction (ddPCR), as described previously10 (Data Supplement). In PCR2023 and cohort A, AR CN was also measured using two different targeted next-generation sequencing (NGS) approaches (Data Supplement). A high agreement was observed between ddPCR and targeted NGS (Data Supplement). AR CN calls from ddPCR were used for the analyses in PCR2023 and Cohort A. AR CN in the BC cohort was estimated using targeted capture NGS, as described previously.12
In PCR2023, serum prostate-specific antigen (PSA) was assessed at screening, at cycle 1, every month for the first 6 months, and every 3 months thereafter. PSA levels in cohort A and the PREMIERE and BC cohorts were measured as previously described.10 Disease was evaluated radiographically using computed tomography scans of the chest, abdomen, and pelvis and technetium whole-body bone scans at the time of screening and every 12 weeks on treatment. Serum lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) were measured at screening or on day 1 of cycle 1.
Outcomes
Plasma samples were collected prospectively in all four studies with the primary aim of studying associations between plasma DNA aberrations and clinical outcome as secondary exploratory objectives. This analysis evaluating plasma AR CN was defined after sample collection. The primary end point for this biomarker analysis in all four cohorts was overall survival (OS). For full definitions of outcome measures, see the Data Supplement.
For exploratory analysis of the association between AR status at development of mCRPC and time on ADT, the outcome measure of time from start of uninterrupted ADT to start of AAP, abiraterone acetate plus dexamethasone (AAD), or enzalutamide was used. Patients on combined androgen blockade and antiandrogens were not excluded.
Statistical Analyses
Multivariable fractional polynomials (MFP) analysis using Cox regression models was performed using STATA/SE version 15.1 (STATA, College Station, TX) to include ALP, LDH, PSA, age, and CN stratified by trial.14 To address AR CN as a continuous variable, maximum log-likelihood statistics were used as a correlative measure along with bootstrapping and cross-validation. Respective CN cut points were superimposed over local hazard ratios (HRs). Local HRs were obtained by exponentiating the differences between log-hazard functions of x (for a given CN) and x + 1. This was determined using RStudio (https://www.rstudio.com/). Time-to-event outcomes were evaluated using Kaplan-Meier survivor estimates and the log-rank test. The association of clinically relevant baseline factors (previously shown to be associated with prognosis15-17 for OS and progression-free survival [PFS]) was examined using univariable and multivariable Cox regression models that were performed with stepwise variable selection to identify the prognostic factors for OS and PFS. Odds ratios of PSA response at 12 weeks from baseline were determined using a 2 × 2 contingency table, and significant differences were determined using Fisher’s exact test. The time on ADT by AR status at mCRPC was compared using the Mann-Whitney U test. All tests were two sided, and an α error of 5% was considered significant.
RESULTS
Clinical Cohorts and Plasma AR Status
