Abstract
PURPOSE
To study whether BRAF V600 mutations in non–small-cell lung cancer (NSCLC) may indicate sensitivity to the BRAF inhibitor vemurafenib, we included a cohort of patients with NSCLC in the vemurafenib basket (VE-BASKET) study. On the basis of observed early clinical activity, we expanded the cohort of patients with NSCLC. We present results from this cohort.
METHODS
This open-label, histology-independent, phase II study included six prespecified cohorts, including patients with NSCLC, and a seventh all-comers cohort. Patients received vemurafenib (960 mg two times per day) until disease progression or unacceptable toxicity. The primary end point of the final analysis was objective response rate (Response Evaluation Criteria in Solid Tumors, version 1.1). Secondary end points included progression-free survival, overall survival, and safety. Because the prespecified clinical benefit endpoint was met in the initial NSCLC cohort, the cohort was expanded.
RESULTS
Sixty-two patients with BRAF V600–mutant NSCLC were enrolled and treated: 13% (n = 8) had received no prior systemic therapy, and 87% (n = 54) had received prior therapies. The objective response rate was 37.1% (95% CI, 25.2% to 50.3%) overall, 37.5% (95% CI, 8.5% to 75.5%) in previously untreated patients, and 37.0% (24.3% to 51.3%) in previously treated patients. Median progression-free survival was 6.5 months (95% CI, 5.2 to 9.0 months), and median overall survival was 15.4 months (95% CI, 9.6 to 22.8 months). The most common all-grade adverse event was nausea (40%). The safety profile of vemurafenib was similar to that observed in melanoma studies.
CONCLUSION
Vemurafenib showed promising activity in patients with NSCLC harboring BRAF V600 mutations. The safety profile of vemurafenib was similar to previous observations in patients with melanoma. Our results suggest a role for single-agent BRAF inhibition in patients with NSCLC and BRAF V600 mutations.
INTRODUCTION
Identification of oncogenic activation of tyrosine kinases in patients with non–small-cell lung cancer (NSCLC), such as mutations in the epidermal growth factor receptor (EGFR) gene and rearrangements of the anaplastic lymphoma kinase (ALK) or ROS1 genes, has enabled the development of targeted treatments for patients with NSCLC.1-3 This has resulted in the recognition of histologically and genetically diverse NSCLC subtypes and led to a targeted therapy approach for selected patients.4 Despite these developments, a considerable proportion of patients fail to benefit from currently available treatment regimens and need new treatment approaches.
BRAF V600 mutations occur in an estimated 1% to 4% of patients with NSCLC.5,6 Among patients with BRAF-mutated NSCLC, the most common aberration is the BRAF V600E mutation, which occurs in 50% of patients.7 In the melanoma setting, where BRAF V600 mutations are common, targeted treatment of patients with BRAF V600 mutation-positive metastatic melanoma using the BRAF kinase inhibitors dabrafenib and vemurafenib was associated with high response rates and improved survival compared with chemotherapy.8-10 Furthermore, superior outcomes were observed with dual inhibition of BRAF and MEK.11,12 Recently, BRAF inhibition was also shown to be effective in patients with BRAF V600–mutated NSCLC in a retrospective cohort study13 and in a clinical study of patients with BRAF V600E–mutated NSCLC.14 Dual BRAF/MEK inhibition has also been investigated as first- and second-line treatment of patients with NSCLC.15,16
We present the results from the expanded NSCLC cohort of the vemurafenib basket (VE-BASKET) trial. This trial assessed the efficacy of vemurafenib in seven cohorts of patients with BRAF V600–mutated malignancies.17
CONTEXT
Key Objective
To establish the efficacy and safety of vemurafenib in patients with BRAF V600 mutation-positive NSCLC who were enrolled in the histology-independent vemurafenib basket (VE-BASKET) trial.
Knowledge Generated
Vemurafenib has prolonged efficacy in patients with BRAF V600–mutant NSCLC (n = 62), as demonstrated by a 37% overall response rate. Response rates were similar in previously treated and untreated patients. Median progression-free survival was 6.5 months, and the median overall survival was 15.4 months; median overall survival was not reached in previously untreated patients. Clinical benefit rates for previously treated and untreated patients were 46% and 63%, respectively. No new safety signals were observed in this expanded cohort of patients with NSCLC.
Relevance
Single-agent vemurafenib has clinically meaningful and durable activity in patients with NSCLC harboring BRAF V600 mutations. This analysis adds to the overall findings of the VE-BASKET trial, which demonstrated clinically relevant activity of vemurafenib in a number of solid tumors.
METHODS
Study Design
The VE-BASKET study was a multicenter, single-arm, phase II study of vemurafenib in patients with a variety of nonmelanoma cancers harboring BRAF V600 mutations. BRAF V600 mutations were identified by means of mutational analysis assays routinely performed at each participating site. The clinical trial did not require central confirmation for this cohort. Six prespecified cohorts were recruited, consisting of patients with NSCLC, ovarian cancer, colorectal cancer, cholangiocarcinoma, breast cancer, and multiple myeloma; all patients with solid tumors other than those mentioned were included in a seventh cohort. Patients were treated with vemurafenib (960 mg orally two times per day) as a single agent. The design of this study has been described in detail elsewhere.17
This trial was performed in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by institutional review boards or human research ethics committees at the participating centers. All patients provided written informed consent.
