INTRODUCTION
Nonrhabdomyosarcoma soft tissue sarcomas (NRSTSs) are a heterogeneous group of tumors comprising approximately 4% of all pediatric cancers.1-3 Histologic diagnosis of NRSTS is challenging, with frequent revisions of the diagnosis on central review.4 Prognosis is largely determined by histologic grade, size, and stage, with the worse outcomes for patients with metastatic disease.2,5 Many subtypes of NRSTS are characterized by different, but highly recurrent, gene fusions like SS18-SSX fusions in synovial sarcoma,6 TPM-ALK fusions in inflammatory myofibroblastic tumors,7,8 and COL1A1-PDGFRB fusions in dermatofibrosarcoma protuberans.9 Irrespective of the genomic basis of these cancers, most patients are treated with surgery, radiation, and/or chemotherapy regimens including alkylators and/or anthracyclines.10 Exceptions include the use of imatinib for dermatofibrosarcoma protuberans and crizotinib for ALK-rearranged inflammatory myofibroblastic tumors.11,12 Undifferentiated sarcomas, a subset of NRSTSs, lack a clear line of differentiation after pathologic evaluation but may also harbor frequent oncogenic fusions.13
The neurotrophin tyrosine kinase receptors TRKA, TRKB, and TRKC are encoded by the NTRK1, NTRK2, and NTRK3 genes, respectively. In normal development, these genes regulate the growth, differentiation, and survival of neurons.14,15 Gene fusions that involve one of these genes (TRK fusions) have been described in a range of pediatric and adult cancers.16-25 In these fusions, the 3′ region of the NTRK gene, which includes the kinase domain, is joined downstream of the promoter and 5′ region of an unrelated gene. The resultant fusion oncoprotein is both aberrantly expressed and constitutively active. Most reported fusions in extracranial solid tumors to date have involved either NTRK1 or NTRK3, with NTRK2 fusions more common in primary CNS tumors.26
Larotrectinib is the first highly selective inhibitor of all three TRK kinases to enter clinical development. Recently, a centrally confirmed 75% objective response rate to larotrectinib (80% by investigator assessment) has been reported in adults and children with TRK fusion-positive solid tumors.27 We report the clinical activity of larotrectinib in the first patient with an NTRK2 fusion.
