Abstract
Drug-related pneumonitis as a result of novel cancer therapy provides new challenges for providers of cancer care in the era of precision medicine. Awareness of this emerging entity and knowledge of its manifestations and management guidelines are essential for state-of-the-art practice of clinical oncology. Here, we provide a detailed review of drug-related pneumonitis that develops during precision cancer therapies using immune-checkpoint inhibitors and molecular targeting agents, and we summarize the emerging data that have been obtained by recent investigations to provide a state-of-the-art overview for clinicians involved in cancer care. We focus on immune-checkpoint inhibitor–related pneumonitis, which is an immune-related adverse event of growing interest and increasing clinical significance in current oncology practice that has rapidly expanding access to these agents. Clinical characteristics, radiographic spectrum, and risk factors and outcome of pneumonitis are described for each class of agents, and current treatment guidelines and monitoring recommendations are discussed. This review also indicates the area of unmet clinical need and provides direction for future investigations, as well as emphasizing the importance of a multidisciplinary approach to further understand the mechanisms, develop methods for accurate diagnosis, and optimize management guidelines of drug-related pneumonitis in the era of precision oncology.
INTRODUCTION
Drug-related pneumonitis is one of the major categories of adverse events during cancer therapy. With recent advances in precision cancer therapy using molecular targeting agents and immune-checkpoint inhibitors (ICIs), pneumonitis in patients who are treated with novel anticancer agents is increasingly recognized as a significant clinical challenge. In addition, rapidly expanding access to ICIs, such as programmed death 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors has brought new and emerging challenges. Recent studies of pneumonitis in patients who are treated with these novel agents have provided important new knowledge and insight that are relevant to cancer care providers across disciplines.
This review focuses on drug-related pneumonitis during precision cancer therapies, including ICIs and molecular targeting agents, and provides an overview for clinicians who are involved in the care of patients with cancer. Clinical characteristics and risk factors for pneumonitis are discussed for each class of agents. The spectrum of radiographic manifestations of pneumonitis is presented in the context of patient management. We also provide up-to-date guidelines and recommendations for the treatment and monitoring of pneumonitis during these therapies. This review is designed to serve as a guide for providers of cancer care in this emerging field of pneumonitis in precision oncology.
DRUG-RELATED PNEUMONITIS AND RADIOGRAPHIC PATTERN-BASED APPROACH
Drug-related pneumonitis is one of the major adverse events in patients who receive systemic anticancer agents and can be a result of direct cytotoxic effects, oxidative stress, and immune-mediated injuries.1 Because drug-related pneumonitis is a manifestation of lung response to these injuries, response patterns are limited to several types of histopathologic manifestations that are commonly observed in the setting of interstitial pneumonias, which have corresponding radiographic patterns on computed tomography (CT).1 Imaging plays a key role in the diagnosis and monitoring of drug-related pneumonitis in patients with cancer, and efforts have been made to characterize the radiographic patterns of pneumonitis caused by various therapeutic agents.1,2
Several recent reports have indicated that the radiographic patterns of drug-related pneumonitis may be categorized according to patterns that correspond to American Thoracic Society/European Respiratory Society (ATS/ERS) classifications of idiopathic interstitial pneumonias and related disorders,3 which are based on CT findings and their extent and distributions on imaging.2,4-8 In these studies of pneumonitis that developed during treatment with molecular targeting agents and ICIs, the commonly noted radiographic patterns of pneumonitis included cryptogenic organizing pneumonia (COP) pattern, nonspecific interstitial pneumonia (NSIP) pattern, hypersensitivity pneumonitis (HP) pattern, and acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS) pattern2,4-10 (Table 1). Clinically, drug-related pneumonitis is evaluated and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (Table 2). Radiographic patterns of pneumonitis have been shown to correlate with the toxicity grades assessed by the Common Terminology Criteria for Adverse Events.5
Table 1.
Radiographic Patterns of Drug-Related Pneumonitis and Imaging Features of Each Pattern
Table 2.
Grading of Drug-Related Pneumonitis in National Cancer Institute Common Terminology Criteria for Adverse Events Version 4
Recent advances in precision cancer therapy have brought the new and emerging challenges of pneumonitis as a result of treatment with novel agents. This review focuses on pneumonitis related to three major groups of agents that are increasingly used in precision oncology settings, including ICIs, mammalian target of rapamycin (mTOR) inhibitors, and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors, and describes their radiographic patterns, clinical manifestations, and management guidelines.
ICI-RELATED PNEUMONITIS
Incidence and Clinical Significance
The clinical application of ICIs has ushered in a new era for the treatment of advanced malignancies. PD-1/PD-L1 inhibitors are the most actively studied groups of agents and have shown marked efficacy in a variety of advanced cancers. Regulatory approvals for these agents have recently been granted, including nivolumab for melanoma, non–small-cell lung cancer (NSCLC), renal cell carcinoma (RCC), and Hodgkin lymphoma; pembrolizumab for melanoma, NSCLC, Hodgkin lymphoma, and squamous cell head and neck cancer; and atezolizumab for urothelial carcinoma and NSCLC. Combination therapy of nivolumab and ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 inhibitor, has also been approved for treatment of advanced melanoma and is actively investigated in NSCLC and other malignancies. As a result, these agents are rapidly expanding their treatment roles in clinical oncology practice, and more agents are in the drug development and testing pipeline.
