INTRODUCTION
Xeroderma pigmentosum (XP) is a rare autosomal recessive disorder caused by a nucleotide excision repair deficit, resulting in sensitivity to UV radiation.1 Individuals with XP are 10,000 times more likely to develop cutaneous squamous cell carcinoma (SCC), with initial presentation at a median age of 9 years.2 Metastatic skin cancer is the leading cause of death (34%), with a median survival of 32 years.2 Few options for effective systemic therapy of advanced cutaneous SCC are available,3,4 and there is no standard therapy for patients with XP who have developed metastatic skin malignancies.5 There is a critical need for effective therapy for advanced skin cancers, and patients with XP represent a unique need because they have the potential to suffer catastrophic adverse effects from standard chemotherapeutic agents.6
Immune checkpoint blockade has demonstrated efficacy in tumors characterized by high mutational burden, including SCC.7,8 Therefore, cutaneous SCC is a good candidate for additional investigation of response to checkpoint inhibitors.9-13 A recent phase I/II clinical trial demonstrated partial response in 41% of patients receiving the programmed cell death 1 (PD-1) inhibitor cemiplimab.14 Case reports in patients with XP and advanced SCC describe response with PD-1 inhibitors, including pembrolizumab.5,15-17 However, these reports do not provide a molecular mechanistic rationale for the use of PD-1 inhibitors specific to this patient population, nor do they provide support for the use of these agents for long-term disease management. We report stable disease for 2 years in response to pembrolizumab in a patient with XP and metastatic cutaneous SCC, along with molecular findings to support the use of PD-1 inhibitors in patients with XP. The case report was categorized as exempt by our institution’s institutional review board, and consent was not obtained because all information included is presented in a deidentified manner.
CASE REPORT
The patient, a product of a consanguineous marriage, initially presented at age 1 year with a benign skin lesion on her forehead. In the first 2 years of life, she developed additional premalignant lesions on her face and body, which recurred despite treatment with laser therapy. She was diagnosed with XP at age 2 years on the basis of clinical presentation and family history. Over the next several years, she developed multiple malignant cutaneous lesions involving her face and right conjunctiva, requiring iterative surgical resections. At age 7 years, she developed right-sided facial weakness and swelling. Magnetic resonance imaging of the brain demonstrated a 2.9 × 2.7 × 1.4 cm well-defined lesion arising from the body of the right sphenoid bone, involving the cavernous sinus, and encasing the right carotid artery; the lesion was concerning for metastatic SCC.