CASE REPORT
An 11-year-old female presented with back and leg pain for 1 month. Computed tomography of her abdomen and pelvis revealed a large (9.6 × 7.4-cm), unresectable, retroperitoneal mass encasing the aorta and involving the vertebral bodies (Fig 1A). Biopsy led to the diagnosis of NRSTS, likely hemangiopericytoma, which upon review at our institution was revised to undifferentiated sarcoma. The patient underwent neoadjuvant chemotherapy of vincristine, cyclophosphamide, and doxorubicin followed by etoposide and ifosfamide. After two cycles of chemotherapy, disease progression continued, and an aortic pseudo-aneurysm developed within the mass, which led to displacement of the inferior vena cava and ureteral impingement (Fig 1B). Hospice was recommended, and the patient was treated with palliative sorafenib, but tumor progression continued.
Fig 1.
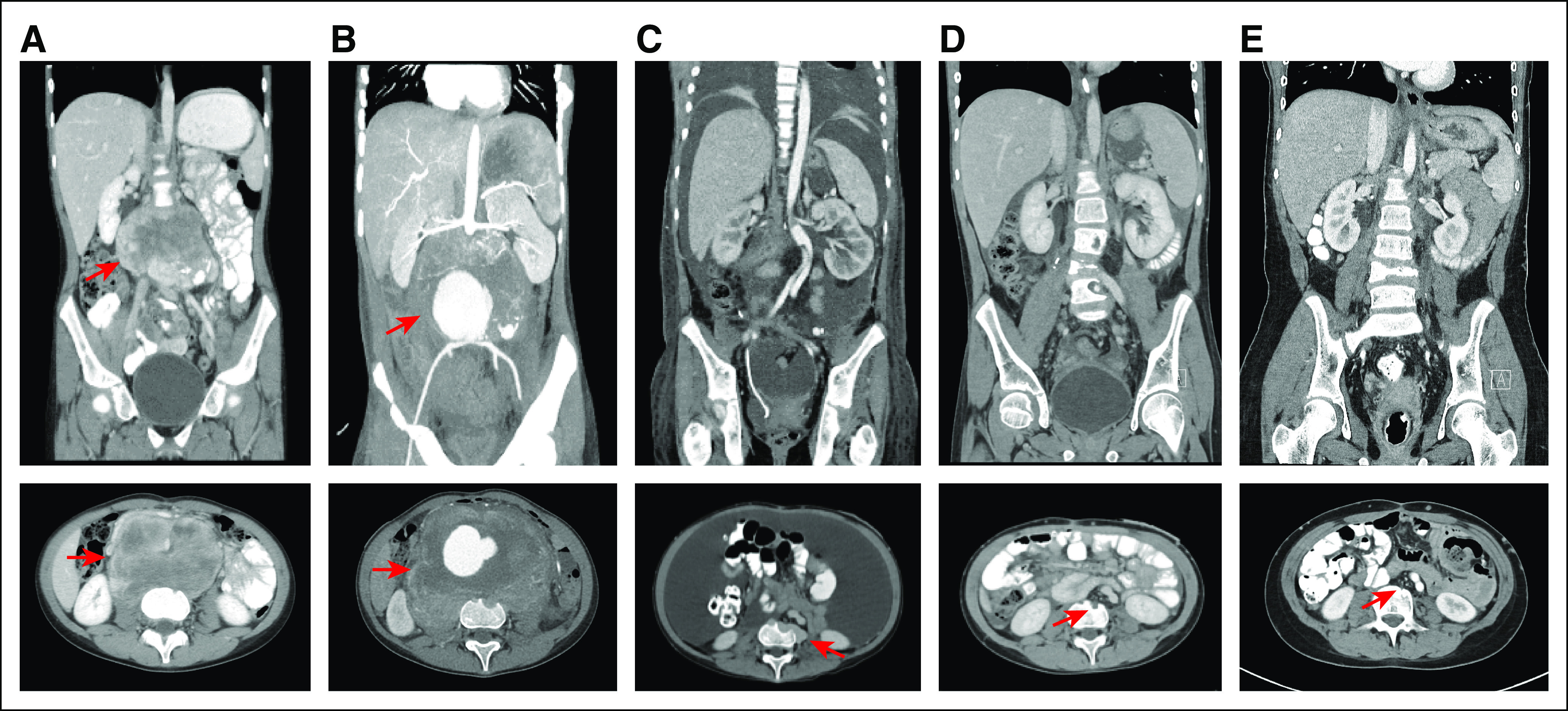
Computed tomography imaging of patient’s abdominal tumor over time. (A) Initial imaging. (B) Development of aortic pseudo-aneurysm. (C) Baseline before enrollment, postsurgery. (D) After 1.6 months of larotrectinib treatment. (E) After 14 months of larotrectinib treatment.
The patient was subsequently referred to our institution, where surgical intervention to debulk the tumor and repair the pseudo-aneurysm was offered. She underwent exploratory laparotomy with resection of a 13.5 × 10.5 × 6.5-cm tumor, resection and reconstruction of the abdominal aorta, ligation and resection of the inferior vena cava, resection of sigmoid colon with end colostomy, and placement of bilateral ureteral stents (Fig 2). Histologically, the tumor consisted of sheets of epithelioid cells with large eccentrically placed nucleus, occasional nucleoli, and abundant eosinophilic cytoplasm, mimicking rhabdoid cells (Fig 3). Immunohistochemically, the cells showed retained INI-1 nuclear positivity and scattered cells positive for S-100 protein and CD34.
Fig 2.