Patients
Patients were eligible for inclusion in the study if they were 16 years of age or older and had histologically confirmed, measurable (Response Evaluation Criteria in Solid Tumors [RECIST], version 1.1), BRAF V600 mutation-positive cancers that were refractory to standard therapy or for which standard or curative therapy did not exist or was not considered appropriate by the investigator. Patients with solid tumors were required to have adequate hematologic, renal, and liver function. Patients with active or untreated CNS metastases were excluded. Prior treatment with a BRAF or MEK inhibitor was not allowed.
Assessments
Response was assessed by the investigators according to RECIST (version 1.1). Assessments were performed using computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis at baseline and then every 8 weeks until disease progression, death, or withdrawal from the study. Adverse events (AEs) were graded by the investigators using National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0) until 28 days after discontinuation of study treatment. AEs of special interest were cutaneous squamous cell carcinoma (SCC; keratoacanthoma, squamous cell carcinoma of the skin, and Bowen disease), fatigue (fatigue and asthenia), liver injury (increased ALT, AST, blood alkaline phosphatase, blood bilirubin, and gamma-glutamyltransferase; hyperbilirubinemia, hepatocellular injury, and cholestatic jaundice), and prolonged QT interval. Patients were assessed for AEs at each clinic visit and as necessary throughout the study.
Outcomes
The primary objective of the study was to evaluate the efficacy of vemurafenib in patients with BRAF V600 mutation-positive cancers. The primary end point for the final analysis in the NSCLC cohort was objective response rate (ORR), defined as the proportion of patients with an objective response (complete response [CR] or partial response [PR]) confirmed on two consecutive occasions 4 or more weeks apart. Efficacy was evaluated by the site investigators according to RECIST (version 1.1). Secondary objectives included assessments of clinical benefit rate (defined as the overall proportion of patients with a CR, PR, or stable disease lasting ≥ 6 months), duration of response, progression-free survival (PFS), overall survival (OS), and safety. Efficacy data were analyzed separately for patients who had received no prior therapy and for those with prior therapies.
Statistical Analysis
This was a modified, two-stage Simon design study. Stage I was complete when seven patients with measurable disease were enrolled and had completed a minimum of 8 weeks of treatment, developed progressive disease, prematurely withdrew, or died. An additional six or 12 patients could be enrolled, to 13 or 19 patients, depending on the results for stage I; if two, three, or four of the initial seven patients responded to treatment, an additional 12 patients could be enrolled in stage II; if five or more of the initial seven patients responded to treatment, an additional six patients were recruited. Recruitment into any cohort/indication could be further expanded up to 70 patients if a response rate was demonstrated in stage II of that cohort, according to the stopping rules defined in the protocol or a clear clinical benefit for patients was observed, as determined by the steering committee. For the NSCLC cohort, with 50 treated patients, the study would have approximately 90% power for the lower bound of the two-sided 95% CI to exclude 20%, given a true ORR of 40%. The lower bound of the 95% CI was set at 20% because established therapy in the second and later lines had an ORR of less than 20% when the study was designed. PFS, OS, and duration of response were calculated using Kaplan-Meier methods. All analyses were performed using SAS (versions 9.2 and 9.4; SAS Institute, Cary, NC).
RESULTS
Patients and Treatment
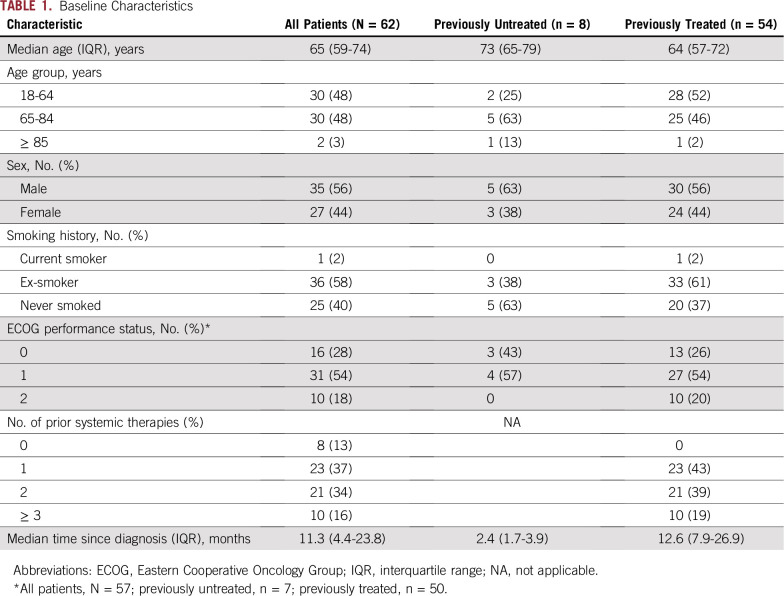
A total of 62 patients with BRAF V600–mutated NSCLC (61 with the V600E mutation and one with an unspecified V600 mutation) were enrolled, eight (13%) of whom were previously untreated (Table 1). Most patients had adenocarcinoma (n = 58; 94%), three patients (4.8%) had CNS metastases, and most were former smokers (n = 36; 58%). Among previously treated patients, the median number of prior systemic regimens was two (interquartile range [IQR], 1 to 2); the most common prior chemotherapies were platinum agents (39 of 54 patients; 72%), pemetrexed (33 of 54 patients; 61%), and taxanes (22 of 54 patients; 41%).
