Abstract
PURPOSE
Next-generation sequencing (NGS) multigene panel testing has become widespread, including the Veterans Affairs (VA), through the VA National Precision Oncology Program (NPOP). The interpretation of genomic alterations remains a bottleneck for realizing precision medicine. We sought to examine the concordance for pathogenicity determination and clinical actionability of annotation services in NPOP.
METHODS
Unique gene variants were generated from NGS gene panel results using two sequencing services. For each unique gene variant, annotations were provided through N-of-One (NoO), IBM Watson for Genomics (WfG), and OncoKB. Annotations for pathogenicity (all three sources) and actionability (WfG and OncoKB) were examined for concordance. Cohen’s kappa statistic was calculated to measure agreement between annotation services.
RESULTS
Among 1,227 NGS results obtained between 2015 and 2017, 1,388 unique variants were identified in 117 genes. The genes with the largest number of variants included TP53 (270), STK11 (92), and CDKN2A (81). The most common cancer type was lung adenocarcinoma (440), followed by colon adenocarcinoma (113). For pathogenic and likely pathogenic variants, there was 30% agreement between WfG and NoO (kappa, −0.26), 76% agreement between WfG and OncoKB (kappa, 0.22), and 42% agreement between NoO and OncoKB (kappa, −0.07). For level 1 drug actionability of gene variant–diagnosis combinations, there was moderate agreement between WfG and OncoKB (96.9%; kappa, 0.44), with 27 combinations identified as level 1 by both services, 58 by WfG alone, and 6 variants by OncoKB alone.
CONCLUSION
There is substantial variability in pathogenicity assessment of NGS variants in solid tumors by annotation services. In addition, there was only moderate agreement in level 1 therapeutic actionability recommendations between WfG and OncoKB. Improvement in the precision of NGS multigene panel annotation is needed.
INTRODUCTION
The framework to implement genomics-driven therapeutic oncology has been rapidly established in leading cancer centers worldwide. An exponential growth in characterized cancer genomes has ensued.1 The results of next-generation sequencing (NGS) assays are intricate and provide a multitude of somatic mutations in hundreds of genes. Mutation rates vary significantly between different cancers.2 Many somatic genetic variants are characterized as variants of unknown significance, and the implication of multiple mutations within a single tumor is poorly defined.3 Moreover, tumors possess intratumoral genetic heterogeneity, adding another layer of complexity to NGS results.4
CONTEXT
Key Objective
Algorithms to interpret the pathogenicity and clinical actionability of next-generation sequencing (NGS)–detected sequence variants have been implemented; however, little is known about their relative performance in clinical practice. The clinical interpretation of genomic alterations remains a major bottleneck for realizing precision medicine. We sought to examine the concordance for pathogenicity and clinical actionability of three annotation services available to the VA Precision Oncology Program (N-of-One, OncoKB, and Watson for Genomics).
Knowledge Generated
We demonstrate that NGS annotation services have wide-ranging agreements in pathogenicity (30%-76%). Moreover, there was moderate agreement (96.9%) in level 1 drug actionability.
Relevance
We anticipate these findings will encourage improvement in the precision of NGS multigene panel annotation. We provide detailed information regarding NGS panels, gene variant pathogenicity, annotation ontology, and level 1 drug actionability by annotation service. This study has significant implications for precision oncology clinical trials and molecular tumor boards.
At many institutions, including the Veterans Affairs (VA) healthcare system, molecular tumor boards (MTBs) have been instituted to interpret the results of NGS and develop treatment recommendations. In some instances the whole genome is sequenced, and in other cases selected subsets of genes are sequenced with smaller panels. Identified alterations that are of particular clinical interest include driver mutations or druggable mutations.1 For use in clinical decision making, identified gene variants must be annotated or interpreted in terms of the likelihood of their tumorigenicity and drug actionability.5 There are many resources to assist with data curation, including OncoKB (Memorial Sloan Kettering Cancer Center, New York, NY),6 My Cancer Genome (Vanderbilt-Ingram Cancer Center, Nashville, TN),7 Precision Medicine Knowledge Base (Weill Cornell Medicine Englander Institute for Precision Medicine, New York, NY),8 Personalized Cancer Therapy (MD Anderson Cancer Center, Houston, TX),9 CIViC (Washington University in St Louis School of Medicine, St Louis, MO),10 Jackson Laboratory Clinical Knowledgebase (Jackson Laboratory, Bar Harbor, ME),11 Cancer Genome Interpreter (Barcelona, Spain),5 Cancer Driver Log (omicX, Le-Petit-Quevilly, France),12 and N-of-One (NoO; Concord, MA).13 The identification of mutations that are pathogenic and actionable is generally performed by multidisciplinary teams who reference genomic databases, published literature, and clinical trials. The development of cognitive computing, such as Watson for Genomics (WfG), has also garnered interest in precision oncology. WfG14 is a cloud-based service that uses computerized models to simulate human thought to analyze large volumes of genome data and generate evidence-based guidelines.
The ultimate objective of gene sequencing is to improve patient outcomes by matching patients to specific treatments that target mutations driving the growth of individual tumors. The landscape of genetic tests has evolved from single mutation to small hotspot mutation panels, large gene panels, and whole exome and whole genome platforms. The interpretation of the clinical significance of these genomic mutations has become a formidable task because of the number of genes tested and limited understanding of normal genetic variation as well as pathogenic gene-to-gene interaction. Although professional societies have recently published consensus guidelines15 for the use and interpretation of NGS, they have not been extensively used in practice. Moreover, traditional clinical trial design approaches of molecularly unselected tumor types may no longer be appropriate because of the molecular heterogeneity of tumors from each primary site (eg, breast). This has led to the development of basket and umbrella trial designs as well as precision genomic oncology trials at the point of care. An urgency exists to examine the various annotation services and clinical support tools available to ensure quality of NGS in oncology.
