INTRODUCTION
Lung cancer is the leading cause of cancer-related death worldwide and is often caused by cigarette smoking. Many cases of non–small-cell lung cancer (NSCLC), particularly adenocarcinomas, harbor at least one oncogenic driver mutation, such as in epidermal growth factor receptor (EGFR) and rearranged anaplastic lymphoma kinase, which are potential targets for therapy. Immune checkpoint inhibitors recently have caused a paradigm shift in the treatment of various types of cancer. In NSCLC, antibodies that target programmed cell death 1 (PD-1) have demonstrated unprecedented durable clinical responses.1,2 Several biomarkers have been proposed, including programmed death-ligand 1 (PD-L1) on the surface of tumor cells,3 tumor mutational burden,4 and DNA mismatch-repair (MMR) deficiency.5
MMR proteins prevent the generation of insertions and deletions at microsatellites by repairing incorrectly paired nucleotides; therefore, MMR deficiency results in microsatellite instability (MSI) with a high tumor mutational burden.6 Clinically, PD-1 blockade was shown to be effective for MSI-high tumors, as these tumors generate neoantigens and can upregulate immunologic checkpoints, including PD-1, in tumor-associated lymphocytes.5,7,8 In NSCLC, little is known about the clinical course of MSI-high tumors because of their rare frequency (0.3% to 0.8%).9-11
Lynch syndrome is an autosomal-dominant inherited syndrome caused by germline variants in MMR genes.12,13 The disorder is associated with colorectal, endometrial, and other epithelial malignancies14; however, Lynch syndrome–associated NSCLC is rare. In a previous study, of 1,952 patients with lung cancer evaluated, 94 were either MSI-intermediate or -high, but none of the patients had Lynch syndrome.15 Only three cases of Lynch syndrome associated with lung cancer have been reported.16-18 Here, we report a case of Lynch syndrome–associated lung adenocarcinoma with intramedullary spinal cord metastasis (ISCM) in a patient who exhibited long-lasting shrinkage of his tumor over 20 subsequent treatment-free months after 15 administrations of nivolumab. Written informed consent was obtained from the patient.
CASE REPORT
A previously healthy 36-year-old man without a history of cigarette smoking presented with a mass in the right upper lung field on chest x-ray. Chest computed tomography revealed a mass in the right upper lobe (Fig 1A). He underwent right upper lobectomy and was diagnosed with pathologic stage IB (T2aN0M0) poorly differentiated lung adenocarcinoma (Figs 1B to 1C). The resected specimen was negative for EGFR mutation and anaplastic lymphoma kinase translocation as determined by droplet digital polymerase chain reaction and fluorescence in situ hybridization analyses, respectively. Immunohistochemical analysis of PD-L1 expression using the murine 22C-3 antibody revealed a tumor proportion score of 1% to 24%. Collection of the patient’s additional history revealed a family history of multiple primary cancers, including colorectal cancer in his sister at age 32 years, father at age 46 and 54 years, paternal uncle at age 40 years, and paternal grandmother at age 41 years, fulfilling the Amsterdam criteria.19 Because of his family history of colorectal cancer, we suspected Lynch syndrome and performed additional analyses. Immunohistochemistry analysis of the resected specimen revealed positive results for MLH2 and MSH6, but negative results for MLH1 or PMS2 in cancer cells (Fig 2A). Moreover, polymerase chain reaction with six microsatellite markers, including the National Cancer Institute panel of five microsatellite markers in the lung tumor and normal epithelium, revealed that DNA from the lung tumor was positive for MSI in three microsatellite markers (BAT40, BAT26, and D2S123; Fig 2B), indicating an MSI-high tumor. After detailed genetic counseling of the patient, direct sequencing for MMR genes using genomic DNA from the blood detected a germline variant in exon 19 of MLH1 (c.2180_2181del, p.His727Hisfs*5), which confirmed the diagnosis of Lynch syndrome associated with lung adenocarcinoma (Fig 2C).
FIG 1.