Two months later, the patient presented to Seattle Children’s Hospital with severe facial pain and cough. Repeat imaging demonstrated an infiltrative mass involving the right parotid and masticator space with bony involvement of the right mandible, as well as infiltration through the pterygopalatine fossa into the right orbital apex, cavernous sinus, and right middle cranial fossa. Extensive perineural spread along the right inferior alveolar nerve and right third through sixth cranial nerves was observed, in addition to leptomeningeal spread into the right aspect of the posterior fossa (Figs 1A to 1D).
FIG 1.
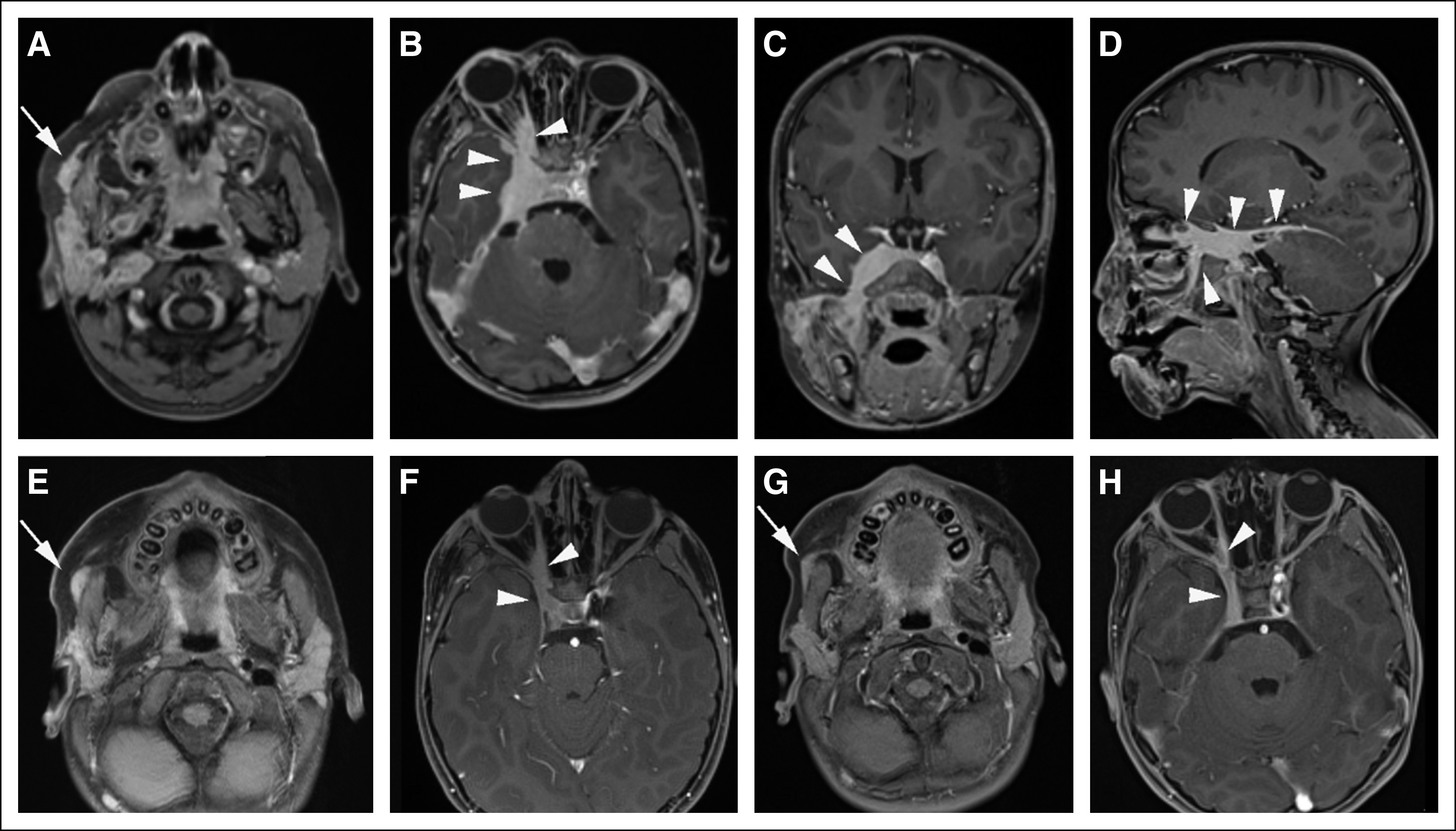
Perineural spread of squamous cell carcinoma (A to D) at time of diagnosis and (E to H) after therapy, shown by contrast-enhanced T1-weighted magnetic resonance imaging. (A) Axial image through face at level of parotid gland shows asymmetric nodular enhancement throughout right parotid gland, as well as more anterior nodular superficial enhancing tissue (arrow). (B) Axial, (C) coronal, and (D) sagittal images through head at level of cavernous sinus and right pterygopalatine fossa show extensive abnormal enhancing tissue within cavernous sinus, extending anteriorly into the right orbital apex, as well as posteriorly toward posterior fossa (arrowheads). (E and F) Five months after initiation of therapy, imaging shows dramatic reduction in tumor burden, both (E, arrow) in superficial tissues of face and (F, arrowheads) within cavernous sinus and orbital apex. (G and H) Thirteen months after initiation of therapy, disease burden had diminished further, both (G, arrow) in superficial tissues of the face and (H, arrowheads) within the cavernous sinus and orbital apex.
Biopsies of sites concerning for malignant skin lesions were performed, including separate left lower eyelid, right conjunctiva and cornea, and right preauricular masses; all were diagnostic of SCC. Biopsy of a right parotid lymph node revealed metastatic SCC. Tumor cells were macrodissected from the non-neoplastic lymphoid cells in the lymph node. Mutational analysis of tumor samples using the University of Washington Laboratory of Medicine UW-OncoPlex Cancer Gene Panel (University of Washington, Seattle, WA;)18 demonstrated ultramutated primary tumors and metastases (total mutation burden ranged from 53 to 460 mutations/Mb) with an excess of C>T and CC>TT low-frequency mutations (Fig 2A), characteristic of UV damage in the setting of nucleotide excision repair deficiency (Catalogue of Somatic Mutations in Cancer signature 7). The non-neoplastic lymphoid tissues were also tested and did not show any unexpected mutations (Data Supplement). Both tumors showed a significant strand bias (P < .001), concordant with the transcription-coupled repair function of the nucleotide excision repair complex (Fig 2B). Intersection of the filtered somatic variants from the tumors revealed that there were two independent primary tumors (conjunctival and corneal), which did not share any variants, as well as two independent metastases to the right parotid lymph node.
