INTRODUCTION
Activating mutations of PIK3CA are found in 25%-40% of estrogen receptor–positive (ER+), HER2-negative (HER2−) breast cancers (BC), and in 8% of ER-negative (ER−) BC.1-5 Two recent studies support the benefit of PI3K inhibition in combination with endocrine therapy. SANDPIPER (study of taselisib plus fulvestrant v placebo plus fulvestrant in participants with advanced or metastatic breast cancer who have disease recurrence or progression during or after aromatase inhibitor therapy) demonstrated a small prolongation in progression-free survival with taselisib,6 and SOLAR-1 (ClinicalTrials.gov identifier: NCT02437318) demonstrated a clinically meaningful improvement in progression-free survival with alpelisib.7 This led to the approval of alpelisib in combination with fulvestrant in PIK3CA-mutant, hormone receptor–positive (HR+), metastatic BC.
Activation of the PI3K pathway is frequent in BC brain metastases, as evidenced by observation of AKT and S6 phosphorylation and loss of PTEN.8 Recent data suggest PIK3CA-activating mutations may be associated with an increased risk of CNS metastases in patients with ER+/HER2− disease.9 In 307 patients with ER+/HER2− metastatic disease, brain metastases were significantly more common in patients with PIK3CA mutations (30.8% v 17.1%; P = .0049). Treatment of brain metastases from ER+ BC remains difficult. A recent retrospective analysis found a clear association of improved survival with continuation of endocrine therapy upon diagnosis of brain metastases.10 Remarkably, the CDK4/6-inhibitor abemaciclib had a clinical benefit rate of 25%.11 Preclinical models of BC brain metastases suggest PI3K pathway inhibition may be effective for treatment of brain metastases.12 Notably, both SANDPIPER and SOLAR-1 excluded patients with untreated or active CNS metastases6,7; however, the precursor to alpelisib, buparlisib, did have brain penetration, which was thought to be the cause for the higher incidence of mood disorders.13 An increase in depression has distinctly not been seen with the α-specific PI3K-inhibitor alpelisib and, in preclinical animal models with intact blood-brain barrier, no significant distribution into the brain was seen (unpublished data). Thus, the activity of alpelisib in brain metastases is unknown.
Herein, we report a case series of 4 patients with ER+/progesterone receptor–positive (PR+)/HER2− metastatic BC with progressive brain metastases (Fig 1) treated with alpelisib. All patients provided consent to publish their information and images.
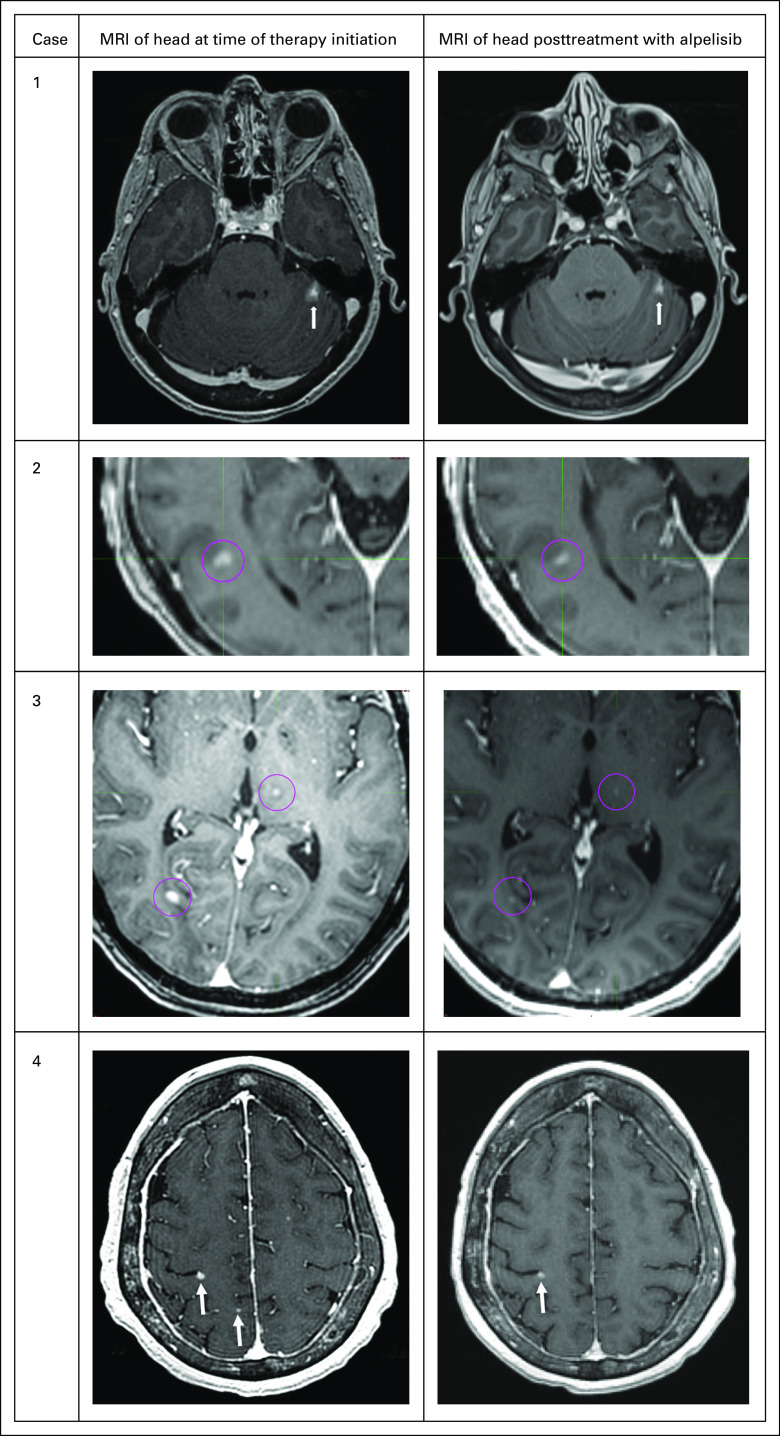
FIG 1.
CNS response measured by magnetic resonance imaging (MRI) of the brain. Pink circles and white arrows represent metastatic disease from breast cancer.
CASE 1