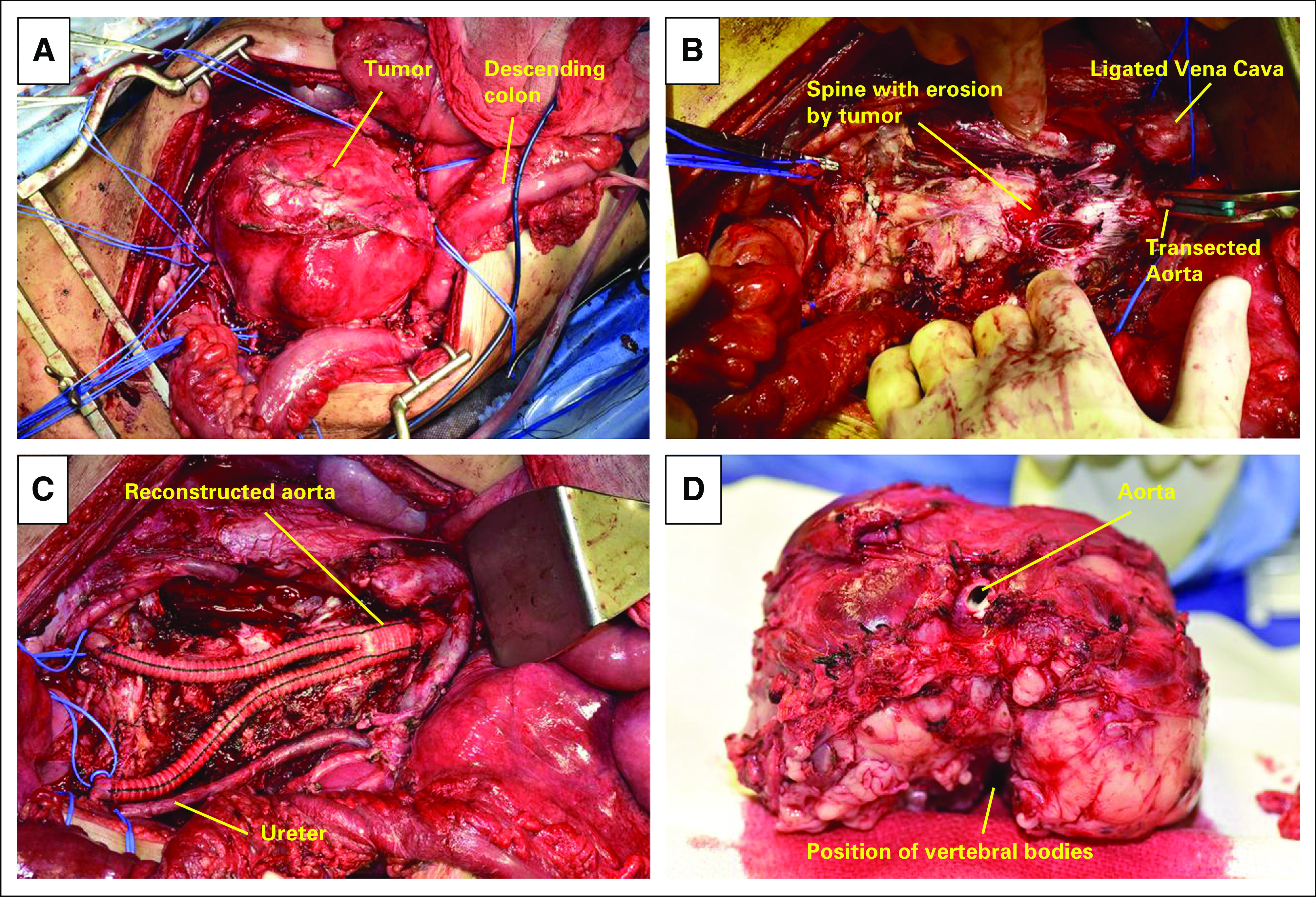
Surgical resection of retroperitoneal sarcoma. (A) Retroperitoneal tumor before resection. (B) Tumor bed after resection, with residual tumor invading the vertebral bodies. (C) Status postreconstruction of the aorta. (D) Excised tumor specimen, including the aortic bifurcation.
Fig 3.
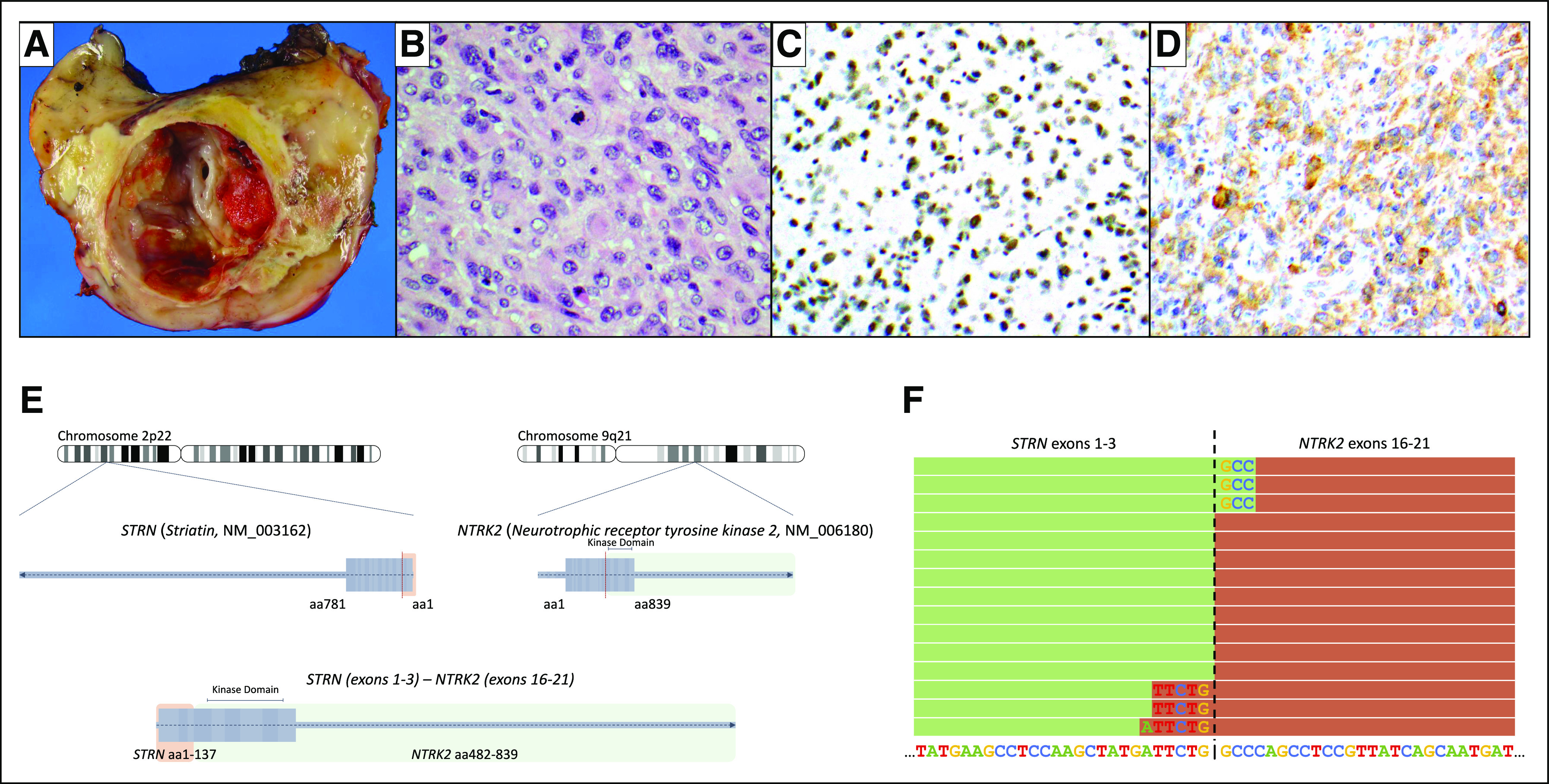
Pathology of patient’s tumor from resection. (A) Transverse section of the mass with aneurysmal cavity around the aorta. (B) Hematoxylin and eosin stain that shows epithelioid/rhabdoid cells with eccentric, pleomorphic nuclei; occasional nucleoli and abundant eosinophilic cytoplasm; and a single mitosis. (C) Immunohistochemistry for INI-1 that shows retained nuclear positivity in the neoplastic cells. (D) Immunohistochemistry for pan-Trk that shows membranous and cytoplasmic positivity in the neoplastic cells. (E) STRN-NTRK2 fusion identified in tumor sample by next generation sequencing. (F) RNA sequencing reads that support the STRN-NTRK2 fusion.
Postoperatively, gross residual tumor remained in the vertebral bodies. Subsequently, the patient developed prolonged ileus, anasarca, and symptomatic ascites that required Denver venous shunt placement. After a prolonged recovery, she was treated with