Between June 2013 and October 2014, 164 patients were recruited onto the PCR2023 study at 22 centers in five countries. Of the 164 intent-to-treat patients, 151 patients consented to the optional biomarker study for the predefined exploratory secondary analysis and donated plasma before start of treatment. In total, 133 patients were available for biomarker analysis. Patient and treatment characteristics of this biomarker population at the time of sample collection are provided in Figure 1A and Appendix Table A1. The median follow-up time for assessment of PFS and OS was 46.03 months. Median radiographic PFS (rPFS) and OS times were 18.66 months (range, 0.5 to 56.64 months) and 40.34 months (range, 0.5 to 56.77 months), respectively.
FIG 1.
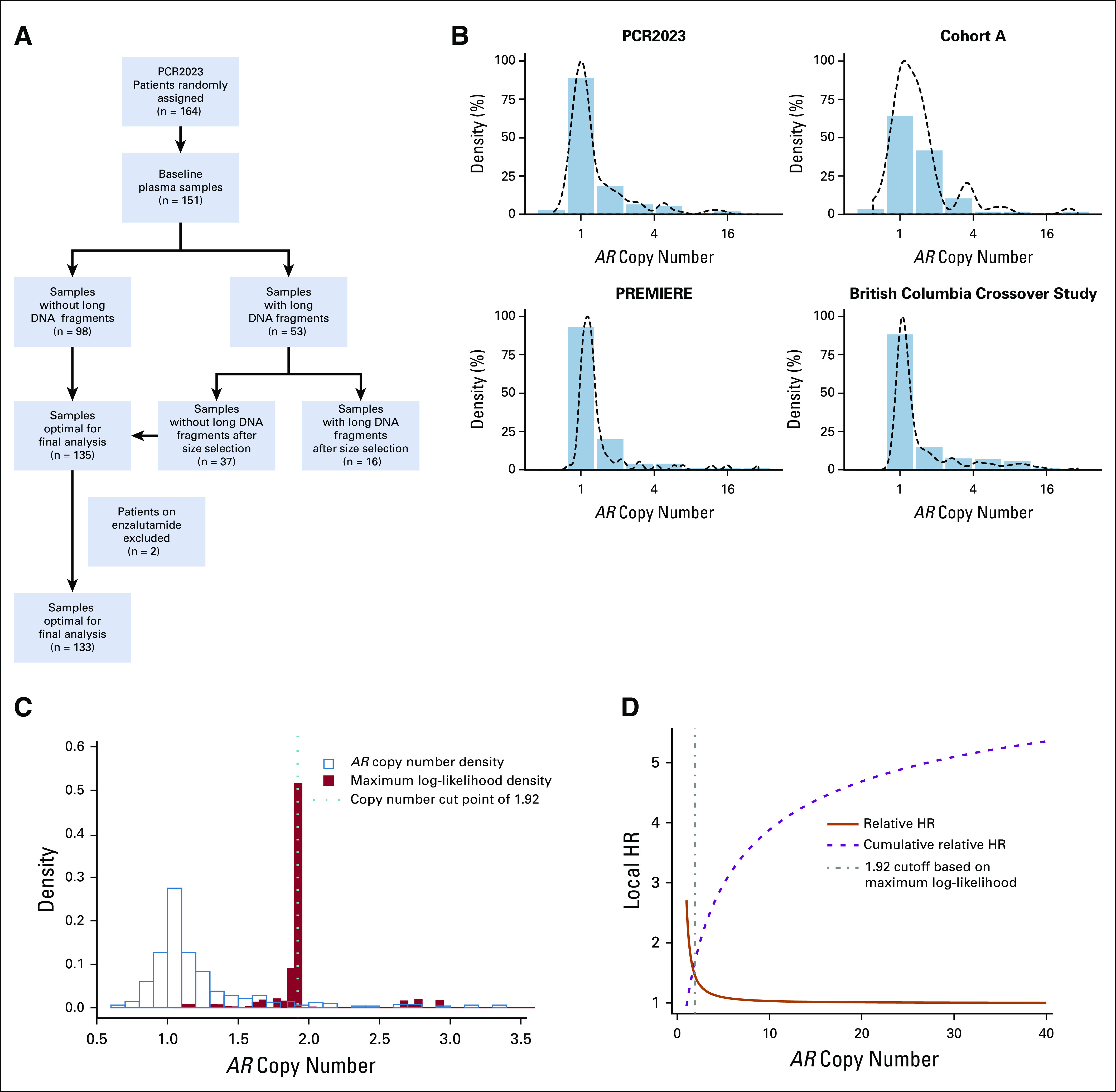
Clinical cohorts, markers of tumor volume, and association with plasma androgen receptor (AR) status. (A) Flow diagram of the patient selection for biomarker evaluation in PCR2023. (B) AR copy number (CN) distribution across the four clinical cohorts (PCR2023, cohort A, PREMIERE, and British Colombia crossover study) included in the pooled analysis (N = 501). Multivariable fractional polynomials analysis was performed using Cox regression models on data pooled from four cohorts including age and CN and stratified by trial. (C) Distributions of AR CN versus maximum log-likelihood statistics. Maximum log-likelihood statistics were used as a correlative measure to identify the optimal cut point of AR CN in association with overall survival outcome. (D) Visual inspection showed that the cut point region overlaps with the turning point of decreasing local hazard ratio (HR) identified via the maximum log-likelihood statistics approach.
We included AR CN data from 501 patients, including new data from the PCR2023 cohort described earlier (n = 133); cohort A, as described previously10 (n = 73); the PREMIERE trial (n = 94); and the BC trial12 (n = 201). The four cohorts have relatively similar eligibility criteria and clinical characteristics (Table 1 and Data Supplement). AR CN distribution for the four cohorts followed a right-skewed distribution (Fig 1B). The median values of AR CN for PCR2023, cohort A, PREMIERE, and the BC cohort were 1.06, 1.23,1.17, and 1.09, respectively; the range across all four cohorts was 0.6 to 28.8 (Appendix Table A1).
TABLE 1.
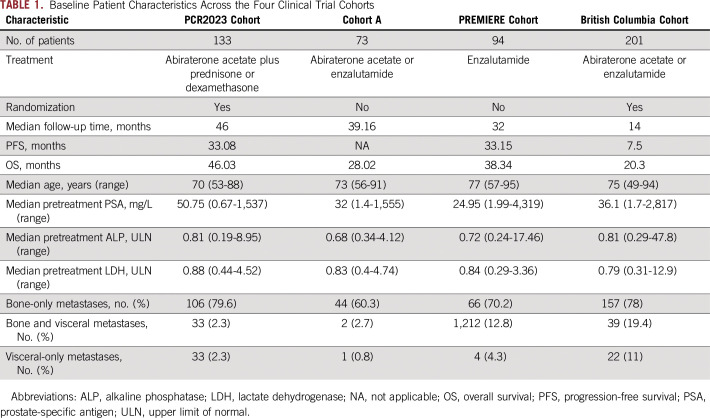
Baseline Patient Characteristics Across the Four Clinical Trial Cohorts
Multitrial and Multivariable Assessment Identifies Nonlinear Relationship Between AR CN and Outcome
To evaluate the relationship between AR CN as a continuous distribution and OS, we performed an MFP analysis using Cox regression models including age, ALP, LDH, and PSA and stratified by trial. At lower levels of AR CN, we found that small incremental increases in CN were associated with large increases to the local HR. However, the added HR was not constant, and the additional impact of increasing AR CN decreased at higher CNs. Using maximum log-likelihood statistics along with bootstrapping and cross-validation, we identified an AR CN of 1.92 as the most optimal cut point associated with poor outcome for OS (Fig 1C). Visual inspection showed that the cut point region overlapped with the turning point of decreasing local HR identified via the maximum log-likelihood statistics approach.14 At increasing CNs, the decreasing trend became so prominent that it reached a plateau close to an HR of 1 (Fig 1D). A similar effect on local HR was seen for PFS (Data Supplement). Using the defined cut-off, we found a statistically significant correlation between plasma AR CN and clinical indices of tumor volume, namely, serum LDH, ALP, PSA, and total circulating DNA yield (Mann-Whitney U test, P ≤ .001 for all four parameters; Fig 2A).
FIG 2.