Immune-checkpoint inhibition of these agents is associated with a unique set of toxicities that are known as immune-related adverse events (irAEs).11-14 Among a variety of irAEs that can affect organs from head to toe, ICI-related pneumonitis is a clinically significant and potentially life-threatening irAE and is therefore recognized as an event of special interest. Phase I trials of PD-1 inhibitors have resulted in pneumonitis-related deaths in patients with advanced solid tumors, including NSCLC, melanoma, and colorectal cancer.15-18 In a report of long-term safety in a NSCLC cohort that was treated in a phase I trial, pneumonitis occurred in 7% (9 of 129 patients), with three pneumonitis-related deaths.15 In a phase II trial of nivolumab in squamous NSCLC, pneumonitis was one of the most common irAEs, occurring in 5% of patients (6 of 117 patients), including four patients with grade 3 pneumonitis.19 More recent data suggest that pneumonitis can be an even more significant issue in patients who are treated with combination therapies. In a phase I study of nivolumab in combination with platinum-based chemotherapy as first-line treatment of advanced NSCLC, pneumonitis was noted in 13% (7 of 56 patients) of patients, including 7% (4 of 56) with grade 3 to 4, and was the most common adverse event responsible for treatment discontinuation (3 [5%] of 56).20 In a recent meta-analysis of 4,496 patients who were treated in 20 trials of PD-1 inhibitors for melanoma, NSCLC, and RCC, overall incidence of pneumonitis was 2.7% during monotherapy and 6.6% during combination therapy21 (Table 3). Higher incidence rates of pneumonitis were noted in patients with NSCLC for all-grade pneumonitis (4.1% v 1.6%; P = .002), grade ≥ 3 pneumonitis (1.8% v 0.2%; P < .001), and in RCC for all-grade pneumonitis (4.1% v 1.6%; P < .001) in reference to melanoma. Pneumonitis was more frequent during combination therapy than with monotherapy for all-grade pneumonitis (6.6% v 1.6%; P < .001) and grade ≥ 3 pneumonitis (1.5% v 0.2%; P = .001).21
Table 3.
Incidence of Pneumonitis in Patients Treated With Programmed Death-1 Inhibitor Therapy in Melanoma, Lung Cancer, and Renal Cell Carcinoma
Newer reports have also provided emerging data for the incidence of PD-L1 inhibitor-related pneumonitis. In a phase II trial of PD-L1 inhibitor atezolizumab versus docetaxel in patients with previously treated NSCLC, 3% (4 of 142) of patients who were treated with atezolizumab developed pneumonitis, including one with a grade 3 to 4 event.22 A recent phase III study of atezolizumab versus docetaxel in patients with advanced NSCLC reported that 1% (6 of 425) of atezolizumab-treated patients experienced pneumonitis, which included four patients with grade 3 events.23 In a phase Ib study of a combination therapy using PD-L1 inhibitor durvalumab and cytotoxic T-cell lymphocyte-4 inhibitor tremelimumab in patients with advanced NSCLC, 5% (5 of 99) of patients experienced drug-related pneumonitis, including four patients (4%) with grade 3 events.24 In a retrospective study of patients who were treated with PD-1/PD-L1 inhibitors at two institutions, incidence of pneumonitis was not significantly different between patients who were treated with PD-1 inhibitor versus those who received PD-L1 (monotherapy:, 4%; 22 of 564 patients v 2%; 2 of 152 patients; P = .13; combination therapy: 10%; 18 of 178 patients v 5%; 1 of 21 patients; P = .70).25
Clinical and Radiographic Manifestations of ICI-Related Pneumonitis
The first report that focused on ICI-related pneumonitis was based on the description of three patients with melanoma who were treated with nivolumab either alone or in combination with ipilimumab.4 As the clinical application of these agents has expanded, more cases of ICI-related pneumonitis in patients with melanoma and NSCLC have recently been described.6,26-30 Although limited to a small number of patients, observations in the initial series indicated that ICI-related pneumonitis has a spectrum of radiographic manifestations that includes different patterns that are described in the ATS/ERS classifications of interstitial pneumonias, including COP pattern, NSIP pattern, and AIP/ARDS pattern4,6,26-30 (Fig 1). Clinical courses of pneumonitis were also variable among patients. Some patients required admission to the intensive care unit and intubation, whereas others were treated successfully with oral corticosteroids on an outpatient basis and were able to restart their PD-1 inhibitor therapy without experiencing recurrent pneumonitis and achieved long-term benefit for treatment of advanced melanoma.4,6
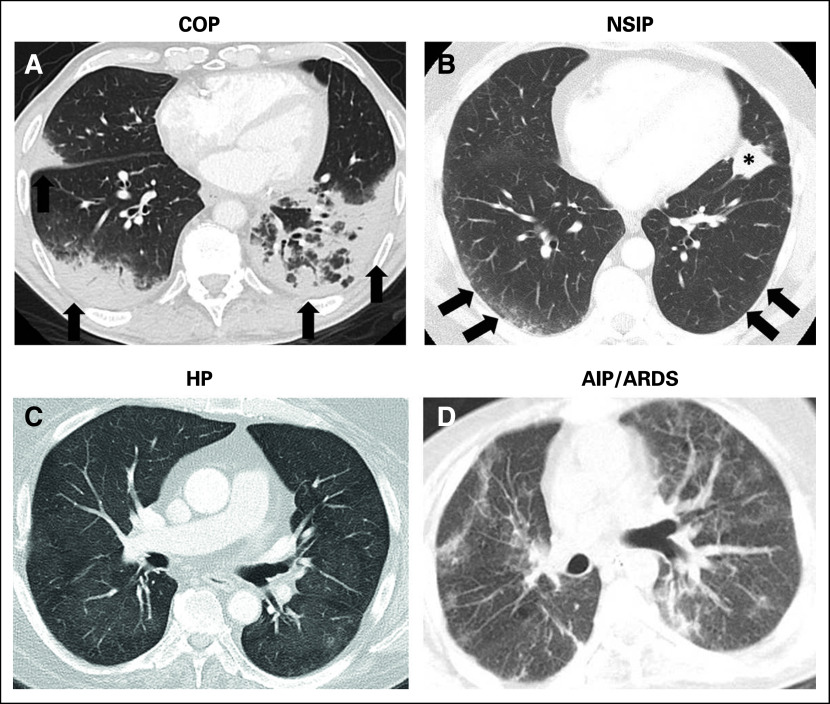
Fig 1.
