Abstract
PURPOSE
Periampullary adenocarcinomas encompass a heterogeneous group of tumors with dismal prognosis and limited treatment options. Emerging evidence shows that tumor morphology (ie, intestinal type [I-type] or pancreatobiliary type [PB-type]) is a more relevant prognostic factor than tumor origin. Knowledge is sparse, however, on whether key mutations differ according to morphology.
MATERIALS AND METHODS
Next-generation sequencing was applied to assess the mutational status of 70 genes in 102 tumors from a retrospective cohort of 175 patients with resected periampullary adenocarcinoma. Brahma-related gene 1 protein expression was examined by immunohistochemistry on tissue microarrays with primary tumors from the original cohort.
RESULTS
APC mutations were significantly more common in I-type than in PB-type tumors (27.5% v 0%; P < .001), as were ERBB3 mutations (20.8% v 4.8%; P = .016), whereas CDKN2A mutations were more common in PB-type than in I-type tumors (19.4% v 2.5%; P = .013). KRAS mutation was an independent factor of poor prognosis in I-type tumors (hazard ratio, 3.73; 95% CI, 1.10 to 12.67). In PB-type tumors, SMARCA4 mutation was an adverse prognostic factor in patients not receiving adjuvant chemotherapy, and there was a significant treatment interaction between expression of Brahma-related gene 1 protein, the protein encoded by SMARCA4, and adjuvant chemotherapy (Pinteraction = .007).
CONCLUSION
To our knowledge, this is the first description of the mutational landscape in the full spectrum of periampullary adenocarcinoma that demonstrates that the distribution and prognostic and predictive significance of commonly mutated genes differ by morphology. The results emphasize that morphology is an important factor to consider in the search for novel biomarkers and targeted personalized treatment of these patients. In addition, the findings support the concept that molecular profiling of these tumors could be of clinical benefit.
INTRODUCTION
Periampullary adenocarcinoma is a collective term for tumors arising in the area surrounding the ampulla of Vater, including the head of the pancreas, the duodenum, and the common bile duct. A morphologic classification into intestinal type (I-type) and pancreatobiliary type (PB-type) has been shown to provide better prognostic information than anatomic origin, with the former having a more favorable clinical outcome.1-3 Although a plethora of mutations has been documented in pancreatic cancer,4-7 the mutational landscape of other periampullary cancers has been less studied. No targeted therapies have proven to be efficient, and adjuvant chemotherapy therefore remains standard of care after resection of these tumors. Hence, there is evident need for additional studies on the distribution and clinical significance of the mutational landscape in the full range of periampullary adenocarcinomas, including pancreatic cancer, to enable a better patient stratification and identify potential responders to targeted therapies. The aim of this study was therefore to map mutations in common cancer-associated genes in tumors from a clinically well-characterized retrospective cohort of patients with resected periampullary adenocarcinoma, with particular reference to morphology and clinical outcome.
MATERIALS AND METHODS
Study Cohort
The original study cohort is a retrospective consecutive series consisting of primary tumors from 175 incident cases of periampullary adenocarcinoma.8,9 The patients underwent pancreaticoduodenectomy at the University Hospitals of Lund and Malmö from January 1, 2001 to December 31, 2011. Follow-up started at the date of surgery and ended at death, or on March 31, 2017, whichever came first. The Swedish National Civil Register was used to obtain information on vital status. Data on adjuvant treatment were obtained from patient charts. All cases underwent thorough histopathological revaluation. The anatomic site of origin of the tumors was 14 duodenal, 70 ampullary, 45 distal bile duct, and 46 pancreatic, whereof 65 tumors were classified as intestinal type (I-type) and 110 as pancreatobiliary type (PB-type).
Context
To what extent does morphology influence the mutational landscape in periampullary adenocarcinomas, and can we find novel prognostic and predictive biomarkers that differ by morphology, so as to better characterize this heterogeneous group of tumors in a clinical context?