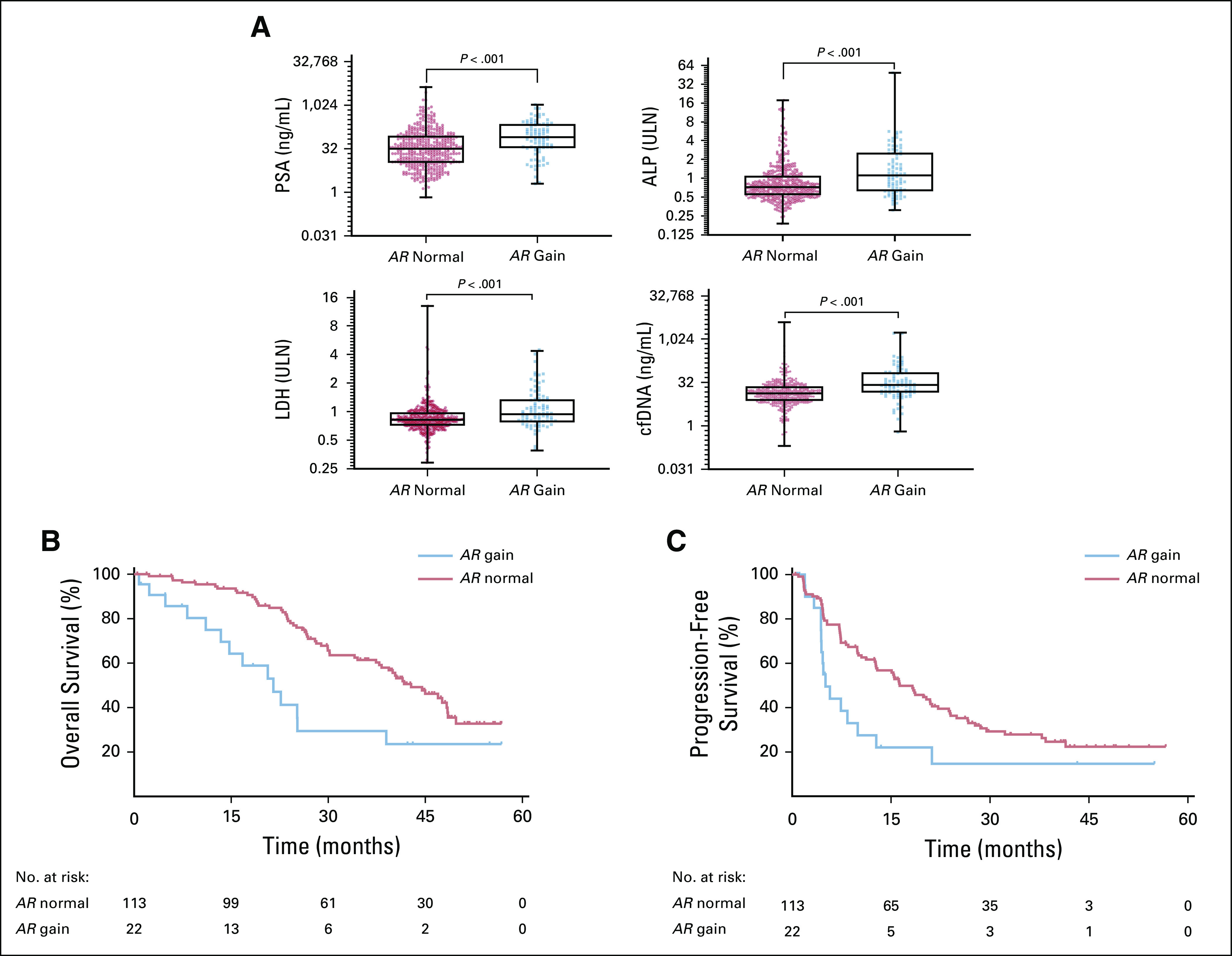
Plasma androgen receptor (AR) copy number (CN) and outcome. (A) Association between estimated plasma AR CN status and four circulating clinical indices of tumor volume markers. Groups were split into plasma AR CN normal or plasma AR CN gain. Cell-free DNA (cfDNA) yield, prostate-specific antigen (PSA), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) levels are reported; ALP and LDH levels are reported relative to upper limit of normal (ULN) The association between outcome and patients dichotomized by a plasma AR CN of 1.92 or greater in PCR2023 is included as an example, showing (C) overall survival and (D) progression-free survival for patients with normal AR CN and AR CN gain.
Plasma AR CN Is Independently Associated With Poor Clinical Outcomes
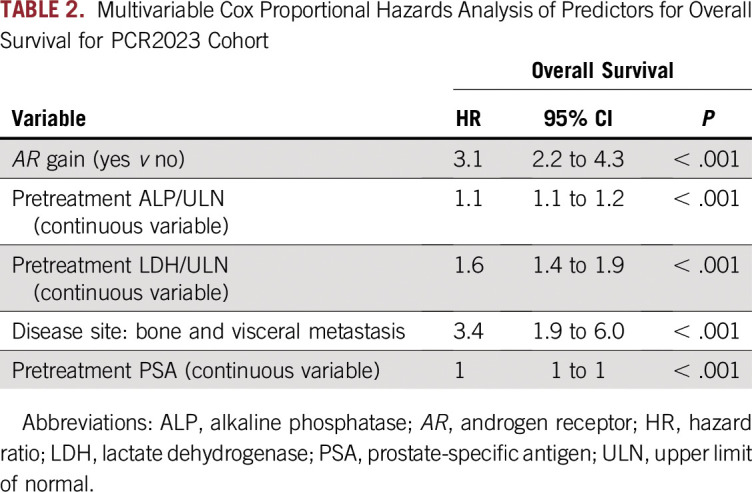
We constructed a multivariable model to evaluate the association between OS and PFS and five clinical prognostic factors (LDH, ALP, PSA, age, and disease site) and plasma AR CN (gain ≥ 1.92) or normal CN as covariates for the PCR2023 cohort. The clinical prognostic factors selected have been previously demonstrated to be strong independent predictors of OS and PFS for AR-targeted therapies.15,17-20 Using stepwise backward elimination, plasma AR gain; high ALP, LDH, and PSA; and the presence of bone and visceral metastases were all independently associated with poorer OS (Table 2). Similarly, in the multivariable model for PFS, plasma AR gain and high LDH and ALP remained independently significantly associated with PFS (Appendix Table A2).
TABLE 2.