irinotecan. Next generation sequencing (NGS) of her tumor was performed using FoundationOneHeme (Foundation Medicine, Cambridge, MA), a combined DNA and RNA sequencing hybrid capture-based assay that has been validated for clinical use without requirement for an orthogonal confirmatory assay.28 Sequencing revealed a novel STRN-NTRK2 fusion in which the kinase domain of NTRK2 is joined in frame to STRN (Figs 3E and 3F). Copy number loss of CDKN2B also was identified.
Two months after surgery, after informed consent, the patient was enrolled in the pediatric phase I trial of larotrectinib.29 She was treated with dose level 1 (100-mg adult equivalent dose by Simcyp modeling [Simcyp, Sheffield, UK]) and received larotrectinib 75 mg twice a day continuously on the basis of her age and body surface area. Pharmacokinetic assessment after the first dose of larotrectinib revealed an estimated 24-hour area under the plasma concentration-time curve (AUC0-24) of 2,980 ng ⋅ h/mL, which was less than the protocol-specified threshold of 3,500 ng ⋅ h/mL that would allow intrapatient dose escalation. Thus, per protocol, the patient’s dose was increased to 100 mg twice a day on day 10 of cycle 1. Pharmacokinetics at this dose demonstrated an estimated AUC0-24 of 3,830 ng ⋅ h/mL.