A spectrum of radiographic patterns of immune-checkpoint inhibitor–related pneumonitis, including (A) cryptogenic organizing pneumonia (COP) pattern, (B) nonspecific interstitial pneumonia (NSIP) pattern, (C) hypersensitivity pneumonitis (HP) pattern, and (D) acute interstitial pneumonia (AIPS)/acute respiratory distress syndrome (ARDS) pattern. (A) COP pattern is characterized by multifocal bilateral parenchymal consolidations with peripheral and lower lung distribution, with ground-glass opacities (GGOs) and reticular opacities (arrows). (B) NSIP pattern demonstrates GGOs and reticular opacities predominantly in peripheral and lower lung distribution (arrows). The asterisk indicates lung tumor burden. (C) HP pattern demonstrates diffuse GGOs and centrilobular nodularities, with scattered areas of air trapping. (D) AIP/ARDS pattern is characterized by diffuse or multifocal GGOs or consolidations, along with lung volume loss and traction bronchiectasis.
A recent systematic study of ICI-related pneumonitis focused on radiographic profiling of patterns of pneumonitis on chest CT using ATS/ERS classifications of interstitial pneumonias.5 Among 170 patients who were treated in 10 trials of nivolumab, either alone or in combination with other ICIs, 20 patients (10 with melanoma, six with lymphoma, and four with lung cancer) developed pneumonitis (grade 1; n = 5; grade 2, n = 10; grade 3, n = 5).5 Time from the initiation of therapy to pneumonitis ranged widely from 0.5 to 11.5 months, with a median of 2.6 months. Time to pneumonitis was shorter in patients with lung cancer compared with others (median time to pneumonitis, 1.1 v 3.1 months, respectively; P = .008). Radiographic pattern of pneumonitis was COP pattern in 13 patients, NSIP pattern in three, HP pattern in two, and AIP/ARDS pattern in two patients. COP pattern was the most common pattern across all tumors and therapeutic regimens. Radiographic pattern was associated with clinical severity of pneumonitis represented by toxicity grades, where AIP/ARDS pattern had the highest grade, followed by COP pattern, whereas NSIP pattern and HP pattern had lower grade (median grade: 3, 2, 1, 1).5 Results indicate that the radiographic pattern–based approach, in addition to early and accurate diagnosis, may help guide patient management and follow-up in the setting of pneumonitis. Another recent study of PD-1/PD-L1 inhibitor-related pneumonitis has also reported the diverse radiographic and pathologic features of the entity,25 further indicating the importance of a standardized common language to describe the findings of ICI-related pneumonitis.10
Treatment, Clinical Course, and Management Guidelines
Treatment of ICI-related pneumonitis consists mainly of holding back on the use ICIs and administering corticosteroids; however, some cases may be refractory to corticosteroids and require additional treatment. In a prior report of 20 patients who developed pneumonitis during nivolumab therapy, most patients (17 of 20; 85%) received corticosteroids, either as oral treatment (n = 12) or as intravenous therapy (n = 5). Three patients (15%) also required infliximab, an anti–TNF-α immunosuppressive agent, in addition to corticosteroids. Thirteen patients were treated on an outpatient basis, whereas seven patients required hospitalization. Seven of 20 patients restarted nivolumab therapy. Among them, two patients developed recurrent pneumonitis during retreatment and both patients were successfully treated again with corticosteroids5 (Fig 2).
Fig 2.
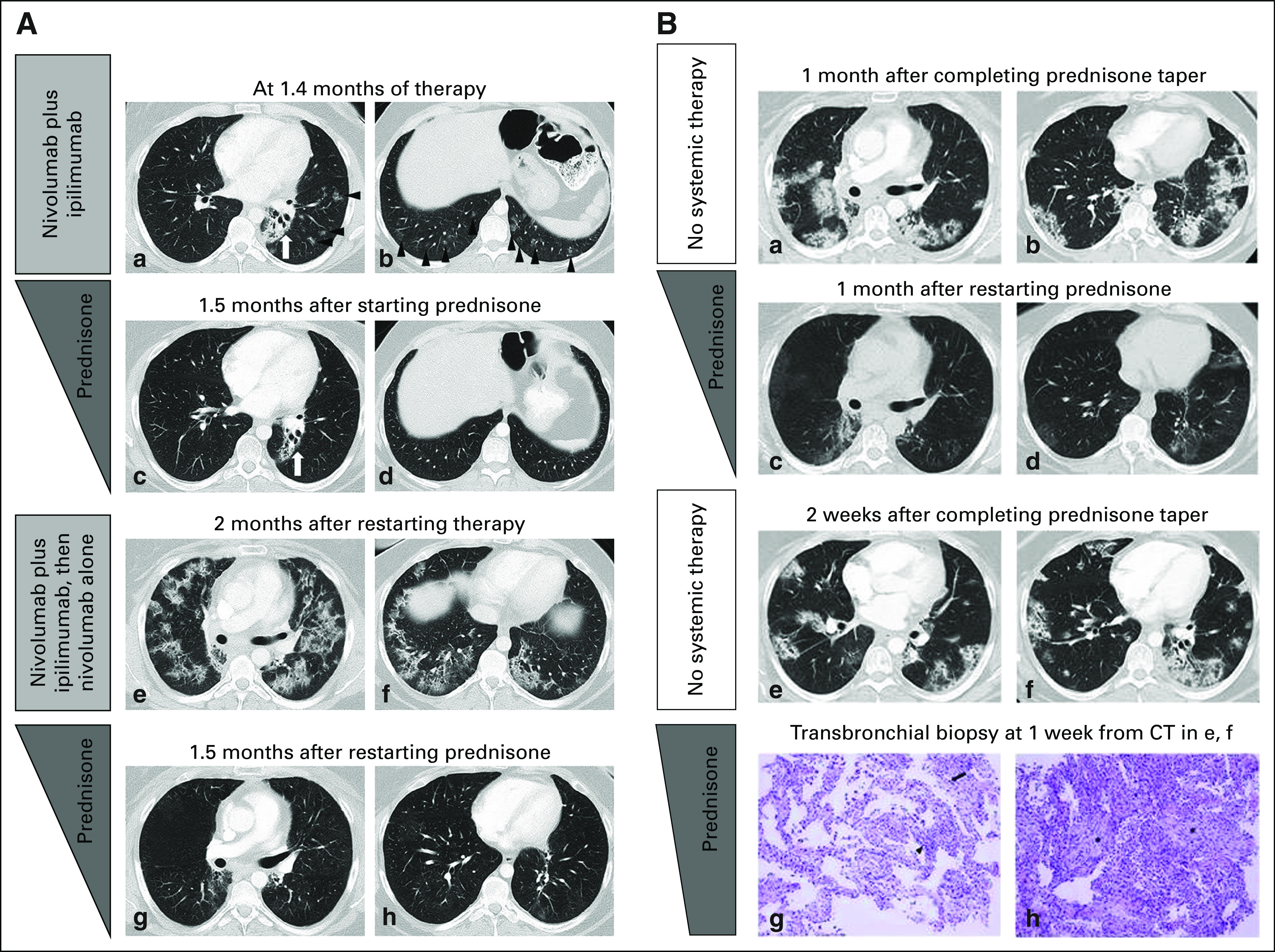
Pneumonitis with a cryptogenic organizing pneumonia (COP) pattern in a 33-year-old female with Hodgkin lymphoma who was treated with nivolumab and ipilimumab combination therapy, with a recurrence during retreatment (2A; a–h) and two episodes of pneumonitis flare after completion of corticosteroid taper (2B; a–h). Reprinted with permission from Nishino et al.5 2A: (a and b) Computed tomography (CT) scan of the chest at 1.4 months of therapy demonstrated ground-glass and reticular opacities and consolidations with multifocal distribution, which are indicative of a COP pattern of pneumonitis (arrowheads). Left perihilar opacity and traction bronchiectasis are a result of prior radiation therapy (arrows). (2A: c and d) The patient was treated with oral prednisone taper, and the findings have resolved on the follow-up scan performed 1.5 month later. (2A: e and f). The patient restarted therapy and received two doses of nivolumab and ipilimumab and two doses of nivolmab monotherapy, then developed recurrent pneumonitis 2 months after restarting therapy. The scan at the time of recurrent pneumonitis demonstrated similar findings with multifocal ground-glass and reticular opacities and consolidations, which again represented a COP pattern. The findings were more extensive than the first episode. (2A: g and h) Nivolumab was held and the patient was treated again with prednisone taper for pneumonitis, with subsequent