Multivariable Cox Proportional Hazards Analysis of Predictors for Overall Survival for PCR2023 Cohort
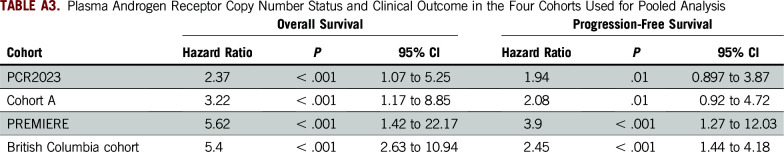
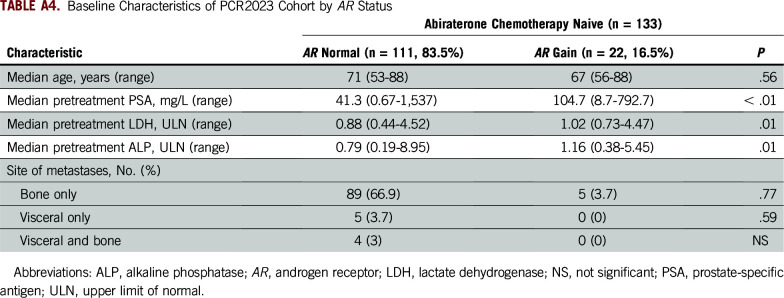
On an individual cohort level, the HRs for AR CN gain versus normal CN are listed in Appendix Table A3. Including the PCR2023 cohort as an example because it has not been reported as a stand-alone study previously, 22 patients (16.5%) were categorized as having AR CN gain (Appendix Table A4). Patients harboring AR gain had a significantly shorter OS when compared with AR normal patients (median OS, 21.52 v 42.81 months, respectively; HR, 2.37; 95% CI, 1.07 to 5.25; P ≤ .001; Fig 2C). We also observed significantly shorter PFS and rPFS in the patients with AR gain compared with those who were AR normal (median PFS, 5.1 v 16.3 months, respectively; HR, 1.94; 95% CI, 0.897 to 3.87; P = .01; and median rPFS, 7.4 v 21.2 months, respectively; HR, 2.0; 95% CI, 0.97 to 4.405; P = .005; Fig 2D; Data Supplement). PSA decline of 50% or greater at 12 weeks was not significantly different for plasma AR normal patients versus patients with AR gain (odds ratio, 1.78; 95% CI, 0.63 to 4.71; P = .29; Data Supplement).
Plasma AR Status at Development of mCRPC and Duration of Benefit From ADT
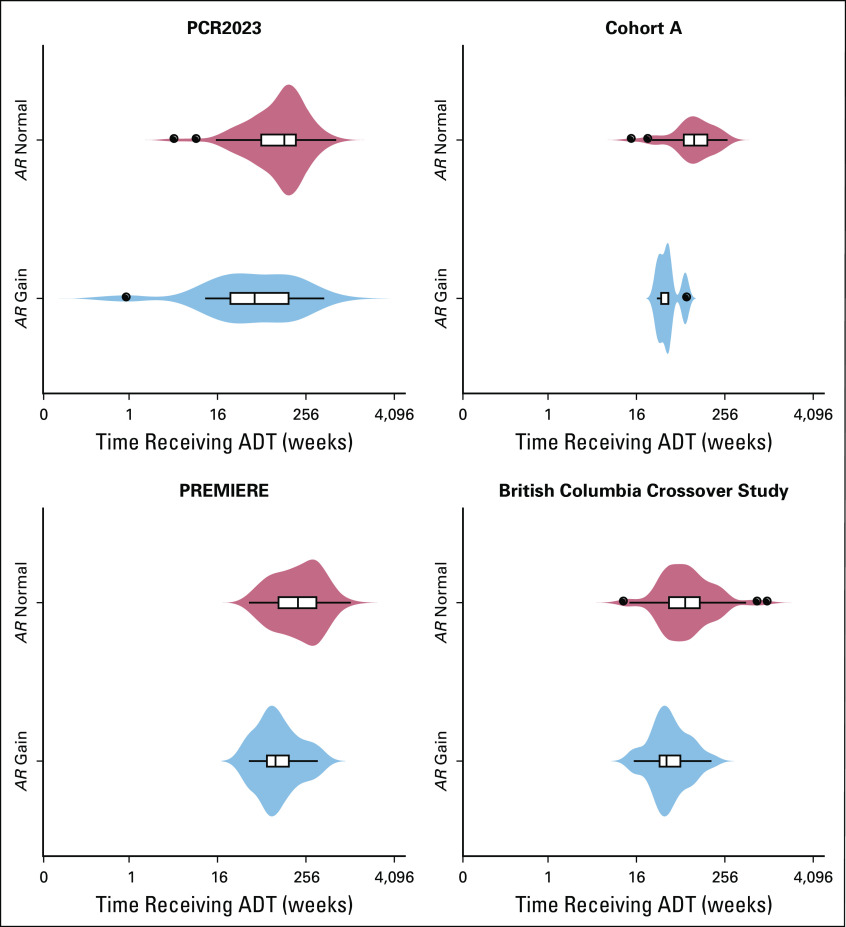
Finally, we performed an exploratory analysis in the PCR2023 cohort evaluating the time from initiation of continuous long-term ADT to start of AA. Start dates of primary ADT were available for 123 patients (92.4%), and all patients were treated with a first-generation antiandrogen before AAP or AAD. The median time on ADT for this population was 128.3 weeks, and disease stage at diagnosis was available for 89 of 123 patients with ADT data. Of these 89 patients, 53 (59.6%) were stage M0 at diagnosis. We observed that AR CN gain (CN ≥ 1.92) in pretreatment AAP or AAD samples was associated with a significantly shorter time on ADT compared normal AR CN (median, 43.1 v 130.2 weeks, respectively; P = .005; Fig 3). We repeated this analysis independently in each of the three cohorts included in the pooled AR CN analysis. Data were available for 52 (70%) of 74 patients from cohort A, all the PREMIERE patients, and 105 (52%) of 201 patients in the BC cohort. For cohort A and the PREMIERE cohort, 69.2% (36 of 52 patients) and 60% (56 of 94 patients) of patients were stage M0 at diagnosis, respectively, whereas in the BC cohort, only men who had metastatic disease detected on computed tomography of the chest, abdomen, and pelvis or whole-body bone scan at start of continuous ADT were included. The median times from start of ADT to second-generation hormone treatment of cohort A and the PREMIERE and BC cohorts were 91, 165, and 64 weeks, respectively. This was significantly shorter for patients with plasma AR gain compared with AR normal patients in all three cohorts (cohort A: median, 41 v 98 weeks, respectively; P = .0026; PREMIERE trial: median, 99 v 198 weeks, respectively; P = .03; and BC cohort: median, 41 v 74 weeks, respectively; P = .003; Mann-Whitney U test; Fig 3).
