Abstract
Experiential negative symptoms—including diminished motivation—have a profound impact on functional outcomes in schizophrenia. Animal research suggests that abnormalities in dopaminergic regulation can negatively impact effort exertion, a translational model that has been applied to individuals with schizophrenia. Paradigms that assess effort-based decision making, for example, suggest less likelihood of choosing high effort tasks that are high in probability of success, and this preference varies with negative symptoms and impaired functioning. Although asociality is another well-documented component of experiential negative symptoms, it is unclear whether diminished motivation for monetary reward extends to the social domain. To test this question, the authors designed the Social Vigor Task (SVT)—a measure of effort exertion in the context of live social encouragement. They further examined the effect of oxytocin, a neuropeptide implicated in social behavior, on vigor. Forty-two individuals with schizophrenia and 43 healthy controls completed the SVT twice: once after intranasal administration of saline placebo and again after oxytocin. Both groups showed similar increases in vigor in response to social encouragement, suggesting effort in the social context is spared in schizophrenia. Group differences in the effect of social encouragement on vigor varied by point-based reward rate and trial length. Oxytocin did not increase vigor during social encouragement in either group. Within the schizophrenia group, clinician-rated passive social withdrawal, but not active social avoidance, was negatively associated with vigor. Results suggest that people with schizophrenia show normative levels of effort in the context of social encouragement; low approach motivation, however, relates to lower effort.
Keywords: schizophrenia, social motivation, effort, oxytocin
General Scientific Summary
Research has shown that people with schizophrenia are less likely than healthy controls to choose tasks that require high effort, particularly when probability of success is high. Less is known about effort exertion in social contexts. This study suggests that effort exertion in the context of social encouragement is generally spared, though related to clinician-rated approach motivation, in schizophrenia.
Motivational impairment—a key component of the experiential domain of negative symptoms—drives a large portion of the psychosocial impairment present in schizophrenia (SZ; Foussias & Remington, 2010). Nonetheless, motivation is a complex, multifaceted construct that is difficult to measure accurately. There has been growing interest in recent years in the development of measures designed to circumvent the limitations posed by self-report instruments, or resource intensive interview-based measures, to more objectively and efficiently capture motivation deficits (Green, Horan, Barch, & Gold, 2015). Standard self-report or interview measures rely on retrospective reporting of motivation, which is biased by current mood state and memory impairment. In addition, these measures typically combine different aspects of motivation, such as anticipatory and consummatory pleasure, and often do not consider the impact of availability of reinforcements in the person’s environment. Development of objective, standardized measures has the potential to vastly improve our understanding of the key components of motivation deficits in SZ and provide meaningful outcome measures for clinical trials.
Recent advances in measure development have capitalized on translational findings that parse out the component parts of motivation, including liking (hedonic experience of reward), wanting (reward seeking), and learning (incorporating information from previous experience to drive current goal-directed behavior; Salamone & Correa, 2012; Treadway & Zald, 2011). Evidence suggests people with SZ have the capacity for emotional experience (i.e., liking; (Cohen & Minor, 2010; Gard, Kring, Gard, Horan, & Green, 2007), but have difficulty using prior affective experience to drive goal-directed behavior (i.e., wanting and learning; (Heerey & Gold, 2007). Measures have been developed in recent years to more objectively and specifically assess deficits in wanting and learning as drivers of goal-directed behavior. One such set of promising measures addresses effort-based decision making, or the willingness to exert physical and mental effort for reward (Fervaha et al., 2013; Reddy et al., 2015; Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009). These measures typically use money as reward, and operationalize low motivation as the tendency to choose tasks that require lower mental or physical effort, and which result in smaller rewards.
The promise in these measures is supported by findings linking a preference for low-effort, low-reward tasks with poorer occupational functioning and lower intrinsic motivation in SZ (Barch, Treadway, & Schoen, 2014; Horan et al., 2015). Findings regarding the role of negative symptoms in effort-based decision making have been inconsistent (Fervaha et al., 2013; Gold, Waltz, & Frank, 2015; McCarthy, Treadway, Bennett, & Blanchard, 2016). These inconsistencies may be due to differences in assessment method (e.g., retrospective report of clinical interviews, conflation of expressive and experiential deficits in some measures), or due to the impact of other explanatory variables, including the effects of antipsychotic medication (Gold et al., 2015). Nonetheless, there appears to be a growing body of evidence in favor of the convergent validity of effort-based decision-making paradigms.
One important potential extension of this work is the development of measures to assess motivation for social rewards. Asociality, the term used to describe an additional key component of experiential negative symptoms of SZ, refers to a lack of motivation to engage in social interaction and/or a preference for being alone. Much of the evidence for asociality in SZ has been derived from self-report and interview-based assessments that combine Items assessing hedonic experience of social interaction (i.e., social anhedonia) with objective indicators of social connection (e.g., number of friends/acquaintances; Blanchard, Mueser, & Bellack, 1998; Chapman, Chapman, & Raulin, 1976). Social anhedonia is considered a robust psychological risk factor for the development of SZ (Kwapil, 1998; Kwapil et al., 2009). Findings of social anhedonia in SZ and analog samples have been supported by experience-sampling (e.g., Ecological Momentary Assessment) and behavioral studies. People high in social anhedonia, for example, report spending more time alone, a preference for being alone, and higher positive affect when alone (L. H. Brown, Silvia, Myin-Germeys, & Kwapil, 2007; Kwapil et al., 2009). They also may show less affiliative behaviors in controlled role-play settings (Llerena, Park, Couture, & Blanchard, 2012). Although asociality due to low desire for social connection is present in some people with SZ, there is emerging evidence that many people with SZ express a desire for more social connection (Gard, Sanchez, Starr, et al., 2014), and patients report improving social outcomes as a key goal in treatment (Byrne, Davies, & Morrison, 2010). In addition, contrary to findings in people with elevated social anhedonia, the experience of positive affect in relation to daily interactions is associated with increased social engagement in people with diagnosed SZ (Granholm, Ben-Zeev, Fulford, & Swendsen, 2013).
Despite reported desire, recent findings continue to suggest that not only do people with SZ set goals that require less effort, but they also are less likely to set goals that are social in nature (Gard, Sanchez, Cooper, et al., 2014; Gard, Sanchez, Starr, et al., 2014). It remains unclear whether asociality is driven by a general lack of interest in interpersonal relationships, or rather, consistent with the effort-based decision-making literature, people with SZ-like social interaction but show difficulty connecting this desire with motivated, goal-directed behavior. Negative symptoms, particularly asociality and anhedonia, might interfere with the drive to form and maintain social bonds. Positive symptoms (viz., paranoia), on the other hand, might prevent social connection through misinterpretation of social cues. A better understanding of the degree to which people with SZ are responsive to social encouragement can help us better design interventions focused on enhancing interpersonal functioning.
Additional contributors to social impairment in SZ are neurocognition and, more specifically, social cognition (Addington & Addington, 2000; Addington, McCleary, & Munroe-Blum, 1998). Social cognition includes abilities such as theory of mind/mentalizing, affect recognition, and causal attributions about the self and others (Penn, Sanna, & Roberts, 2007). Deficits in social cognition predict substantial variance in social functioning impairment (Couture, Penn, & Roberts, 2006; Fett et al., 2011). Research suggests that social–cognitive deficits lead to poorer functional outcome through their negative influence on motivation (Gard, Fisher, Garrett, Genevsky, & Vinogradov, 2009; Green, Hellemann, Horan, Lee, & Wynn, 2012). Nonetheless, although clearly important for understanding skills and abilities in the social domain, it is unknown whether impairments in social cognition are informative in regards to willingness to exert effort in the service of forming or maintaining social bonds in SZ.
Despite the importance of clarifying contributors to social impairment in SZ, there is surprisingly little research examining objective indicators of drive for social connection. Recent studies examining motivation in individuals high in social anhedonia suggest diminished responsivity to social, but not monetary, reward (Xie et al., 2015); however, tasks that purportedly measure social motivation either use stimuli that are only social inasmuch as they include cartoon images or photographs of faces (e.g., social incentive delay task; (Spreckelmeyer et al., 2009)). These tasks shed light on individual differences in the experience of social stimuli as rewarding (i.e., the hedonic, liking quality), but may not tell us much about the extent to which this liking translates into effort exertion in social contexts. As there are currently no available measures designed to assess effort in the context of social reinforcement, a primary aim of the current study was to develop a task that incorporated social encouragement as reinforcement for effortful behavior.