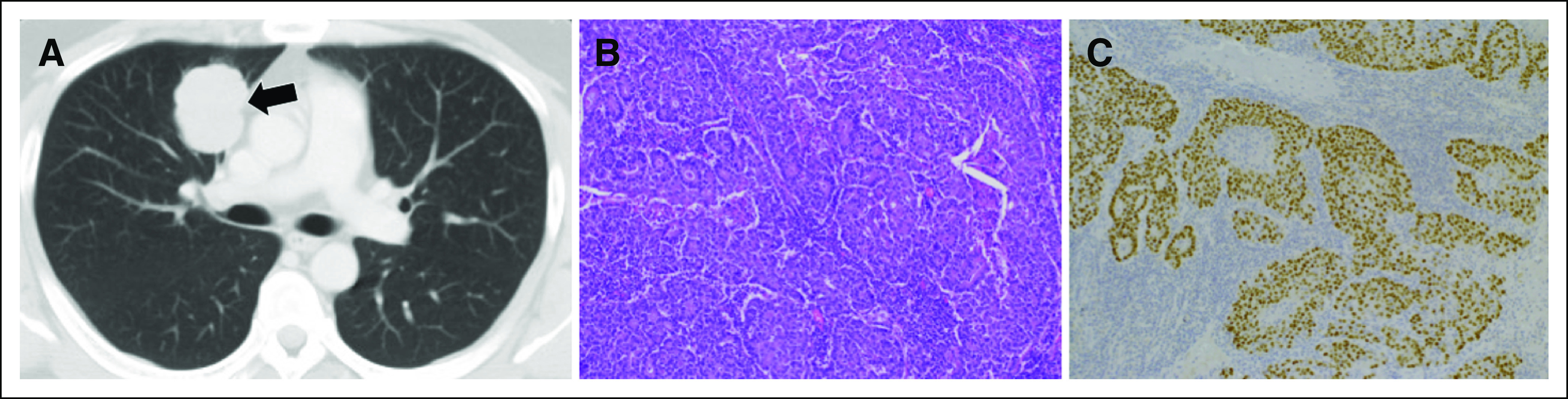
(A) Chest computed tomography revealing a mass in the right upper lobe (arrow). (B) Hematoxylin and eosin–stained biopsy specimen showing poorly differentiated adenocarcinoma (magnification, ×200). (C) Immunohistochemical analysis showing thyroid transcription factor-1–positive staining (magnification, ×200).
FIG 2.
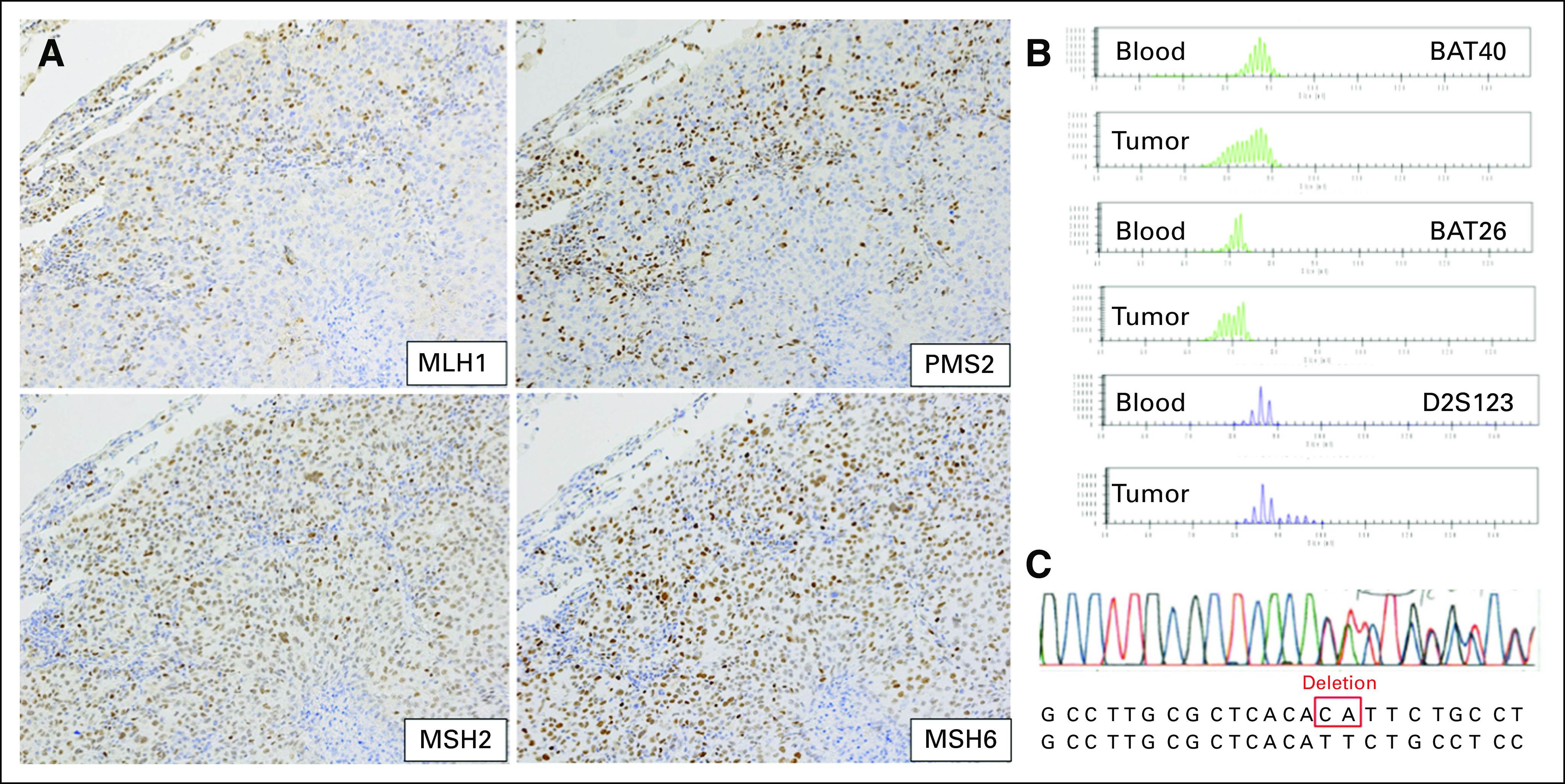
(A) Absence of MLH1 and PMS2 immunohistochemical staining in the tumor cell nuclei, and presence of MSH2 and MSH6 staining in the tumor cell nuclei (magnification, ×200). (B) Arrows depict aberrant peaks in tumorous tissues compared with that in the normal epithelium, indicating high microsatellite instability. (C) Sequence chromatogram containing the mutation (MLH1 c.2180_2181del, p.His727Hisfs*5). N, normal epithelium; T, tumor.
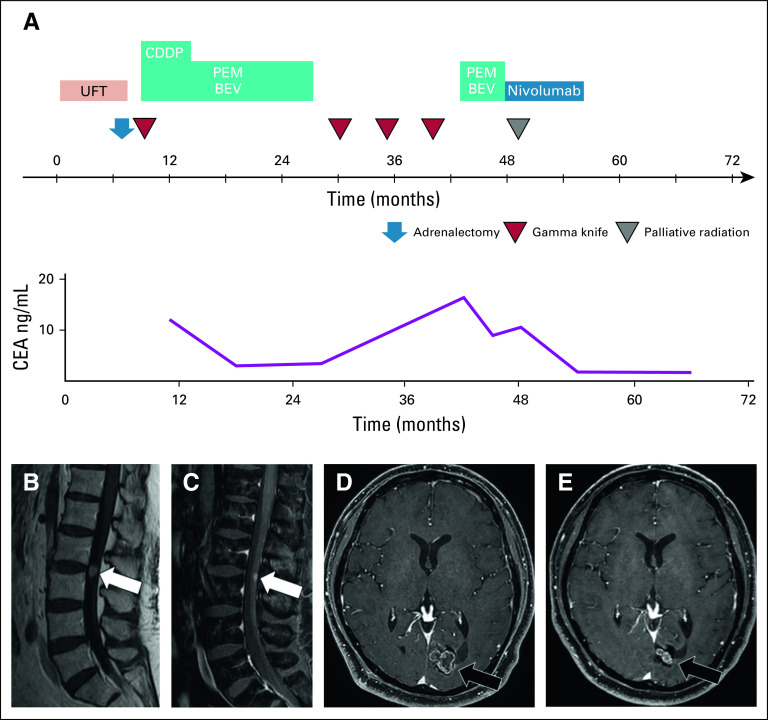
The timeline of the patient’s anticancer treatment is shown in Figure 3A. He was administered tegafur-uracil as adjuvant chemotherapy; however, after 6 months, metastasis of the left adrenal gland and right cerebral hemisphere was detected. He was then administered anticancer chemotherapy that consisted of cisplatin 75 mg/m2, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks after left adrenalectomy and gamma knife therapy for brain metastasis. After the completion of 4 cycles of the regimen, we conducted 16 cycles of continuous maintenance therapy with pemetrexed and bevacizumab. After discontinuation of this regimen, he underwent three rounds of gamma knife therapy because of recurrence in the brain metastases. After 15 months of discontinuation of maintenance therapy, the patient restarted the same regimen. After four cycles of this treatment, he developed back pain. Magnetic resonance imaging revealed ISCM at the L2 to L3 spinal level and the recurrence of brain metastasis in the left occipital lobe (Figs 3B and 3D). We began administration of nivolumab 3 mg/kg every 2 weeks, which was approved as a second-line treatment of patients with NSCLC in Japan, with palliative radiation therapy for ISCM. These therapies resulted in improvement of his back pain, ISCM, and brain metastasis (Figs 3C and 3E), and his carcinoembryonic antigen level was decreased. After 15 cycles of the regimen (30 weeks), treatment was discontinued upon the patient’s request. Twenty months after the discontinuation of treatment, the size of the tumor continued to shrink without evidence of systemic progression or elevated levels of carcinoembryonic antigen.