FIG 3.
Time from start of androgen-deprivation therapy (ADT) to start of abiraterone acetate or enzalutamide divided by androgen receptor (AR) copy number (CN) of 1.92 or greater (AR CN gain) or AR CN of less than 1.92 (AR CN normal). AR CN normal is demonstrated by the blue violin plots, whereas AR CN gain is demonstrated by the light red violin plots. Box plots within the violin plots indicate the median and the upper and lower quartiles, with whiskers extending from the shortest to longest time on ADT. The x-axis (weeks) is scaled as log2.
DISCUSSION
Our pooled analysis confirms a nonlinear relationship between AR CN and outcome, with an incremental increase in HR to a CN value of 1.92 that then becomes progressively less at higher values. This supports the use of a fixed cut point as a pragmatic way to clinically implement AR CN as a liquid biopsy biomarker to dichotomize patients into prognostically distinct groups. Importantly, this analysis identifies a cut point higher than would be expected using the technical limits for defining gain that have been used in most previous studies.10,12,21,22 Using the technical limits would result in misclassification of patients with a better prognosis who have an AR CN between approximately 1.3 and 1.92. Furthermore, multivariable analyses suggest that plasma AR provides information independent from well-established poor prognostic biomarkers and, as a result of the underlying biology, could provide predictive information. The plasma AR CN cut point generated from this analysis incorporates data from both targeted NGS and ddPCR because we have demonstrated a strong agreement between these two techniques; thus, this study robustly confirms the applicability of this cut point regardless of methodology undertaken to obtain AR CN. In addition, to account for differences in frequency of PSA and imaging measurements, end points, follow-up times, and treatment, the MFP was stratified by trial. Despite the clinical heterogeneity of the cohorts, the association between plasma AR CN and outcome was seen across all of the cohorts.
The wide variability in the median time on ADT in the four cohorts is likely attributed to the differences in the proportion of patients with metastatic disease at diagnosis. Importantly, we nonetheless observed a shorter time on primary ADT for men who had plasma AR CN gain at development of mCRPC. This finding may offer a biologic explanation for the observation of a lower response rate to second-generation AR-targeting agents when the response to ADT was shorter than 12 months.23-25
To appropriately interrogate our main aim, we focused our genomic analysis solely on AR. Future studies could integrate AR CN dichotomization with other molecular markers that have been suggested to be associated with worse outcome, including aberrations of TP5312,21 or WNT signaling26 or AR splice variant detection.27,28 These studies would require larger cohorts of patients to allow multiple testing. ARV-7 status may be more challenging to detect in lower volume patients similar to the population in our study as a result of the rarity of circulating tumor cells.27 In addition, we did not include AR somatic point mutations because they are rarely detected (at allelic frequencies > 0.01) in this population.10,12
We used AR CN values not controlled for circulating tumor DNA (ctDNA) fraction. AR CN has a wide range in a patient and is heterogenous across metastatic clones.8,12,21,29 In this disease setting, low-volume disease often precludes biopsy of multiple metastases, and it is not feasible to derive tumor biopsy CN values for comparison. Plasma AR is a representation of the overall CN with putatively varying contributions from individual clones. We have hypothesized that correcting AR CN on the basis of total circulating tumor fraction would not be reflective of true AR CN in individual clones. Accepting this limitation of plasma tumor DNA assessment, we propose to use actual AR CN values for classification. It is probable that the actual AR CN at a cellular level that results in treatment resistance is manyfold higher than our cut point; the latter may be the value that is most likely to detect the presence of a high CN clone that rapidly expands after treatment initiation, leading to resistance.
Patients with AR gain have a higher ctDNA fraction, and in fact, in this prechemotherapy setting where overall ctDNA fractions are lower, the two are closely linked and do not provide independent prognostic information.12,21,29 Nonetheless, AR CN gain can be detected at low fractions, probably as a result of the high level of AR gain observed in mCRPC. In liquid biopsy tests for a molecular aberration, there is an increased chance of detection at higher tumor load. This introduces a prognostic bias derived from the worse outcomes expected as a result of high ctDNA fraction.11,12 This could be the case for AR CN. Most importantly for clinical utility is whether a test is prognostic or predictive. This analysis and previous studies of AR CN are single-arm studies and, therefore, have not tested the predictive value of AR CN. In an exploratory analysis in two similar but nonrandomized cohorts, we reported that the outcome of patients with mCRPC at a similar stage as patients in our study population but who were treated with taxane chemotherapy was worse in patients with higher circulating DNA but not those with plasma AR gain.30 Randomized studies are required to investigate whether AR gain can be used for treatment selection. Our pooled analyses identify a higher cut point than used in previous studies as most strongly associated with outcome. Although technically justified by detection limits of the assays used, splitting patients into AR CN gain or CN normal groups using lower cut points may be less sensitive for detecting clinically relevant associations. Future studies that evaluate the utility of assessing plasma AR CN should use the higher CN cut point for dichotomization of patients.
ACKNOWLEDGMENT
We thank the study participants and study centers. We acknowledge all the staff at the Spanish Oncology Genitourinary Group for their support in running the PREMIERE trial and Apoyo a la Investigacion Clinica en Espana for data management.
APPENDIX
TABLE A1.
Plasma AR CN Across All Four Clinical Cohorts
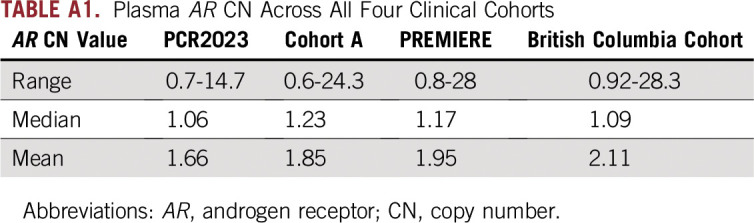
TABLE A2.