The VA National Precision Oncology Program (NPOP)16 initially used two commercial vendors for NGS testing, Personal Genome Diagnostics (PGDx, Baltimore, MD) and Personalis (Menlo Park, CA), that used the NoO commercial annotation service. In addition, WfG was available to the VA through a gift from IBM. Until NPOP accrues substantial outcomes data, treatment recommendations are largely based on biomarker panels, annotations, and relevant patient characteristics.17 NPOP also references open data sources, including OncoKB (an evidence source, not an annotation provider), when interpreting pathogenicity and actionability of gene variants. For some variants and cases, annotation is assisted by a molecular pathologist. The primary objective of this study was to assess the concordance for pathogenicity determination and clinical actionability for the annotation services available to NPOP.
METHODS
Sequencing data from all patients who underwent NGS testing under NPOP since its genesis in 2015 through 2017 were analyzed. The work described here was conducted to assess the quality of annotation services available to the NPOP, a clinical operational program that is not research and does not require institutional review board review; permission to publish was obtained from the appropriate VA authority. Details of the NPOP have been published previously.16 The goal is to use NGS testing to facilitate patient access to US Food and Drug Administration (FDA)–approved targeted therapies and immune checkpoint inhibitors and increase participation in clinical trials (Data Supplement). Clinical trials have been the recommended option in > 50% of cases for which there was no FDA-approved therapy. NGS results were generated through two contracted vendors: PGDx18 (CancerSELECT 125, PlasmaSELECT 64, CancerSELECT 88, and CancerSELECT 203) and Personalis (ACE Cancer Plus 181)19 (Data Supplement).
We matched gene variants for the two contracted vendors, PGDx and Personalis, to compare vendor recommendations as well as commercial annotation services for the same unique gene variant. NGS-detected variants were annotated by the sequencing vendor using the commercial annotation service NoO. We examined NoO as implemented by the vendors. NPOP staff re-annotated DNA sequence results using WfG and OncoKB. IBM donated use of WfG to the VA; OncoKB is created and maintained by Memorial Sloan Kettering Cancer Center (MSKCC) and available online.6
After completion of sequencing, results were uploaded by an NPOP data scientist to WfG (including tumor type, a list of sequence variants as a variant call file, presence of gene fusions, and gene copy number variation). Results were routinely uploaded into WfG as part of routine test interpretation workflow. Batch analysis was performed approximately weekly using a custom-built informatics workflow and a packaging tool provided by IBM. WfG assigned a pathogenicity label to each variant or fusion. Drug matching for pathogenic variants is also part of the WfG analysis pipeline. Variants that are pathogenic or likely pathogenic are matched with FDA-approved drugs and actively recruiting clinical trials using levels of evidence (level 1, 2A, 2B, 3A, 3B, 4, or R1; Tables 1 and 2).3 For actionability, WfG uses a subset of National Cancer Institute (NCI) thesaurus terms for diagnosis coding.20 WfG was performed on all samples as part of NPOP workflow, and OncoKB analysis was performed on an ad hoc basis in response to NPOP consults and/or MTB cases.
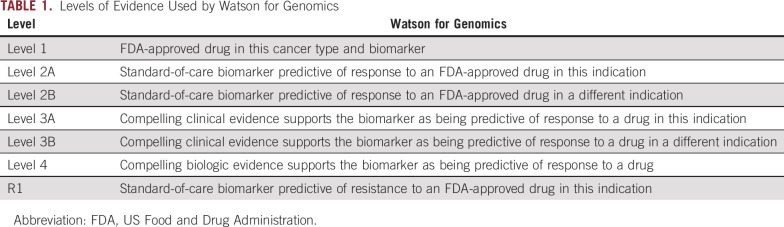
TABLE 1.
Levels of Evidence Used by Watson for Genomics
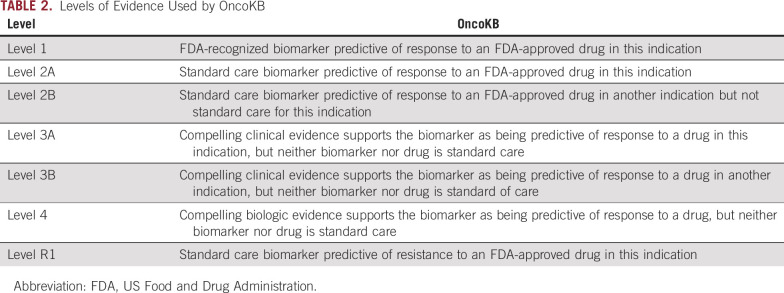
TABLE 2.
Levels of Evidence Used by OncoKB
Using the same unique gene variants annotated by NoO and WfG, we queried all variants using the OncoKB curated database for the current study (Data Supplement). The public database21 includes information on the clinical actionability for each somatic gene variant organized by indication and four-tier levels of evidence (Tables 1 and 2) which are ultimately incorporated into cBioPortal22 for Cancer Genomics to aid physicians and cancer researchers.6 Many genes involved in tumorigenesis are not targetable with currently available drugs. More than 90% of alterations in OncoKB have biologic effects and are classified as oncogenic but not actionable. For actionability, tumor ontology is considered. OncoKB contains 43 tumor types with biomarker-drug associations and uses an open-source ontology, OncoTree,23 which was developed at MSKCC (699 tumor types). The Clinical Genomics Annotation Committee (CGAC) reviews the OncoKB alteration across 22 disease management teams. Curation reviews occur every 3 months, and CGAC recommendations and feedback are updated in real time.6
For this study, levels of evidence 2A and 2B were consolidated to level 2 and levels 3A and 3B to level 3 for both WfG and OncoKB. For actionability annotation comparisons, we mapped ontology from WfG to OncoKB to ensure that gene variants were properly annotated with their respective tumor type. In addition, for actionability annotation comparisons, we excluded microsatellite instability (MSI), as the MSI status was not entered into WfG and we do not have MSI information from WfG.