TABLE 1.
Baseline Characteristics
This analysis was performed after a median duration of follow-up of 10.7 months (IQR, 4.3 to 17.1 months). Reasons for vemurafenib discontinuation were progressive disease (41 of 62 patients; 66%), AEs (six of 62 patients; 10%), death (four of 62 patients; 6%), withdrawal by the patient (two of 62 patients; 3%), physician decision (two of 62 patients; 3%), and other reasons in the case of seven patients (11%), six (10%) of whom rolled over into an extension study and one of whom withdrew from the study.
Efficacy
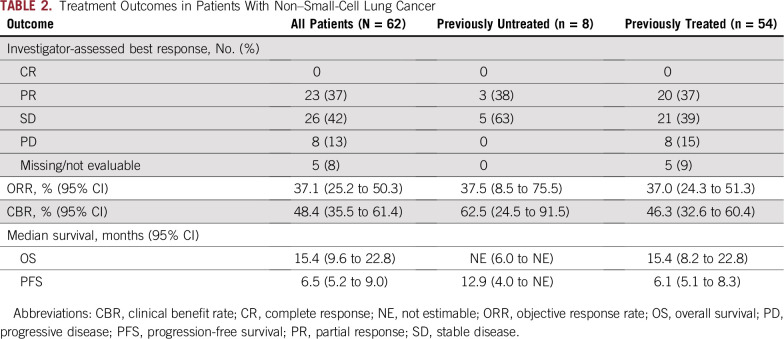
Response to treatment is listed in Table 2 and shown in Figure 1. Overall, the investigator-determined ORR was 37% (95% CI, 25% to 50%), and the clinical benefit (CR plus PR plus stable disease lasting ≥ 6 months) rate was 48% (95% CI, 36% to 61%). Clinical benefit rates for previously treated and untreated patients were 46% (95% CI, 33% to 60%) and 63% (95% CI, 24% to 91%), respectively.
TABLE 2.
Treatment Outcomes in Patients With Non–Small-Cell Lung Cancer
FIG 1.
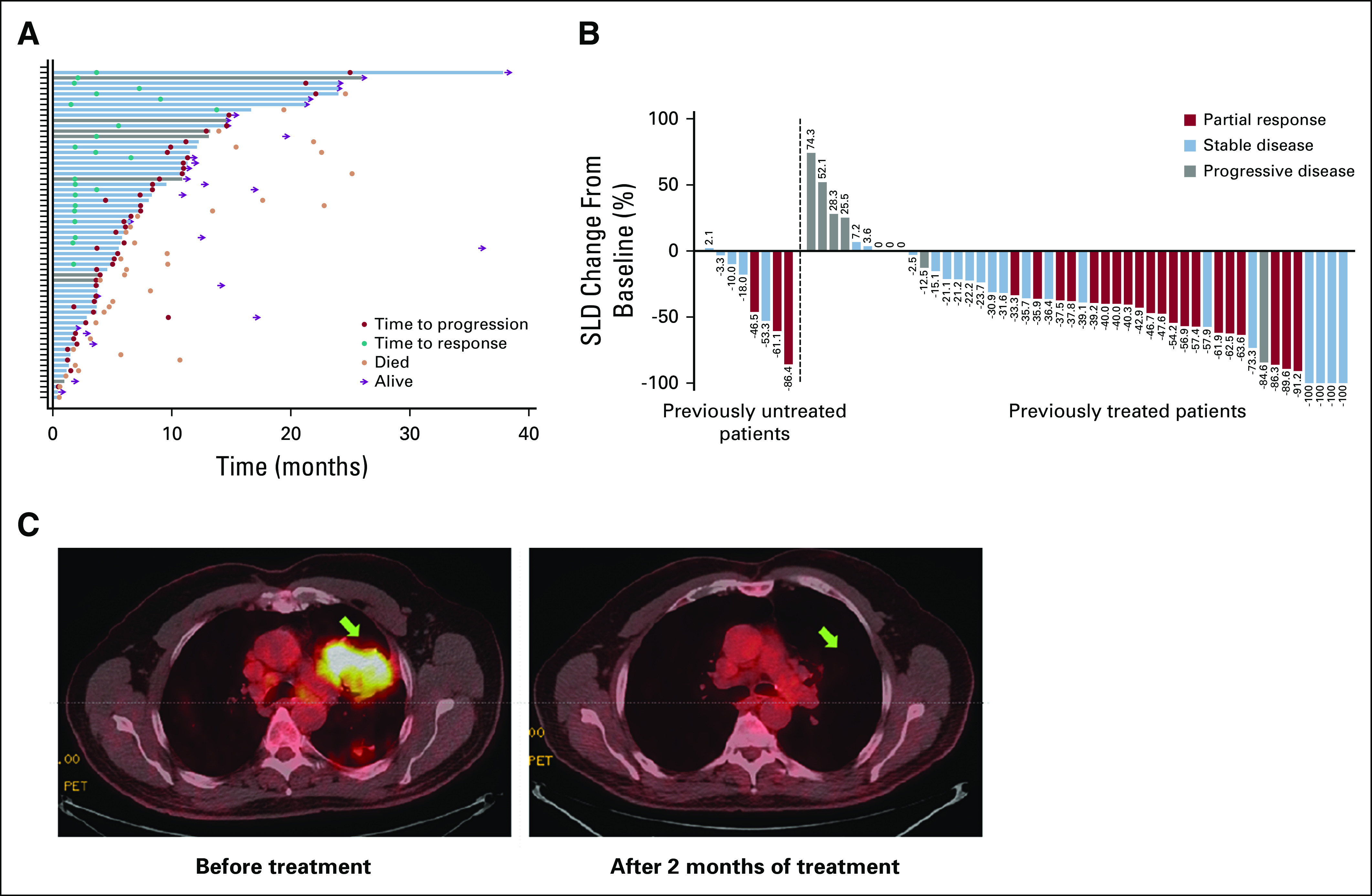
Tumor response to vemurafenib in patients with non–small-cell lung cancer (NSCLC). (A) Plot of time to progression, time to response, and death in individual patients with NSCLC. Blue bars indicate previously treated patients; gray bars indicate previously untreated patients. (B) Waterfall plot of maximum percent decrease from baseline in the sum of diameters of target tumors on the basis of investigator assessment: best overall response in individual patients. (C) Pretherapy and post-therapy 18F-fluorodeoxyglucose positron emission tomography images of a chemotherapy-naive patient with BRAF V600E mutation-positive NSCLC. The patient continues to respond to date. SLD, sum of the longest diameters.