A secondary goal of this study was to test the effects of a manipulation of the oxytocin system—a neuropeptide implicated in social behavior—on effort in the context of social encouragement. Given its presence on receptors that populate the ventral tegmental area and nucleus accumbens (Skuse & Gallagher, 2009), oxytocin is believed to interact with dopamine to influence social salience (Shamay-Tsoory & Abu-Akel, 2016; Theodoridou, Penton-Voak, & Rowe, 2013). For example, dopamine has been shown to modulate the effect of exogenous oxytocin on responsivity of reward regions of the brain during presentation of social stimuli (Montag, Sauer, Reuter, & Kirsch, 2013). Thus, it has been postulated that activation of oxytocin increases the salience of social stimuli through its influence on the dopaminergic system (Groppe et al., 2013; Rademacher, Schulte-Rüther, Hanewald, & Lammertz, 2017). Because of the clear role dopamine plays in effort-based decision making and motivated behavior (Arias-Carrión & Pöppel, 2007; Berridge & Robinson, 1998; Salamone, Correa, Farrar, & Mingote, 2007), it is possible that exogenous oxytocin, through its interaction with dopamine, might increase effortful behavior in social contexts. As such, an additional goal of the current study was to determine the extent to which oxytocin might influence vigor during social encouragement, in relation to an absence of social encouragement, as would be implied by theories suggesting the impact of oxytocin on social approach behavior (Kemp & Guastella, 2011; Scheele et al., 2013).
The role of the oxytocin system in the etiology and course of SZ and other disorders characterized by social impairment (e.g., autism, anxiety) has seen a surge in research in recent years (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Feifel et al., 2010; Labuschagne et al., 2010). Some of this research was prompted by animal models suggesting that manipulation of the oxytocin system could serve as a useful model for understanding positive and negative symptoms of SZ (for a review, see Feifel, Shilling, & MacDonald, 2016). Early work in humans, however, showed no difference in endogenous oxytocin levels between people with SZ and healthy controls (HCs; Glovinsky, Kalogeras, Kirch, Suddath, & Wyatt, 1994). Other studies since have shown associations between lower endogenous oxytocin levels and negative symptoms (Keri, Kiss, & Kelemen, 2008; Sasayama et al., 2012). In an examination of the association between endogenous oxytocin and social approach and avoidance behavior in SZ, Brown and colleagues (E. C. Brown et al., 2014) found that patients with higher levels of oxytocin were quicker to avoid angry faces. This avoidance was positively correlated with PANSS positive symptoms and self-reported paranoia. Despite the above findings, the extent to which peripheral measures of endogenous oxytocin are informative in regard to functioning of the oxytocin system in the brain is unclear (see (McCullough, Churchland, & Mendez, 2013; Szeto et al., 2011). As such, findings regarding differences in blood plasma levels of oxytocin should be interpreted with caution.
Findings regarding the impact of exogenous (i.e., intranasally delivered) oxytocin on symptoms, cognition, and other outcomes in SZ are decidedly mixed. Although the state of the knowledge suggests that exogenous oxytocin does not impact positive or negative symptoms in SZ (Oya, Matsuda, Matsunaga, Kishi, & Iwata, 2016; Williams & Bürkner, 2017), some studies have found that oxytocin may serve to enhance higher-level social–cognitive functions, such as mentalizing (Bradley & Woolley, 2017; Feifel et al., 2016; J. D. Woolley et al., 2014). It is important to note that age, sex, medication status, and even early life experiences (e.g., attachment) can all moderate the effects of oxytocin on social behavior (Bradley & Woolley, 2017). Given the theory that oxytocin may enhance social salience, it makes sense that it can facilitate both affiliative and hostile reactions, depending on the context (Bartz, Zaki, Bolger, & Ochsner, 2011; Tabak, 2013). Despite variable findings, the oxytocin system is clearly implicated in human and mammal social behavior to some degree and is an important area of inquiry in SZ, given characteristic social impairments (Rosenfeld, Lieberman, & Jarskog, 2010).
Drawing on the above findings, in the current study we administered the Social Vigor Task (SVT; described below) to examine effort in the context of social reward in SZ. The SVT was administered twice to each participant: once in the context of placebo, and once following intranasal administration of oxytocin, using a within-subjects, double-blind crossover design. Our primary hypotheses were that (a) people with SZ would exert significantly less effort in the context of social encouragement than would HCs and (b) oxytocin would increase effort during social encouragement in both groups. A secondary hypothesis was that people with SZ would exert less effort than HCs during longer (vs. shorter) trials and during lower (vs. higher) reward rate trials. In regards to within-group predictions, we hypothesized that (a) lower perceived social affiliation, as measured by self-reported empathy and attachment, would be associated with less effort during social encouragement in both groups; (b) higher social cognition, as measured by accuracy of lie/sarcasm detection, would be associated with higher effort during social encouragement in both groups; and (c) higher positive and negative symptoms would be associated with less effort during social encouragement in the SZ group.
Method
Participants
Participants were 42 individuals with SZ and 43 age- and gender-matched HCs. These individuals volunteered for a study on social processes in SZ and were recruited through outpatient facilities in the San Francisco Bay Area and through online advertisements. Thirty-four (81%) of the SZ participants were taking antipsychotic medications. Chlorpromazine equivalents were computed using a standardized conversion table (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010). See Table 1 for demographic and clinical characteristics of the two groups. The University of California, San Francisco Human Research Protection Program approved all study procedures (Protocol #10–02262). Written informed consent was obtained from each participant and all were compensated.
Table 1.
Sample Characteristics
| Group | |||||
|---|---|---|---|---|---|
| Healthy controls (n = 43) | Schizophrenia (n = 42) | ||||
| Characteristic | M | SD | M | SD | p |
| Demographics | |||||
| Age | 41.30 | 14.57 | 42.74 | 14.88 | .65 |
| Gender (% male) | 67.4% | 73.8% | .42 | ||
| Race (% non-Caucasian) | 62.8% | 52.4% | .94 | ||
| Years education | 15.58 | 1.95 | 13.64 | 2.44 | <.001 |
| Medication status | |||||
| Typical AP (%) | n/a | 7.1% | n/a | ||
| Atypical AP (%) | n/a | 61.9% | n/a | ||
| Typical and atypical AP (%) | n/a | 11.9% | n/a | ||
| Chlorpromazine equivalent | n/a | 206.68 | 231.42 | n/a | |
| Clinical ratings | |||||
| PANSS positive symptoms | n/a | 15.31 | 6.01 | n/a | |
| PANSS negative symptoms | n/a | 13.81 | 5.28 | n/a | |
| PANSS disorganized thought | n/a | 10.88 | 3.40 | n/a | |
| Self-reports | |||||
| EQ | 45.98 | 9.97 | 38.37 | 12.02 | .02 |
| ECR—Global Avoidance | 2.93 | .94 | 2.69 | 1.28 | .33 |
| ECR—Global Anxiety | 2.07 | 1.07 | 2.48 | 1.29 | .12 |
| Social cognitiona | |||||
| TASIT—Lie (placebo) | .82 | .10 | .78 | .13 | .11 |
| TASIT—Sarcasm (placebo) | .74 | .17 | .66 | .19 | .10 |
| TASIT—Lie (oxytocin) | .82 | .12 | .77 | .12 | .11 |
| TASIT—Sarcasm (oxytocin) | .77 | .15 | .70 | .15 | .09 |
Note. AP = antipsychotics; ECR = Experience in Close Relationships = Scale; EQ = Empathy Quotient; PANSS = Positive and Negative Syndrome Scale; TASIT = The Awareness of Social Inference Test.
Schizophrenia group, n = 28; healthy controls, n = 31.
Measures
SVT.
The SVT was designed to assess effort exertion for reward in the context of social encouragement and nonsocial reinforcement. Our aim in the development of the SVT was to assess motivated behavior that differed from existing effort-based decision-making tasks (e.g., Effort Expenditure for Rewards Task [Treadway et al., 2009]; deck choice task [Kool, McGuire, Rosen, & Botvinick, 2010]; grip effort task [Cléry-Melin et al., 2011]; balloon effort task [Gold et al., 2013]) in two important ways. For one, given our goal of assessing social motivation, the primary manipulation was the presence of social encouragement. We developed a standard set of positive statements (e.g., “Good,” “Keep going!,” “Awesome. You’re doing great!”). In half the trials, trained research assistants (RAs) delivered these statements at 10 second intervals with increasing intensity across task completion while they sat beside the participant. RAs were instructed to give encouragement even when participants were not engaged in the task. In the other half of trials (points only), the RA quietly filled out study forms on a clipboard. To provide participants with some indication of task progress, and to increase motivation for task completion, reward was presented in both conditions: points accumulated on the screen as participants rapidly pressed the key. Participants were instructed that the number of points earned was associated with social adeptness (as determined in previous studies). Explicit mention of what number of points was considered “good” was purposefully omitted as, consistent with motivation intensity theory (Brehm, 1999), research suggests that uncertainty of performance can increase effort mobilization for tasks with moderate difficulty (Brinkmann, Franzen, Rossier, & Gendolla, 2014).