RESULTS
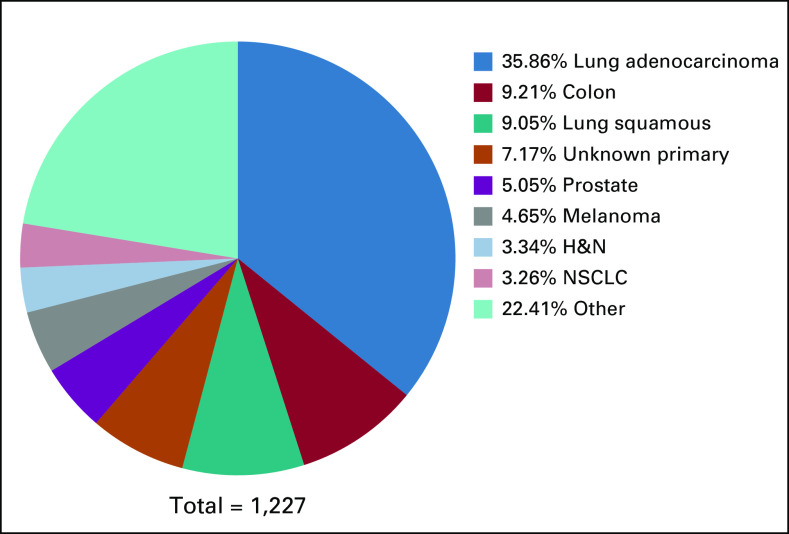
Among 1,227 NGS results, 1,388 unique variants were observed in 117 genes. The entire set of unique gene variants is shown in the Data Supplement. The genes with the largest number of variants included were: TP53 (270), STK11 (92), CDKN2A (81), ATM (67), PTEN (52), NF1 (46), and BRCA2 (45). The most common cancer was lung adenocarcinoma in 35.86% (440). Other cancer types included colon adenocarcinoma 9.21% (113) and lung squamous cell carcinoma 9.05% (111). The complete list of cancer types is shown in Figure 1.
FIG 1.
Next-generation sequencing (NGS) tumor types. Among 1,227 NGS results, 1,388 unique gene variants were identified. The NGS solid tumor type distribution is indicated. H&N, head and neck; NSCLC, non–small-cell lung cancer.
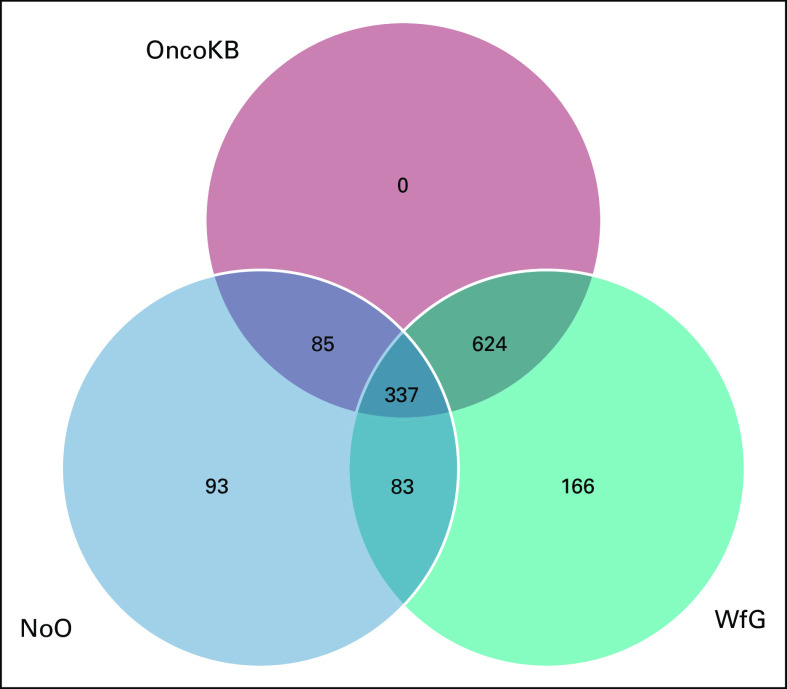
Of the 1,388 unique gene variants, 1,082 were identified only by PGDx (a larger proportion of the samples were sequenced by PGDx), and 480 were identified only by Personalis, whereas 174 gene variants were generated by both vendors in different specimens. NoO classification should be similar for both vendors. The unique gene variants generated by each vendor are shown in the Data Supplement. The genes included in the gene panels are listed in the Data Supplement. We also list the well-characterized genes of each panel as well as the genes for which copy analysis is performed. For panel distribution by NGS samples, see the Data Supplement. All unique gene variants were annotated by NoO, WfG, and OncoKB. Out of the 1,388 unique gene variants, 337 (24.2%) were variants of unknown significance by OncoKB. Out of the 1,388 unique gene variants, 270 (19.4%) were variants of unknown significance by WfG.