improvement. (2B: a and b) The patient completed 2 months of corticosteroid taper, and after 1 month, experienced another episode of pneumonitis with a similar radiographic pattern, without nivolumab retreatment or other systemic therapy, which indicated a pneumonitis flare. (2B: c and d) Another course of corticosteroid taper was administered, with subsequent improvement. (2B: e and f) The 2.7-month course of corticosteroid taper was completed, and after 2 weeks, the patient again developed a pneumonitis flare with a similar radiographic pattern as prior episodes. (2B: g and h) The sampled fragments of lung that were obtained by transbronchial biopsies showed interstitial pneumonitis that evolved to organizing pneumonia. Findings included lymphocyte-predominant interstitial pneumonitis (arrowhead, 2B[g], hematoxylin and eosin stain, ×200) with rare eosinophils (arrow, 2B [g]), and areas of organizing pneumonia with fibroblast plugs and foamy macrophages filling the airspaces (asterisks, 2B [h], hematoxylin and eosin stain, ×200). No tumor cells, microorganisms, or viral cytopathic changes were identified. The patient started another course of prednisone taper with subsequent clinical improvement and is schedule for a follow-up CT scan.
Another recent study has described the management and clinical course of 43 patients who were treated in therapeutic trials or expanded access programs of PD-1/PD-L1 inhibitor therapy for various malignancies.25 Time to onset of pneumonitis ranged widely from 9 days to 19.2 months, with a median of 2.8 months, and tended to be shorter in patients who were treated with combination therapy than in those who were treated with monotherapy (median, 2.7 v 4.6 months; P = .02).25 Treatment was holding of the drug in 15 patients (all grade 1), corticosteroids in 23 patients (two grade 1, 14 grade 2, and seven grade 3 cases), and corticosteroids with additional immunosuppression in five patients with pneumonitis of grade ≥ 3 (three with infliximab and two with both infliximab plus cyclophosphamide).25 In 28 patients who received corticosteroids, 61% (17 of 28) began with oral treatment and 39% (11 of 28) began with intravenous treatment.25 Five patients worsened clinically and died. Although the cause of death was solely attributed to pneumonitis in one patient alone, the serious outcome in 12% (5 of 43) of patients again emphasizes the clinical significance of the entity.25 Twelve patients were retreated with ICIs after complete clinical resolution of initial pneumonitis, and three patients experienced recurrent pneumonitis, which again resolved by drug holding (n = 1) or oral corticosteroids (n = 2) as in the course of their initial pneumonitis treatment.25
A recently published guideline of pneumonitis management is in agreement with the observations in these reports.31 The guideline recommends oral corticosteroid treatment, including prednisone 1 to 2 mg/kg/d or methylprednisolone 0.5 to 1 mg/kg/d in mild to moderate cases. In mild to moderate cases, bronchoscopy is recommended to exclude infectious etiologies before starting immunosuppression.31 In severe cases, hospitalization is necessary, with treatment to include high-dose corticosteroids, such as methylprednisolone 2 to 4 mg/kg/d, and additional immunosuppression, including mycophenolate mofetil, cyclophosphamide; infliximab can be administered if necessary.31
Although limited to a small percentage of patients, an additional unique phenomenon of pneumonitis flare has been reported where ICI-related pneumonitis recurs after the termination of corticosteroid taper and in the absence of ICI retreatment after the initial episode of pneumonitis has been successfully treated (Fig 2). This was first reported in a patient with NSCLC who was treated with commercial nivolumab and who experienced pneumonitis.6 The patient was successfully treated with corticosteroids for the initial pneumonitis; however, the patient experienced another episode of pneumonitis after completing corticosteroid taper without resuming PD-1 inhibitor therapy or starting any other therapy, indicating a pneumonitis flare.6 Although similar to the initial presentation both clinically and radiographically, flare pneumonitis tends to be more severe and extensive than the initial episode. Such a phenomenon has not been described in the setting of pneumonitis related to other anticancer agents and this that indicates the clinical course of ICI-related pneumonitis may be more complex than other drug-related pneumonitis in some patients. In a series of 20 patients with ICI-related pneumonitis, one patient with lymphoma experienced two episodes of pneumonitis flare in which pneumonitis came back with similar but more extensive radiographic presentations compared with the initial episode after completion of corticosteroid taper without PD-1 retreatment5 (Fig 2). This observation further confirms the phenomenon, which is likely unique to irAEs and may involve autoimmune mechanisms.