Each trial displayed the number of seconds remaining (indicating if it was a short [30-s] or long [60-s] trial), and whether it was a trial with low (“50 presses = 10 points”) or high (“5 presses = 20 points”) reward rate. Our rationale for varying trial length and reward rate was based on models of vigor and reinforcement learning that suggest that vigor is influenced by the opportunity cost of time and the rate of experienced reward, both of which are moderated by tonic levels of dopamine (Guitart-Masip, Beierholm, Dolan, Duzel, & Dayan, 2011; Niv, Daw, Joel, & Dayan, 2007). In varying trial length, we aimed to examine the extent to which fatigue (or predicted fatigue) influenced vigor, and the potential moderating influence of social encouragement, and how this varied by group. Thirty and 60 s were chosen based on pilot testing of the task, in which users experienced these lengths as subjectively short and long, respectively. Similarly, we varied reward rate to examine the extent to which higher rates might increase vigor (and lower rates decrease vigor), and how the effect of reward rate on vigor might vary by social context and between groups.
A second primary difference from existing effort-based decision-making tasks was that we minimized decision making requirements that might tax various cognitive resources (e.g., attention, working memory, executive function), eliminating the forced-choice nature of these tasks—participants were not required to select from a hard or easy task, nor were they presented with information on probability of winning or amount of potential reward. We did this to both minimize the cognitive burden on participants with SZ, who are known to experience these deficits, as well in an attempt to isolate pure motivation intensity, or vigor, from the more cognitively complex decision-making process.
Study procedures were kept as standardized as possible across participants. Each participant was consented by the RA who administered the task. They were also provided instructions from this RA for computer tasks that preceded the SVT. A different RA completed clinical assessments in the week prior to task administration (see below). The larger study included a total of roughly 2–3 hours of testing, and the SVT occurred at the beginning and end of each session.
We implemented a systematic process to ensure all RAs were sufficiently trained and kept to the detailed script provided, while maintaining as much believability as possible in delivering encouragement. The script included a list of 12 prompts for each 10-s interval. RAs were trained to advance through the list to cover the available prompts across the different trials. The script included built-in prompts to reflect increasing intensity of encouragement. For example, one prompt at 50 s remaining would be “You’re off to a good start.” At 20 seconds remaining this would advance to “Great!” By 10 s remaining the prompt would intensify to “Wow, amazing!” RAs were trained not only on how to deliver the prompts in a standard way across time and participants, but also in how much emphasis and enthusiasm to display at each increasing level of encouragement over time. In regard to timing, the SVT includes a countdown timer display. RAs followed this display to ensure consistent timing of prompt delivery. The training process involved (a) watching several videotaped examples, (b) at least three practice rounds with a trained RA serving as subject, and (c) independent filming of one full practice session, viewed by trained study staff and examined for quality control. Only once all the three steps had been successfully completed were RAs cleared to run participants. There was also regular review of videotaped sessions by study staff to ensure adherence to the protocol.
Clinical ratings.
Diagnoses were based on a Structured Clinical Interview for the DSM–IV–TR (First, Spitzer, Gibbon, & Williams, 1995). Discussion of diagnostic consensus occurred in groups of masters-level interviewers on a weekly basis. All participants were in good general health, had no neurological disorders or substance dependence within the last 6 months, and had a negative urine toxicology test at each visit. SZ participants were on a stable dose of psychiatric medications for at least one month and throughout the study. HCs were excluded for any self-reported personal or family history of psychosis or bipolar disorder. Clinical symptoms were assessed at a separate session in the week prior to the first testing session using the Positive and Negative Syndrome Scale (PANSS; (Kay, Fiszbein, & Opler, 1987). We included factor analytically derived positive and negative symptom scores in the analyses (Marder, Davis, & Chouinard, 1997). The SZ group, as a whole, was clinically stable: roughly 62% were functioning at a level better than “mildly ill” (based on links between the Clinical Global Impression and PANSS total score; see Leucht et al., 2005); the remaining 38% of participants were considered “mildly ill.”
Self-report measures of attachment and empathy.
Participants completed the following individual difference measures designed to assess social affiliation: (a) the Empathy Quotient (EQ; Baron-Cohen & Wheelwright, 2004; Lawrence, Shaw, Baker, Baron-Cohen, & David, 2004) and (b) the Experience of Close Relationships scale (ECR; Brennan, Clark, & Shaver, 1998). The EQ is a 60-item questionnaire designed to measure empathy. Participants select how strongly they agree or disagree with a list of statements (e.g., “I really enjoy caring for other people;” “It doesn’t bother me too much if I am late meeting a friend”). People with autism spectrum disorders (Baron-Cohen & Wheelwright, 2004) and those with SZ (Bora, Gökçen, & Veznedaroglu, 2008) have been shown to score significantly lower than HCs on this measure. The ECR assesses attachment anxiety (i.e., sensitivity to rejection and/or abandonment) and avoidance (i.e., discomfort with, and avoidance of, interpersonal closeness). Participants are asked to report how much they agree or disagree with each item on a seven-point scale. Questions cover relationships with family members, romantic partners, and close friends. Attachment anxiety as measured by the ECR has been found to moderate the effect of oxytocin on recollections of maternal care (e.g., Bartz et al., 2010). Both the EQ and ECR were included in the current study as individual difference variables that might explain variance in social motivation on the SVT.
Social and nonsocial cognition.
Social cognition was measured using The Awareness of Social Inference Test (TASIT; McDonald, 2002). We included the Lie and Sarcasm subscales as tests of mentalizing in the current study, given the potential associations with social motivation. Participants view short video clips of human social interaction and are asked to identify the presence of lying or sarcasm through a series of follow up questions. The primary outcome is the proportion of correct responses across the trials. Because the TASIT was implemented partway through the current study, data are available and presented on 31 HC and 28 SZ participants. Working memory was assessed using the letter-number sequencing task (Wechsler, 1997).
Procedures
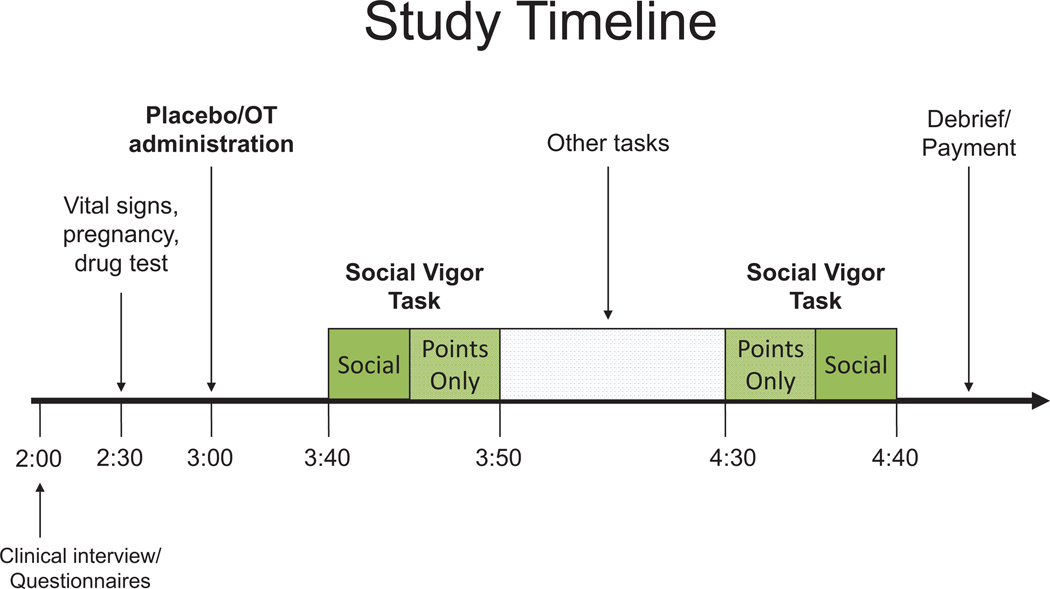
The SVT was completed twice in the context of a randomized, double-blind, placebo-controlled cross-over study design, with the two testing days separated by at least one week (median number of days between sessions = 11). Participants were presented with 56 trials of the SVT on each testing day. For each session, they were presented with four separate 5-min blocks (two of which are presented back-to-back), each with one set of trials of social and points-only conditions, for a total of 20 min spent on the task each testing day (see Figure 1). Order presentation for the social/points-only blocks was randomly selected and counterbalanced. All participants were presented with eight short/six long (trial length), and eight low/six high (reward rate) trials in each block.