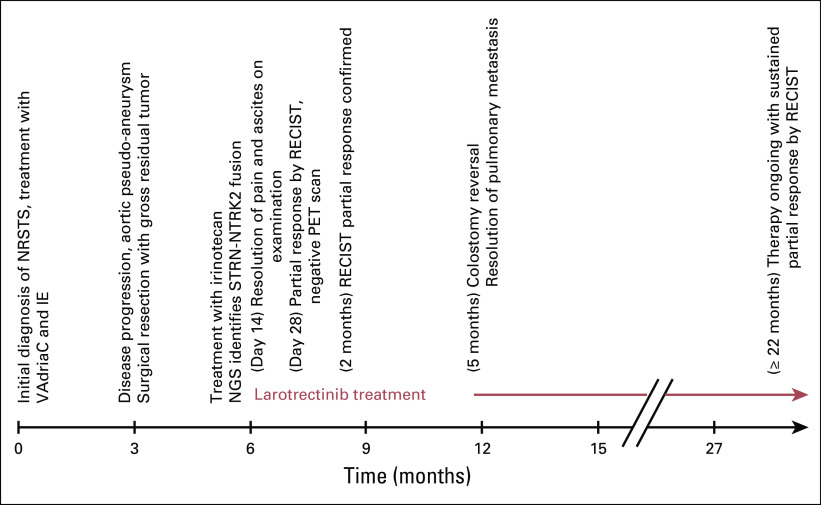
At the time of initiation of larotrectinib, the patient’s Lansky play-performance score (LPS) was 60. She had a distended firm abdomen with significant ascites and back pain that required daily narcotic pain medication. Baseline disease evaluation revealed the presence of a pulmonary metastasis, multiple enlarging positron emission tomography (PET)–avid tumors (largest 1.5 × 5.3 cm) within the retroperitoneal surgical bed, and massive ascites that was presumed to be malignant (Fig 1C). By 14 days after initiation of larotrectinib, the patient was noticeably less fatigued (LPS of 70), the ascites had resolved by physical examination, and her back pain had resolved (Fig 4). Repeat imaging at the end of cycle 1, which was confirmed after cycle 2, demonstrated resolution of PET avidity of all tumors, resolution of ascites, and significant reduction in the size of the residual pulmonary metastases and retroperitoneal tumors consistent with a partial response by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (Figs 1D and 1E). Five months after starting larotrectinib, the patient underwent uncomplicated closure of her colostomy and removal of her Denver shunt.
Fig 4.
Timeline. IE, ifosfamide and etoposide; NGS, next-generation sequencing; NRSTS, nonrhabdomyosarcoma soft tissue sarcoma; PET, positron emission tomography; RECIST, Response Evaluation Criteria in Solid Tumors; VAdriaC, vincristine, doxorubicin, and cyclophosphamide.
Despite heavy pretreatment, the patient has tolerated larotrectinib well, with the only drug-related adverse events consisting of mild (Common Terminology Criteria for Adverse Events [version 4] grade 1) fatigue and intermittent grade 1 to 2 cytopenias. She has not required dose reduction or interruption for toxicity. Her response continues to deepen with complete resolution of her pulmonary metastasis and only residual PET-negative tissue at the site of tumor invasion of the vertebral bodies after 22 months of larotrectinib therapy as of February 2018. Currently, the patient’s LPS is 90, and she has returned to school.