FIG 2.
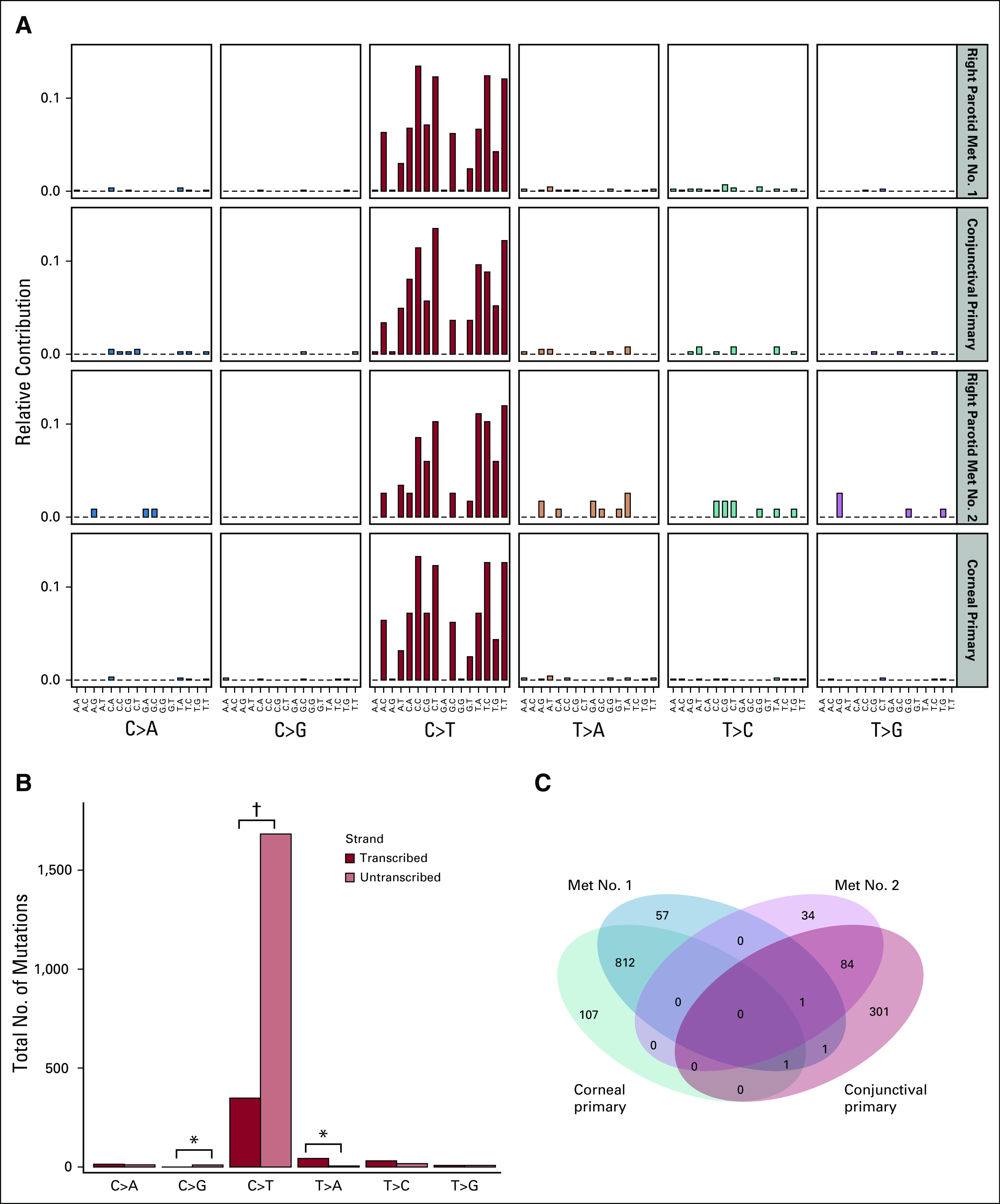
Mutation profile analysis of the corneal and conjunctival primary tumors, along with their parotid lymph node metastases (Met), was performed on identified somatic single nucleotide variants.19 (A) All four tumors show mutation patterns consistent with base excision repair deficiency (Catalogue of Somatic Mutations in Cancer mutation signature 7). (B) Analysis of mutation signatures based on the transcribed gene strand shows a strong bias of C>T mutations on the untranscribed strand (1,683 v 348 mutations on the transcribed strand), consistent with the transcription-coupled nature of the nucleotide excision repair pathway. (C) Comparison and set overlap of somatic variants identified in four sequenced samples reveals two unrelated primary tumors (corneal and conjunctival), each with unique metastases to the parotid lymph nodes. (*) P < .01. (†) P < .001.