The median duration of response was 7.2 months (95% CI, 5.5 to 18.4 months) in the overall population and 6.1 months (95% CI, 5.5 to 18.4 months) in previously treated patients. The median duration of response was not estimable (NE) in previously untreated patients. Median time to response was 7.3 months (95% CI, 3.7 months to NE) in the overall population and 7.3 months (95% CI, 3.7 to 13.7 months) in previously treated patients; median time to response was NE in previously untreated patients. The three previously untreated patients who responded to vemurafenib treatment had responses lasting 24.0, 7.2, and 9.1 months. At the time of study closure, there was no record of reported disease progression in six responders, including four previously treated and two previously untreated patients.
The median OS was 15.4 months (95% CI, 9.6 to 22.8 months) in the overall population, 15.4 months (95% CI, 8.2 to 22.6 months) in previously treated patients, and NE in previously untreated patients (Fig 2A). OS durations in the five previously untreated patients with censored observations were 26.1, 19.6, 14.6, 11.2, and 1.9 months; OS durations were 6.0, 13.9, and 4.0 months for the three patients who had died at the time of the analysis, all of whom had a best overall response of stable disease.
FIG 2.
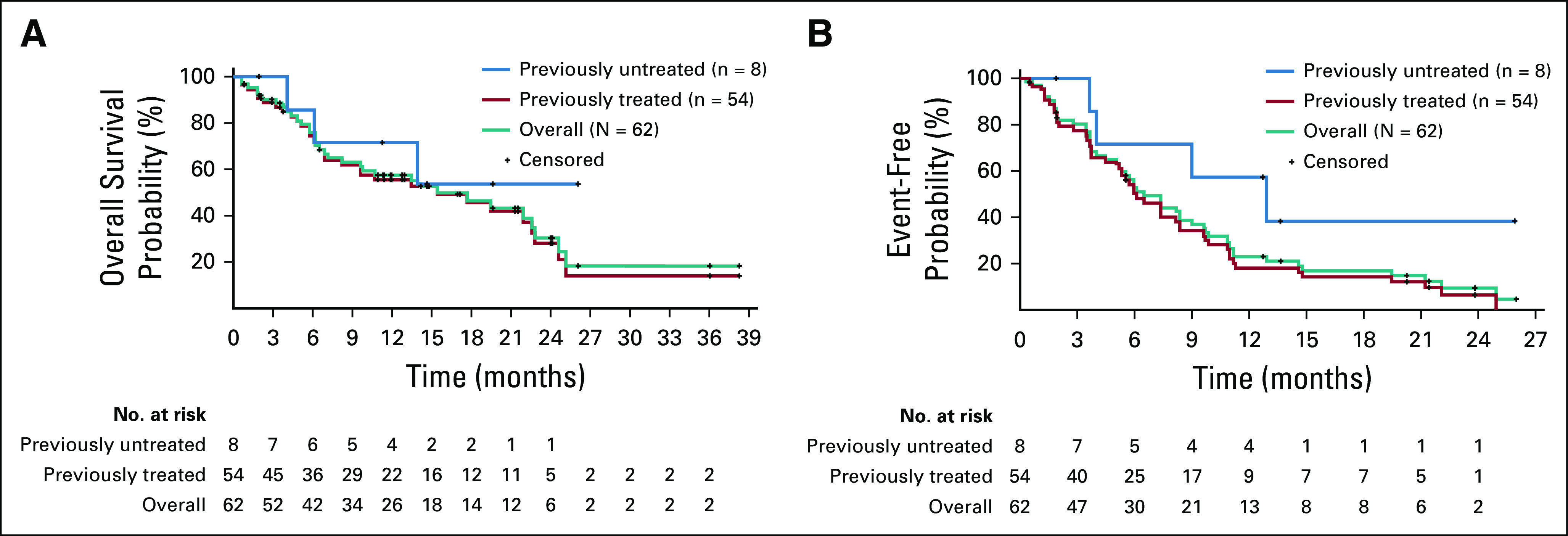
(A) Overall survival and (B) progression-free survival in patients with non–small-cell lung cancer.