A 55-year-old woman presented with a large breast ulceration, biopsy specimen–diagnosed invasive ductal carcinoma (IDC), grade 3, ER+/PR+/HER2−. Computed tomography (CT) scan revealed pulmonary nodules, osseous lesions, and hypodense lesions within the right hepatic lobe. Brain magnetic resonance imaging (MRI) showed a 10-mm mass in the left cerebellar hemisphere; this was treated with stereotactic radiosurgery with initial shrinkage to 8 mm and stabilization on follow-up. Tumor sequencing showed an activating PIK3CA mutation H1047R and amplification of PIK3C2B. Disease progressed after 4 months of treatment with fulvestrant and palbociclib. Brain MRI showed an increase of the left cerebellar lesion to 12 mm, judged by neuroradiology and radiation oncology to be more compatible with progression than with radiation-induced tissue necrosis. Palbociclib was switched for alpelisib (300 mg daily) with continuation of fulvestrant and zoledronic acid. The patient’s body mass index (BMI) was 25 (calculated as kilograms divided by square of height in meters) and Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0. Subsequently, the patient’s chest wound started to heal with epithelization and closure of the wound. Brain MRI after 6 weeks showed a reduction in the left cerebellar lesion to 9 mm (62% reduction of bidimensional areas, 35% reduction of sum of longest distances). Follow-up MRI 2 months later revealed stability of the CNS lesion. Subsequent brain MRI at 3, 4, and 6 months showed stable disease without changes in measurements. This was compatible with a partial response per Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria.14
CASE 2
A 71-year-old woman was diagnosed in 2010 with pT2N0 IDC of the right breast, grade 3, ER+/PR+/HER2−. She was treated with breast-conserving surgery followed by adjuvant doxorubicin plus cyclophosphamide therapy and radiation. She completed 5 years of adjuvant tamoxifen in 2016. In 2017, she had a recurrence in the right breast, pleural effusion, and bone metastases and was treated with multiple regimens: letrozole and palbociclib, fulvestrant and palbociclib, and capecitabine and paclitaxel. Brain MRI showed multiple (> 10) subcentimeter metastases. Testing of primary tumor had demonstrated a PIK3CA H1047R mutation and TP53 mutation (H179Q). Because the patient was asymptomatic from the brain metastases, whole-brain radiotherapy (WBRT) was deferred and she was administered treatment with fulvestrant plus alpelisib (300 mg daily). Her BMI was 23 and ECOG PS score was 0. Brain MRI 4 weeks later demonstrated minor regressions of nonmeasurable, subcentimeter brain metastases without new or progressive lesions. Brain MRI performed 10 weeks after initiation of therapy demonstrated stability of CNS lesions; however, CT scan demonstrated progression of liver metastases, which prompted change of therapy to eribulin. A liquid biopsy specimen at that time was notable for continued presence of PIK3CA H1047R and TP53 H179Q, and an acquired ESR1 (Y537N) mutation (minor allele frequency, 1.4%).