The results presented here demonstrate that the distribution and prognostic impact of mutations in key genes indeed differ according to morphology and that emphasis on morphology rather than anatomy is of importance. Of particular potential clinical relevance are the findings that assessment of KRAS mutation may add value to prognostication of patients with intestinal type tumors and that SMARCA4/BRG1 expression may be of use as a predictive biomarker for patients with pancreatobiliary-type tumors receiving chemotherapy.
Thus, this study further underlines that molecular profiling in combination with assessment of tumor morphology may be a useful tool for improved treatment stratification of patients with adenocarcinoma in the periampullary region.
Ethics Approval and Consent to Participate
All national and European Union regulations and requirements for handling human samples have been fully complied with during the conduct of this study, ie, decision number 1110/94/EC of the European Parliament and of the Council (OJL126 18,5,94), the Helsinki Declaration on ethical principles for medical research involving human subjects, and the European Union Council Convention on human rights and biomedicine. Approval for the study was obtained from the ethics committee of Lund University (reference number 445/07), whereby the committee waived the need for consent other than the option to opt ;out.
Next-Generation Sequencing
Tissue cores of 1-mm diameter were taken from tumor cell–enriched formalin-fixed paraffin-embedded tissue. DNA extraction was performed using the Qiagen GeneRead (Qiagen, Hilden, Germany) kit for formalin-fixed paraffin-embedded tissue according to the manufacturer’s instructions. In total, 102 (58.3%) cases had a sufficient number of tumor cells for analysis. The anatomic site of origin of these tumors was 10 duodenal, 42 ampullary, 30 distal bile duct, and 20 pancreatic. Forty-one (39.8%) were classified as I-type and 62 (60.2%) as PB-type.
A panel of 70 cancer-associated genes was put together for this study and characterized using Illumina TruSeq custom amplicon assay (Illumina, San Diego, CA) with a MiSeq instrument according to manufacturer’s instructions. The gene panel was selected to include known cancer-associated genes and is detailed in the Data Supplement. The panel was designed to be bidirectional and to target the most cancer-relevant parts of the selected genes. Only exon parts of the aforementioned genes were sequenced. Alignment, quality filtering, variant calling, and variant annotation were performed using the supplier’s standard analysis pipeline (Illumina, San Diego, CA). Only nonsynonymous variants with variant frequency of 4% or more were not included. Detected mutations were screened against the COSMIC and ExAC databases, to filter single-nucleotide polymorphisms commonly reported in different populations.
Immunohistochemical Analysis of Brahma-Related Gene 1 Protein Expression
For immunohistochemical analysis of Brahma-related gene 1 (BRG1) protein expression, 4-μm tissue microarray sections were automatically pretreated in the PT-link system (Dako, Glostrup, Denmark) and stained in an automated immunostainer (Autostainer Plus, Dako) using the Dako EnVision FLEX+ Detection System, Peroxidase/DAB, Rabbit/Mouse with the monoclonal anti-BRG1 antibody clone G-7 (Santa Cruz Biotechnology, Dallas, TX), diluted 1:25.
BRG1 was expressed in the tumor cell nuclei and present in the majority of tumor cells in positive cases. Therefore, only the intensity was annotated as 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. Each tissue microarray core was evaluated separately, and the lowest and highest scores were denoted for each case. For the statistical analyses, a total score (0 to 6) was calculated from the sum of the lowest and highest scores. On the basis of visual inspection of Kaplan-Meier curves for the entire cohort and in strata according to morphology and adjuvant treatment, the total score was dichotomized into low (0 or 1) versus high (> 1) BRG1 staining.