Median PFS was 6.5 months (95% CI, 5.2 to 9.0 months) in the overall population and 6.1 months (95% CI, 5.1 to 8.3 months) in previously treated patients (Fig 2B). The median PFS was 12.9 months (95% CI, 4.0 months to NE) in previously untreated patients, four of whom were censored at the time of study closure (PFS: 26.0, 13.6, 1.9, and 12.7 months at study closure).
Safety
The median treatment duration for all patients was 6.0 months (IQR, 2.8 to 11.5 months); the median treatment duration was 5.7 months (IQR, 2.8 to 11.2 months) for previously treated patients and 12.0 months (IQR, 4.0 to 13.9 months) for previously untreated patients. The median relative dose intensity achieved was 78% (IQR, 64% to 91%) overall.
All 62 patients experienced at least one any-cause AE; grade 3 or 4 AEs occurred in 48 patients (77%), and two patients had grade 5 AEs (3%; one patient with sepsis, one with a pulmonary embolism and respiratory failure; both patients had been previously treated, and none of the events were considered to be related to vemurafenib). Table 3 lists all-cause and grade 3 or greater AEs occurring in 20% or more of patients.
TABLE 3.
AEs Occurring in ≥ 20% of Patients Overall (N = 62)
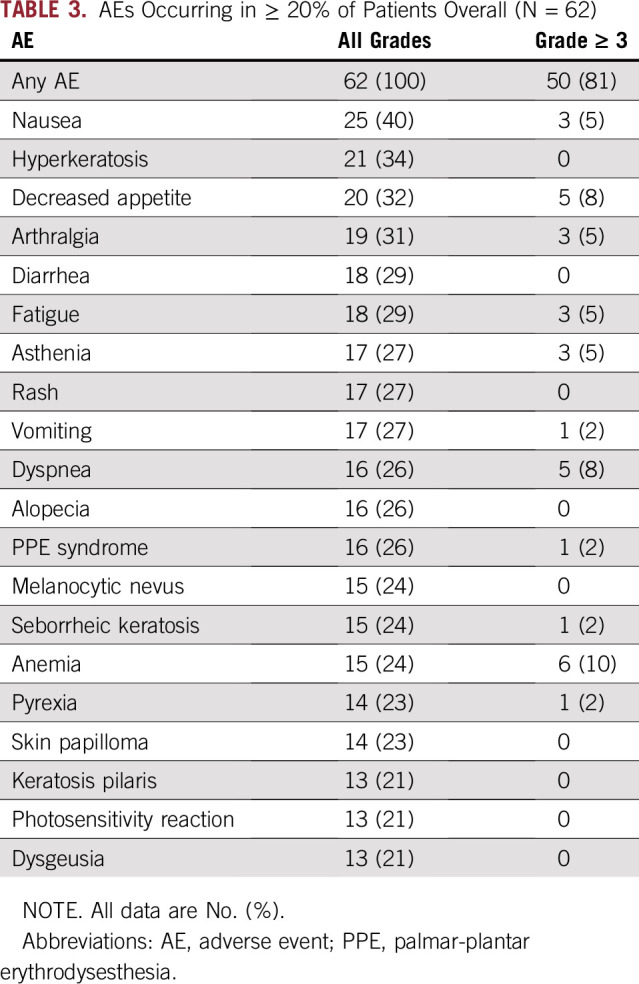
AEs leading to treatment interruption occurred in 25 of 62 patients (40%). The most common of these were sepsis (n = 3; 5%), vomiting (n = 3; 5%), bronchitis (n = 2; 3%), pneumonia (n = 2; 3%), nausea (n = 2; 3%), acute coronary syndrome (n = 2; 3%), and dyspnea (n = 2; 3%). AEs leading to dose reduction occurred in 38 of 62 patients (61%). The most common of these events were arthralgia (n = 6; 10%), fatigue (n = 5; 8%), and decreased appetite (n = 4; 6%). Six patients had AEs that resulted in treatment discontinuation: chronic kidney disease (two of 62 patients; 3%); acute kidney injury (one of 62 patients; 2%); renal failure (one of 62 patients; 2%); lower respiratory tract infection (one of 62 patients; 2%), and oropharyngeal candidiasis and nausea (one of 62 patients; 2%).