Figure 1.
Study timeline. The Social Vigor Task included a total of 56 trials on each testing day. See the online article for the color version of this figure.
At the beginning of each testing day, 40 IU of oxytocin (Syntocin, Novartis, Switzerland) or saline placebo was self-administered via nasal spray. Insufflations were alternated every 15 s between each nostril over 5 min (Feifel et al., 2010). Intranasally administered oxytocin travels to the brain through the olfactory and trigeminal nerves, with passive diffusion into the cerebrospinal fluid through the nasal epithelium (Veening & Olivier, 2013). Previous work suggests that oxytocin delivered intranasally begins to have physiological effects within 30 min and lasts for at least 90 min (Norman et al., 2011). As such, we initiated behavioral testing 30 min postadministration and completed within 120 min in the current study.
We chose a dosage of 40 IU of oxytocin as we have used this dosage in previous published studies and found to be safe and well-tolerated (J. D. Woolley et al., 2014, 2017; Josh D. Woolley et al., 2016). Most published studies of intranasally administered oxytocin in humans involve doses between 20 and 40 IU (Mac-Donald et al., 2011). Importantly, a review of 38 oxytocin administration trials concluded that doses between 18 and 40 IU are undetectable by participants and produce no consistent side effects or adverse outcomes in controlled research settings (Mac-Donald et al., 2011).
Data Analyses
Because the SVT measures outcomes repeatedly within participants (i.e., 56 trials across eight blocks), we used generalized estimating equations (GEEs) to accommodate for correlations among the observations originating from the same participant. Normal distribution GEEs were used to model continuous responding of the outcome variable (key presses). Unstructured correlation matrices were chosen based on no a priori theoretical assumptions about specific temporal dependencies among observations. For outcomes, vigor was defined as average key presses per second during task completion. Significant interactions were followed up with pairwise comparisons using Sidak correction. Outcomes are presented as parameter estimates and differences in beta values for pairwise comparisons. We included group (SZ, control) as a between-subjects factor. Reward type (social, points-only), reward rate (low, high), and trial length (30 s, 60 s) were included as within-subjects factors. To account for order and practice/fatigue effects, we included trial number and Session Day (1 or 2) as covariates in all models.
To explore the fundamental question of whether people with SZ were less willing than HCs to exert effort in the context of social encouragement, we first conducted analyses focused on the placebo day only. Then, to determine whether OT increased effort exertion in the context of social encouragement across groups, we examined task performance during placebo and OT, including drug (oxytocin vs. placebo) as an additional within-subjects factor in the model. We included tests of interactions in our models to further explore group- and condition-level findings.
Demographic variables (i.e., age, gender, race and ethnicity), self-report forms, symptom measures, and social and nonsocial cognitive scores were examined as correlates of changes in vigor using correlation and regression, separately by group. As done in Bartz et al. (Bartz et al., 2010), we group-mean centered the ECR Attachment Anxiety and Avoidance scales and examined their associations (including their interaction) with difference in vigor on the SVT between OT and placebo days, and between social and points-only trials. Demographic variables and scores on the EQ, TASIT, and letter-number sequencing were also examined as correlates of change in vigor between OT and placebo days, and between social and points-only trials. Any significant associations among these measures and change in vigor on the SVT were explored as covariates in GEE models.
We conducted sets of sensitivity and exploratory analyses. As a sensitivity analysis, we examined potential gender differences in task performance, including gender of the RA as a potential moderator of outcomes. We also examined the association between specific negative symptoms (passive/apathetic social withdrawal and active social avoidance from the PANSS) and task performance in the SZ group as an exploratory analysis.
Results
Groups did not differ in age, gender, race, or ethnicity. As consistent with other studies of community-dwelling participants, the SZ group had lower personal education than the HC group. Among the self-report measures, the SZ group reported significantly lower empathy on the EQ, but no significant differences on the ECR (see Table 1).
Associations Between Vigor and Demographic Variables, Empathy, Attachment, and Social and Nonsocial Cognition
Participant age, education, ethnicity, and race were unrelated to either the difference in vigor between social and points-only trials (rs range = −0.17 – 0.18, ps = > 0.05) or to change in vigor from placebo to OT day (rs range = 0.05 – 0.07, ps = > 0.05) on the SVT. Participant gender was significantly positively associated with change in vigor from placebo to OT day, but only in the SZ group, r = .44, p <.001. Gender was thus included as a covariate in the GEE model examining drug effects. Self-reported empathy (EQ) and attachment (ECR) were unrelated to changes in vigor. In addition, neither the TASIT subscales nor the letter-number sequencing tasks were associated with changes in vigor.
Group Differences in Vigor
Results from GEE analyses on placebo day are displayed in Table 2. Groups did not differ in overall vigor (p = .179): On average, HC participants pressed the keys approximately 112 times, and SZ approximately 111 times, per 30-s trial (average for 60-s trials: HC = 229, SZ = 222). There was a significant main effect of reward type (social vs. points-only). Across groups, participants displayed higher vigor during social encouragement than during points-only trials (p < .001). There were no main effects of reward rate or trial length. Across groups and reward conditions, vigor was equal during low and high rate trials and long and short trials.
Table 2.
Generalized Estimating Equations—Placebo Day
| Variable | b | SE | p |
|---|---|---|---|
| Trial number | −.006 | .002 | .001 |
| Testing day | −.096 | .154 | .534 |
| Group (HC, SZ) | −.228 | .169 | .179 |
| Reward type (points-only, social) | −.086 | .022 | <.001 |
| Reward rate (low, high) | −.057 | .030 | .062 |
| Trial length (short, long) | −.024 | .021 | .258 |
| Group × Reward Type | .187 | .053 | <.001 |
| Group × Reward Rate | .108 | .098 | .269 |
| Group × Trial Length | −.002 | .051 | .969 |
| Group × Reward Type × Trial Length | −.152 | .054 | .005 |
| Group × Reward Type × Reward Rate | −.185 | .051 | <.001 |
Note. HC = healthy control; SZ = schizophrenia.
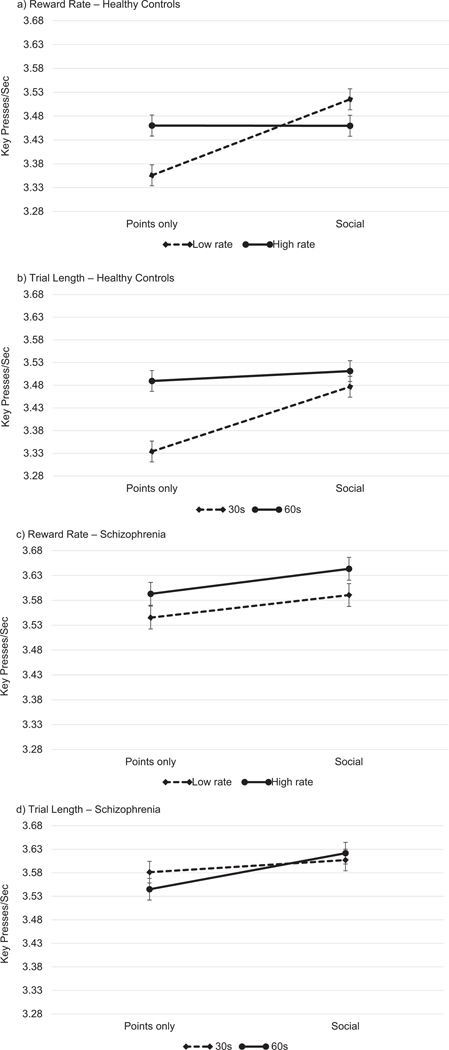
To explore whether group performance on the SVT varied by reward type, trial length, or reward rate, we included the interaction terms in the model. Although there was a significant Group × Reward Type interaction, planned pairwise comparisons revealed that both SZ (b = −0.052, p <.05) and HC (b = −0.067, p <.05) participants displayed more vigor during social trials than during points-only trials. There were no interactions between Group and Trial Length and Reward Rate. A significant three-way interaction among Group, Reward Type, and Reward Rate suggested that social encouragement significantly increased vigor during low rate trials only in the HC group (b = −0.159, p <.05; see Figure 2a); in the SZ group, social encouragement significantly increased vigor during high rate trials only (b = 0.059, p <.05) (see Figure 2c). An additional significant 3-way interaction among group, reward type, and trial length suggested social encouragement significantly increased vigor during short (30 s) trials only in the HC group (b = −0.143, p <.05; see Figure 2b); in the SZ group, social encouragement significantly increased vigor during long (60 s) trials only (b = 0.079, p <.05; see Figure 2d).