FIG 3.
(A) Clinical course after diagnosis of NSCLC. (B) Sagittal T1-weighted contrast-enhanced magnetic resonance imaging (MRI) showing a solitary mass (arrow) in the intramedullary spinal cord at the L2-L3 spinal level. (C) After 9 weeks of radiation and nivolumab treatment, sagittal T1-weighted contrast-enhanced MRI with fat suppression showed significant regression of the leptomeningeal metastasis (arrow). (D) Axial T1-weighted contrast-enhanced MRI showing brain metastasis in the left occipital lobe. (E) After 9 weeks of radiation and nivolumab treatment, axial T1-weighted contrast-enhanced MRI revealed regression of the brain metastasis. BEV, bevacizumab; CDDP, cisplatin; CEA, carcinoembryonic antigen; PEM, pemetrexed; UFT, tegafur-uracil.
DISCUSSION
We observed a nonsmoking, young male patient with suspected Lynch syndrome–associated lung adenocarcinoma diagnosed on the basis of his family history of colorectal cancer and germline genetic testing. PD-1 blockade therapy resulted in long-lasting shrinkage of his tumor, including ISCM.
In this case, Lynch syndrome–associated lung adenocarcinoma was diagnosed without preceding cancers of other organs. Only three cases of lung cancer associated with Lynch syndrome have been reported.16-18 One case was the Muir-Torre variant of Lynch syndrome with the loss of MSH2 expression,16 and the second was a germline variant in MSH2 in which the tumor was immunohistochemically deficient for MSH2 and MSH6 with MSI-high.17 The third case consisted of a rearrangement in the MSH2 germline gene, with the tumor immunohistochemically deficient for MSH2 and MSH6.18 These three patients were diagnosed with lung adenocarcinoma during follow-up for colorectal cancer, while our patient showed no history of any cancers. Germline mutations in EGFR and HER2,20 and rare variants of BRCA2,21 TP53,22 and STK11,23 are associated with an increased risk for lung cancer, despite the low incidence rate. Germline TP53 and STK11 variants have been reported in other familial cancer syndromes, such as Li-Fraumeni syndrome24 and Peutz-Jeghers syndrome,25 respectively. Thus, clinicians should be alert to the importance of family history, not only of lung cancer, but also of other cancers.
The case of Lynch syndrome–associated lung adenocarcinoma demonstrated long-lasting shrinking of the tumor as a result of an immune checkpoint inhibitor, even after discontinuation of the treatment. Previous studies of NSCLC have suggested that better prognostic factors for immune checkpoint inhibitors include high mutation burden, the molecular smoking signature, higher neoantigen burden, and DNA repair pathway mutations.26 In a recent study of MSI-high or MMR-deficient colorectal cancer, nivolumab demonstrated durable responses and disease control.8 Moreover, for MSI-high or MMR-deficient tumors without NSCLC, pembrolizumab showed an overall response rate of 39.6%, with the effects lasting for 6 months or longer in 78% of patients who achieved a response.7 A recent report similarly described a case of metastatic MMR-deficient lung adenocarcinoma in a patient with Lynch syndrome who experienced a prolonged response to nivolumab after discontinuation because of drug-induced interstitial lung disease.18 This patient had a 50-pack-year history of smoking, and the lung tumor decreased in size after treatment with nivolumab. In contrast, our patient had never smoked and responded to nivolumab against CNS metastases, including brain metastasis and ISCM. Immune checkpoint inhibitors may be effective options for patients with MSI-high NSCLC, such as Lynch syndrome.