CASE 3
A 55-year-old woman was diagnosed in 2009 with pT2N2 IDC of the left breast, grade 2, ER+/PR+/HER2−. She was treated with mastectomy followed by dose-dense doxorubicin plus cyclophosphamide and paclitaxel followed by radiation. She completed 5 years of tamoxifen therapy and then letrozole. Workup for hip pain in 2015 demonstrated metastatic ER+/PR−/HER2− BC. She was serially treated with fulvestrant and palbociclib, radiation to the base of skull to treat cranial nerve symptoms, and with exemestane and everolimus, capecitabine, and abemaciclib. For the first time, brain MRI demonstrated multiple parenchymal metastases and she was treated with hippocampal-sparing WBRT. Subsequently, treatment was changed from abemaciclib to liposomal doxorubicin for progression in the brain, followed by gemcitabine. She continued to have progressive CNS parenchymal disease. Testing of primary breast tumor demonstrated a PIK3CA E545K mutation. She was administered treatment with fulvestrant and alpelisib (300 mg); her BMI was 23 and ECOG PS score was 0. Brain MRI 6 weeks later demonstrated a 14% reduction in the sum of longest distances of measurable brain metastases (24% reduction in bidimensional areas) and regressions of nonmeasurable brain metastases. Repeated brain MRI at the 4-month mark showed mixed response, still compatible with stable disease by RANO-BM criteria,14 and treatment was continued. The most recent brain MRI at the 6-month mark revealed progressive parenchymal disease requiring change of therapy.
CASE 4
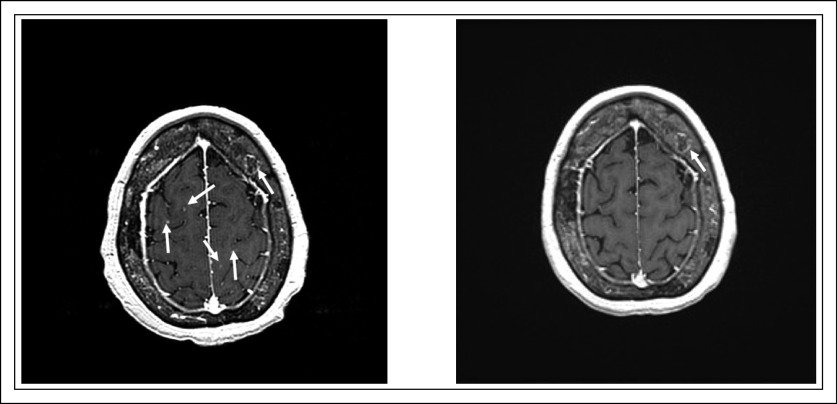
A 70-year-old woman was diagnosed in 2013 with T1N1 IDC of the right breast, grade 3, ER+/PR+/HER2−. She was treated with breast-conserving surgery, adjuvant docetaxel plus cyclophosphamide, radiation, and anastrozole. In 2018, metastatic disease developed to the lung, liver, and bone while she received adjuvant anastrozole. Examination of a liver biopsy specimen confirmed recurrent metastatic ER+/PR−/HER2− BC. Brain MRI performed for dizziness revealed millimeter-size lesions in the right parietal and left posterior frontal lobes and the left frontal lobe. The patient received stereotactic radiation followed by therapy with fulvestrant, palbociclib, and denosumab. At 3 months, capecitabine was started to treat progressing liver metastases. Follow-up MRI demonstrated at least 15 new intraparenchymal lesions and several progressing dural-based lesions, so the patient proceeded to undergo WBRT and restarted capecitabine therapy. Restaging 6 weeks later revealed disease progression in the lungs, liver, and brain parenchyma. Examination of a liquid biopsy specimen revealed multiple PI3K mutations (PIK3CA: H1047R, E81K, and E563K) and she was administered alpelisib (300 mg) with exemestane. Her BMI was 22 and ECOG PS score was 1. The patient’s pretreatment HbA1c value was 6.3%; despite avoiding sugars, she required admission for hyperglycemia after starting alpelisib treatment and glucose levels have been difficult to control. Restaging after 6 weeks of therapy revealed substantial disease regression in the lungs and liver as well as interval resolution of punctate cerebellar and cerebral metastasis and reduction of a dural metastasis (Appendix Fig A1), compatible with stable disease per RANO-BM criteria.14 Repeated brain MRI at the 3- and 5-month marks showed stable disease.