AEs of special interest included arthralgia (19 of 62 patients; 31%), cutaneous SCC (including keratoacanthoma; 16 of 62 patients; 26%), fatigue (34 of 62 patients; 55%), prolonged QT interval (11 of 62 patients; 18%), and liver injury (increased ALT, AST, blood alkaline phosphatase, bilirubin, and gamma-glutamyltransferase, as well as the hepatobiliary disorders hyperbilirubinemia, hepatocellular injury, and cholestatic jaundice; 16 of 62 patients; 26%). A total of 82 serious AEs occurred in 39 patients (63%), the most common of which were SCC of the skin (nine patients; 15%) and keratoacanthoma (nine patients; 15%), which was defined as a serious AE. Basal cell carcinoma was observed in one patient (2%). In total, 25 patients (40%) had serious AEs considered by the investigator to be caused by vemurafenib (keratoacanthoma, n = 9; SCC of the skin, n = 9; basal cell carcinoma, n = 1; Bowen disease, n = 1; acute kidney injury, n = 4; pericarditis, n = 1; stomatitis, n = 1; pyrexia, n = 1; hypersensitivity, n = 1; sepsis, n = 1; and dehydration, n = 1); serious AEs not considered to be related to vemurafenib included pneumonia (n = 2), bronchitis (n = 2), dyspnea (n = 3), pericardial effusion (n = 1), sepsis (n = 3), pulmonary embolism (n = 2), and lung infection (n = 2).
DISCUSSION
Targetable oncogenic drivers in NSCLC with robust clinical validation include EGFR mutations and ALK and ROS1 fusions, but identifying other targetable, clinically important subgroups of NSCLC is a high priority. In this context, we found that patients with BRAF V600E–mutated NSCLC treated with vemurafenib had an ORR of 37%, with similar response rates in previously treated and untreated patients. Median OS was 15 months in the overall patient population, but had not been reached in the group of previously untreated patients after 12 months of follow-up. Similarly, our previously untreated patients had a median PFS of 12.9 months, which was considerably longer than the 6.5 months observed in patients who had received prior therapies. This may be explained either by small patient numbers or by increased acquisition of resistance mechanisms with prior therapy. This might suggest that targeted treatment in earlier lines of patients with a driver mutation could be more effective. The safety profile of vemurafenib in our group of patients with NSCLC was similar to that seen in patients with melanoma.10,18 No new safety signals were observed in this population. There were three patients with CNS metastases. Because response assessment in neuro-oncology–based criteria were not collected for CNS metastases, we do not have data on responses. This is one of the limitations of the study.
Our results provide evidence for the value of targeting BRAF with single-agent vemurafenib in patients with NSCLC. Although cross-study comparisons are made with caution, the OS we observed with single-agent vemurafenib (median, 15.4 months; 95% CI, 9.6 to 22.8 months) seems similar to that observed with the combination of dabrafenib and trametinib (median, 18.2 months; 95% CI, 14.3 months to NE), which was approved in 2017 by the US Food and Drug Administration and the European Medicines Agency for the treatment of patients with BRAF V600E mutation-positive NSCLC.16 With this approval, combination therapy consisting of a BRAF inhibitor and an MEK inhibitor has now become standard of care for patients with BRAF mutation-positive NSCLC, as is the case for patients with BRAF mutation-positive melanoma, adding to the range of targeted therapies now available for selected patients with NSCLC. We suggest that future studies should examine additional combinations in patients with BRAF mutation-positive NSCLC.
In conclusion, the results of the present cohort analysis suggest a role for BRAF inhibition in patients with NSCLC with BRAF mutations. The prolonged OS (median, 15.4 months) in the NSCLC population represents promising durability of effect with single-agent BRAF inhibition. The apparent increase in median PFS in previously untreated patients compared with previously treated patients warrants additional investigation of earlier treatment in this patient population.
ACKNOWLEDGMENT
The investigators thank the patients who participated in this study and their families.
Footnotes
Presented, in part, at the 2017 American Society of Clinical Oncology Annual Meeting, June 2 to 6, Chicago, IL.
Supported by F. Hoffmann-La Roche (Basel, Switzerland) and in part by the National Institutes of Health (Grant No. P30 CA008748). University of Texas MD Anderson Cancer Center Precision Oncology Decision Support Core was funded through CPRIT RP150535. Editorial support, provided by Miller Medical Communications (Brindle, United Kingdom), was funded by F. Hoffmann-La Roche.
ClinicalTrials.gov identifier: NCT01524978.