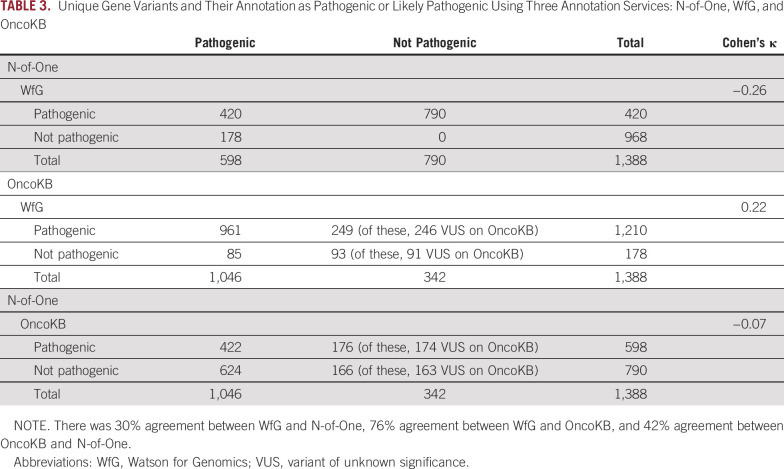
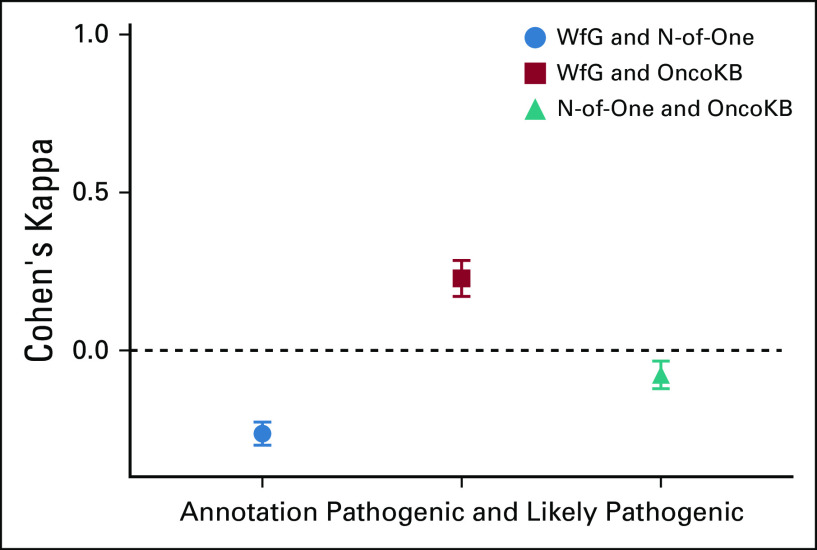
For pathogenicity annotation, (ie, pathogenic and likely pathogenic variants v all other variants), there was fair agreement between WfG and OncoKB (76%; kappa, 0.22) and no agreement between WfG and NoO (30%; kappa, −0.26) as well as NoO and OncoKB (42%; kappa, −0.07; Table 3; Fig 2; Appendix Fig A1).
TABLE 3.
Unique Gene Variants and Their Annotation as Pathogenic or Likely Pathogenic Using Three Annotation Services: N-of-One, WfG, and OncoKB
FIG 2.
Agreement of pathogenicity annotation: Watson for Genomics (WfG) and N-of-One good strength agreement, WfG and OncoKB fair agreement, and N-of-One and OncoKB no agreement. Cohen’s kappa coefficient of 1 indicates perfect agreement, and 0 indicates that any agreement is due to chance. The number of samples in each group is shown in Table 3.
There were 91 unique gene variant–diagnosis combinations identified as having level 1 drug actionability recommendations identified by WfG, OncoKB, or both (not available from NoO). As part of actionability annotation, we mapped diagnosis ontology from WfG to OncoKB for each observed diagnosis (Data Supplement).
There was moderate agreement between WfG and OncoKB (96.9%; kappa, 0.445), with 58 variants identified only by WfG as level 1 and 6 variants identified only by OncoKB as level 1 (Table 4). The complete set of level 1 drug recommendations for WfG and OncoKB are shown in the Data Supplement. When both annotation services had level 1 drug actionability, the recommended drugs were overwhelmingly identical. An example of a unique gene variant with drug actionability concordance is the exon 19 deletion in lung adenocarcinoma, EGFR-L747_S752del, with level 1 evidence for use of erlotinib, afatinib, or gefitinib using both WfG and OncoKB annotation services. Response rates of tumors with EGFR exon 19 deletions at L747 have been reported as high as 83.3%.24
TABLE 4.