Statistical Analyses, Data Processing, and Data Availability Statement
The χ2 test was applied to compare the distribution of clinicopathological factors in cases with information on mutational status and cases without information. Classification and regression tree analysis was applied to find an optimal prognostic cutoff for mutation burden. Kaplan-Meier analysis log-rank test was applied to illustrate any difference in 5-year overall survival (OS), and Cox regression proportional hazard models were used to estimate hazard ratios (HRs) for death within 5 years in both univariable and multivariable analysis. Multivariable Cox regression included adjustment for age (continuous), T-stage (T1 or T2 v T3 or T4), N-stage (negative v positive nodal status), grade (well to moderate v poor), morphology (I-type v PB-type) in the entire cohort, adjuvant chemotherapy (none v any), invasion into vascular and lymphatic structures, and perineural growth. The proportional hazard (PH) assumption was tested using Cox regression with a time-dependent covariate analysis, whereby the PH assumption was considered to be satisfied when the factor × time interaction was nonsignificant. The PH assumption was also evaluated graphically using log-minus-log plots. In the entire cohort, three cases were excluded in the survival analyses: one patient with a PB-type tumor who was lost to follow-up because of emigration, and two patients with I-type tumors who died as a result of complications from the initial surgical treatment. In the group with information on mutational status, one patient who died as a result of complications from surgery was censored. Two additional patients with PB-type tumors having received neoadjuvant chemotherapy were excluded from the survival analyses related to BRG1 expression. To estimate the interaction effect between adjuvant treatment and selected biomarkers, the following interaction variable was constructed: any adjuvant treatment (+/−) × biomarker (+/−). Genes that were mutated in less than 10% of the cases were excluded from the survival analyses to obtain reasonable statistical power. All calculations were performed with SPSS version 24.0 (SPSS, Chicago, IL). OncoPrinter at cBioPortal (http://www.cbioportal.org/) was used to illustrate the data with heat maps.10,11 All statistical tests were two-sided, and P-values < 0.05 were considered significant. All data generated or analyzed during this study are included in this published article (Data Supplement).