DISCUSSION
We describe a patient in whom tumor sequencing led to the identification of a novel NTRK2 fusion in a chemotherapy-refractory, metastatic, unresectable, undifferentiated sarcoma and successful treatment with larotrectinib. The identification of an NTRK2 fusion is consistent with other reports of oncogenic fusions in this histology.13,30-39 A wide range of tumors that harbor TRK fusions has been reported to respond to larotrectinib.27 The activity of larotrectinib is striking in children, with a 93% confirmed objective response rate and all patients with TRK fusions experiencing tumor regression.29
This patient’s tumor harbored a fusion of NTRK2, and she was the sole patient in the initial 55-patient data set to have a fusion of this gene.27 Other rare NTRK2 fusions have been described in gliomas, specifically with VCL, AGBL4, QKI, and NACC2,20,40 but to our knowledge, this report is the first of an NTRK2 fusion in a pediatric extracranial solid tumor. The morphologic similarity of the patient’s tumor to soft tissue sarcomas that harbor fusions of NTRK1 and NTRK3 suggests that these sarcomas should be evaluated for fusions of all three NTRK genes.41
STRN on chromosome 2p22.2 encodes striatin, a ubiquitously expressed calmodulin-binding protein. In thyroid cancer, it is a common 5′ fusion partner for ALK also located on 2p.42 However, STRN has not previously been reported as an NTRK fusion partner, which is consistent with the broad diversity of 5′ fusion partners seen in TRK fusion-positive cancers27 and is an important consideration when designing testing strategies for TRK fusions. Tests such as reverse transcriptase polymerase chain reaction only detect known fusion partners and are likely to miss tumors with uncommon or unique NTRK fusion genes, which can respond to TRK inhibition. Testing that uses hybrid capture–based NGS has the potential to detect a diversity of NTRK fusion partners, but careful attention must be paid to the initial substrate (DNA v RNA), the target enrichment strategy (hybrid capture v amplicon v anchored multiplex polymerase chain reaction), and the detailed probe design.
The profound clinical response seen in this STRN-NTRK2 fusion patient to larotrectinib establishes that the TRK fusion is the key driver for this patient’s tumor. Her duration of response (ongoing at 22 months) compares favorably with that seen with other tyrosine kinase inhibitors, such as BRAF inhibitors for BRAFV600E mutant melanoma. In the latter, despite high initial response rates, nearly all patients treated with vemurafenib or dabrafenib experience disease progression within 12 to 18 months of starting therapy.43,44 Durable responses also have been observed in patients with fusions that involve NTRK1 and NTRK3 treated with larotrectinib.27
In conclusion, NGS of the undifferentiated sarcoma in our patient was critical in identifying a highly actionable kinase fusion (STRN-NTRK2), which resulted in a profound and durable clinical response on matched treatment. Genomic and molecular testing of tumors have become increasingly useful for treatment through the identification of potential targets for novel therapies irrespective of histology. Such testing should be considered for all patients with advanced cancer, especially those with NRSTS, because both classic and novel fusion oncogenes can be detected without a prior therapeutic hypothesis.
ACKNOWLEDGMENT
We thank the patient and her family.
Footnotes
Supported by Cancer Prevention and Research Institute of Texas Grants No. RP120685-AC and RP120685-P2, Loxo Oncology, Bayer AG, and the Children’s Cancer Fund.
Clinical trial information: NCT02637687.
AUTHOR CONTRIBUTIONS
Conception and design: Lawrence W. Wu, Michael C. Cox, Andrew Martin, Theodore W. Laetsch
Financial support: Michael C. Cox, Theodore W. Laetsch
Provision of study material or patients: Tara Pavlock, Steve Megison, Theodore W. Laetsch
Collection and assembly of data: Lawrence W. Wu, Tara Pavlock, Alison Patterson, Anne Post, Caitlyn Ambrose, Dean C. Pavlick, Matthew Cooke, Siraj M. Ali, Steven Smith, Theodore W. Laetsch
Data analysis and interpretation: Lawrence W. Wu, Tara Pavlock, Veena Rajaram, Matthew Cooke, Vincent A. Miller, Lee A. Albacker, Siraj M. Ali, Steven Smith, Michael C. Cox, Steve Megison, Theodore W. Laetsch
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Lawrence W. Wu
No relationship to disclose
Tara Pavlock
No relationship to disclose
Alison Patterson
No relationship to disclose
Anne Post
No relationship to disclose
Caitlyn Ambrose
No relationship to disclose
Veena Rajaram
No relationship to disclose
Dean C. Pavlick
Employment: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine
Matthew Cooke