Level 1 Drug Actionability Comparing IBM WfG and Open Academic Somatic Variant Database OncoKB
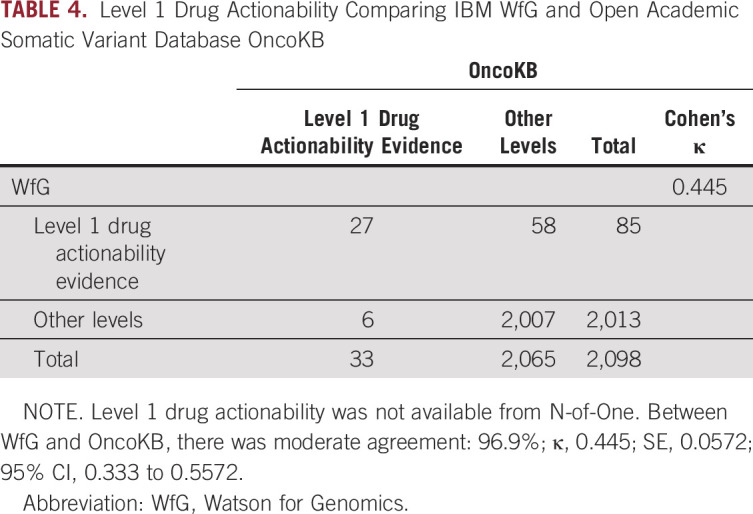
Among the 33 unique gene variant diagnoses identified as level 1 by OncoKB, WfG classification was level 1 in 27; for the 6 cases with discordant classification in WfG, 5 cases were lung adenocarcinoma EGFR mutations and a melanoma BRAF-V600R mutation. For the BRAF-V600R mutation in melanoma, WfG had no level 1 recommendations, whereas OncoKB had 5 drug or drug combinations as level 1. BRAF-V600R mutations constitute approximately 3%-7% of all BRAF mutations and were not included in the original BRAF/MEK inhibitors clinical trials.25 Although V600R is not listed on the BRAF/MEK FDA label, National Comprehensive Cancer Network panel guidelines from as early as 2016 consider single-agent BRAF inhibitor monotherapy and BRAF/MEK inhibitor combination therapy an appropriate treatment of all activating BRAF mutations, including V600R, V600D, and others.26 For EGFR mutations in lung adenocarcinoma (EGFR-A750P, EGFR-E746_T751delinsI, EFGR-G719C, EGFR-L861Q, and EGFR-S7681), WfG had no recommendations in 3 variants and had level 2A for EGFR-G719C and level 3B for both EGFR-A740P and EGFR L861Q for drugs afatinib, erlotinib, and gefitinib. For EGFR-L861Q, an uncommon EGFR mutation, the afatinib FDA label expanded to include EGFR-L861Q in January of 2018, which was after our annotation analysis. These approvals were based on findings from the phase 2 LUX-Lung 2 trial as well as the phase 3 LUX-Lung3 and LUX-Lung 6 trials that showed an objective response rate of 66% (95% CI, 47% to 81%).27
Among the 85 unique gene variant diagnoses identified as level 1 by WfG, OncoKB classification was level 1 in 27, and 58 had discordant classification. Most of the gene variant diagnoses (40/58 [68.9%]) with discordant recommendations were mismatch repair (MMR) genes, such as MLH1, MSH2, MSH6, and PMS2. The remaining gene variant diagnoses (18/58; 31.0%) with discordant recommendations included genes CDK4, EGFR, FGFR1, FLT4, KIT, PDGFRA, PIK3CA, POLE, PTEN, TSC1, TSC2, VEGFA, and VHL. For a lung adenocarcinoma case with MLH1-A42, which is part of DNA MMR genes and known to have increased MSI, WfG provide a level 1 recommendation for atezolizumab, nivolumab, and pembrolizumab. Although MMR status predicts the clinical benefit of immune checkpoint blockade in multiple tumor types,28,29 none of these drugs are FDA approved for MLH1 mutants. Similarly, for other MMR gene variants, WfG provided level 1 recommendations in the absence of an FDA on-label indication.
DISCUSSION
Precision medicine has promoted the development of biomarker-driven treatment strategies. There has been a surge in the genetic testing market, with a 10% annual increase in new genetic tests and a 20% annual increase in gene-based diagnostic tests.15,30 NGS generates massive amounts of data, and different biologic questions require the development of specific bioinformatics pipelines, which are frequently platform specific.30,31 The processing of raw sequence data has a profound effect on patient care and outcomes, and NGS test validation is necessary.32 Until recently, there were no established uniform technical standards for reporting tumor-derived NGS gene panel sequencing results.33 In this analysis, we examine a large number of clinically observed unique gene variants and compare annotations for pathogenicity and actionability through two commercial services, NoO and WfG, as well as an open database, OncoKB.
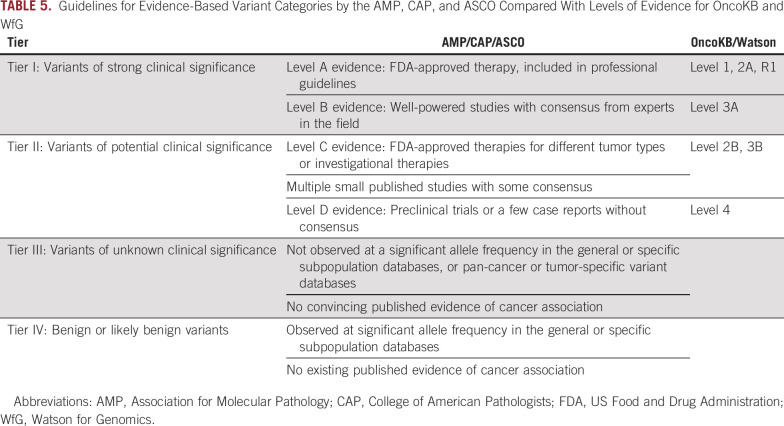
In efforts to establish guidelines for NGS gene panel interpretation of somatic variants, the Association of Molecular Pathology (AMP) and College of American Pathologists (CAP) issued a joint consensus of classification (Table 5).32,33 The four-tier system has different nomenclature and classification as compared with WfG and OncoKB (Tables 1 and 2). The WfG and OncoKB levels of evidence are similar and compatible, but not identical, to the AMP/CAP/ASCO evidence levels. The OncoKB evidence levels were mapped to the evidence levels for AMP/CAP/ASCO tier I and II variants (Table 5).34 We used the WfG and OncoKB classification systems to compare their levels to each other, and we found that variants classified as pathogenic had a wide range of concordance, from 30% to 76% (Fig 2; Table 3; Appendix Fig A1). In routine clinical practice, only gene variants that are pathogenic or likely pathogenic are considered for actionability, so misclassification in pathogenicity could readily affect provider prescribing. For drug actionability, there was moderate-strength agreement 96.9% (kappa, 0.445) for levels 1. In practice, levels of evidence 1 and 2A both frequently result in use of a targeted drug, so concordance in level 2A may be similar to level 1, and their combination may improve concordance among annotation services for actionability. However, we expect greater discordance among levels of evidence 2B to 4.
