INTRODUCTION
Lung cancer is a worldwide, known disease with a high mortality rate and is often related to nicotine abuse. Family-related lung cancer has been reported occasionally, but little is known about the genetic backgrounds. With routine analysis for targetable mutations, unexpected potentially pathogenic mutations may be found. Here, we present a case study of a patient with a targetable somatic EGFR mutation and a novel EGFR germline variant, which also occurred in other family members with lung cancer.
INDEX PATIENT
Our female index patient of Surinam origin, a former smoker with a 10 pack-year history, was diagnosed at the age of 57 years with non–small-cell lung cancer (NSCLC), stage IIIA cT2bN2M0 adenocarcinoma. Initial treatment included concurrent chemotherapy and radiotherapy, and palliative chemotherapy after recurrence (the treatment timeline is shown in Fig 1). After a second recurrence, a new biopsy of a lymph node metastasis was obtained for EGFR mutation analysis by next-generation sequencing. This analysis revealed a well-known oncogenic L858R mutation along with a V834L variant, which are both EGFR exon 21 substitutions and are present in the same allele (in cis). The V834L variant seemed to be present in the germline, because both normal adjacent lymph node tissue and lymphocyte-derived DNA from whole blood of the index patient showed this variant.
Fig 1.
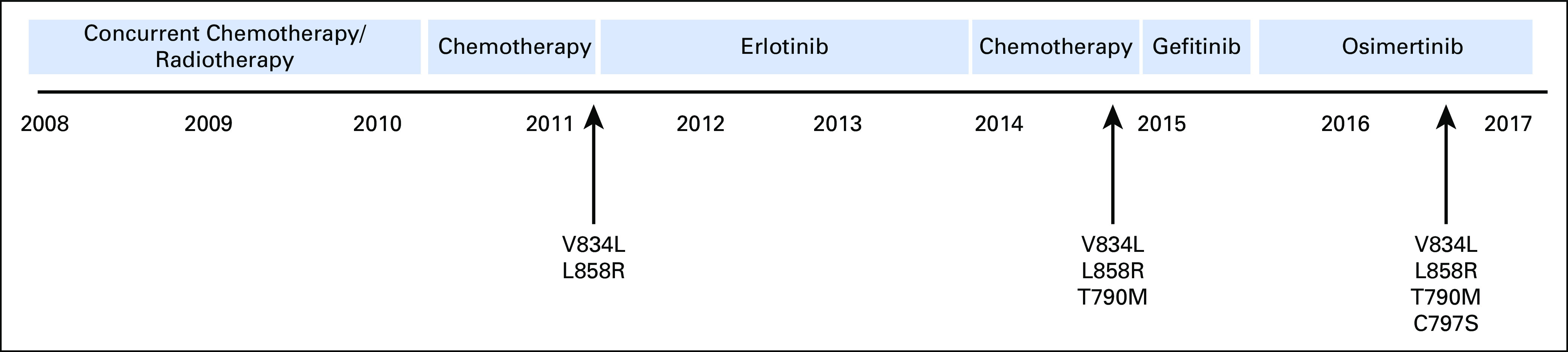
Timeline of treatment and detection of acquired somatic EGFR mutations of the index patient. The germline EGFR variant V834L is indicated at all time points.
The patient was treated with the EGFR tyrosine kinase inhibitor (TKI) erlotinib. At the fourth progression of the disease, the patient was retreated with chemotherapy. After the fifth progression of the disease, a new biopsy revealed a first-generation TKI-resistance EGFR T790M mutation, in addition to L858R and V834L. Therefore, treatment was switched to the third-generation EGFR TKI osimertinib. Because of progression after 1 year of osimertinib treatment, a liquid biopsy was taken and cell-free DNA was isolated. Subsequent mutation analysis showed that the progression was probably because of the development of EGFR resistance mutation C797S in cis with T790M (data not shown). Performance of the patient was declining, and there were no remaining therapeutic options. Best supportive care was given, and the patient eventually died.
PATIENT’S FAMILY HISTORY WAS POSITIVE FOR LUNG CANCER
The patient’s younger brother, who was 57 years old and a current smoker, was diagnosed in 2016 with stage IV, EGFR L858R mutation–positive NSCLC. He was treated with erlotinib, and his disease progressed after 3 months. A new biopsy was obtained, revealing no targetable secondary resistance mechanism, such as T790M mutation or MET amplification. Standard chemotherapy was started, but the patient died because of disease progression and decline in performance status.