DISCUSSION
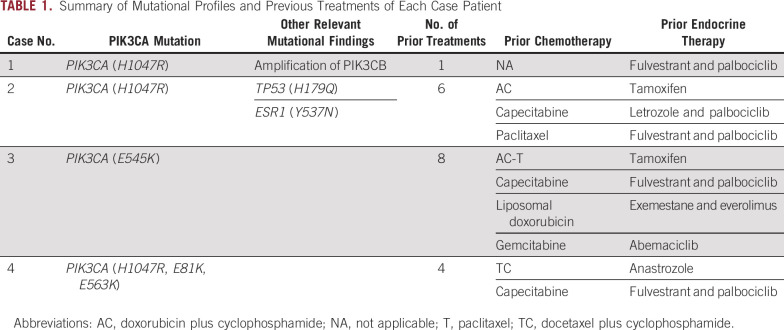
Activating PIK3CA mutations occur early in breast carcinogenesis and are typically not lost or acquired during clonal evolution in later stages of the disease—features that suggest these are driver mutations. Table 1 provides a summary of mutational profile and previous treatments. Cases 1 and 2 harbored the activating mutation PIK3CA H1047R in the coding exon 20, a kinase-activating mutation and the most common mutation in BC.14 Case 1 also had PIK3C2B amplification, which occurs in 13% to 25% of patients.15 PIK3C2B is a class II PI3-kinase.16 Whether its amplification in conjunction with PIK3CA mutation deepens or attenuates PI3K-pathway dependence of cancer and whether PIK3C2B’s kinase activity is inhibited by alpelisib is unknown. However, understanding this relationship appears to be important because activating PIK3CA mutations and copy number gain of PIK3C2B do co-occur in BC.15 Case 3 harbored the PIK3CA E545K mutation, affecting the helical domain of p110-α that activates signaling because of its detachment from the inhibitory p85 subunit of PI3K.17 Helical domain mutations are the second most frequent in BC, with an incidence of 6.4%.14 Case 4 had 3 mutations in PIK3CA; E81K and H1047R are activating mutations and E563K is novel. This combination of a major (H1047R) and a minor (E81K) PIK3CA mutation is thought to amplify PI3K signaling and predict for responsiveness to PI3K inhibition.18
TABLE 1.
Summary of Mutational Profiles and Previous Treatments of Each Case Patient
This patient had a comutation of PIK3CA mutations and ESR1. These are uncommon and accounted for 10% in a small study with 86 ER+ endocrine therapy–resistant metastatic BC.19,20 Our query of the 453 samples of metastatic BC available via cBioPortal (Memorial Sloan Kettering Cancer Center, New York, NY), indicated a tendency for co-occurrence of these 2 mutations (log2 odds ratio, 1.518; P = .003).21 Data specific to brain metastasis were not available for such analysis. This case was also notable for discordance at the time of progression: liver lesions progressed whereas brain lesions did not, possibly a result of the outgrowth of clones with alterations that convey resistance such as loss of PTEN.22
In the SOLAR-1 study, the majority of PIK3CA mutations were activating mutations in p110α H1047(X) (54%), and although the hazard ratio was similarly favorable for all mutations, the CIs in the H1047(X) group were the most narrow, meaning these patients most reliably responded to alpelisib.7 Consistently, 75% of patients in our series also had a H1047(X) mutation.