TABLE 5.
Guidelines for Evidence-Based Variant Categories by the AMP, CAP, and ASCO Compared With Levels of Evidence for OncoKB and WfG
Although no direct comparisons between NoO, WfG, and OncoKB have been previously performed, WfG has been compared with MTBs.3 The utility of WfG was compared with MTBs by examining 1,018 cases analyzed by the University of North Carolina’s Human-MTB. WfG identified an additional 8 actionable genes (7 of which passed actionability criteria by Human-MTB). Mutations in these 8 genes were identified in 231 and 96 patients out of 703 and 315 patients with already identified actionable and no actionable mutations, respectively.3 Of the 7 actionable genes identified by WfG, 3 had no clinical trial available, and 4 were made potentially eligible for a biomarker-selected clinical trial. The reason for the added genes by WfG was believed to be the opening of several clinical trials within weeks of the WfG analysis. Most genes identified and reclassified as actionable, however, have yet to demonstrate their utility as predictive biomarkers of response to the recommended therapy. These actionable mutations identified by WfG were found retrospectively in 323 patients, of whom only 47 (4.6%) had active disease requiring further therapy. Moreover, none of the patients had treatment altered based on WfG’s recommendation. Cognitive computing may supplement MTBs, and multiple studies have shown concordance of > 90%.35,36 WfG may provide additional decision support in the current era of rapid generation of information from clinical trials.
Currently, existing evidence does not support population-based universal tumor sequencing.37 The SHIVA trial (ClinicalTrials.gov identifier: NCT01771458) examined patients with any metastatic solid tumor refractory to standard treatment and randomly assigned 195 to receive treatment on the basis of molecular profile (n = 99) versus standard treatment (n = 95). Median progression-free survival was 2.3 months (95% CI, 1.7 to 3.8 months) in the experimental group versus 2.0 months (95% CI, 1.8 to 2.1 months) in the control group (hazard ratio, 0.88; 95% CI, 0.65 to 1.19; P = .41). The study used a variety of tumor types and histologies with single molecular alterations as a predictor for efficacy of targeted agents in heavily pretreated patients, possibly reducing their effectiveness. Nevertheless, there were no differences in progression-free survival or overall survival, and off-label use of targeted agents was discouraged.38 The benefit of off-label use of molecularly targeted agents is largely debated in oncology; however, most agree that clinical trial enrollment should be encouraged to identify predictive druggable biomarkers.
Other prospective studies, including EXACT,39 MOSCATO-01 (ClinicalTrials.gov identifier: NCT01566019),40,41 and MD Anderson’s Phase I Clinic42,43 have shown promising results for targeted therapy on previously treated solid tumors. Additional studies examining the efficacy of off-label use of targeted therapy on the basis of NGS test results are underway: ASCO’s Targeted Agent and Profiling Utilization Registry (TAPUR) study (ClinicalTrials.gov identifier: NCT02693535),44 NCI-MATCH (Molecular Analysis for Therapy Choice) trial (ClinicalTrials.gov identifier: NCT02465060),45 and Pediatric MATCH (ClinicalTrials.gov identifier: NCT03155620). Results from NCI-MATCH for patients with PIK3CA mutations treated with taselisib show 0% objective response rate,46 whereas patients with FGFR pathway mutations treated with AZD4547 showed a 5% objective response rate.47 Currently, there are no randomized controlled trial results supporting a universal NGS-based treatment paradigm. Despite this, NGS testing continues to be incorporated into the clinic.48 In a recent survey of 1,281 US oncologists, 75.6% reported using NGS tests to guide treatment decisions. Of these, 34% used them for patients with advanced refractory disease, and 17.5% used them for decisions on off-label use of FDA-approved drugs.49 More than 50% reported that NGS tests were difficult to interpret either often or sometimes, which is a separate but related challenge facing personalized medicine.49 The use of NGS will likely continue to grow, with providers facing uncertainty as to how to integrate its use into the clinic. Studies like ours are critical and further highlight the current shortcomings in precisely interpreting results of NGS gene panels for use in clinical management.
APPENDIX
Veterans Affairs National Precision Oncology Program.
National Precision Oncology Program (NPOP) services are available to all Veterans Affairs (VA) facilities and as of June 2018, over 72 different VA facilities submitted tumor samples. Following sequencing, a formal report of identified genomic aberrations is collated, annotated and included in patient records. The turnaround time is 14 days. NPOP provides a molecular oncology consultation service to assist VA clinicians with treatment decisions, and a case-based education-focused molecular tumor board. The users of NPOP are VA general oncologists and pathologists. Specialized oncologists and molecular pathologists oversee the program.
Panel information.
Panels were constructed to identify base/missense substitutions, insertions/deletions in protein-encoding regions, copy number variations, selected gene fusions/rearrangements and microsatellite instability (only on CancerSELECT 125 panel). There was overlap between the panels and a minimum of 500X DNA sequence coverage was required for all assays.
OncoKB pathogenicity and actionability.
Annotation of variant pathogenicity was executed using OncoKB support and through the API (http://oncokb.org/). Annotation of actionability as defined by the OncoKB Levels of Evidence was executed using OncoKB support and are available through the OncoKB annotator (https://github.com/). Data regarding all variants annotated as actionable per OncoKB at the time of the study are publicly available at https://github.com/.
Panel breakdown of the NGS samples.