A sister and daughter of our index patient, 46 and 42 years old, respectively, both nonsmokers, were diagnosed with stage IV NSCLC before the knowledge and treatment options of EGFR mutations. They were both treated with palliative chemotherapy but died as a result of disease progression. The patient’s father died at a young age as the result of massive hemoptysis, without a diagnosis.
After written consent and permission were obtained from the index patient and family members, EGFR mutation and germline variation analyses were performed on tumor samples and healthy tissue, respectively, of the three affected relatives. Because our study started after the deaths of the index patient’s brother, sister, and daughter, no blood was available; only archival formalin-fixed paraffin-embedded normal and tumor tissues were available.
Like our index patient, all three affected family members had a heterozygous EGFR V834L germline variant in normal tissue and the same somatic EGFR L858R mutation in cis with the V834L variant in their respective tumor tissues. No tissue samples from the patient’s father were available.
Further germline research was performed in four healthy family members who did not have signs of lung cancer. One of the index patient’s children, who was 36 years old, showed the same heterozygous EGFR V834L germline variant. All other investigated family members were wild type at this position in EGFR (Fig 2).
Fig 2.
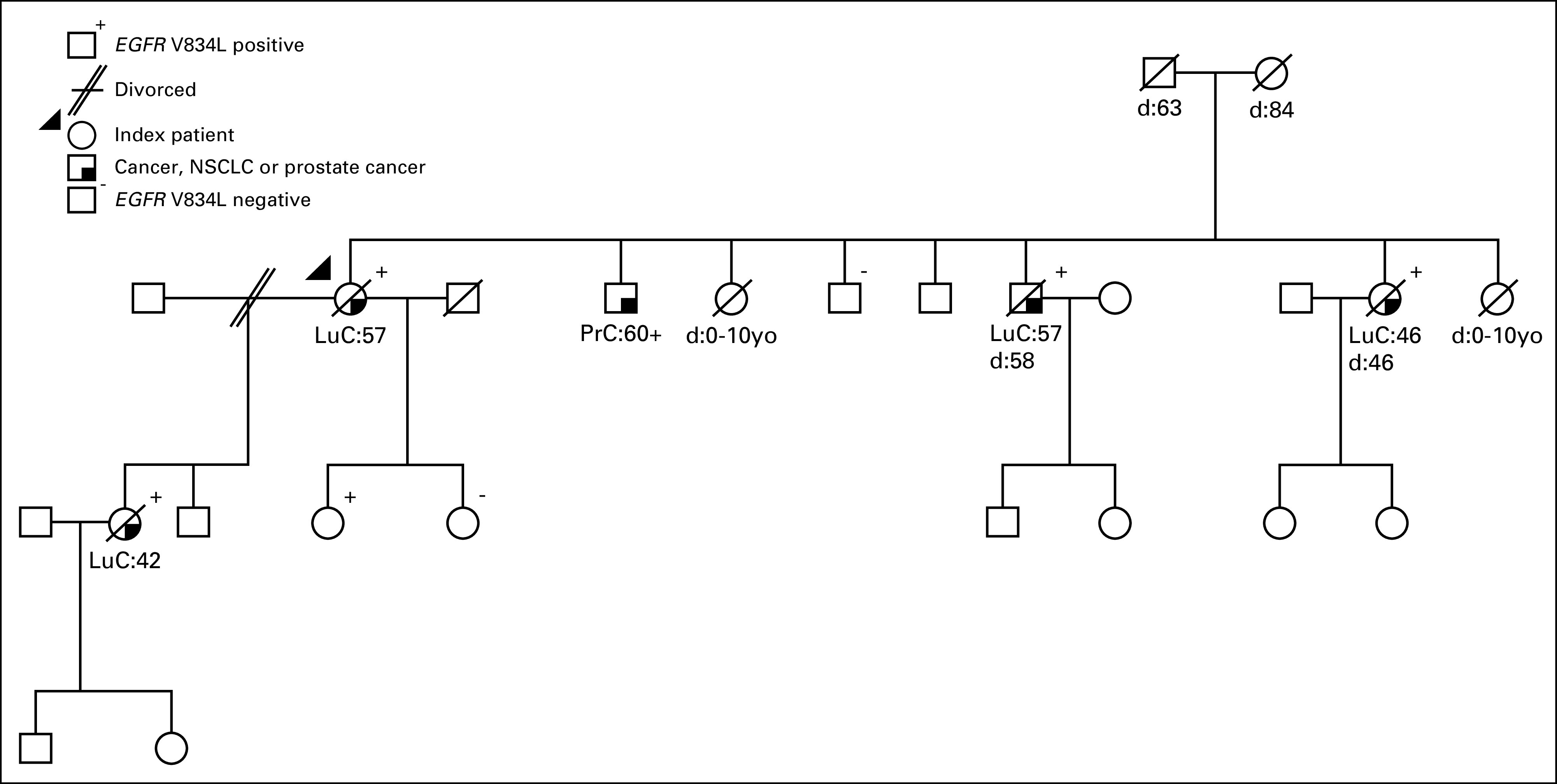
Pedigree of a family with non–small-cell lung cancer (NSCLC) associated with the presence of an EGFR V834L germline variant. Squares and circles represent male and female family members, respectively. Diagonal strikes represent deceased family members. Age at diagnosis of the indicated cancer or at death (d) is indicated below the squares and circles. LuC, lung cancer; PrC, prostate cancer; yo, years old.