Figure 2.
The impact of social encouragement on vigor as a function of reward rate (low vs. high) and trial length (30 s vs. 60 s).
Effects of Oxytocin on Vigor
We then tested a GEE model with drug (oxytocin vs. placebo) included as an additional within-subjects factor. This analysis revealed no main effect of drug (b = 0.018, p <.721), nor was there a Group × Drug interaction (b = 0.063, p <.330). Drug also did not interact with reward rate (b = −0.004, p <.969) or trial length (b = 0.046, p <.590). There was a significant Drug × Reward Type interaction (b = −0.077, p <.005). A visual inspection of marginal means suggested a difference in vigor between social and points-only trials that was present during placebo (Ms = 3.65 for social trials vs. 3.57 for points-only trials), but not during oxytocin (Ms = 3.64 for social trials vs. 3.62 for points-only trials); however, none of the post hoc pairwise comparisons were significant (ps = 0.115–0.127).
Sensitivity Analysis
Because of the potential for gender differences in both response to, and delivery of, social encouragement, as well as in the effects of oxytocin, we ran two sets of sensitivity analyses using the parallel GEE models above, but with only male participants and female research assistants delivering encouragement (ns: HC = 29, SZ = 26). The placebo day analysis revealed that both the main effect of Reward Type (b = −0.066, p <.05) and the Group × Reward Type interaction (b = 0.115, p 0.05) remained significant. The Group × Reward Type × Reward Rate interaction was similar in magnitude to the primary analysis but did not reach significance (b = 0.100, p <.06), likely due to reduced power. The Group × Reward Type × Reward Length remained significant (b = −0.23, p <.05). The analysis including oxytocin also resulted in no main effect of drug, nor were there any drug interactions.
Associations Between Vigor and Symptom Ratings, Medication, and Working Memory in SZ
Neither chlorpromazine medication equivalent levels, nor presence of typical versus atypical antipsychotics, were related to changes in vigor. Changes in vigor were also unrelated to working memory or PANSS negative symptoms across all trial conditions (r’s range = −0.25 to 0.29). There was, however, a significant negative association between PANSS positive symptoms and the social encouragement-points-only difference on vigor during placebo day (r = −0.33, p < 0.05). That is, higher positive symptoms were associated with a diminished impact of social encouragement on vigor during the placebo day. Given this finding, we included PANSS positive symptoms in the GEE models in two ways (based on previous studies examining the association between symptoms and effort-based decision making; see Horan et al., 2015): one as a dimensional covariate, and another as a factor based on a median split. In these models, PANSS positive symptoms were not significant predictors of vigor on the SVT.
Exploratory Analyses: Links Between Vigor and Specific Symptoms
To examine more specific associations between symptoms and task outcomes in the SZ group, we conducted exploratory analyses with data from the placebo only testing session. Given the lack of specificity in negative symptom assessment of the PANSS, we included two specific symptoms that tap into social/motivational processes: passive/apathetic social withdrawal and active social avoidance. In these analyses, higher passive/apathetic social withdrawal was associated with significantly less overall vigor on the SVT (b = −0.35, p <.001), whereas active social avoidance was not significantly related to vigor (b = −0.09, p =.14). These exploratory results suggest that diminished approach motivation influenced performance on the SVT among people with SZ.
Discussion
Our primary goal in the current study was to develop a test of social motivation focused on objectively measured individual differences in effort exertion (i.e., vigor) in a social context. We also sought to examine potential differences in vigor between people with SZ and HCs. Finally, we explored the potential moderating role of exogenous oxytocin on vigor in these groups.
Groups did not differ in overall vigor. Although a burgeoning literature suggests abnormalities in effort-based decision making in the context of monetary reward in people with SZ (Barch et al., 2014; Gold et al., 2015; Horan et al., 2015), it may be that continuously reinforced effortful behavior, such as that assessed in the SVT using both points and social encouragement, is spared in SZ. There were also no group differences in the impact of social encouragement on vigor—both groups increased their vigor in the social context. The latter finding suggests that the SVT was successful in manipulating effort in a social context, and may serve as a useful objective measure of social motivation. Importantly, this also suggests that people with SZ may not show impairment in at least one aspect of social motivation—effort in the context of encouragement—a finding that may help improve our understanding of contributors to asociality and social functioning impairments in the disorder.
A secondary hypothesis was that people with SZ would exert less effort than HCs during trials that were longer and associated with a lower reward rate, given these trials were ostensibly more costly (i.e., providing less reinforcement and requiring longer continuous effort exertion). Groups did not differ in vigor by reward rate or trial length, suggesting that there was no general tendency to exert less effort when task demands varied. It is possible that the social context attenuated potential costs of effort exertion in these trials, even when there was no active social encouragement. That is, the fact that the RA was present during task completion, even when not delivering encouragement, could have minimized the effect of task demands on vigor. It is also possible that our manipulations of task demands were not robust enough to elicit higher perceived costs.
There were, however, group differences in vigor based on reward rate and trial length as a function of social encouragement. That is, the impact of social encouragement on increased vigor was specific to low reward rate trials in HCs; conversely, the impact of social encouragement on increased vigor was specific to high reward rate trials in participants with SZ. In addition, the impact of social encouragement on increased vigor was specific to short trials in HCs, and to long trials in participants with SZ. It is important to note that while these interactions were significant, the effects were in the same direction in both groups, and the group differences were small. As such, it would be important to replicate these findings in future studies.
If findings are replicated, there are potential clinical implications. The fact that people with SZ increased vigor in the social context to the same extent as HCs suggests that augmenting monetary or “earning-based” reward with social encouragement may be particularly effective in increasing effortful behavior in interventions designed to improve motivation deficits in SZ. It may also be the case that social encouragement is particularly effective for people with SZ when less sustained effort is required (or when performance-based rewards are constant and bountiful). Direct, high intensity social reinforcement may serve to influence choices to exert effort during task-based learning, such as in cognitive remediation for SZ (Medalia & Saperstein, 2011; Silverstein, 2010). Incorporating continuous and sufficient social encouragement as delivered in the current study in such interventions could increase effort exertion and associated benefit of practice/rehearsal.
An additional hypothesis was that oxytocin would increase social motivation in both groups given its purported role in modulating expression of mesolimbic dopamine (Love, 2014). We hypothesized, more specifically, that this effect would be particularly strong in the SZ group, given previous findings linking oxytocin administration with reductions in negative symptoms and improved social cognition (Feifel et al., 2010; J. D. Woolley et al., 2014). We found no main effect of drug on vigor in either group, suggesting oxytocin did not influence overall vigor. Although there was a significant interaction between drug and reward type (social vs. points-only), a lack of any significant post hoc pairwise comparisons precludes interpretation of the potential effect of oxytocin on vigor in the context of social encouragement. It is possible that the potential impact of oxytocin on vigor is subtle and would emerge if tested in a larger sample.
Examination of potential confounds and correlates of vigor indicated that perceived social affiliation, as measured by self-reported empathy and attachment, was unrelated to vigor in the social context. Items covered in these scales, which assess more general affiliative tendencies and experiences in current relationships, may not be reflective of effort-based social motivation. Interestingly, participant gender was significantly positively associated with the difference in vigor between oxytocin and placebo days in the SZ group. That is, oxytocin increased vigor, but only in females with SZ. Previous literature suggests sexual dimorphism in both levels of endogenous oxytocin and in the effects of exogenous oxytocin administration on behavior (Kramer, Cushing, Carter, Wu, & Ottinger, 2004; Toufexis, Davis, Hammond, & Davis, 2005). Given we had fewer females than males in the SZ group, it is possible that we may have seen group differences in drug effects with more female participants. Nonetheless, GEE models revealed no significant main effect of drug, nor an interaction between drug and reward type; as such, it is impossible to interpret a potential gender difference in the effect of oxytocin on social motivation. Future research with larger samples of female participants could help elucidate the potential for this association.