Multivariate Cox Proportional Hazards Analysis of Predictors for Progression-Free Survival for PCR2023 Cohort
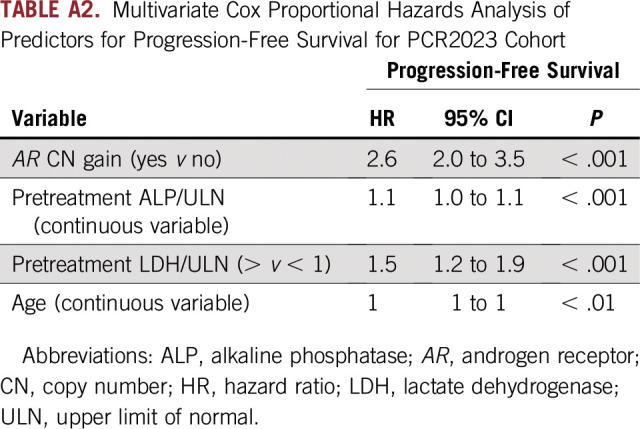
TABLE A3.
Plasma Androgen Receptor Copy Number Status and Clinical Outcome in the Four Cohorts Used for Pooled Analysis
TABLE A4.
Baseline Characteristics of PCR2023 Cohort by AR Status
Footnotes
Presented in part at the 54th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018; and the Prostate Cancer Foundation 25th Annual Scientific Retreat, Carlsbad, CA, October 26-28, 2018.
The PCR2023 study was supported by Janssen. The PREMIERE trial was sponsored by the Spanish Oncology Genitourinary Group, which received a grant from Astellas to support the conduct of the trial. The BC Cancer Agency study was supported by Canadian Cancer Society Research Institute Innovation Grant No. 702837 (K.N.C. and A.W.W.), Prostate Cancer Canada through Movember Discovery Grants No. D2015-06 (A.W.W. and K.N.C.) and D2014-13 (K.N.C. and A.W.W.), the Movember Rising Star in Prostate Cancer research program (A.W.W.), the Emil Aaltonen Foundation (M.A.), the Prostate Cancer Foundation (A.W.W. and K.N.C.), Terry Fox New Frontiers Program Project Grant No. TFF116129 (A.W.W. and K.N.C.), and clinical trials funding from Janssen and Astellas. G.A. is supported by a Cancer Research UK Advanced Clinician Scientist Fellowship. A.J. is supported by a Medical Research Council Clinical Research Training Fellowship. V.C. was supported by a European Society of Medical Oncology Translational Clinical Research Fellowship. M.A. is supported by the Jane and Aatos Erkko Foundation. A.W.W. is supported by Prostate Cancer Foundation and the Canadian Institutes of Health Research.
AUTHOR CONTRIBUTIONS
Conception and design: Anuradha Jayaram, Gerhardt Attard
Financial support: Shibu Thomas, Dong Shen, Kim N. Chi, Ugo De Giorgi, Enrique Gonzalez-Billalabeitia, Alexander Wyatt, Gerhardt Attard
Administrative support: Florence Lefresne, Kim N. Chi, Ugo De Giorgi, Enrique Gonzalez-Billalabeitia, Alexander W. Wyatt, Gerhardt Attard
Provision of study materials or patients: Vincenza Conteduca, Lorraine Barwell, Susan Feyerabend, Enrique Grande, Albert Font, Alfredo Berruti, Cora N. Sternberg, Rob Jones, Shibu Thomas, Dong Shen, Kim N. Chi, Ugo De Giorgi, Enrique Gonzalez-Billalabeitia, Alexander W. Wyatt, Gerhardt Attard
Collection and assembly of data: Anuradha Jayaram, Anna Wingate, Daniel Wetterskog, Vincenza Conteduca, Lorraine Barwell, Susan Feyerabend, Alberto Martinez-Carrasco, Albert Font, Alfredo Berruti, Cora N. Sternberg, Rob Jones, Dong Shen, Deborah Ricci, Michael Gormley, Axel S. Merseburger, Bertrand Tombal, Kim N. Chi, Enrique Gonzalez-Billalabeitia, Alexander W. Wyatt, Gerhardt Attard
Data analysis and interpretation: Anuradha Jayaram, Anna Wingate, Daniel Wetterskog, Daniel Khalaf, Mansour Taghavi Azar Sharabiani, Fabio Calabrò, Susan Feyerabend, Enrique Grande, Cora N. Sternberg, Florence Lefresne, Marjolein Lahaye, Shibu Thomas, Shilpy Joshi, Dong Shen, Deborah Ricci, Michael Gormley, Axel S. Merseburger, Bertrand Tombal, Matti Annala, Ugo De Giorgi, Gerhardt Attard
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Anna Wingate
Research Funding: Janssen (Inst)
Travel, Accommodations, Expenses: Janssen
Vincenza Conteduca
Consulting or Advisory Role: Bayer, Astellas Pharma, Janssen-Cilag, Sanofi
Travel, Accommodations, Expenses: Bayer, Astellas Pharma, Janssen-Cilag, Sanofi
Daniel Khalaf
Consulting or Advisory Role: Bayer
Fabio Calabrò
Consulting or Advisory Role: Pfizer
Susan Feyerabend
Consulting or Advisory Role: Janssen-Cilag
Enrique Grande
Honoraria: Pfizer, Bristol-Myers Squibb, Ipsen, Roche, Eisai, Eusa Pharma, MSD, Genzyme, Adacap, Novartis, Pierre Fabre, Lexicon, Celgene, Janssen-Cilag, Astellas Pharma
Consulting or Advisory Role: MSD, Pfizer, Ipsen
Research Funding: MTEM/Threshold (Inst), Roche, Pfizer (Inst), AstraZeneca (Inst), Ipsen (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Genentech, Pfizer, Janssen-Cilag
Alfredo Berruti
Consulting or Advisory Role: Janssen-Cilag, Astellas Pharma
Speakers' Bureau: Janssen-Cilag
Research Funding: Astellas Pharma (Inst), Janssen-Cilag (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Sanofi
Cora N. Sternberg
Honoraria: Pfizer, Astellas Pharma, Sanofi, Ipsen, AstraZeneca. Janssen
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Bayer, Eisai, MSD, Clovis Oncology, Pfizer, Roche, Ipsen, Incyte, AstraZeneca, Sanofi, Merck, Medscape, UroToday