A recent study showed that PIK3CA-mutant BC not only has a higher probability to metastasize to the brain than nonmutant BC (31% v 17%) but also has shorter median overall survival after the diagnosis of CNS metastasis (0.5 v 1.1 year).9 The high frequency of brain metastases and their poor prognosis raise the question whether PI3K inhibitors can be beneficial for patients with active brain metastases. The earlier pan-PI3K inhibitor buparlisib was tested in 4 patients with treatment-refractory CNS lymphoma, 1 of whom achieved a partial remission.23 In animal models, systemically administered buparlisib showed activity against BC brain metastases.24-26 Patients with active brain metastases were excluded from the pivotal SOLAR-1 and SANDPIPER studies.6,7
Conclusions from our observations are limited by the small number of patients selected on the basis of their surprising response. The RANO-BM criteria consider lesions < 10 mm as nonmeasurable.14 Here, we show examples with resolution of small lesions, which are clinically meaningful but classified as stable disease per the criteria. Nevertheless, our observations suggest the presence of CNS metastases may lead to sufficient disruption of the blood-brain barrier to enable drug activity in the CNS. Additional investigation to prospectively evaluate alpelisib in patients with BC and CNS involvement may be justified.
In conclusion, we have described cases of regression or stabilization of progressive CNS lesions in patients with HR+/HER2− metastatic BC treated with the PI3K inhibitor alpelisib. On the basis of these observations, we believe additional clinical investigation of PI3K inhibition in patients with brain metastases is warranted.
Appendix
FIG. A1.
Additional cuts showing further evidence of response in case 4. Left image was taken before treatment with alpelisib and exemestane; the right image was taken posttreatment. White arrows are directed to lesions, most of which were no longer visible after treatment.
Footnotes
Supported by the Breast Cancer Research Foundation (G.W. and N.U.L.), the National Comprehensive Cancer Network and Pfizer Independent Grants for Learning & Change (N.U.L.), and the National Institutes of Health (Grant No. NIH R01 1R01CA226776 [G.W.]).
AUTHOR CONTRIBUTIONS
Conception and design: Felipe Batalini, Stacy L. Moulder, Eric P. Winer, Nancy U. Lin, Gerburg M. Wulf
Financial support: Gerburg M. Wulf
Administrative support: Nancy U. Lin
Provision of study material or patients: Felipe Batalini, Stacy L. Moulder, Hope S. Rugo, Nancy U. Lin, Gerburg M. Wulf
Collection and assembly of data: Felipe Batalini, Stacy L. Moulder, Hope S. Rugo, Nancy U. Lin, Gerburg M. Wulf
Data analysis and interpretation: Felipe Batalini, Stacy L. Moulder, Hope S. Rugo, Nancy U. Lin, Gerburg M. Wulf
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Stacy Moulder
Research Funding: Oncothyreon (Inst), Pfizer (Inst), Novartis (Inst), Genentech (Inst), Takeda (Inst), Bayer (Inst), EMD Serono (Inst), Genentech (Inst)
Eric P. Winer
Stock and Other Ownership Interests: Verastem
Honoraria: Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Eli Lilly, Genomic Health, G1 Therapeutics
Research Funding: Genentech (Inst), Novartis (Inst)
Hope S. Rugo
Consulting or Advisory Role: Ionis, Celtrion
Research Funding: Macrogenics (Inst), OBI Pharma (Inst), Eisai (Inst), Pfizer (Inst), Novartis (Inst), Eli Lilly (Inst), Genentech (Inst), Merck (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Daiichi Sankyo (Inst), Seattle Genetics (Inst)
Travel, Accommodations, Expenses: Pfizer, Puma Biotechnology, Mylan, Amgen, AstraZeneca, Macrogenics, Daiichi Sankyo, Merck, Novartis, OBI Pharma
Open Payments Link: https://openpaymentsdata.cms.gov/summary
Nancy U. Lin
Consulting or Advisory Role: Roche, Seattle Genetics, Puma Biotechnology, Novartis, Daiichi Sankyo
Research Funding: Genentech, Pfizer, Seattle Genetics, Merck
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties from Jones & Bartlett
Gerburg M. Wulf
Stock and Other Ownership Interests: Selecta Biosciences (I)