Although overall levels of positive and negative symptoms in the SZ group were unrelated to vigor, an exploratory analysis revealed that passive/apathetic social withdrawal was associated with significantly less vigor across task conditions. Examination of this PANSS item reveals that it covers not only social withdrawal, but also general levels of approach motivation (i.e., the extent to which withdrawal results in neglect of activities of daily living). That passive/apathetic social withdrawal was associated with less vigor suggests diminished approach motivation (e.g., lack of initiative) in SZ may limit drive to engage in effortful behavior. The lack of association between vigor on the SVT and overall positive and negative symptoms is not particularly surprising, given the low levels of positive symptoms in our sample, as well as the fact that the PANSS does not adequately cover the experiential domain of negative symptoms. Although one might expect an association between active social avoidance and vigor, a possible explanation is the restricted range on that item—the mean score was 1.90, with only two participants scoring above a 3 (mild); the mean score for passive/apathetic social withdrawal, on the other hand, was 2.69, with 10 participants scoring a 4 (moderate) or 5 (moderately severe). Another interpretation is that, unlike diminished approach motivation, heightened avoidance motivation may not interfere with effortful behavior.
Limitations of the current study should be mentioned. Although a strength of the SVT is its potentially higher ecological validity than other computer-based tasks of effort-based decision making and exertion, with this higher ecological validity comes less control over standardization of stimulus delivery. Research staff were thoroughly trained on administering the task, and they followed a standard script of reinforcing statements delivered at specified times. Nonetheless, the delivery of encouragement could have been subtly influenced by unobserved interaction dynamics between the participant and the experimenter. Although the sensitivity analysis including only female RAs and male participants showed generally the same primary findings, we were underpowered to detect potential influences of gender match (i.e., male RAs with male participants, female RAs with female participants) given the majority of pairs consisted of a female RA and male participant. Another limitation is that we did not include comprehensive measures of negative symptoms (as in, e.g., the Clinical Assessment Interview for Negative Symptoms [CAINS; Kring, Gur, Blanchard, Horan, & Reise, 2013) or psychosocial functioning in the current study.
One explanation for the finding of more vigor during social encouragement trials is that demand characteristics were at play. Demand characteristics are defined as the extent to which knowledge of the experimenters’ hypothesis leads a participant to perform in a manner designed to confirm the hypothesis (Orne, 2009). Although it is possible that increases in vigor occurred to at least some extent because participants were aware that our goal was to manipulate vigor, we do not believe this possibility undermines the validity of the task as a measure of social motivation. Increased vigor in response to encouragement could have been due to a number of participant motivations, including a desire to gain experimenter approval, a fear of negative evaluation, or simply wanting to be a “good” participant. Yet any of these reasons can be considered socially motivated. In research directly testing demand characteristics, positive attitudes toward the experiment or experimenter influence whether a subject exhibits these characteristics, and this effect is independent of individual differences in socially desirable responding, suggesting it is more about a desire to affiliate (or avoid censure) than it is about conforming to social norms (Nichols & Maner, 2008; Silverman, Shulman, & Wiesenthal, 1970). As such, if increased vigor occurred because of demand characteristics, we still believe this increase was socially motivated.
We aim to test additional variations of the SVT in future research, and to include a more refined symptom and functional assessment battery. Inclusion of negative feedback, for example, could improve our understanding of sensitivity to punishment/rejection in SZ, and the extent to which within- and between-groups differences in task performance are associated with varying types of social functioning impairments (e.g., low approach motivation vs. high avoidance motivation). It would also be informative to include a condition in which the RA is not present in the testing room, so as to minimize social influence on participant behavior even when active encouragement is not provided. To address discrepant findings between the current study and other studies of motivation in SZ, future research should directly compare effort-based decision making in the context of continuous versus delayed reinforcement, as well as the potentially differential impact of social encouragement on effort between these reinforcement contingencies. In addition, although average key press rate per second serves as a straightforward indicator of vigor, modeling within-trial changes in key press rate (i.e., slowing down, speeding up in relation to encouragement) would provide more information about the temporal associations between encouragement and effort. Inclusion of a comprehensive assessment of negative symptoms, such as the CAINS, could help identify more robust symptom-based correlates of task performance variability that may have not been captured by the PANSS. In regard to ecological validity, the social manipulation could have been more realistic by incorporating reinforcement based on successful completion of a task or activity one would more readily encounter in their daily life (e.g., positive feedback delivered following successful completion of an activity of daily living). Finally, data on psychosocial functioning may have also improved our understanding of the impact of task performance on real-world outcomes. In particular, the use of Ecological Momentary Assessment could provide a more sensitive index of daily psychosocial functioning outcomes (e.g., presence and enjoyment of social interactions, participation in occupations).
In sum, in the current study we developed an objective measure of social motivation, including an ecologically meaningful manipulation of social encouragement that influenced vigor in both participants with SZ and HCs. Participants with SZ did not show an overall deficit in effortful behavior, nor did they differ from HCs in their response to social encouragement. Group differences in the relative associations of reward rate and trial length on social motivation suggest directions for future research. As we continue to improve our understanding of the multifaceted nature of motivational deficits in SZ, work from the current study suggests certain domains of motivation (e.g., effort in the context of social encouragement) may be spared. Future studies should evaluate the impact of similar types of social encouragement on daily occupational goals, or in the context of interventions such as social skills training, cognitive remediation, or social cognition and interaction training. Such studies might help elucidate the influence of social motivation on task efforts with more direct implications for treatment and social and occupational functioning outcomes.
Acknowledgments
In the past 3 years, Michael Treadway has served as a paid consultant to Boston Consulting Group, NeuroCog Trials, Avanir Pharmaceuticals, and Blackthorn Therapeutics. Michael Treadway is a coinventor of the Effort Expenditure for Rewards Task, which is discussed in this review. Emory University and Vanderbilt University licensed this software to BlackThorn Therapeutics. Under the IP Policies of both universities, Michael Treadway Treadway receives licensing fees and royalties from BlackThorn Therapeutics. Additionally, Michael Treadway Treadway has a paid consulting relationship with BlackThorn. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors.
We thank Jennifer Akazawa, Ellen Bradley, and Win Huynh for their help coordinating this study, and all of the participants for their time and effort. We also thank Charles Carver, David Gard, and Kim Mueser for their thoughtful comments on earlier versions of this article.
Contributor Information
Daniel Fulford, Departments of Occupational Therapy and Psychological & Brain Sciences, Boston University.
Michael Treadway, Department of Psychology, Emory University.
Joshua Woolley, San Francisco Veterans Affairs Medical Center, San Francisco, California, and University of California, San Francisco.