Research Funding: Janssen (Inst), Genentech (Inst), Bayer (Inst), Sanofi (Inst), Medivation (Inst), Exelixis (Inst), Genzyme (Inst), Aragon Pharmaceuticals (Inst), Array BioPharma (Inst), Aveo (Inst), AstraZeneca (Inst), Bristol-Myers Squibb (Inst), Boehringer Ingelheim (Inst), Clovis Oncology (Inst), Eisai (Inst), Genentech (Inst), GlaxoSmithKline (Inst), Eli Lilly (Inst), Incyte (Inst), Merck (Inst), Millennium (Inst), Myovant Sciences (Inst), Nektar (Inst), Pfizer (Inst), Clovis Atlas (Inst)
Rob Jones
Honoraria: Astellas Pharma, Janssen, AstraZeneca, MSD Oncology, Bristol-Myers Squibb, Pfizer, Novartis, Ipsen, Seattle Genetics, Sanofi, Bayer, Genentech, Eusa Pharma, Pharmacyclics
Consulting or Advisory Role: Clovis Oncology
Research Funding: Roche (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Pfizer (Inst), Novartis (Inst), Exelixis (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Ipsen, Bayer, Janssen, Astellas Pharma, MSD Oncology
Florence Lefresne
Employment: Johnson & Johnson
Stock and Other Ownership Interests: Johnson & Johnson
Honoraria: Johnson & Johnson
Research Funding: Johnson & Johnson
Travel, Accommodations, Expenses: Johnson & Johnson
Marjolein Lahaye
Employment: Janssen-Cilag
Leadership: Janssen-Cilag
Stock and Other Ownership Interests: Janssen-Cilag
Honoraria: Janssen-Cilag
Consulting or Advisory Role: Janssen-Cilag
Shibu Thomas
Employment: Janssen Research & Development
Leadership: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Research Funding: Janssen Oncology
Patents, Royalties, Other Intellectual Property: Four patents (Inst)
Travel, Accommodations, Expenses: Janssen Research & Development
Dong Shen
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Janssen Research & Development
Deborah Ricci
Employment: Janssen
Stock and Other Ownership Interests: Janssen
Patents, Royalties, Other Intellectual Property: Janssen (Inst)
Travel, Accommodations, Expenses: Janssen
Michael Gormley
Employment: Janssen Research & Development
Stock and Other Ownership Interests: Johnson & Johnson
Patents, Royalties, Other Intellectual Property: I have several patents pending where I am listed as the inventor of things that have been developed in the course of my work at Janssen Research and Development (Inst)
Travel, Accommodations, Expenses: Janssen Research & Development
Axel S. Merseburger
Honoraria: Janssen-Cilag, Astellas Pharma, Ipsen, Roche, Bristol-Myers Squibb, Eisai, Takeda, Pfizer, Novartis
Consulting or Advisory Role: MSD Oncology, Bristol-Myers Squibb, Janssen-Cilag, Astellas Pharma, Ipsen
Speakers' Bureau: Ipsen
Research Funding: Novartis (Inst), AstraZeneca (Inst), Janssen-Cilag (Inst), Bristol-Myers Squibb (Inst), Clovis Oncology (Inst)
Travel, Accommodations, Expenses: Janssen-Cilag, Astellas Pharma, Ipsen
Bertrand Tombal
Honoraria: Amgen, Astellas Pharma, Bayer, Ferring, Sanofi, Janssen, Pfizer, Myovant Sciences
Consulting or Advisory Role: Astellas Pharma, Bayer, Ferring, Janssen, Takeda, Steba Biotech, Sanofi
Speakers' Bureau: Amgen, Janssen
Research Funding: Ferring (Inst)
Travel, Accommodations, Expenses: Amgen, Astellas Pharma, Bayer, Ferring, Janssen, Sanofi
Kim N. Chi
Honoraria: Sanofi, Janssen, Astellas Pharma, Bayer
Consulting or Advisory Role: Essa, Astellas Pharma, Janssen, Sanofi, Amgen, Bayer, AstraZeneca, Roche
Research Funding: Janssen (Inst), Astellas Pharma (Inst), Bayer (Inst), Sanofi (Inst), Tokai Pharmaceuticals (Inst), Eli Lilly/ImClone (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Roche (Inst)
Ugo De Giorgi
Consulting or Advisory Role: Pfizer, Janssen, Astellas Pharma, Sanofi, Bristol-Myers Squibb, Bayer, Ipsen, Merck
Research Funding: Sanofi (Inst), AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Ipsen, Janssen, Pfizer
Enrique Gonzalez-Billalabeitia
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Pfizer, Bristol-Myers Squibb, Janssen-Cilag, Astellas Pharma, Sanofi, Roche, Pfizer
Alexander W. Wyatt
Consulting or Advisory Role: Genzyme
Speakers' Bureau: Janssen
Research Funding: Janssen
Gerhardt Attard
Honoraria: Janssen, Astellas Pharma, Janssen (I)
Consulting or Advisory Role: Janssen-Cilag, Veridex, Ventana Medical Systems, Astellas Pharma, Medivation, Novartis, Millennium, Abbott Laboratories, ESSA, Bayer, Pfizer
Speakers' Bureau: Janssen, Astellas Pharma, Takeda, Sanofi, Ventana Medical Systems
Research Funding: Janssen (Inst), Arno Therapeutics (Inst), Innocrin Pharma (Inst)
Patents, Royalties, Other Intellectual Property: I am on the Institute of Cancer Research Rewards to Inventors list of abiraterone acetate
Travel, Accommodations, Expenses: Janssen, Astellas Pharma, Medivation, Ventana Medical Systems, Abbott Laboratories, Bayer, ESSA, Janssen (I), Astellas Pharma (I), Pfizer
Other Relationship: Institute of Cancer Research
No other potential conflicts of interest were reported.