RESULTS
Distribution of Mutations According to Morphologic Type
There were no significant differences in the distribution of clinicopathological characteristics between cases with known and unknown mutational status (Data Supplement). The frequency of mutations according to morphologic type is shown in Figure 1 and further outlined in the Data Supplement. In the entire cohort, only nine out of 70 genes were mutated in more than 10% of the cases; the most common were TP53 (n = 51; 50.0%) and KRAS (n = 47; 46.1%). APC mutations were significantly more common in I-type compared to PB-type tumors (27.5% v 0%; P < .001), and CDKN2A mutations were significantly more common in PB-type compared to I-type tumors (19.4% v 2.5%; P = .013). Furthermore, ERBB3 mutations were more frequent in I-type compared with PB-type tumors (20.0% v 4.8%; P = .016). Human epidermal growth factor receptor 3 (HER3) protein overexpression has previously also been found to be significantly more frequent in I-type compared with PB-type tumors,9 but there was no significant association between HER3 expression and ERBB3 mutational status (P = .67). The frequency of mutations according to anatomic origin is shown in the Data Supplement.
FIG 1.
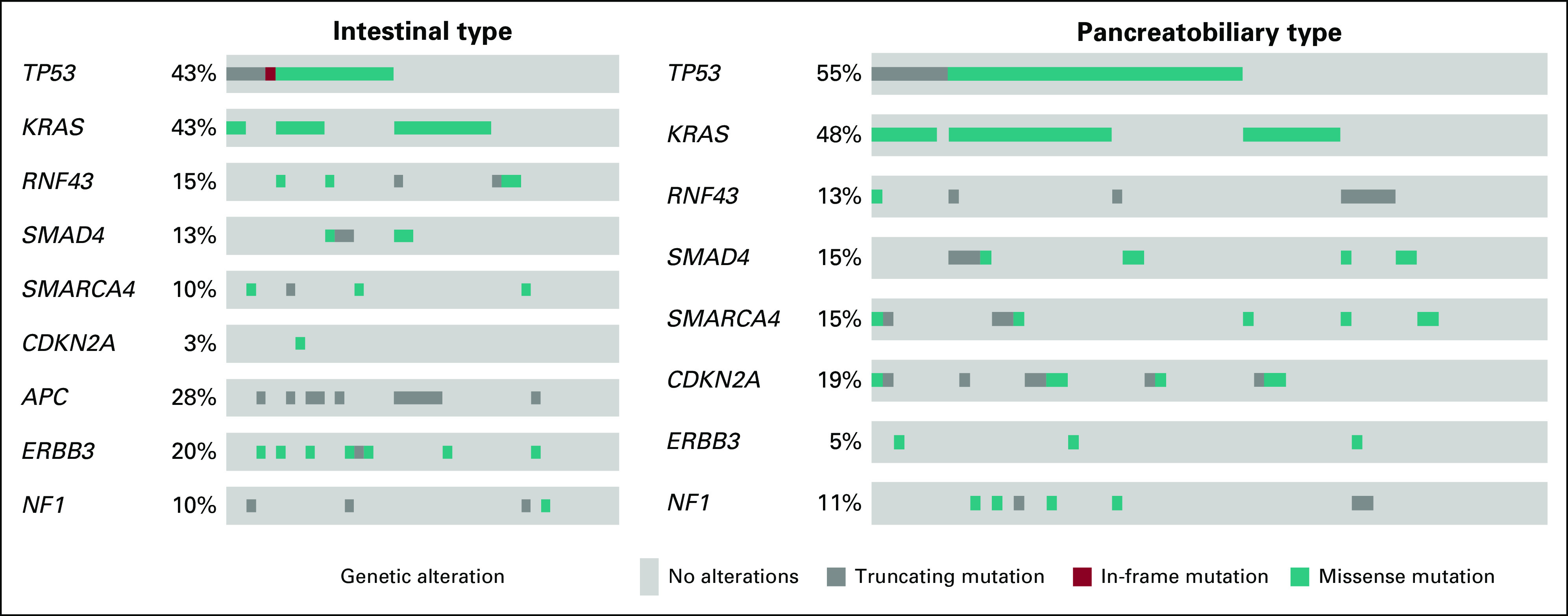
The frequency and type of mutations by morphologic type. Heat maps illustrate the frequency and type of mutations in intestinal-type and pancreatobiliary-type tumors. The genetic alterations were classified as either truncating, missense, or in-frame mutations.
Prognostic Impact of the Most Frequent Mutations
Kaplan-Meier analyses demonstrate a significantly prolonged OS for patients with I-type compared with patients with PB-type tumors (Data Supplement). When stratifying for anatomic origin, the survival curves cluster into two groups concordant with morphology (Data Supplement).
Of the nine most frequently mutated genes outlined above, only APC, ERBB3, KRAS, and SMARCA4 were shown to confer a prognostic value, depending on the context. Both APC and ERBB3 mutations were significantly associated with a prolonged OS in the entire cohort in univariable, but not in multivariable, analysis (HR, 0.30; 95% CI, 0.11 to 0.82; and HR, 0.34; 95% CI, 0.12 to 0.92, respectively). APC mutation was not prognostic in I-type tumors (data not shown), and ERBB3 mutation was not prognostic in analyses stratified for morphology (data not shown).