Unmet Clinical Needs That Require Additional Investigations
Although several recent reports provide important knowledge and observations of ICI-related pneumonitis, significant knowledge gaps still exist for this emerging entity. Because early and accurate diagnosis is key for this mostly treatable condition—with good response to corticosteroids in the majority of the cases—additional studies are needed to identify risk factors and early markers for pneumonitis to improve diagnostic accuracy. Treatment and clinical management guidelines need to be further optimized as the immune-oncology community accumulates the experience of this entity. ICI retreatment after resolution of pneumonitis—thus far without sufficient data—is a challenging issue, but requires evidence-based guidelines as these patients have advanced cancers without many other treatment options for their cancer.
In addition, awareness of pneumonitis related to ICIs other than PD-1/PD-L1 inhibitors is becoming increasingly important. Prior studies have shown pneumonitis to be associated with ipilimumab monotherapy, occurring in 5% to 7% of patients and radiographically manifesting the COP or NSIP pattern.12,32 Given the similarities of radiographic manifestation of pneumonitis associated with different ICIs, strategies for detecting and monitoring these findings and patterns on imaging are needed for optimal patient management during ICI therapies and during the use of other novel immunotherapeutic agents, such as agonist antibodies.
PNEUMONITIS DURING mTOR INHIBITOR THERAPY
mTOR is a serine/threonine protein kinase that plays a key role in the phosphatidylinositol 3-kinase/Akt/mTOR pathway, which is an established oncogenic driver in human cancers.33 Everolimus and temsirolimus are specific inhibitors of mTOR and are used as anticancer therapeutic agents. Everolimus is approved for the treatment of several cancers, including advanced RCC, advanced pancreatic neuroendocrine tumors (NETs), subependymal giant cell astrocytomas, and advanced hormone receptor–positive, human epidermal growth factor receptor 2–negative breast cancer (in combination with exemestane). Temsirolimus is approved for the treatment of advanced RCC.
Drug-related pneumonitis is a recognized class effect toxicity of mTOR inhibitors and has been described in detail in the context of advanced RCC treatment. In a study by Maroto et al,34 among 178 patients with RCC who were treated with single-agent temsirolimus, 52 (29%) had radiographically identified drug-related pneumonitis. Of these, 16 patients (31%) had respiratory symptoms at the time of the onset of radiographically detected pneumonitis, whereas others (36 of 52; 69%) were asymptomatic and thus may not be clinically diagnosed for pneumonitis. The estimated cumulative probability of radiologically identified drug-related pneumonitis was 21% (95% CI, 15% to 29%) at 8 weeks, 31% (95% CI, 24% to 40%) at 16 weeks, and 45% (95% CI, 36% to 57%) at 13 months.34 Another study of 46 patients with RCC who were treated with temsirolimus or everolimus reported that 14 patients (30%) developed drug-related pneumonitis.35 Median time of the onset of radiologic manifestations of pneumonitis was 56 days (range, 31 to 214 days). These 14 patients more frequently achieved stable disease than did others, which indicates a possibility of pneumonitis as a treatment benefit marker.35
Reports of mTOR inhibitor–related pneumonitis in tumors other than RCC indicate a somewhat different incidence of pneumonitis according to tumor types. Soria et al36 studied 64 patients with advanced NSCLC who were treated with everolimus and reported that 24 patients (25%) had newly occurring or worsening radiographic changes suggestive of drug-related pneumonitis. Another recent study of the entity in patients with Waldenström macroglobulinemia (WM) who were treated in trials of everolimus reported a higher incidence of pneumonitis—noted in 58% (23 of 40) of patients, with a median time to onset of pneumonitis of 5.7 months.8 Conversely, among patients with advanced NET who were treated with mTOR inhibitors, pneumonitis was noted in 21% (14 of 66 patients), which is slightly lower than the incidence in other tumors, with a time to pneumonitis that ranged from 1.0 to 27.7 months (estimate of 25th percentile,16.0 months).7 It remains to be investigated whether patients with different tumor types have different susceptibilities to the development of pneumonitis during mTOR inhibitor therapy.
Despite apparent differences in incidence, the radiographic appearance of mTOR inhibitor–related pneumonitis is similar across different cohorts with different tumors, which further supports the concept of pneumonitis as a class effect of mTOR inhibitors. Radiographic patterns of mTOR inhibitor–related pneumonitis have been characterized in patients with WM and in those with advanced NET.7,8 In both cohorts, the most common findings were bilateral ground-glass opacities (GGOs) and reticular opacities, sometimes with consolidations, in a peripheral and lower distribution. COP pattern was the leading pattern observed in the majority of cases (70% in WM and 57% in NET), followed by NSIP pattern (30% in WM and 36% in NET), whereas one patient in the NET cohort demonstrated the HP pattern.7,8 Similar imaging manifestations are noted in other cohorts with mTOR inhibitor–related pneumonitis.34,35 Recognition of the characteristic radiographic patterns of pneumonitis during mTOR inhibitor therapy helps with the early detection and accurate diagnosis of this entity.