References
- Addington J, & Addington D (2000). Neurocognitive and social functioning in schizophrenia: A 2.5 year follow-up study. Schizophrenia Research, 44, 47–56. 10.1016/S0920-9964(99)00160-7 [DOI] [PubMed] [Google Scholar]
- Addington J, McCleary L, & Munroe-Blum H (1998). Relationship between cognitive and social dysfunction in schizophrenia. Schizophrenia Research, 34, 59–66. 10.1016/S0920-9964(98)00079-6 [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, & Ho B-C (2010). Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biological Psychiatry, 67, 255–262. 10.1016/j.biopsych.2009.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Carrión O, & Poˇppel E (2007). Dopamine, learning, and rewardseeking behavior. Acta Neurobiologiae Experimentalis, 67, 481–488. [DOI] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, & Schoen N (2014). Effort, anhedonia, and function in schizophrenia: Reduced effort allocation predicts amotivation and functional impairment. Journal of Abnormal Psychology, 123, 387–397. 10.1037/a0036299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, & Wheelwright S (2004). The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders, 34, 163–175. 10.1023/B:JADD.0000022607.19833.00 [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, & Ochsner KN (2011). Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences, 15, 301–309. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, & Lydon JE (2010). Effects of oxytocin on recollections of maternal care and closeness. Proceedings of the National Academy of Sciences of the United States of America, 107, 21371–21375. 10.1073/pnas.1012669107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28, 309–369. 10.1016/S0165-0173(98)00019-8 [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Mueser KT, & Bellack AS (1998). Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophrenia Bulletin, 24, 413–424. 10.1093/oxfordjournals.schbul.a033336 [DOI] [PubMed] [Google Scholar]
- Bora E, Gökçen S, & Veznedaroglu B (2008). Empathic abilities in people with schizophrenia. Psychiatry Research, 160, 23–29. 10.1016/j.psychres.2007.05.017 [DOI] [PubMed] [Google Scholar]
- Bradley ER, & Woolley JD (2017). Oxytocin effects in schizophrenia: Reconciling mixed findings and moving forward. Neuroscience and Biobehavioral Reviews, 80, 36–56. 10.1016/j.neubiorev.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm JW (1999). The intensity of emotion. Personality and Social Psychology Review, 3, 2–22. 10.1207/s15327957pspr0301_1 [DOI] [PubMed] [Google Scholar]
- Brennan KA, Clark CL, & Shaver PR (1998). Self-report measurement of adult romantic attachment: An integrative overview In Simpson JA & Rholes WS (Eds.), Attachment theory and close relationships (pp. 46–76). New York, NY: Guilford Press. [Google Scholar]
- Brinkmann K, Franzen J, Rossier C, & Gendolla GHE (2014). I don’t care about others’ approval: Dysphoric individuals show reduced effort mobilization for obtaining a social reward. Motivation and Emotion, 38, 790–801. 10.1007/s11031-014-9437-y [DOI] [Google Scholar]
- Brown EC, Tas C, Kuzu D, Esen-Danaci A, Roelofs K, & Brüne M (2014). Social approach and avoidance behaviour for negative emotions is modulated by endogenous oxytocin and paranoia in schizophrenia. Psychiatry Research, 219, 436–442. 10.1016/j.psychres.2014.06.038 [DOI] [PubMed] [Google Scholar]
- Brown LH, Silvia PJ, Myin-Germeys I, & Kwapil TR (2007). When the need to belong goes wrong: The expression of social anhedonia and social anxiety in daily life. Psychological Science, 18, 778–782. 10.1111/j.1467-9280.2007.01978.x [DOI] [PubMed] [Google Scholar]
- Byrne R, Davies L, & Morrison AP (2010). Priorities and preferences for the outcomes of treatment of psychosis: A service user perspective. Psychosis: Psychological, Social and Integrative Approaches, 2, 210–217. 10.1080/17522430903456913 [DOI] [Google Scholar]
- Chapman LJ, Chapman JP, & Raulin ML (1976). Scales for physical and social anhedonia. Journal of Abnormal Psychology, 85, 374–382. http://dx.doi.org/10.1037/0021–843X.85.4.374 [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, & Schultz RT (2012). The social motivation theory of autism. Trends in Cognitive Sciences, 16, 231–239. 10.1016/j.tics.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cléry-Melin M-L, Schmidt L, Lafargue G, Baup N, Fossati P, & Pessiglione M (2011). Why don’t you try harder? An investigation of effort production in major depression. PLoS ONE, 6(8), e23178. 10.1371/journal.pone.0023178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, & Minor KS (2010). Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophrenia Bulletin, 36, 143–150. 10.1093/schbul/sbn061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture SM, Penn DL, & Roberts DL (2006). The functional significance of social cognition in schizophrenia: A review. Schizophrenia Bulletin, 32(Suppl. 1), S44–S63. 10.1093/schbul/sbl029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, . . . Hadley A (2010). Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry, 68, 678–680. 10.1016/j.biopsych.2010.04.039 [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, & MacDonald K (2016). A review of oxytocin’s effects on the positive, negative, and cognitive domains of schizophrenia. Biological Psychiatry, 79, 222–233. 10.1016/j.biopsych.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, & Remington G (2013). Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. Journal of Psychiatric Research, 47, 1590–1596. 10.1016/j.jpsychires.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Fett A-KJ, Viechtbauer W, Dominguez MD, Penn DL, van Os J, & Krabbendam L (2011). The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neuroscience and Biobehavioral Reviews, 35, 573–588. 10.1016/j.neubiorev.2010.07.001 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1995). Structured clinical interview for DSM–IV axis I disorders—Patient ed. (SCID-I/P, Version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Foussias G, & Remington G (2010). Negative symptoms in schizophrenia: Avolition and Occam’s razor. Schizophrenia Bulletin, 36, 359–369. 10.1093/schbul/sbn094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Fisher M, Garrett C, Genevsky A, & Vinogradov S (2009). Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophrenia Research, 115, 74–81. 10.1016/j.schres.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, & Green MF (2007). Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93(1–3), 253–260. 10.1016/j.schres.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, & Vinogradov S (2014). Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? Journal of Abnormal Psychology, 123, 771–782. 10.1037/abn0000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Starr J, Cooper S, Fisher M, Rowlands A, & Vinogradov S (2014). Using self-determination theory to understand motivation deficits in schizophrenia: The ‘why’ of motivated behavior. Schizophrenia Research, 156, 217–222. 10.1016/j.schres.2014.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glovinsky D, Kalogeras KT, Kirch DG, Suddath R, & Wyatt RJ (1994). Cerebrospinal fluid oxytocin concentration in schizophrenic patients does not differ from control subjects and is not changed by neuroleptic medication. Schizophrenia Research, 11, 273–276. 10.1016/0920-9964(94)90021-3 [DOI] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, & Frank MJ (2013). Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biological Psychiatry, 74, 130–136. 10.1016/j.biopsych.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, & Frank MJ (2015). Effort cost computation in schizophrenia: A commentary on the recent literature. Biological Psychiatry, 78, 747–753. 10.1016/j.biopsych.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Ben-Zeev D, Fulford D, & Swendsen J (2013). Ecological Momentary Assessment of social functioning in schizophrenia: Impact of performance appraisals and affect on social interactions. Schizophrenia Research, 145, 120–124. 10.1016/j.schres.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, & Wynn JK (2012). From perception to functional outcome in schizophrenia: Modeling the role of ability and motivation. JAMA Psychiatry, 69, 1216–1224. 10.1001/archgenpsychiatry.2012.652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Horan WP, Barch DM, & Gold JM (2015). Effort-based decision making: A novel approach for assessing motivation in schizophrenia. Schizophrenia Bulletin, 41, 1035–1044. 10.1093/schbul/sbv071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, Gründer G, & Spreckelmeyer KN (2013). Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biological Psychiatry, 74, 172–179. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Beierholm UR, Dolan R, Duzel E, & Dayan P (2011). Vigor in the face of fluctuating rates of reward: An experimental examination. Journal of Cognitive Neuroscience, 23, 3933–3938. 10.1162/jocn_a_00090 [DOI] [PubMed] [Google Scholar]
- Heerey EA, & Gold JM (2007). Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology, 116, 268–278. 10.1037/0021-843X.116.2.268 [DOI] [PubMed] [Google Scholar]
- Horan WP, Reddy LF, Barch DM, Buchanan RW, Dunayevich E, Gold JM, . . . Green MF (2015). Effort-based decision-making paradigms for clinical trials in schizophrenia: Part 2—external validity and correlates. Schizophrenia Bulletin, 41, 1055–1065. http://dx.doi.org/10.1093/schbul/sbv090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, & Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13, 261–276. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Kemp AH, & Guastella AJ (2011). The role of oxytocin in human affect a novel hypothesis. Current Directions in Psychological Science, 20, 222–231. 10.1177/0963721411417547 [DOI] [Google Scholar]
- Kéri S, Kiss I, & Kelemen O (2008). Sharing secrets: Oxytocin and trust in schizophrenia. Social Neuroscience, 4, 287–293. 10.1080/17470910802319710 [DOI] [PubMed] [Google Scholar]