REFERENCES
- 1.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabernero J, Lenz HJ, Siena S, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: A retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16:937–948. doi: 10.1016/S1470-2045(15)00138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi K, Uchida J, Nishino K, et al. Quantitative detection of EGFR mutations in circulating tumor DNA derived from lung adenocarcinomas. Clin Cancer Res. 2011;17:7808–7815. doi: 10.1158/1078-0432.CCR-11-1712. [DOI] [PubMed] [Google Scholar]
- 6.Gevensleben H, Garcia-Murillas I, Graeser MK, et al. Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin Cancer Res. 2013;19:3276–3284. doi: 10.1158/1078-0432.CCR-12-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50:645–651. doi: 10.1038/s41588-018-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Coleman I, Morrissey C, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22:369–378. doi: 10.1038/nm.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kallio HML, Hieta R, Latonen L, et al. Constitutively active androgen receptor splice variants AR-V3, AR-V7 and AR-V9 are co-expressed in castration-resistant prostate cancer metastases. Br J Cancer. 2018;119:347–356. doi: 10.1038/s41416-018-0172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conteduca V, Wetterskog D, Sharabiani MTA, et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: A multi-institution correlative biomarker study. Ann Oncol. 2017;28:1508–1516. doi: 10.1093/annonc/mdx155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romanel A, Tandefelt DG, Conteduca V, et al. doi: 10.1126/scitranslmed.aac9511. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med 7:312re10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annala M, Vandekerkhove G, Khalaf D, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8:444–457. doi: 10.1158/2159-8290.CD-17-0937. [DOI] [PubMed] [Google Scholar]
- 13. doi: 10.1001/jamaoncol.2019.1011. Attard G, Merseburger AS, Arlt W, et al: Assessment of the safety of glucocorticoid regimens in combination with abiraterone acetate for metastatic castration-resistant prostate cancer: A randomized, open-label phase 2 study. JAMA Oncol 5:1159-1167, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1002/sim.1333. Mazumdar M, Smith A, Bacik J: Methods for categorizing a prognostic variable in a multivariable setting. Stat Med 22:559-571, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2014;32:671–677. doi: 10.1200/JCO.2013.52.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. doi: 10.1093/annonc/mdy406. Armstrong AJ, Lin P, Higano CS, et al: Development and validation of a prognostic model for overall survival in chemotherapy-naive men with metastatic castration-resistant prostate cancer (mCRPC) from the phase III PREVAIL clinical trial. J Clin Oncol 35, 2017 (suppl 6; abstr 138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi KN, Kheoh T, Ryan CJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol. 2016;27:454–460. doi: 10.1093/annonc/mdv594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. doi: 10.1016/j.eururo.2014.02.056. Rathkopf DE, Smith MR, de Bono JS, et al: Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 66:815-825, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong AJ, Lin P, Higano CS, et al. Development and validation of a prognostic model for overall survival in chemotherapy-naïve men with metastatic castration-resistant prostate cancer. Ann Oncol. 2018;29:2200–2207. doi: 10.1093/annonc/mdy406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. doi: 10.1158/1078-0432.CCR-18-1943. De Laere B, Oeyen S, Mayrhofer M, et al: TP53 outperforms other androgen receptor biomarkers to predict abiraterone or enzalutamide outcome in metastatic castration-resistant prostate cancer. Clin Cancer Res 25:1766-1773, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Laere B, van Dam PJ, Whitington T, et al. Comprehensive profiling of the androgen receptor in liquid biopsies from castration-resistant prostate cancer reveals novel intra-AR structural variation and splice variant expression patterns. Eur Urol. 2017;72:192–200. doi: 10.1016/j.eururo.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Loriot Y, Eymard JC, Patrikidou A, et al. Prior long response to androgen deprivation predicts response to next-generation androgen receptor axis targeted drugs in castration resistant prostate cancer. Eur J Cancer. 2015;51:1946–1952. doi: 10.1016/j.ejca.2015.06.128. [DOI] [PubMed] [Google Scholar]
- 24.Bellmunt J, Kheoh T, Yu MK, et al. Prior endocrine therapy impact on abiraterone acetate clinical efficacy in metastatic castration-resistant prostate cancer: Post-hoc analysis of randomised phase 3 studies. Eur Urol. 2016;69:924–932. doi: 10.1016/j.eururo.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung J, Taylor AR, Divine GW, et al. The effect of time to castration resistance on outcomes with abiraterone and enzalutamide in metastatic prostate cancer. Clin Genitourin Cancer. 2016;14:381–388. doi: 10.1016/j.clgc.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 26. doi: 10.1093/annonc/mdx689. Wang L, Dehm SM, Hillman DW, et al: A prospective genome-wide study of prostate cancer metastases reveals association of wnt pathway activation and increased cell cycle proliferation with primary resistance to abiraterone acetate-prednisone. Ann Oncol 29:352-360, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antonarakis ES, Lu C, Luber B, et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35:2149–2156. doi: 10.1200/JCO.2016.70.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scher HI, Graf RP, Schreiber NA, et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 2018;4:1179–1186. doi: 10.1001/jamaoncol.2018.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torquato S, Pallavajjala A, Goldstein A, et al. Genetic alterations detected in cell-free DNA are associated with enzalutamide and abiraterone resistance in castration-resistant prostate cancer. JCO Precis Oncol. doi: 10.1200/PO.18.00227. 10.1200/PO.18.00227, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conteduca V, Jayaram A, Romero-Laorden N, et al. Plasma androgen receptor and docetaxel for metastatic castration-resistant prostate cancer. Eur Urol. doi: 10.1016/j.eururo.2018.09.049. 75:368-373, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]