Clinical management of mTOR inhibitor–related pneumonitis mostly depends on the severity of clinical symptoms. In general, asymptomatic patients with radiographic changes alone (grade 1) may continue mTOR inhibitor therapy with close monitoring for respiratory symptoms.34 Symptomatic patients with concurrent radiographic changes should hold mTOR inhibitors while they undergo evaluations, such as pulmonary function tests and bronchoscopy, and initiate empirical treatment, such as corticosteroids and antibiotics.34 A systematic review of mTOR inhibitor–related pneumonitis by Albiges et al37 has proposed subdividing grade 2 pneumonitis into grade 2a and 2b, where grade 2a patients have mild-to-moderate cough and are close to grade 1, and grade 2b have severe cough or dyspnea and are close to grade 3. Albiges et al recommend that grade 2a patients continue mTOR inhibitor therapy under close monitoring, with or without dose adjustment depending on benefit-risk assessments. Patients with grade 2b pneumonitis pose a management dilemma given the concerns over the progressive worsening of pneumonitis to higher grades; however, Albiges et al recommend a dose reduction of mTOR inhibitors—from 10 mg/d oral dose to 5 mg/d for everolimus and withholding of 1 to 2 injections for temsirolimus—and close monitoring, with the possible addition of corticosteroids if no improvement is noted and infection has been ruled out.37 For patients with grade ≥ 3, mTOR inhibitor therapy should be interrupted, bronchoscopy and bronchoalveolar lavage are strongly recommended, and corticosteroids should be administered after ruling out infection.37
PNEUMONITIS DURING EGFR TYROSINE KINASE INHIBITOR THERAPY
EGFR tyrosine kinase inhibitors (TKIs) play major roles in the treatment of patients with advanced NSCLC with sensitizing EGFR mutations, which markedly respond to erlotinib, gefitinib, and afatinib.38-42 Recently, a third-generation EGFR-TKI, osimertinib, has also been approved for treatment in patients with resistance to conventional EGFR-TKIs.43 Although the incidence is relatively low, pneumonitis during EGFR-TKI therapy is also recognized as a class effect and has been studied mostly in the context of erlotinib and gefitinib.44,45 The first report, a Japanese study, described four patients with advanced NSCLC who developed severe acute interstitial pneumonia as a result of treatment with gefitinib; two patients recovered with corticosteroids, whereas the other two died of progressive respiratory dysfunction as a result of diffuse alveolar damage (DAD) as evaluated on autopsy.45 A subsequent large cohort study in Japanese patients with NSCLC who were treated with gefitinib reported a cumulative incidence rate of 4.0% at the end of a 12-week follow-up and a mortality rate of 27.9%.46 The study also identified risk factors, including old age, smoking history, pre-existing interstitial lung disease, and poor performance status.46 The risk was higher with gefitinib than chemotherapy, mainly in the first 4 weeks of therapy.46 Similar incidence and mortality rates were reported in an erlotinib-treated Japanese cohort (4.3% and 35.7%, respectively).47 In a recent meta-analysis of 16 trials of EGFR-TKIs, pneumonitis was the most common cause of death attributed to EGFR-TKI toxicity.48
Different radiographic patterns can be noted in EGFR-TKI–related pneumonitis, including COP pattern, HP pattern, NSIP pattern, and DAD/AIP pattern.49 Among them, DAD/AIP pattern, which is characterized by nonsegmental GGO or consolidation with traction bronchiectasis and volume loss, was associated with a higher mortality (65%).47 Treatment of pneumonitis during EGFR-TKI therapy is mainly supportive and includes supplemental oxygen, empirical antibiotics, and mechanical ventilation in severe cases. EGFR-TKI therapy should be discontinued, and systemic corticosteroids are usually administered.49
Although EGFR-TKI–related pneumonitis has mostly been a specific concern among the Japanese population and has often had a low clinical impact in other populations, a recent observation from a trial of a novel ICI and third-generation EGFR-TKI, osimertinib, raises a new concern in the setting of combination therapy. In a multiarm phase Ib trial of PD-L1 inhibitor, durvalumab, plus osimertinib in EGFR-mutant NSCLC, a significantly high rate of lung toxicity was reported with an overall incidence of 38% (13 of 34 patients) and a rate of grade 3 to 4 events of 15% (5 of 34 patients), which resulted in a suspension of enrollment in this arm of the study.50 In addition, a recent observation from Japan reported that eight patients with advanced NSCLC who were previously treated with nivolumab developed severe interstitial lung disease during subsequent treatment using EGFR-TKIs, which led to death in three cases.51 Although the risk of combination or sequential use of ICIs and EGFR-TKIs in patients with lung cancer must be further studied, these observations may indicate a need for increased awareness and caution for lung toxicity with EGFR-TKIs in the era of rapidly increasing use of ICIs in the clinical setting.