- Kool W, McGuire JT, Rosen ZB, & Botvinick MM (2010). Decision making and the avoidance of cognitive demand. Journal of Experimental Psychology: General, 139, 665–682. 10.1037/a0020198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, & Ottinger MA (2004). Sex and species differences in plasma oxytocin using an enzyme immunoassay. Canadian Journal of Zoology, 82, 1194–1200. 10.1139/z04-098 [DOI] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, & Reise SP (2013). The clinical assessment interview for negative symptoms (CAINS): Final development and validation. The American Journal of Psychiatry, 170, 165–172. 10.1176/appi.ajp.2012.12010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR (1998). Social anhedonia as a predictor of the development of schizophrenia-spectrum disorders. Journal of Abnormal Psychology, 107, 558–565. 10.1037/0021-843X.107.4.558 [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Silvia PJ, Myin-Germeys I, Anderson AJ, Coates SA, & Brown LH (2009). The social world of the socially anhedonic: Exploring the daily ecology of asociality. Journal of Research in Personality, 43, 103–106. 10.1016/j.jrp.2008.10.008 [DOI] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, . . . Nathan PJ (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology, 35, 2403–2413. 10.1038/npp.2010.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EJ, Shaw P, Baker D, Baron-Cohen S, & David AS (2004). Measuring empathy: Reliability and validity of the Empathy Quotient. Psychological Medicine, 34, 911–919. http://dx.doi.org/10.1017/S0033291703001624 [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, & Engel RR (2005). What does the PANSS mean? Schizophrenia Research, 79, 231–238. 10.1016/j.schres.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Llerena K, Park SG, Couture SM, & Blanchard JJ (2012). Social anhedonia and affiliation: Examining behavior and subjective reactions within a social interaction. Psychiatry Research, 200, 679–686. 10.1016/j.psychres.2012.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM (2014). Oxytocin, motivation and the role of dopamine. Pharmacology, Biochemistry, and Behavior, 119, 49–60. 10.1016/j.pbb.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac-Donald E, Dadds MR, Brennan JL, Williams K, Levy F, & Cauchi AJ (2011). A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology, 36, 1114–1126. 10.1016/j.psyneuen.2011.02.015 [DOI] [PubMed] [Google Scholar]
- Marder SR, Davis JM, & Chouinard G (1997). The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. The Journal of Clinical Psychiatry, 58, 538–546. 10.4088/JCP.v58n1205 [DOI] [PubMed] [Google Scholar]
- McCarthy JM, Treadway MT, Bennett ME, & Blanchard JJ (2016). Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophrenia Research, 170, 278–284. 10.1016/j.schres.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough ME, Churchland PS, & Mendez AJ (2013). Problems with measuring peripheral oxytocin: Can the data on oxytocin and human behavior be trusted? Neuroscience and Biobehavioral Reviews, 37, 1485–1492. 10.1016/j.neubiorev.2013.04.018 [DOI] [PubMed] [Google Scholar]
- McDonald S (2002). Awareness of Social Inference Test: Manual. Retrieved from https://www.pearsonclinical.ca/en/products/product-master/item-38.html
- Medalia A, & Saperstein A (2011). The role of motivation for treatment success. Schizophrenia Bulletin, 37(Suppl. 2), S122–S128. 10.1093/schbul/sbr063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Sauer C, Reuter M, & Kirsch P (2013). An interaction between oxytocin and a genetic variation of the oxytocin receptor modulates amygdala activity toward direct gaze: evidence from a pharmacological imaging genetics study. European archives of psychiatry and clinical neuroscience, 263, 169–175. [DOI] [PubMed] [Google Scholar]
- Nichols AL, & Maner JK (2008). The good-subject effect: Investigating participant demand characteristics. Journal of General Psychology, 135, 151–166. 10.3200/GENP.135.2.151-166 [DOI] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, & Dayan P (2007). Tonic dopamine: Opportunity costs and the control of response vigor. Psychopharmacology, 191, 507–520. 10.1007/s00213-006-0502-4 [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Karelina K, Malarkey WB, Devries AC, & Berntson GG (2011). Selective influences of oxytocin on the evaluative processing of social stimuli. Journal of Psychopharmacology, 25, 1313–1319. 10.1177/0269881110367452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orne MT (2009). Demand characteristics and the concept of quasicontrols In Rosenthal R, Rosnow RL, & Kazdin AE(Eds.), Artifacts in behavioral research: Robert Rosenthal and Ralph L. Rosnow’s classic books (pp. 110–137). Oxford, UK: Oxford University Press. [Google Scholar]
- Oya K, Matsuda Y, Matsunaga S, Kishi T, & Iwata N (2016). Efficacy and safety of oxytocin augmentation therapy for schizophrenia: An updated systematic review and meta-analysis of randomized, placebo-controlled trials. European Archives of Psychiatry and Clinical Neuroscience, 266, 439–450. 10.1007/s00406-015-0634-9 [DOI] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, & Roberts DL (2007). Social cognition in schizophrenia: An overview. Schizophrenia Bulletin, 34, 408–411. 10.1093/schbul/sbn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Schulte-Rüther M, Hanewald B, & Lammertz S (2017). Reward: From basic reinforcers to anticipation of social cues. Current Topics in Behavioral Neuroscience, 30, 207–221. 10.1007/7854_2015_429 [DOI] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Barch DM, Buchanan RW, Dunayevich E, Gold JM, . . . Green MF (2015). Effort-based decision-making paradigms for clinical trials in schizophrenia: Part 1—psychometric characteristics of 5 paradigms. Schizophrenia Bulletin, 41, 1045–1054. 10.1093/schbul/sbv089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld AJ, Lieberman JA, & Jarskog LF (2010). Oxytocin, dopamine, and the amygdala: A neurofunctional model of social cognitive deficits in schizophrenia. Schizophrenia Bulletin, 37, 1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, & Correa M (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron, 76, 470–485. 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, & Mingote SM (2007). Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology, 191, 461–482. 10.1007/s00213-006-0668-9 [DOI] [PubMed] [Google Scholar]
- Sasayama D, Hattori K, Teraishi T, Hori H, Ota M, Yoshida S, . . . Kunugi H (2012). Negative correlation between cerebrospinal fluid oxytocin levels and negative symptoms of male patients with schizophrenia. Schizophrenia Research, 139, 201–206. 10.1016/j.schres.2012.06.016 [DOI] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O, . . . Hurlemann R (2013). Oxytocin enhances brain reward system responses in men viewing the face of their female partner. PNAS Proceedings of the National Academy of Sciences of the United States of America, 110, 20308–20313. 10.1073/pnas.1314190110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79, 194–202. 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Silverman I, Shulman AD, & Wiesenthal DL (1970). Effects of deceiving and debriefing psychological subjects on performance in later experiments. Journal of Personality and Social Psychology, 14, 203–212. 10.1037/h0028852 [DOI] [Google Scholar]
- Silverstein SM (2010). Bridging the gap between extrinsic and intrinsic motivation in the cognitive remediation of schizophrenia. Schizophrenia Bulletin, 36, 949–956. 10.1093/schbul/sbp160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH, & Gallagher L (2009). Dopaminergic-neuropeptide interactions in the social brain. Trends in Cognitive Science, 13, 27–35. 10.1016/j.tics.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, . . . Gründer G (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4, 158–165. 10.1093/scan/nsn051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, . . . Mendez AJ (2011). Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine, 73, 393–400. 10.1097/PSY.0b013e31821df0c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak BA (2013). Oxytocin and social salience: A call for gene-environment interaction research. Frontiers in Neuroscience, 7 10.3389/fnins.2013.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoridou A, Penton-Voak IS, & Rowe AC (2013). A direct examination of the effect of intranasal administration of oxytocin on approach-avoidance motor responses to emotional stimuli. PLoS ONE, 8(2), e58113. 10.1371/journal.pone.0058113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis D, Davis C, Hammond A, & Davis M (2005). Sex differences in hormonal modulation of anxiety measured with light-enhanced startle: Possible role for arginine vasopressin in the male. The Journal of Neuroscience, 25, 9010–9016. 10.1523/JNEUROSCI.0127-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, & Zald DH (2009). Worth the “EEfRT”? The Effort Expenditure for Rewards Task as an objective measure of motivation and anhedonia. PLoS ONE, 4(8), e6598. 10.1371/journal.pone.0006598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, & Zald DH (2011). Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews, 35, 537–555. 10.1016/j.neubiorev.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JG, & Olivier B (2013). Intranasal administration of oxytocin: Behavioral and clinical effects, a review. Neuroscience and Biobehavioral Reviews, 37, 1445–1465. 10.1016/j.neubiorev.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1997). WAIS-III: Wechsler adult intelligence scale. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Williams DR, & Bürkner P-C (2017). Effects of intranasal oxytocin on symptoms of schizophrenia: A multivariate Bayesian meta-analysis. Psychoneuroendocrinology, 75, 141–151. 10.1016/j.psyneuen.2016.10.013 [DOI] [PubMed] [Google Scholar]
- Woolley JD, Arcuni PA, Stauffer CS, Fulford D, Carson DS, Batki S, & Vinogradov S (2016). The effects of intranasal oxytocin in opioid-dependent individuals and healthy control subjects: A pilot study. Psychopharmacology, 233, 2571–2580. 10.1007/s00213-016-4308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Fussell C, Scherer S, Biagianti B, Fulford D, . . . Vinogradov S (2017). Intranasal oxytocin increases facial expressivity, but not ratings of trustworthiness, in patients with schizophrenia and healthy controls. Psychological Medicine, 47, 1311–1322. 10.1017/S0033291716003433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Lam O, Lai W, O’Donovan A, Rankin KP, . . . Vinogradov S (2014). Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology, 47, 116–125. 10.1016/j.psyneuen.2014.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WZ, Yan C, Ying XY, Zhu SY, Shi HS, Wang Y, . . . Chan RCK (2015). Domain-specific hedonic deficits towards social affective but not monetary incentives in social anhedonia. Scientific Reports, 4, 4056 10.1038/srep04056 [DOI] [PMC free article] [PubMed] [Google Scholar]