AUTHOR CONTRIBUTIONS
Conception and design: Vivek Subbiah, Jean-Yves Blay, Igor Puzanov, Noopur S. Raje, Todd Riehl, Jose Baselga, David M. Hyman
Financial support: David M. Hyman
Administrative support: Vivek Subbiah
Provision of study materials or patients: Vivek Subbiah, Gregory Riely, Antoine Hollebecque, Jean-Yves Blay, Enriqueta Felip, Martin Schuler, Anthony Gonçalves, Antonio Italiano, Igor Puzanov, Funda Meric-Bernstam
Collection and assembly of data: Vivek Subbiah, Radj Gervais, Gregory Riely, Antoine Hollebecque, Jean-Yves Blay, Martin Schuler, Antonio Italiano, Vicki Keedy, Igor Puzanov, Funda Meric-Bernstam, Martina Makrutzki, Todd Riehl, David M. Hyman
Data analysis and interpretation: Vivek Subbiah, Radj Gervais, Antoine Hollebecque, Jean-Yves Blay, Enriqueta Felip, Martin Schuler, Anthony Gonçalves, Antonio Italiano, Vicki Keedy, Ian Chau, Igor Puzanov, Noopur S. Raje, Martina Makrutzki, Todd Riehl, Bethany Pitcher, Jose Baselga, David M. Hyman
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Vivek Subbiah
Consulting or Advisory Role: MedImmune
Research Funding: Novartis (Inst), GlaxoSmithKline (Inst), NanoCarrier (Inst), Northwest Biotherapeutics (Inst), Genentech (Inst), Berg Pharma (Inst), Bayer (Inst), Incyte (Inst), Fujifilm (Inst), PharmaMar (Inst), D3 Oncology Solutions (Inst), Pfizer (Inst), Amgen (Inst), AbbVie (Inst), Multivir (Inst), Blueprint Medicines (Inst), Loxo (Inst), Vegenics (Inst), Takeda (Inst), Alfasigma (Inst), Agensys (Inst), Idera (Inst), Boston Biomedical (Inst), Inhibrx (Inst), Exelixis (Inst)
Travel, Accommodations, Expenses: PharmaMar
Expenses: Bayer
Gregory Riely
Research Funding: Novartis (Inst), Genentech (Inst), Millennium (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Infinity Pharmaceuticals (Inst), Ariad (Inst), Mirati Therapeutics (Inst), Merck (Inst)
Patents, Royalties, Other Intellectual Property: Patent application submitted covering pulsatile use of erlotinib to treat or prevent brain metastases (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Other Relationship: Pfizer, Genentech, Takeda
Antoine Hollebecque
Honoraria: Merck Serono
Consulting or Advisory Role: Amgen, AstraZeneca/MedImmune (Inst), Gritstone Oncology, Incyte (Inst), Eli Lilly (Inst), Spectrum Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Amgen, Servier, Eli Lilly, AstraZeneca/MedImmune
Other Relationship: AbbVie, Agios, Amgen, Argenx, Arno Therapeutics, Astex Pharmaceuticals, AstraZeneca, Aveo, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Chugai Pharma, Clovis Oncology, Daiichi Sankyo, Debiopharm Group, Eisai, Exelixis, Forma Therapeutics, GamaMabs Pharma, Genentech, GlaxoSmithKline, H3 Biomedicine, Innate Pharma, Janssen-Cilag, Kyowa Hakko Kirin, Loxo, Lytix Biopharma, MedImmune, Menarini, Merck Sharp & Dohme, Merrimack, Merus, Millennium Pharmaceuticals, Nanobiotix, Nektar, Novartis, Octimet, Oncoethix, Onyx, Orion Pharma, Oryzon Genomics, Pfizer, Pierre Fabre, Genentech, Sanofi/Aventis, Taiho Pharmaceutical, Tesaro, Xencor, Roche, Servier, Eli Lilly
Jean-Yves Blay
Leadership: Innate Pharma
Honoraria: Roche, Novartis Bayer, GlaxoSmithKline, Pharmamar, Eli Lilly
Consulting or Advisory Role: Roche, Novartis, GlaxoSmithKline, Bayer, PharmaMar, Merck, GlaxoSmithKline (Inst), PharmaMar (Inst), Novartis (Inst), Bayer (Inst), Roche (Inst)
Other Relationship: Innate Pharma
Enriqueta Felip
Consulting or Advisory Role: Pfizer, Roche, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, Celgene, Guardant Health, Novartis, Takeda, AbbVie, Blueprint Medicines, Eli Lilly, Merck, Merck Sharp & Dohme
Speakers' Bureau: AstraZeneca, Bristol-Myers Squibb, Novartis, Boehringer Ingelheim, Merck Sharp & Dohme, Roche, Pfizer, AbbVie, Eli Lilly, Merck, Takeda
Research Funding: Fundación Merck Salud (Inst), EMD Serono (Inst)
Martin Schuler
Honoraria: AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, MSD, Novartis, Pierre Fabre, Eli Lilly, Celgene
Consulting or Advisory Role: AstraZeneca Boehringer Ingelheim, Bristol-Myers Squibb, Novartis, Roche
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst), Boehringer Ingelheim (Inst), AstraZeneca (Inst)
Patents, Royalties, Other Intellectual Property: Highly sensitive method for mutation detection by polymerase chain reaction (Inst)
Anthony Gonçalves
Research Funding: MSD (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), Cascadian Therapeutics (Inst), Nektar (Inst), Boehringer Ingelheim (Inst), Eli Lilly (Inst), AbbVie (Inst), Genentech (Inst), AstraZeneca (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis, Genentech, Celgene, Boehringer Ingelheim, MSD, AstraZeneca