CHALLENGES AND OUTSTANDING ISSUES
Despite accumulating data and increasing knowledge of drug-related pneumonitis during precision cancer therapy, many challenges and issues remain to be solved and require additional investigation. The pathophysiology of drug-related pneumonitis has not yet been elucidated for most agents, although suggested mechanisms include cell-mediated autoimmune response or T cell–mediated delayed hypersensitivity, which have been proposed not only in ICI-related pneumonitis but also in pneumonitis related to mTOR and EGFR inhibitors.37,49,52 In addition, the signaling pathways of these molecular targeting agents may limit, in part, the destructive remodeling of the lung and impair the lung’s ability to respond to injury.37,49,52 These possible mechanisms are also likely modified by various host and environmental factors, which further complicates the issue.52 Likewise, risk factors for pneumonitis need to be defined, especially for ICI-related pneumonitis, to allow for optimal patient selection with regard to treatment safety. The risk of recurrent pneumonitis after reintroduction of therapy is another important debate for these agents, as they may be the only available or effective therapy for patients with advanced cancers.5,37
The impact of radiotherapy on pneumonitis is a major topic, given a number of ongoing combination therapy trials that use ICI and radiotherapy.53 In some studies of patients with advanced NSCLC who were treated with EGFR-TKIs, prior thoracic irradiation and concomitant radiotherapy have been noted as risk factors for pneumonitis.49,54 Although the exact effect of radiotherapy on ICI-related pneumonitis awaits additional data, prior chest radiation has influenced the radiographic appearance of PD-1 pneumonitis in patients with lymphoma, which indicates a need for caution in treatment monitoring.30 Prior reports have often focused on radiation to the chest; however, radiotherapy in the extrathoracic sites may require more attention given the concept of abscopal effects, which are increasingly discussed in the context of ICI and radiation treatment.53
In the clinical setting, drug-related pneumonitis is ultimately a diagnosis of clinical correlation and exclusion, and there is no specific test for the entity.55 Among various lung conditions that can affect patients with advanced cancer who undergo systemic therapy, infection is an important differential diagnosis that should be excluded before starting corticosteroid therapy. Fiberoptic bronchoscopy is helpful for this purpose as it allows for targeted and deep sampling of specimens for microbiology, cytology, and histology via bronchoalveolar lavage and transbronchial biopsy.55 A minority of patients may present with respiratory distress in the absence of radiographic confirmation, thereby leading to delays in appropriate management. Of note, some of the findings of pneumonitis, such as GGOs, are not adequately evaluated on chest radiographs, and thus diagnostic chest CT scans are needed to sensitively detect and fully characterize the lung abnormalities in the setting of suspected pneumonitis.
In conclusion, knowledge of drug-related pneumonitis has increasingly emerged in recent investigations, providing important clues for accurate diagnosis, patient management, and monitoring. It should be emphasized that, although it is uncommon overall, pneumonitis can be a severe and potentially fatal adverse event with ICI therapies and molecular targeting agents. ICI-related pneumonitis is a newly recognized irAE of considerable clinical significance, which requires increased awareness and familiarity among providers of cancer care. A radiographic pattern-based approach offers a practical aid to recognize and characterize drug-related pneumonitis and may help guide treatment decisions. A multidisciplinary approach is essential to better understand the mechanisms, establish methods for early detection and accurate diagnosis, and optimize the clinical management and outcome in drug-related pneumonitis during precision cancer therapy.
Footnotes
Supported by National Cancer Institute Grants No. 5K23-CA157631 and 1R01-CA203636 (M.N.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Mizuki Nishino, F. Stephen Hodi
Data analysis and interpretation: Mizuki Nishino, Hiroto Hatabu, Nikhil H. Ramaiya
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Drug-Related Pneumonitis in the Era of Precision Cancer Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or po.ascopubs.org/site/ifc.
Mizuki Nishino
Honoraria: Bayer Yakuhin
Consulting or Advisory Role: Bristol-Myers Squibb, WorldCare Clinical, Toshiba Medical Systems
Research Funding: Merck (Inst)
Hiroto Hatabu
Consulting or Advisory Role: Toshiba Medical Systems
Research Funding: Toshiba Medical Systems, Konica Minolta, Canon
F. Stephen Hodi
Employment: Dana-Farber Cancer Institute
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech, Amgen, EMD Serono
Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy, patent pending royalties received on MICA-related disorders application to institution per institutional policy
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb
Other Relationship: Bristol-Myers Squibb, Genentech
Nikhil H. Ramaiya
No relationship to disclose
REFERENCES
- 1.Erasmus JJ, McAdams HP, Rossi SE: High-resolution CT of drug-induced lung disease. Radiol Clin North Am 40:61-72, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Müller NL, White DA, Jiang H, et al. : Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer 91:S24-S30, 2004. (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis WD, Costabel U, Hansell DM, et al. : An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188:733-748, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino M, Sholl LM, Hodi FS, et al. : Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med 373:288-290, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishino M, Ramaiya NH, Awad MM, et al. : PD-1 inhibitor-related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin Cancer Res 22:6051-6060, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishino M, Chambers ES, Chong CR, et al. : Anti-PD-1 inhibitor-related pneumonitis in non–small-cell lung cancer. Cancer Immunol Res 4:289-293, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Brais LK, Brooks NV, et al. : Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy in patients with neuroendocrine tumors: A radiographic pattern-based approach. Eur J Cancer 53:163-170, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishino M, Boswell EN, Hatabu H, et al. : Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy: Radiographic pattern-based approach in Waldenström macroglobulinemia as a paradigm. Oncologist 20:1077-1083, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishino M, Itoh H, Hatabu H: A practical approach to high-resolution CT of diffuse lung disease. Eur J Radiol 83:6-19, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. doi: 10.1200/JCO.2016.71.0434. Nishino M, Hatabu H: Programmed death-1/programmed death ligand-1 inhibitor-related pneumonitis and radiographic patterns. J Clin Oncol 35:1625-1626, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Nishino M, Tirumani SH, Ramaiya NH, et al. : Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment. Eur J Radiol 84:1259-1268, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tirumani SH, Ramaiya NH, Keraliya A, et al. : Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 3:1185-1192, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michot JM, Bigenwald C, Champiat S, et al. : Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer 54:139-148, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Weber JS, Dummer R, de Pril V, et al. : Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: Detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 119:1675-1682, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Gettinger SN, Horn L, Gandhi L, et al. : Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer. J