Employment: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine
Travel, Accommodations, Expenses: Foundation Medicine
Vincent A. Miller
Employment: Foundation Medicine
Leadership: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Periodic royalties related to T790M patent awarded to Memorial Sloan Kettering Cancer Center
Lee A. Albacker
Employment: Foundation Medicine
Stock and Other Ownership Interests: Foundation Medicine
Siraj M. Ali
Employment: Foundation Medicine
Leadership: Incyte
Stock and Other Ownership Interests: Exelixis, Blueprint Medicines, Agios Pharmaceuticals, Genocea Biosciences
Patents, Royalties, Other Intellectual Property: Patents through Foundation Medicine, patents through Seres Therapeutics on microbiome in non-neoplastic disease (I)
Steven Smith
Consulting or Advisory Role: Loxo Oncology
Michael C. Cox
Employment: Bayer AG, Loxo Oncology, Merck, Amgen
Stock and Other Ownership Interests: Loxo Oncology, Bayer AG, Merck, Amgen
Patents, Royalties, Other Intellectual Property: US patent 62/318,041 issued to Loxo Oncology (Inst)
Andrew Martin
No relationship to disclose
Steve Megison
No relationship to disclose
Theodore W. Laetsch
Consulting or Advisory Role: Novartis, Loxo Oncology, Eli Lilly, Bayer AG
Research Funding: Pfizer (Inst)
REFERENCES
- 1.Alaggio R, Coffin CM. The evolution of pediatric soft tissue sarcoma classification in the last 50 years. Pediatr Dev Pathol. 2015;18:481–494. doi: 10.2350/15-07-1666-MISC.1. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari A, Sultan I, Huang TT, et al. Soft tissue sarcoma across the age spectrum: A population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer. 2011;57:943–949. doi: 10.1002/pbc.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo VY, Fletcher CD. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology. 2014;46:95–104. doi: 10.1097/PAT.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171:950–965.e28. doi: 10.1016/j.cell.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappo AS, Devidas M, Jenkins J, et al. Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group study. J Clin Oncol. 2005;23:4031–4038. doi: 10.1200/JCO.2005.03.209. [DOI] [PubMed] [Google Scholar]
- 6.Clark J, Rocques PJ, Crew AJ, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto H, Yoshida A, Taguchi K, et al. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology. 2016;69:72–83. doi: 10.1111/his.12910. [DOI] [PubMed] [Google Scholar]
- 8.Antonescu CR, Suurmeijer AJ, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39:957–967. doi: 10.1097/PAS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon MP, Pedeutour F, Sirvent N, et al. Deregulation of the platelet-derived growth factor B-chain gene via fusion with collagen gene COL1A1 in dermatofibrosarcoma protuberans and giant-cell fibroblastoma. Nat Genet. 1997;15:95–98. doi: 10.1038/ng0197-95. [DOI] [PubMed] [Google Scholar]
- 10.Spunt SL, Skapek SX, Coffin CM. Pediatric nonrhabdomyosarcoma soft tissue sarcomas. Oncologist. 2008;13:668–678. doi: 10.1634/theoncologist.2007-0182. [DOI] [PubMed] [Google Scholar]
- 11.Theilen TM, Soerensen J, Bochennek K, et al. Crizotinib in ALK+ inflammatory myofibroblastic tumors—Current experience and future perspectives. Pediatr Blood Cancer. 2018;65:65. doi: 10.1002/pbc.26920. [DOI] [PubMed] [Google Scholar]
- 12.Mossé YP, Voss SD, Lim MS, et al. Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: A Children’s Oncology Group study. J Clin Oncol. 2017;35:3215–3221. doi: 10.1200/JCO.2017.73.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laetsch TW, Roy A, Xu L, et al. Undifferentiated sarcomas in children harbor clinically-relevant oncogenic fusions and gene copy-number alterations: A report from the Children’s Oncology Group. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-18-0672. 10.1158/1078-0432.CCR-18-0672 [epub ahead of print on April 24, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawara A. Trk receptor tyrosine kinases: A bridge between cancer and neural development. Cancer Lett. 2001;169:107–114. doi: 10.1016/s0304-3835(01)00530-4. [DOI] [PubMed] [Google Scholar]
- 15.Rubin JB, Segal RA. Growth, survival and migration: The Trk to cancer. Cancer Treat Res. 2003;115:1–18. doi: 10.1007/0-306-48158-8_1. [DOI] [PubMed] [Google Scholar]
- 16.Créancier L, Vandenberghe I, Gomes B, et al. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma. Cancer Lett. 2015;365:107–111. doi: 10.1016/j.canlet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- 18.Stransky N, Cerami E, Schalm S, et al. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlick D, Schrock AB, Malicki D, et al. Identification of NTRK fusions in pediatric mesenchymal tumors. Pediatr Blood Cancer. 2017;64:e26433. doi: 10.1002/pbc.26433. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng WQ, Hisaoka M, Okamoto S, et al. Congenital-infantile fibrosarcoma. A clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin-embedded tissues. Am J Clin Pathol. 2001;115:348–355. doi: 10.1309/3H24-E7T7-V37G-AKKQ. [DOI] [PubMed] [Google Scholar]