Germline genetic testing found homozygous NM_004628.4 c.2116-1G>A in the XPC gene. There was no evidence of other well-described mechanisms of ultramutation, such as somatic POLE or POLD1 exonuclease domain proofreading mutations, in any tumor tested. There was also no evidence of microsatellite instability (MSI) in any tumor (all tumors were MSI negative) using the microsatellite instability by next-generation sequencing method.20
The case was reviewed at the Seattle Children’s Hospital multidisciplinary tumor board, which recommended against larger resection, based on extent of disease and anticipated morbidity, and against systemic therapy with traditional chemotherapeutic agents, because they have not demonstrated efficacy in advanced SCC. On the basis of encouraging reported results in MSI-high malignancies to immune checkpoint blockade, pembrolizumab (2 mg/kg intravenously every 3 weeks) was initiated 1 month after diagnosis, using the recent US Food and Drug Administration indication for MSI-high malignancies to support insurance approval.
After five cycles of pembrolizumab, follow-up imaging demonstrated a considerable decrease in tumor bulk and resolution of leptomeningeal disease along the right pons and cerebellum (Figs 1E to 1H). The patient experienced resolution of the chronic cough and reported substantial improvement in the facial pain (initially the pain required scheduled opioid medications, but they have now been discontinued without pain). Presently, the patient has received pembrolizumab for 24 months, with stable imaging findings for more than 18 months and no clinically significant adverse effects.
Although the patient’s oculocutaneous (conjunctival) lesions responded to therapy after incomplete resection, her right corneal tumor persisted and showed mild progression on clinical exam. Mutational analyses demonstrated that the ocular lesions had different mutations but a similar mutational burden compared with her metastatic tumor (Data Supplement). Mutations implicated in PD-1 resistance, including WNT, were not identified in analysis of the patient’s tumors.21-23 Topical fluorouracil, which is efficacious in cutaneous SCC associated with XP, was initiated in the affected eye, and no additional tumor progression was noted after 1 month of local treatment.
DISCUSSION
We report the case of a pediatric patient with XP who demonstrated long-term partial response of advanced cutaneous SCC to pembrolizumab with development of no clinically significant adverse effects. Although a small number of prior reports have discussed the use of checkpoint blockade for advanced SCC in the setting of XP, this report adds:
1. The molecular justification of the use of PD-1 inhibitors specific to this patient population;
2. Support for the use of PD-1 inhibitors in long-term disease management of advanced SCC, and;
3. Acknowledges the potential limitation of PD-1 inhibitors for the treatment of SCC involving immune-privileged sites, such as the cornea.
XP represents a deficit in DNA nucleotide excision repair, resulting in sensitivity to UV radiation. Loss of XPC (or XPE) is consistent with deficient global genomic repair (GGR) but relatively preserved transcription-coupled repair (TCR); this contrasts with loss of XPA, XPB, or XPD, which results in a GGR-negative/TCR-negative phenotype.24,25 The TCR-negative/GGR-positive mutational signature of XPC is demonstrated in this patient with a greater burden of C>T mutations on the untranscribed strand (Fig 2B). The novel report of our patient’s mutational signature enriches our understanding of XP and further supports the potential efficacy of immune checkpoint blockade with PD-1 inhibitors in the treatment of advanced SCC among patients with XP. Primarily, immune checkpoint inhibitors depend on neoantigen presentation via major histocompatibility complex 1. Theoretically, patients with an intact TCR, such as those with XPC mutations, would potentially have a less robust response to immune checkpoint inhibitors. Our experience disagrees with this, providing reassurance that this class of agents remains promising for this patient population. In addition, we were able to use molecular methods to demonstrate the coincidental development of two independent primary lesions with two paired metastatic lesions to better understand the origin of this patient’s metastatic disease.