The panel breakdown of the 1,227 NGS samples was: Personalis ACE CancerPlus (n = 404); Personal Genome Diagnostics (PGDx) CancerSELECT 125 (n = 286); PGDx CancerSELECT 203 (n = 75); PGDx CancerSELECT 88 (n = 176); PGDx CancerSELECT 64 (n = 1); PGDx unknown panel (n = 285).
FIG A1.
Venn diagram showing the relationship between pathogenicity annotation for N-of-One (NoO), Watson for Genomics (WfG), and OncoKB.
This work was conducted as a clinical operational analysis of the National Oncology Program Office, Office of Specialty Care Services, Department of Veterans Affairs. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. Presented at the ASCO Annual Meeting, Chicago, IL, June 1-5, 2018.
Supported by the Department of Veterans Affairs, Office of Specialty Care Services.
AUTHOR CONTRIBUTIONS
Conception and design: Evangelia Katsoulakis, Michael J. Kelley
Provision of study material or patients: Bradley Hintze
Collection and assembly of data: Evangelia Katsoulakis, Jill E. Duffy, Bradley Hintze
Data analysis and interpretation: Evangelia Katsoulakis, Bradley Hintze, Neil L. Spector, Michael J. Kelley
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Evangelia Katsoulakis
Stock and Other Ownership Interests: AbbVie, Alexion Pharmaceuticals, Biogen
Research Funding: Advantagene (Inst)
Neil L. Spector
Stock and Other Ownership Interests: Eydis Bio, Bessor Pharma
Research Funding: Immunolight
Patents, Royalties, Other Intellectual Property: I am on a patent related to my work with the company Immunolight; Patent through Eydis Bio
Michael J. Kelley
Consulting or Advisory Role: AstraZeneca, Eisai, IBM Japan
Research Funding: Bavarian Nordic, Novartis, AstraZeneca, Bristol-Myers Squibb
Other Relationship: IBM
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hayes DN, Kim WY. The next steps in next-gen sequencing of cancer genomes. J Clin Invest. 2015;125:462–468. doi: 10.1172/JCI68339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel NM, Michelini VV, Snell JM, et al. Enhancing next-generation sequencing-guided cancer care through cognitive computing. Oncologist. 2018;23:179–185. doi: 10.1634/theoncologist.2017-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris LG, Riaz N, Desrichard A, et al. Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget. 2016;7:10051–10063. doi: 10.18632/oncotarget.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamborero D, Rubio-Perez C, Deu-Pons J, et al. Cancer Genome Interpreter annotates the biological and clinical relevance of tumor alterations. Genome Med. 2018;10:25. doi: 10.1186/s13073-018-0531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chakravarty D, Gao J, Phillips SM, et al: OncoKB: A precision oncology knowledge base. JCO Precis Oncol 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed]
- 7.Taylor AD, Micheel CM, Anderson IA, et al. The path(way) less traveled: A pathway-oriented approach to providing information about precision cancer medicine on My Cancer Genome. Transl Oncol. 2016;9:163–165. doi: 10.1016/j.tranon.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang L, Fernandes H, Zia H, et al. The cancer precision medicine knowledge base for structured clinical-grade mutations and interpretations. J Am Med Inform Assoc. 2017;24:513–519. doi: 10.1093/jamia/ocw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumbrava EI, Meric-Bernstam F. Personalized cancer therapy—leveraging a knowledge base for clinical decision-making. Cold Spring Harb Mol Case Stud. 2018;4:a001578. doi: 10.1101/mcs.a001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith M, Spies NC, Krysiak K, et al. CIViC is a community knowledgebase for expert crowdsourcing the clinical interpretation of variants in cancer. Nat Genet. 2017;49:170–174. doi: 10.1038/ng.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patterson SE, Liu R, Statz CM, et al. The clinical trial landscape in oncology and connectivity of somatic mutational profiles to targeted therapies. Hum Genomics. 2016;10:4. doi: 10.1186/s40246-016-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damodaran S, Miya J, Kautto E, et al. Cancer Driver Log (CanDL): Catalog of potentially actionable cancer mutations. J Mol Diagn. 2015;17:554–559. doi: 10.1016/j.jmoldx.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. N-of-One: A Qiagen Company. Clinical interpretation solutions. https://n-of-one.com/
- 14.Rhrissorrakrai K, Koyama T, Parida L. Watson for Genomics: Moving personalized medicine forward. Trends Cancer. 2016;2:392–395. doi: 10.1016/j.trecan.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;33:3660–3667. doi: 10.1200/JCO.2015.63.0996. [DOI] [PubMed] [Google Scholar]
- 16. doi: 10.1200/PO.19.00075. Poonnen PJ, Duffy JE, Hintze B, et al: Genomic analysis of metastatic solid tumors in veterans: Findings from the VHA National Precision Oncology Program. JCO Precis Oncol 10.1200/PO.19.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiore LD, Brophy MT, Turek S, et al. The VA point-of-care precision oncology program: Balancing access with rapid learning in molecular cancer medicine. Biomark Cancer. 2016;8:9–16. doi: 10.4137/BIC.S37548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Personal Genome Diagnostics (PGDx): CLIA certified. https://www.personalgenome.com/
- 19. Personalis, Inc: A leading precision medicine company focused on advanced NGS based services for cancer and clinical trials and translational research. https://www.personalis.com/
- 20.Fragoso G, de Coronado S, Haber M, et al. Overview and utilization of the NCI thesaurus. Comp Funct Genomics. 2004;5:648–654. doi: 10.1002/cfg.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. doi: 10.1200/PO.17.00011. OncoKB: Precision oncology knowledge base. https://www.oncokb.org/ [DOI] [PMC free article] [PubMed]
- 22.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. OncoTree: Displaying: oncotree_latest_stable. http://oncotree.mskcc.org/#/home.