In our patient, immune checkpoint inhibitor with radiation therapy for ISCM resulted in long-lasting shrinkage of his tumor. ISCM of NSCLC is rare but characterized by poor prognosis.27,28 Although the optimum treatment of ISCM of NSCLC remains unknown, surgical treatment,29 radiotherapy,28 and tyrosine kinase inhibitors30 may contribute to improving survival, whereas chemotherapy can be poorly effective for ISCM because of the low drug permeability through the blood–spinal cord barrier.31 Only one case of successful treatment has been reported for asymptomatic ISCM of NSCLC by nivolumab.32 The potential benefit of combining immune checkpoint inhibitors with radiotherapy has been demonstrated previously.33 Although the mechanism of the synergistic effect is not completely understood, upregulation of PD-L1 has been observed in tumor models after radiation therapy.34 Combination therapy with radiation and an immune checkpoint inhibitor may be effective for treating symptomatic ISCM of Lynch syndrome–associated lung adenocarcinoma.
In summary, we saw a patient with a rare case of Lynch syndrome–associated lung adenocarcinoma that showed a long-lasting response after treatment with nivolumab. Physicians should consider a patient’s family history to assess the likelihood of hereditary cancer syndromes. Moreover, immune checkpoint inhibitors may be an effective option for the treatment of Lynch syndrome–associated lung adenocarcinoma.
ACKNOWLEDGMENT
The authors thank Hiroshi Kawachi and Tomoyo Kakita for assistance with immunohistochemistry.
AUTHOR CONTRIBUTIONS
Conception and design: Keita Masuzawa, Takanori Asakura, Hiroyuki Yasuda, Ichiro Kawada, Sosuke Takaoka, Kenzo Soejima
Financial support: Kenzo Soejima
Administrative support: Hiroyuki Yasuda, Kenzo Soejima
Provision of study materials or patients: Ichiro Kawada, Yuichiro Hayashi, Takeshi Nakajima, Masami Arai, Kenzo Soejima
Collection and assembly of data: Keita Masuzawa, Takanori Asakura, Sosuke Takaoka, Takeshi Nakajima, Masami Arai, Kenzo Soejima
Data analysis and interpretation: Keita Masuzawa, Shinnosuke Ikemura, Yuichiro Hayashi, Koichi Fukunaga, Kenzo Soejima
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHOR' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kenzo Soejima
Honoraria: AstraZeneca, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, MSD Oncology, Eli Lilly, Novartis
Research Funding: Nippon Boehringer Ingelheim, Taiho Pharmaceutical, AstraZeneca
No other potential conflicts of interest were reported.
REFERENCES
- 1.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015;5:16–18. doi: 10.1158/2159-8290.CD-14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshleman JR, Lang EZ, Bowerfind GK, et al. Increased mutation rate at the hprt locus accompanies microsatellite instability in colon cancer. Oncogene. 1995;10:33–37. [PubMed] [Google Scholar]
- 7.Lemery S, Keegan P, Pazdur R. First FDA approval agnostic of cancer site: When a biomarker defines the indication. N Engl J Med. 2017;377:1409–1412. doi: 10.1056/NEJMp1709968. [DOI] [PubMed] [Google Scholar]
- 8.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takamochi K, Takahashi F, Suehara Y, et al. DNA mismatch repair deficiency in surgically resected lung adenocarcinoma: Microsatellite instability analysis using the Promega panel. Lung Cancer. 2017;110:26–31. doi: 10.1016/j.lungcan.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Warth A, Körner S, Penzel R, et al. Microsatellite instability in pulmonary adenocarcinomas: A comprehensive study of 480 cases. Virchows Arch. 2016;468:313–319. doi: 10.1007/s00428-015-1892-7. [DOI] [PubMed] [Google Scholar]
- 11.Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22:1342–1350. doi: 10.1038/nm.4191. [Erratum: Nat Med 23:1241, 2017; Nat Med 24:525, 2018] [DOI] [PubMed] [Google Scholar]