This report describes long-term sustained stable disease, including response of significant burden of disease in the CNS. Of the existing published reports using PD-1 inhibitors in patients with XP, the longest reported time to follow-up was 18 months,16 with other studies describing therapy for no more than 1 year.5,15,17 Our patient’s sustained response supports the use of PD-1 inhibitors for long-term disease management in this setting. This is important because few options for alternative therapies exist in patients with advanced SCC that are promising for increasing progression-free survival. Moreover, PD-1 inhibitors have the potential to do this with minimal adverse effects, providing a quality-of-life benefit, which is an important consideration for individuals with advanced cancer.
Our patient’s right corneal lesion demonstrated discordant poor response. The cornea is immune privileged26 and does not have T-cell immunosurveillance, potentially limiting the ability of immune checkpoint blockade to treat malignancies in the cornea and other immune-privileged sites, such as the testis. This concept is particularly intriguing given the lack of response seen with immune checkpoint blockade in cisplatin-resistant testicular germ cell tumors.27 In conclusion, our experience and molecular findings support the use of PD-1 inhibitors in advanced cutaneous SCC, especially in patients with XP, and highlights the need for additional research.
APPENDIX
Molecular Methods
Tumor and germline samples were processed, sequenced, and analyzed using the University of Washington Laboratory Medicine UW-OncoPlex Cancer Gene Panel (University of Washington, Seattle, WA).18 Single nucleotide variants and indels in targeted regions were called from aligned BAM files using the Genome Analysis Toolkit (Broad Institute, Cambridge, MA) and the Variant Detection in Massively Parallel Sequencing Data—VarScan. Common artifacts and polymorphisms were filtered by limiting analyzed variants to those with low population frequency (not present in the Phase 3 1000 Genomes [http://phase3browser.1000genomes.org/index.html]; < 0.01%, 0.005%, and 0.1% frequency in the National Heart, Lung, and Blood Institute Exome Sequencing Project, Exome Aggregation Consortium, and an in-house database, respectively) and variant allele fraction greater than 1%. Germline variants were removed from all tumor samples. Mutation signatures and figures were created from selected variants using R and the MutationalPatterns package.19
Footnotes
Supported by T32 Training Grant No. 5T32CA009351-40 (A.S.) and a Damon Runyon/Sohn Pediatric Cancer Foundation Fellowship (J.F.S.).
AUTHOR CONTRIBUTIONS
Conception and design: Angela Steineck, Jay F. Sarthy, Colin C. Pritchard, Andrew W. Stacey, Nicholas A. Vitanza, Bonnie Cole
Financial support: Colin C. Pritchard
Administrative support: Bonnie Cole
Provision of study materials or patients: Teresa Chapman, Bonnie Cole
Collection and assembly of data: Angela Steineck, Colin C. Pritchard, Teresa Chapman, Bonnie Cole
Data analysis and interpretation: Angela Steineck, Niklas Krumm, Colin C. Pritchard, Teresa Chapman, Nicholas A. Vitanza, Bonnie Cole
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Niklas Krumm
Stock and Other Ownership Interests: Reference Genomics
Andrew W. Stacey
Honoraria: Immunocore
Travel, Accommodations, Expenses: Immunocore
No other potential conflicts of interest were reported.