- 24.Su J, Zhong W, Zhang X, et al. Molecular characteristics and clinical outcomes of EGFR exon 19 indel subtypes to EGFR TKIs in NSCLC patients. Oncotarget. 2017;8:111246–111257. doi: 10.18632/oncotarget.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein O, Clements A, Menzies AM, et al. BRAF inhibitor activity in V600R metastatic melanoma. Eur J Cancer. 2013;49:1073–1079. doi: 10.1016/j.ejca.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Coit DG, Thompson JA, Algazi A, et al. NCCN guidelines insights: Melanoma, version 3.2016. J Natl Compr Canc Netw. 2016;14:945–958. doi: 10.6004/jnccn.2016.0101. [DOI] [PubMed] [Google Scholar]
- 27.Wu TH, Hsiue EH, Lee JH, et al. New data on clinical decisions in NSCLC patients with uncommon EGFR mutations. Expert Rev Respir Med. 2017;11:51–55. doi: 10.1080/17476348.2017.1267569. [DOI] [PubMed] [Google Scholar]
- 28.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gargis AS, Kalman L, Bick DP, et al. Good laboratory practice for clinical next-generation sequencing informatics pipelines. Nat Biotechnol. 2015;33:689–693. doi: 10.1038/nbt.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy S, Coldren C, Karunamurthy A, et al. Standards and guidelines for validating next-generation sequencing bioinformatics pipelines: A joint recommendation of the Association for Molecular Pathology and the College of American Pathologists. J Mol Diagn. 2018;20:4–27. doi: 10.1016/j.jmoldx.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Jennings LJ, Arcila ME, Corless C, et al. Guidelines for validation of next-generation sequencing-based oncology panels: A joint consensus recommendation of the Association for Molecular Pathology and College of American Pathologists. J Mol Diagn. 2017;19:341–365. doi: 10.1016/j.jmoldx.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: A joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wagner AH, Walsh B, Mayfield G, et al: A harmonized meta-knowledgebase of clinical interpretations of cancer genomic variants. Nat Genet (in press) [DOI] [PMC free article] [PubMed]
- 35.Somashekhar SP, Sepúlveda MJ, Puglielli S, et al. Watson for Oncology and breast cancer treatment recommendations: Agreement with an expert multidisciplinary tumor board. Ann Oncol. 2018;29:418–423. doi: 10.1093/annonc/mdx781. [DOI] [PubMed] [Google Scholar]
- 36.Kim YY, Oh SJ, Chun YS, et al. Gene expression assay and Watson for Oncology for optimization of treatment in ER-positive, HER2-negative breast cancer. PLoS One. 2018;13:e0200100. doi: 10.1371/journal.pone.0200100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West HJ. No solid evidence, only hollow argument for universal tumor sequencing: Show me the data. JAMA Oncol. 2016;2:717–718. doi: 10.1001/jamaoncol.2016.0075. [DOI] [PubMed] [Google Scholar]
- 38.Le Tourneau C, Delord JP, Gonçalves A, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16:1324–1334. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 39.Prager GW, Unseld M, Waneck F, et al. Results of the extended analysis for cancer treatment (EXACT) trial: A prospective translational study evaluating individualized treatment regimens in oncology. Oncotarget. 2019;10:942–952. doi: 10.18632/oncotarget.26604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. doi: 10.1016/j.ejca.2017.10.013. Verlingue L, Malka D, Allorant A, et al: Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer 87:122-130, 2017 [Erratum: Eur J Cancer 93:156-157, 2018] [DOI] [PubMed] [Google Scholar]
- 41.Massard C, Michiels S, Ferté C, et al. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: Results of the MOSCATO 01 trial. Cancer Discov. 2017;7:586–595. doi: 10.1158/2159-8290.CD-16-1396. [DOI] [PubMed] [Google Scholar]
- 42.Tsimberidou AM, Iskander NG, Hong DS, et al. Personalized medicine in a phase I clinical trials program: The MD Anderson Cancer Center initiative. Clin Cancer Res. 2012;18:6373–6383. doi: 10.1158/1078-0432.CCR-12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsimberidou AM, Wen S, Hong DS, et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: Validation and landmark analyses. Clin Cancer Res. 2014;20:4827–4836. doi: 10.1158/1078-0432.CCR-14-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mangat PK, Halabi S, Bruinooge SS, et al: Rationale and Design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis Oncol 10.1200/PO.18.00122. [DOI] [PMC free article] [PubMed]
- 45.Moore KN, Mannel RS. Is the NCI MATCH trial a match for gynecologic oncology? Gynecol Oncol. 140:161-166, 2016. [DOI] [PubMed]
- 46. Krop I, Jegede O, Grilley-Olson J, et al: Results from Molecular Analysis for Therapy Choice (MATCH) arm I: Taselisib for PIK3CA-mutated tumors. J Clin Oncol 36:101, 2018 (15_suppl; abstr 101)
- 47. Chae YK Vaklavas C, Cheng HH, et al: Molecular analysis for therapy choice (MATCH) arm W: Phase II study of AZD4547 in patients with tumors with aberrations in the FGFR. J Clin Oncol 36:2503, 2018 (15_suppl; abstr 2503)
- 48. doi: 10.3390/jpm8030030. Morash M, Mitchell H, Beltran H, et al: The role of next-generation sequencing in precision medicine: A review of outcomes in oncology. J Pers Med . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freedman AN, Klabunde CN, Wiant K, et al. Use of next-generation sequencing tests to guide cancer treatment: Results from a nationally representative survey of oncologists in the United StatesJCO Precis Oncol 10.1200/PO.18.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]