Antonio Italiano
Honoraria: Bayer, Daiichi Sankyo, Eli Lilly, Epizyme, Novartis, Roche
Consulting or Advisory Role: Roche, Daiichi Sankyo, Immune Design, Epizyme, Bayer, Eli Lilly
Research Funding: Roche, Bayer, AstraZeneca/MedImmune, PharmaMar, MSD Oncology, Merck Serono, Merck Serono
Vicki Keedy
Consulting or Advisory Role: Karyopharm Therapeutics
Research Funding: Medpacto (Inst), Plexxikon (Inst), Roche (Inst), Daiichi Sankyo (Inst), Eli Lilly (Inst), BioMed Valley Discoveries (Inst), Immune Design (Inst), GlaxoSmithKline (Inst), Tracon Pharma (Inst), Advenchen Laboratories (Inst)
Ian Chau
Honoraria: Eli Lilly
Consulting or Advisory Role: Eli Lilly, Bristol-Myers Squibb, MSD Oncology, Merck Serono, Genentech, Bayer, AstraZeneca, Oncologie International, Pierre Fabre
Research Funding: Janssen-Cilag (Inst), Sanofi (Inst), Merck Serono (Inst), Eli Lilly (Inst)
Travel, Accommodations, Expenses: MSD, Merck Serono, Eli Lilly, Bristol-Myers Squibb
Igor Puzanov
Consulting or Advisory Role: Amgen, Genentech, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Amgen, Merck
Noopur S. Raje
Consulting or Advisory Role: Amgen, Celgene, Takeda, Novartis, Bristol-Myers Squibb, Merck, Janssen Oncology
Research Funding: AstraZeneca (Inst)
Funda Meric-Bernstam
Honoraria: Sumitomo Group, Dialectica
Consulting or Advisory Role: Genentech, Inflection Biosciences, Pieris Pharmaceuticals, Clearlight Diagnostics, Darwin Health, Samsung Bioepis, Spectrum Pharmaceuticals, Aduro Biotech, Origimed, Xencor, Debiopharm Group, Mersana
Research Funding: Novartis, AstraZeneca, Taiho Pharmaceutical, Genentech, Calithera Biosciences, Debiopharm Group, Bayer, Aileron Therapeutics, Puma Biotechnology, CytomX Therapeutics, Jounce Therapeutics, Zymeworks, Curis, Pfizer, Effector Therapeutics, AbbVie, Boehringer Ingelheim (I), Guardant Health (Inst), Daiichi Sankyo, GlaxoSmithKline
Martina Makrutzki
Employment: Roche
Todd Riehl
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Bethany Pitcher
Employment: Hoffmann-La Roche
Jose Baselga
Leadership: Infinity Pharmaceuticals, Varian Medical Systems, Bristol-Myers Squibb, Foghorn
Stock and Other Ownership Interests: PMV Pharma, Juno Therapeutics, Grail, Tango, Venthera, Northern Biologics, Apogen Biotechnologies, Aura Biosciences
Consulting or Advisory Role: Novartis
Patents, Royalties, Other Intellectual Property: Combination therapy using PDK1 and PI3K inhibitors, use of phosphoinositide 3-kinase inhibitors for treatment of vascular malformations, inhibition of KMT2D for the treatment of cancer
Travel, Accommodations, Expenses: Roche, Daiichi Sankyo
David M. Hyman
Consulting or Advisory Role: Chugai Pharma, CytomX Therapeutics, Boehringer Ingelheim, AstraZeneca, Pfizer, Bayer, Genentech
Research Funding: AstraZeneca, Puma Biotechnology, Loxo, Bayer
Travel, Accommodations, Expenses: Genentech, Chugai Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Solomon BJ, Mok T, Kim D-W, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas A, Liu SV, Subramaniam DS, et al. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol. 2015;12:511–526. doi: 10.1038/nrclinonc.2015.90. [DOI] [PubMed] [Google Scholar]
- 5. My Cancer Genome: BRAF c.1799T>A (V600E) mutation in non-small cell lung cancer. https://www.mycancergenome.org/content/disease/lung-cancer/braf/54/
- 6.Cui G, Liu D, Li W, et al. A meta-analysis of the association between BRAF mutation and nonsmall cell lung cancer. Medicine (Baltimore) 2017;96:e6552. doi: 10.1097/MD.0000000000006552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 9.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): Extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 12.Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 13.Gautschi O, Milia J, Cabarrou B, et al. Targeted therapy for patients with BRAF-mutant lung cancer: Results from the European EURAF cohort. J Thorac Oncol. 2015;10:1451–1457. doi: 10.1097/JTO.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 14.Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: A single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:642–650. doi: 10.1016/S1470-2045(16)00077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. doi: 10.1016/S1470-2045(16)30146-2. Planchard D, Besse B, Groen HJM, et al: Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: An open-label, multicentre phase 2 trial. Lancet Oncol 17:P984-P993, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 17.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: An open-label, multicentre, safety study. Lancet Oncol. 2014;15:436–444. doi: 10.1016/S1470-2045(14)70051-8. [DOI] [PubMed] [Google Scholar]