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: Pin1 as a marker for abnormal cell growth (Patent No.: 8129131)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loi S, Michiels S, Baselga J, et al. Correction: PIK3CA genotype and a PIK3CA mutation-related gene signature and response to everolimus and letrozole in estrogen receptor positive breast cancer. PLoS One. 2019;14:e0216175. doi: 10.1371/journal.pone.0216175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christgen M, Noskowicz M, Schipper E, et al. Oncogenic PIK3CA mutations in lobular breast cancer progression. Genes Chromosomes Cancer. 2013;52:69–80. doi: 10.1002/gcc.22007. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez-Ardila DE, Helmijr JC, Look MP, et al. Hotspot mutations in PIK3CA associate with first-line treatment outcome for aromatase inhibitors but not for tamoxifen. Breast Cancer Res Treat. 2013;139:39–49. doi: 10.1007/s10549-013-2529-7. [DOI] [PubMed] [Google Scholar]
- 5.Kalinsky K, Jacks LM, Heguy A, et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15:5049–5059. doi: 10.1158/1078-0432.CCR-09-0632. [DOI] [PubMed] [Google Scholar]
- 6.Baselga J, Cortes Castan J, De Laurentiis M, et al. SANDPIPER: Phase III study of the PI3-kinase (PI3K) inhibitor taselisib (GDC-0032) plus fulvestrant in patients (pts) with oestrogen receptor (ER)-positive, HER2-negative locally advanced or metastatic breast cancer (BC) enriched for pts with PIK3CA-mutant tumours. Ann Oncol. 2016;27:vi99. (suppl 6) [Google Scholar]
- 7.André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 8.Adamo B, Deal AM, Burrows E, et al. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res. 2011;13:R125. doi: 10.1186/bcr3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald DM, Muzikansky A, Pinto C, et al. Association between PIK3CA mutation status and development of brain metastases in HR+/HER2- metastatic breast cancer. Ann Oncol. 2019;30:v110. (suppl 5) [Google Scholar]
- 10.Bergen ES, Berghoff AS, Medjedovic M, et al. Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res. 2019;25:2737–2744. doi: 10.1158/1078-0432.CCR-18-1968. [DOI] [PubMed] [Google Scholar]
- 11.Tolaney SM, Lin NU, Thornton D, et al. Abemaciclib for the treatment of brain metastases (BM) secondary to hormone receptor positive (HR+), HER2 negative breast cancer. J Clin Oncol. 2017;35(15 suppl):1019. [Google Scholar]
- 12.Ippen FM, Alvarez-Breckenridge CA, Kuter BM, et al. The dual PI3K/mTOR pathway inhibitor GDC-0084 achieves antitumor activity in PIK3CA-mutant breast cancer brain metastases. Clin Cancer Res. 2019;25:3374–3383. doi: 10.1158/1078-0432.CCR-18-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campone M, Im S-A, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: Overall survival results from BELLE-2. Eur J Cancer. 2018;103:147–154. doi: 10.1016/j.ejca.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015;16:e270–e278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga The Cancer Genome Atlas Program. 2018.
- 16.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maffucci T, Cooke FT, Foster FM, et al. Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J Cell Biol. 2005;169:789–799. doi: 10.1083/jcb.200408005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leontiadou H, Galdadas I, Athanasiou C, et al. Insights into the mechanism of the PIK3CA E545K activating mutation using MD simulations. Sci Rep. 2018;8:15544. doi: 10.1038/s41598-018-27044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasan N, Razavi P, Johnson JL, et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science. 2019;366:714–723. doi: 10.1126/science.aaw9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, et al. Analysis of ESR1 and PIK3CA mutations in plasma cell-free DNA from ER-positive breast cancer patients. Oncotarget. 2017;8:52142–52155. doi: 10.18632/oncotarget.18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. https://www.cbioportal.org/results/oncoprint?Action=Submit&RPPA_SCORE_THRESHOLD=2.0&Z_SCORE_THRESHOLD=2.0&cancer_study_list=brca_igr_2015%2Cbrca_mbcproject_wagle_2017&case_set_id=all&data_priority=0&gene_list=ESR1%253AMUT%250APIK3CA%253AMUT&geneset_list=%20&profileFilter=0&tab_index=tab_visualize cBioPortal for Cancer Genomics.
- 22.Juric D, Castel P, Griffith M, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature. 2015;518:240–244. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grommes C, Pentsova E, Nolan C, et al. Phase II study of single agent buparlisib in recurrent/refractory primary (PCNSL) and secondary CNS lymphoma (SCNSL) Ann Oncol. 2016;27:vi106. (suppl 6) [Google Scholar]
- 24.Maira S-M, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Ide JL, Norton I, et al. Molecular imaging of drug transit through the blood-brain barrier with MALDI mass spectrometry imaging. Sci Rep. 2013;3:2859. doi: 10.1038/srep02859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendell JC, Rodon J, Burris HA, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]