REFERENCES
- 1. Kraemer KH, DiGiovanna JJ: Xeroderma pigmentosum, in Adam MP, Ardinger HH, Pagon RA, et al (eds): GeneReviews. Seattle, WA, University of Washington, 2003. [PubMed] [Google Scholar]
- 2.Bradford PT, Goldstein AM, Tamura D, et al. Cancer and neurologic degeneration in xeroderma pigmentosum: Long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48:168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarkowski A, III, Hare R, Loud P, et al. Systemic therapy in advanced cutaneous squamous cell carcinoma (CSCC): The Roswell Park experience and a review of the literature. Am J Clin Oncol. 2016;39:545–548. doi: 10.1097/COC.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 4.Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–3426. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 5.Deinlein T, Lax SF, Schwarz T, et al. Rapid response of metastatic cutaneous squamous cell carcinoma to pembrolizumab in a patient with xeroderma pigmentosum: Case report and review of the literature. Eur J Cancer. 2017;83:99–102. doi: 10.1016/j.ejca.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Sumiyoshi M, Soda H, Sadanaga N, et al. Alert regarding cisplatin-induced severe adverse events in cancer patients with xeroderma pigmentosum. Intern Med. 2017;56:979–982. doi: 10.2169/internalmedicine.56.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology: Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: Results from a single-arm, phase II study. J Clin Oncol. 2017;35:1542–1549. doi: 10.1200/JCO.2016.70.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang AL, Kim J, Luciano R, et al. A case report of unresectable cutaneous squamous cell carcinoma responsive to pembrolizumab, a programmed cell death protein 1 inhibitor. JAMA Dermatol. 2016;152:106–108. doi: 10.1001/jamadermatol.2015.2705. [DOI] [PubMed] [Google Scholar]
- 11.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase IB KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34:3838–3845. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falchook GS, Leidner R, Stankevich E, et al. Responses of metastatic basal cell and cutaneous squamous cell carcinomas to anti-PD1 monoclonal antibody REGN2810. J Immunother Cancer. 2016;4:70. doi: 10.1186/s40425-016-0176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevenson ML, Wang CQ, Abikhair M, et al. Expression of programmed cell death ligand in cutaneous squamous cell carcinoma and treatment of locally advanced disease with pembrolizumab. JAMA Dermatol. 2017;153:299–303. doi: 10.1001/jamadermatol.2016.5118. [DOI] [PubMed] [Google Scholar]
- 14.Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 15.Chambon F, Osdoit S, Bagny K, et al. Dramatic response to nivolumab in xeroderma pigmentosum skin tumor. Pediatr Blood Cancer. 2018;65:e26837. doi: 10.1002/pbc.26837. [DOI] [PubMed] [Google Scholar]
- 16.Salomon G, Maza A, Boulinguez S, et al. Efficacy of anti-programmed cell death-1 immunotherapy for skin carcinomas and melanoma metastases in a patient with xeroderma pigmentosum. Br J Dermatol. 2018;178:1199–1203. doi: 10.1111/bjd.16270. [DOI] [PubMed] [Google Scholar]
- 17.Hauschild A, Eichstaedt J, Möbus L, et al. Regression of melanoma metastases and multiple non-melanoma skin cancers in xeroderma pigmentosum by the PD1-antibody pembrolizumab. Eur J Cancer. 2017;77:84–87. doi: 10.1016/j.ejca.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 2014;16:56–67. doi: 10.1016/j.jmoldx.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blokzijl F, Janssen R, van Boxtel R, et al. MutationalPatterns: Comprehensive genome-wide analysis of mutational processes. Genome Med. 2018;10:33. doi: 10.1186/s13073-018-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salipante SJ, Scroggins SM, Hampel HL, et al. Microsatellite instability detection by next generation sequencing. Clin Chem. 2014;60:1192–1199. doi: 10.1373/clinchem.2014.223677. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24987110&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 22.Spranger S, Gajewski TF. A new paradigm for tumor immune escape: β-Catenin-driven immune exclusion. J Immunother Cancer. 2015;3:43. doi: 10.1186/s40425-015-0089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruizinga R, Scherer-Rath M, Schilderman JB, et al. The Life in Sight Application study (LISA): Design of a randomized controlled trial to assess the role of an assisted structured reflection on life events and ultimate life goals to improve quality of life of cancer patients. BMC Cancer. 2013;13:360. doi: 10.1186/1471-2407-13-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford JM. Regulation of DNA damage recognition and nucleotide excision repair: Another role for p53. Mutat Res. 2005;577:195–202. doi: 10.1016/j.mrfmmm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Hanawalt PC, Ford JM, Lloyd DR. Functional characterization of global genomic DNA repair and its implications for cancer. Mutat Res. 2003;544:107–114. doi: 10.1016/j.mrrev.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Medawar PB. Immunity to homologous grafted skin; the relationship between the antigens of blood and skin. Br J Exp Pathol. 1946;27:15–24. [PMC free article] [PubMed] [Google Scholar]
- 27.Adra N, Einhorn LH, Althouse SK, et al. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: A Hoosier Cancer Research Network Study GU14-206. Ann Oncol. 2018;29:209–214. doi: 10.1093/annonc/mdx680. [DOI] [PubMed] [Google Scholar]