- 22.Bourgeois JM, Knezevich SR, Mathers JA, et al. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol. 2000;24:937–946. doi: 10.1097/00000478-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Knezevich SR, Garnett MJ, Pysher TJ, et al. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998;58:5046–5048. [PubMed] [Google Scholar]
- 24.El Demellawy D, Cundiff CA, Nasr A, et al. Congenital mesoblastic nephroma: A study of 19 cases using immunohistochemistry and ETV6-NTRK3 fusion gene rearrangement. Pathology. 2016;48:47–50. doi: 10.1016/j.pathol.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Prasad ML, Vyas M, Horne MJ, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–1107. doi: 10.1002/cncr.29887. [DOI] [PubMed] [Google Scholar]
- 26.Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1:e000023. doi: 10.1136/esmoopen-2015-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He J, Abdel-Wahab O, Nahas MK, et al. Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood. 2016;127:3004–3014. doi: 10.1182/blood-2015-08-664649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: Phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19:705–714. doi: 10.1016/S1470-2045(18)30119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierron G, Tirode F, Lucchesi C, et al. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet. 2012;44:461–466. doi: 10.1038/ng.1107. [DOI] [PubMed] [Google Scholar]
- 31.Italiano A, Sung YS, Zhang L, et al. High prevalence of CIC fusion with double-homeobox (DUX4) transcription factors in EWSR1-negative undifferentiated small blue round cell sarcomas. Genes Chromosomes Cancer. 2012;51:207–218. doi: 10.1002/gcc.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peters TL, Kumar V, Polikepahad S, et al. BCOR-CCNB3 fusions are frequent in undifferentiated sarcomas of male children. Mod Pathol. 2015;28:575–586. doi: 10.1038/modpathol.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada Y, Kuda M, Kohashi K, et al. Histological and immunohistochemical characteristics of undifferentiated small round cell sarcomas associated with CIC-DUX4 and BCOR-CCNB3 fusion genes. Virchows Arch. 2017;470:373–380. doi: 10.1007/s00428-017-2072-8. [DOI] [PubMed] [Google Scholar]
- 34.Li WS, Liao IC, Wen MC, et al. BCOR-CCNB3-positive soft tissue sarcoma with round-cell and spindle-cell histology: A series of four cases highlighting the pitfall of mimicking poorly differentiated synovial sarcoma. Histopathology. 2016;69:792–801. doi: 10.1111/his.13001. [DOI] [PubMed] [Google Scholar]
- 35.Hofvander J, Tayebwa J, Nilsson J, et al. Recurrent PRDM10 gene fusions in undifferentiated pleomorphic sarcoma. Clin Cancer Res. 2015;21:864–869. doi: 10.1158/1078-0432.CCR-14-2399. [DOI] [PubMed] [Google Scholar]
- 36.O’Meara E, Stack D, Phelan S, et al. Identification of an MLL4-GPS2 fusion as an oncogenic driver of undifferentiated spindle cell sarcoma in a child. Genes Chromosomes Cancer. 2014;53:991–998. doi: 10.1002/gcc.22208. [DOI] [PubMed] [Google Scholar]
- 37.Kao YC, Sung YS, Zhang L, et al. Recurrent BCOR internal tandem duplication and YWHAE-NUTM2B fusions in soft tissue undifferentiated round cell sarcoma of infancy: Overlapping genetic features with clear cell sarcoma of kidney. Am J Surg Pathol. 2016;40:1009–1020. doi: 10.1097/PAS.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Specht K, Zhang L, Sung YS, et al. Novel BCOR-MAML3 and ZC3H7B-BCOR gene fusions in undifferentiated small blue round cell sarcomas. Am J Surg Pathol. 2016;40:433–442. doi: 10.1097/PAS.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugita S, Arai Y, Aoyama T, et al. NUTM2A-CIC fusion small round cell sarcoma: A genetically distinct variant of CIC-rearranged sarcoma. Hum Pathol. 2017;65:225–230. doi: 10.1016/j.humpath.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Jones DT, Hutter B, Jäger N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45:927–932. doi: 10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudzinski ER, Lockwood CM, Stohr BA, et al. Pan-Trk immunohistochemistry identifies NTRK-rearrangements in pediatric mesenchymal tumors. Am J Surg Pathol. 2018;42:927–935. doi: 10.1097/PAS.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 42.Kelly LM, Barila G, Liu P, et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A. 2014;111:4233–4238. doi: 10.1073/pnas.1321937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 44.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]