DISCUSSION
Little is known about hereditary lung cancer. This is probably in part because lung cancer is strongly associated with smoking and therefore a familial association may be easily missed. To our knowledge, this is the first report of a family with multiple cases of NSCLC associated with germline transmission of an EGFR V834L germline variant; all tumors in the four family members additionally harbored a somatic EGFR L858R mutation in cis. All except one of the known V834L carriers developed lung cancer during their lifespan, but so far none of the investigated noncarriers.
There are only a few reports documenting families with proven inherited EGFR variants over generations that probably conferred predisposition to lung cancer.1,2 In a family with EGFR V843I mutations, four family members in two generations (three of them with proven germline variants) developed lung adenocarcinoma.1 As in the family we studied, all of these adenocarcinomas displayed a somatically-acquired L858R mutation in cis with V843I. The index patient in the family with EGFR V843I mutations was treated with erlotinib and gefitinib, but without response, even though the L858R mutation was present. Also, in vitro, the V843I substitution conferred TKI resistance. Yu et al2 described a family carrying a germline T790M mutation. In their study, both the index patient and her mother developed lung adenocarcinomas. Interestingly, multiple distinct synchronous tumors of the index patient displayed different additional EGFR mutations (ie, deletions of 3 and 15 bp in exon 19 and L858R).2 A report by Bell et al3 describes a family with five members affected by lung cancer. Proven germline EGFR T790M mutations were present in three patients in two generations.3 In distinct lesions in two of these patients, the mutation co-occurred with different EGFR substitutions (L858R, L747-T751del, and G716A), all in cis with T790M. Because T790M is a well-known TKI resistance mutation, no response was observed upon gefitinib treatment in the only TKI-treated patient of this family, as expected.
Incidental cases of germline EGFR variants in patients with lung cancer have been reported. These have included variants such as V769M, R776H, and T790M substitutions in exon 20 and R831C, V843I, and P848L substitutions in exon 21.4-9 However, in the absence of family histories, it is unclear whether these variants predisposed these patients to lung cancer. Importantly, as described above, these germline variants often co-occur in the tumor with other, mostly well-known, actionable EGFR mutations. This strongly indicates that somatically acquired secondary EGFR mutations are indispensable for tumorigenesis.
We hypothesize that presence of the EGFR V834L germline variant is causative for the high incidence and early onset of lung cancer in the family we studied. So far, all lung cancers in this family have been found to harbor both the EGFR V834L variant and the well-described oncogenic EGFR L858R mutation. Although this is a relatively small cohort, this may indicate that the V834L variant is not or only weakly oncogenic and by itself is not sufficient to drive uncontrolled growth. However, when a second oncogenic mutation in cis is present, cell growth accelerates more than proportionally.
This hypothesis is in line with eight described EGFR V834L variants found in the Catalogue Of Somatic Mutations In Cancer database.9 The V834L variant in the lung tumors of all of these patients, with unknown germline status, is accompanied by the L858R mutation (seven times) or by an exon 19 deletion.10-12 The reason for the apparent preference of V834L for L858R is unclear. Such a preference is not unique (eg, the germline variant EGFR V769M preferentially associates with mutations of codon G719 and/or S768). This is possibly because the energy balance of the EGFR V769M–substituted protein does not favor combination with L858R.9 Structural and functional analysis of the V834L variant alone and/or in combination with strong oncogenic driver mutations can provide additional mechanistic information on its pathogenicity and preferred association with other oncogenic EGFR mutations.