Clin Oncol 33:2004-2012, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garon EB, Rizvi NA, Hui R, et al. : Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med 372:2018-2028, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443-2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postow MA, Chesney J, Pavlick AC, et al. : Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372:2006-2017, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizvi NA, Mazières J, Planchard D, et al. : Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non–small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol 16:257-265, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizvi NA, Hellmann MD, Brahmer JR, et al. : Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol 34:2969-2979, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino M, Giobbie-Hurder A, Hatabu H, et al. : Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol 2:1607-1616, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Fehrenbacher L, Spira A, Ballinger M, et al. : Atezolizumab versus docetaxel for patients with previously treated non–small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 387:1837-1846, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Rittmeyer A, Barlesi F, Waterkamp D, et al. : Atezolizumab versus docetaxel in patients with previously treated non–small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonia S, Goldberg SB, Balmanoukian A, et al. : Safety and antitumour activity of durvalumab plus tremelimumab in non–small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol 17:299-308, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naidoo J, Wang X, Woo KM, et al. : Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 35:709-717, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiset PO, Shapera S, Butler MO, et al. : Anti-PD-1-associated organizing pneumonia in a responding melanoma patient. Ann Oncol 27:1649-1650, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Fragkou P, Souli M, Theochari M, et al. : A case of organizing pneumonia (OP) associated with pembrolizumab. Drug Target Insights 10:9-12, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gounant V, Brosseau S, Naltet C, et al. : Nivolumab-induced organizing pneumonitis in a patient with lung sarcomatoid carcinoma. Lung Cancer 99:162-165, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Sano T, Uhara H, Mikoshiba Y, et al. : Nivolumab-induced organizing pneumonia in a melanoma patient. Jpn J Clin Oncol 46:270-272, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Nishino M, Ramaiya NH, Hatabu H, et al. : PD-1 inhibitor-related pneumonitis in lymphoma patients treated with single-agent pembrolizumab therapy. Br J Haematol, 10.1111/bjh.14441 [epub ahead of print on November 11, 2016] [DOI] [PubMed] [Google Scholar]
- 31.Friedman CF, Proverbs-Singh TA, Postow MA: Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol 2:1346-1353, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Hodi FS, Lee S, McDermott DF, et al. : Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA 312:1744-1753, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghobrial IM, Gertz M, Laplant B, et al. : Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenström macroglobulinemia. J Clin Oncol 28:1408-1414, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maroto JP, Hudes G, Dutcher JP, et al. : Drug-related pneumonitis in patients with advanced renal cell carcinoma treated with temsirolimus. J Clin Oncol 29:1750-1756, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Dabydeen DA, Jagannathan JP, Ramaiya N, et al. : Pneumonitis associated with mTOR inhibitors therapy in patients with metastatic renal cell carcinoma: Incidence, radiographic findings and correlation with clinical outcome. Eur J Cancer 48:1519-1524, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Soria JC, Shepherd FA, Douillard JY, et al. : Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol 20:1674-1681, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Albiges L, Chamming’s F, Duclos B, et al. : Incidence and management of mTOR inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Ann Oncol 23:1943-1953, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Jänne PA, Gurubhagavatula S, Yeap BY, et al. : Outcomes of patients with advanced non–small cell lung cancer treated with gefitinib (ZD1839, “Iressa”) on an expanded access study. Lung Cancer 44:221-230, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Lynch TJ, Bell DW, Sordella R, et al. : Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med 350:2129-2139, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Paez JG, Jänne PA, Lee JC, et al. : EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 304:1497-1500, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Nishino M, Hatabu H, Johnson BE, et al. : State of the art: Response assessment in lung cancer in the era of genomic medicine. Radiology 271:6-27, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishino M, Jackman DM, Hatabu H, et al. : Imaging of lung cancer in the era of molecular medicine. Acad Radiol 18:424-436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jänne PA, Yang JC, Kim DW, et al. : AZD9291 in EGFR inhibitor-resistant non–small-cell lung cancer. N Engl J Med 372:1689-1699, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Burotto M, Manasanch EE, Wilkerson J, et al. : Gefitinib and erlotinib in metastatic non–small cell lung cancer: A meta-analysis of toxicity and efficacy of randomized clinical trials. Oncologist 20:400-410, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue A, Saijo Y, Maemondo M, et al. : Severe acute interstitial pneumonia and gefitinib. Lancet 361:137-139, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Kudoh S, Kato H, Nishiwaki Y, et al. : Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case-control study. Am J Respir Crit Care Med 177:1348-1357, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Gemma A, Kudoh S, Ando M, et al. : Final safety and efficacy of erlotinib in the phase 4 POLARSTAR surveillance study of 10 708 Japanese patients with non–small-cell lung cancer. Cancer Sci 105:1584-1590, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding PN, Lord SJ, Gebski V, et al. : Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: A meta-analysis of clinical trials of gefitinib, erlotinib, and afatinib in advanced EGFR-mutated non–small cell lung cancer. J Thorac Oncol 12:633-643, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Min JH, Lee HY, Lim H, et al. : Drug-induced interstitial lung disease in tyrosine kinase inhibitor therapy for non–small cell lung cancer: A review on current insight. Cancer Chemother Pharmacol 68:1099-1109, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Ahn M, Yang J, Yu H, et al. : Osimertinib combined with durvalumab in EGFR-mutant non–small cell lung cancer: Results from the TATTON phase Ib trial. J Thorac Oncol 11:S115,2016. (suppl 4) [Google Scholar]
- 51. Precautions relating to interstitial lung disease during administration of epidermal growth factor receptor tyrosine kinase inhibitors. Pharmaceuticals and Medical Devices Safety Information No. 336. https://www.pmda.go.jp/files/000214024.pdf#page=4.
- 52.Kubo K, Azuma A, Kanazawa M, et al. : Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig 51:260-277, 2013 [DOI] [PubMed] [Google Scholar]
- 53.De Ruysscher D, Reynders K, Van Limbergen E, et al. : Radiotherapy in combination with immune checkpoint inhibitors. Curr Opin Oncol 29:105-111, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Hotta K, Kiura K, Tabata M, et al. : Interstitial lung disease in Japanese patients with non–small cell lung cancer receiving gefitinib: An analysis of risk factors and treatment outcomes in Okayama Lung Cancer Study Group. Cancer J 11:417-424, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Powell CA: Pulmonary infiltrates in a patient with advanced melanoma. J Clin Oncol 35:705-708, 2017 [DOI] [PubMed] [Google Scholar]