- 12.Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasen HF, Mecklin JP, Khan PM, et al. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC) Dis Colon Rectum. 1991;34:424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- 14.Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 15.Latham A, Srinivasan P, Kemel Y, et al. Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol. 2019;37:286–295. doi: 10.1200/JCO.18.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nolan L, Eccles D, Cross E, et al. First case report of Muir-Torre syndrome associated with non-small cell lung cancer. Fam Cancer. 2009;8:359–362. doi: 10.1007/s10689-009-9247-7. [DOI] [PubMed] [Google Scholar]
- 17.Canney A, Sheahan K, Keegan D, et al. Synchronous lung tumours in a patient with metachronous colorectal carcinoma and a germline MSH2 mutation. J Clin Pathol. 2009;62:471–473. doi: 10.1136/jcp.2008.063008. [DOI] [PubMed] [Google Scholar]
- 18.Kawashima Y, Nishikawa S, Ninomiya H, et al. Lung adenocarcinoma with Lynch syndrome and the response to nivolumab. Intern Med. 2019;58:1479–1484. doi: 10.2169/internalmedicine.1673-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 20.Lu S, Yu Y, Li Z, et al. EGFR and ERBB2 germline mutations in Chinese lung cancer patients and their roles in genetic susceptibility to cancer. J Thorac Oncol. 2019;14:732–736. doi: 10.1016/j.jtho.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, McKay JD, Rafnar T, et al. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet. 2014;46:736–741. doi: 10.1038/ng.3002. [Erratum: Nat Genet 49:651, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park KJ, Choi HJ, Suh SP, et al. Germline TP53 mutation and clinical characteristics of Korean patients with Li-Fraumeni syndrome. Ann Lab Med. 2016;36:463–468. doi: 10.3343/alm.2016.36.5.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez P, Carretero J, Medina PP, et al. Distinctive gene expression of human lung adenocarcinomas carrying LKB1 mutations. Oncogene. 2004;23:5084–5091. doi: 10.1038/sj.onc.1207665. [DOI] [PubMed] [Google Scholar]
- 24.Bachinski LL, Olufemi SE, Zhou X, et al. Genetic mapping of a third Li-Fraumeni syndrome predisposition locus to human chromosome 1q23. Cancer Res. 2005;65:427–431. [PubMed] [Google Scholar]
- 25.van Lier MG, Wagner A, Mathus-Vliegen EM, et al. High cancer risk in Peutz-Jeghers syndrome: A systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258–1264, author reply 1265. doi: 10.1038/ajg.2009.725. [DOI] [PubMed] [Google Scholar]
- 26.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conill C, Marruecos J, Verger E, et al. Clinical outcome in patients with intramedullary spinal cord metastases from lung cancer. Clin Transl Oncol. 2007;9:172–176. doi: 10.1007/s12094-007-0031-6. [DOI] [PubMed] [Google Scholar]
- 28.Potti A, Abdel-Raheem M, Levitt R, et al. Intramedullary spinal cord metastases (ISCM) and non-small cell lung carcinoma (NSCLC): Clinical patterns, diagnosis and therapeutic considerations. Lung Cancer. 2001;31:319–323. doi: 10.1016/s0169-5002(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 29.Dam-Hieu P, Seizeur R, Mineo JF, et al. Retrospective study of 19 patients with intramedullary spinal cord metastasis. Clin Neurol Neurosurg. 2009;111:10–17. doi: 10.1016/j.clineuro.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Wagner M, Besse B, Balleyguier C, et al. Leptomeningeal and medullary response to second-line erlotinib in lung adenocarcinoma. J Thorac Oncol. 2008;3:677–679. doi: 10.1097/JTO.0b013e3181757a8b. [DOI] [PubMed] [Google Scholar]
- 31.Gazzeri R, Galarza M, Neroni M, et al. Failure rates and complications of interspinous process decompression devices: A European multicenter study. Neurosurg Focus. 2015;39:E14. doi: 10.3171/2015.7.FOCUS15244. [DOI] [PubMed] [Google Scholar]
- 32.Phillips KA, Gaughan E, Gru A, et al. Regression of an intramedullary spinal cord metastasis with a checkpoint inhibitor: A case report. CNS Oncol. 2017;6:275–280. doi: 10.2217/cns-2017-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]