Until now, lung cancer surveillance programs have been mainly recommended for active smokers or patients with a smoking history of 30 pack-years but who have stopped within the last 15 years. Although smoking is unmistakably associated with lung cancer development, increased awareness of genetic factors that predispose individuals to lung cancer is necessary. Currently, the prevalence of germline EGFR T790M mutations in patients with lung cancer and their relatives is being studied in the INHERIT EGFR trial (NCT01754025), to further investigate the role of EGFR mutations in lung cancer development. For people with a germline EGFR mutation and/or a strong family history of lung cancer, clinical follow-up may be recommended.
In conclusion, we describe a novel EGFR V834L germline mutation illustrating that genetic factors may play an important role in lung cancer predisposition and should be evaluated to optimize surveillance and clinical genetic counseling.
AUTHOR CONTRIBUTIONS
Conception and design: Joachim G. Aerts, Winand N.M. Dinjens, Hendrikus J. Dubbink
Provision of study material or patients: Cor van der Leest, Anja Wagner
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Cor van der Leest
Honoraria: Roche, Boehringer Ingelheim, Bristol-Myers Squibb
Consulting or Advisory Role: Roche, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb
Anja Wagner
No relationship to disclose
Rute M. Pedrosa
No relationship to disclose
Joachim G. Aerts
Stock and Other Ownership Interests: Amphera
Consulting or Advisory Role: Eli Lilly, Genentech, Bristol-Myers Squibb, MSD Oncology, Boehringer Ingelheim, Amphera
Speakers’ Bureau: AstraZeneca
Patents, Royalties, Other Intellectual Property: An allogenic lysate for vaccination (Inst)
Winand N.M. Dinjens
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, AstraZeneca (Inst)
Hendrikus J. Dubbink
Consulting or Advisory Role: Pfizer
Research Funding: AstraZeneca
REFERENCES
- 1.Ohtsuka K, Ohnishi H, Kurai D, et al. Familial lung adenocarcinoma caused by the EGFR V843I germ-line mutation. J Clin Oncol. 2011;29:e191–e192. doi: 10.1200/JCO.2010.31.4492. [DOI] [PubMed] [Google Scholar]
- 2.Yu HA, Arcila ME, Harlan Fleischut M, et al. Germline EGFR T790M mutation found in multiple members of a familial cohort. J Thorac Oncol. 2014;9:554–558. doi: 10.1097/JTO.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell DW, Gore I, Okimoto RA, et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat Genet. 2005;37:1315–1316. doi: 10.1038/ng1671. [DOI] [PubMed] [Google Scholar]
- 4.van Noesel J, van der Ven WH, van Os TA, et al. Activating germline R776H mutation in the epidermal growth factor receptor associated with lung cancer with squamous differentiation. J Clin Oncol. 2013;31:e161–e164. doi: 10.1200/JCO.2012.42.1586. [DOI] [PubMed] [Google Scholar]
- 5.Prudkin L, Tang X, Wistuba II. Germ-line and somatic presentations of the EGFR T790M mutation in lung cancer. J Thorac Oncol. 2009;4:139–141. doi: 10.1097/JTO.0b013e3181915f92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prim N, Legrain M, Guerin E, et al. Germ-line exon 21 EGFR mutations, V843I and P848L, in nonsmall cell lung cancer patients. Eur Respir Rev. 2014;23:390–392. doi: 10.1183/09059180.00009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demierre N, Zoete V, Michielin O, et al. A dramatic lung cancer course in a patient with a rare EGFR germline mutation exon 21 V843I: Is EGFR TKI resistance predictable? Lung Cancer. 2013;80:81–84. doi: 10.1016/j.lungcan.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Chung KP, Shih JY, Yu CJ. Favorable response to gefitinib treatment of lung adenocarcinoma with coexisting germline and somatic epidermal growth factor receptor mutations. J Clin Oncol. 2010;28:e701–e703. doi: 10.1200/JCO.2010.28.6260. [DOI] [PubMed] [Google Scholar]
- 9.Hellmann MD, Hayashi T, Reva B, et al. Identification and functional characterization of EGFR V769M, a novel germline variant associated with multiple lung adenocarcinomas. JCO Precis Oncol. doi: 10.1200/PO.16.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu SG, Chang YL, Hsu YC, et al. Good response to gefitinib in lung adenocarcinoma of complex epidermal growth factor receptor (EGFR) mutations with the classical mutation pattern. Oncologist. 2008;13:1276–1284. doi: 10.1634/theoncologist.2008-0093. [DOI] [PubMed] [Google Scholar]
- 12.Tam IY, Chung LP, Suen WS, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res. 2006;12:1647–1653. doi: 10.1158/1078-0432.CCR-05-1981. [DOI] [PubMed] [Google Scholar]