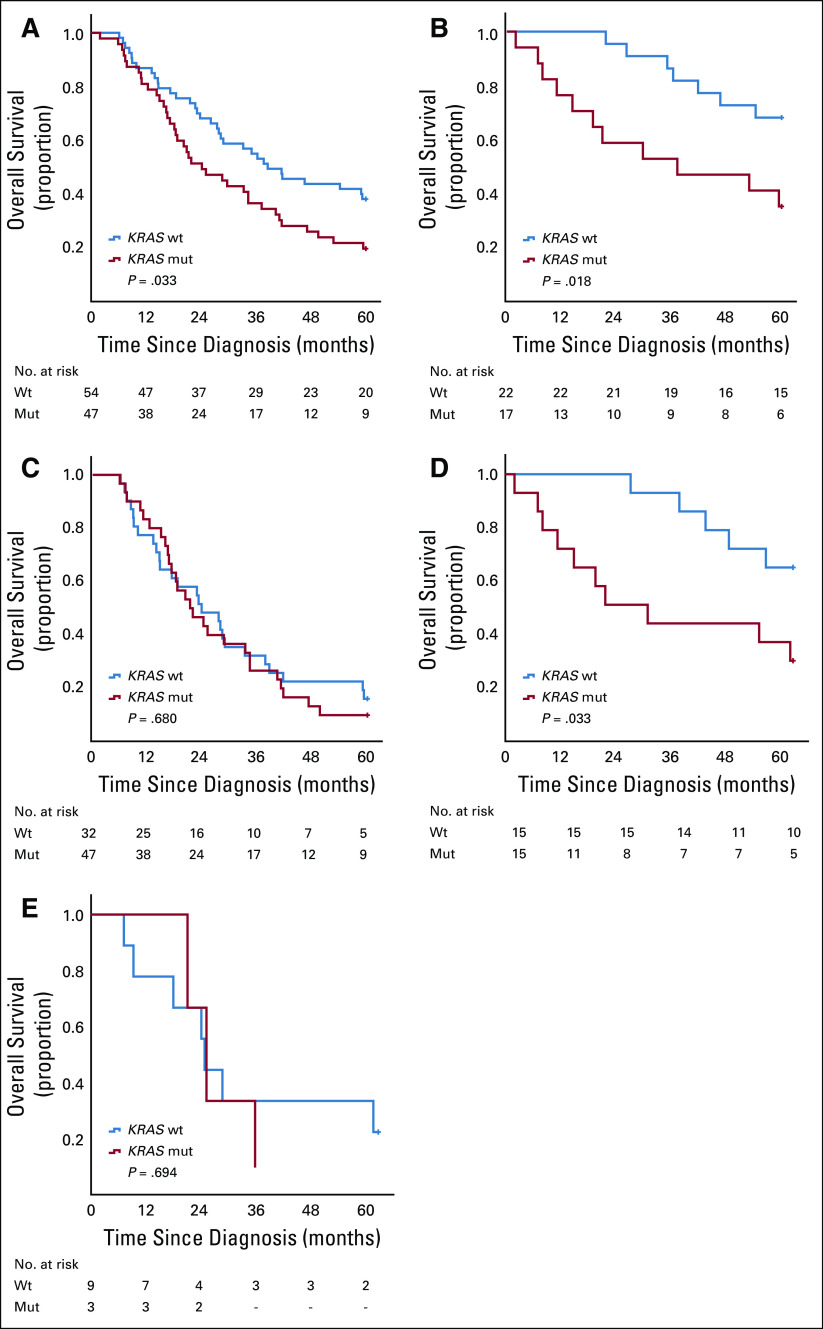
KRAS mutation was the strongest predictor of survival, as visualized in Figure 2. KRAS mutation was significantly associated with a reduced OS in the entire cohort (P = .029; Fig 2A). This association was confirmed in univariable Cox regression analysis (HR, 1.68; 95% CI, 1.05 to 2.68) and remained significant in multivariable analysis (HR, 1.67; 95% CI, 1.01 to 2.73). KRAS mutation was also a significant factor for reduced OS in I-type tumors (P = .018; Fig 2B), but not in PB-type tumors (Fig 2C). This association was confirmed in univariable Cox regression analysis (HR, 3.00; 95% CI, 1.16 to 7.52) and remained significant in multivariable analysis (HR, 3.73; 95% CI, 1.10 to 12.67). When stratifying for ampullary origin, KRAS mutation remained a prognostic factor in I-type tumors (P = 0.033; Fig 2D) but was not prognostic in PB-type tumors (Fig 2E). This association was confirmed in univariable Cox regression analysis (HR, 3.06; 95% CI, 1.04 to 9.00) and remained significant in multivariable analysis (HR, 7.76; 95% CI, 1.37 to 43.95).
FIG 2.
Relationship of KRAS mutation status with overall survival in the entire cohort and according to morphology. Kaplan-Meier curves visualize differences in 5-year overall survival for patients with KRAS-mutated (mut) and wild-type (wt) tumors in (A) the entire cohort, (B) intestinal-type tumors, (C) pancreatobiliary-type tumors, (D) intestinal-type ampullary tumors, and (E) pancreatobiliary-type ampullary tumors.
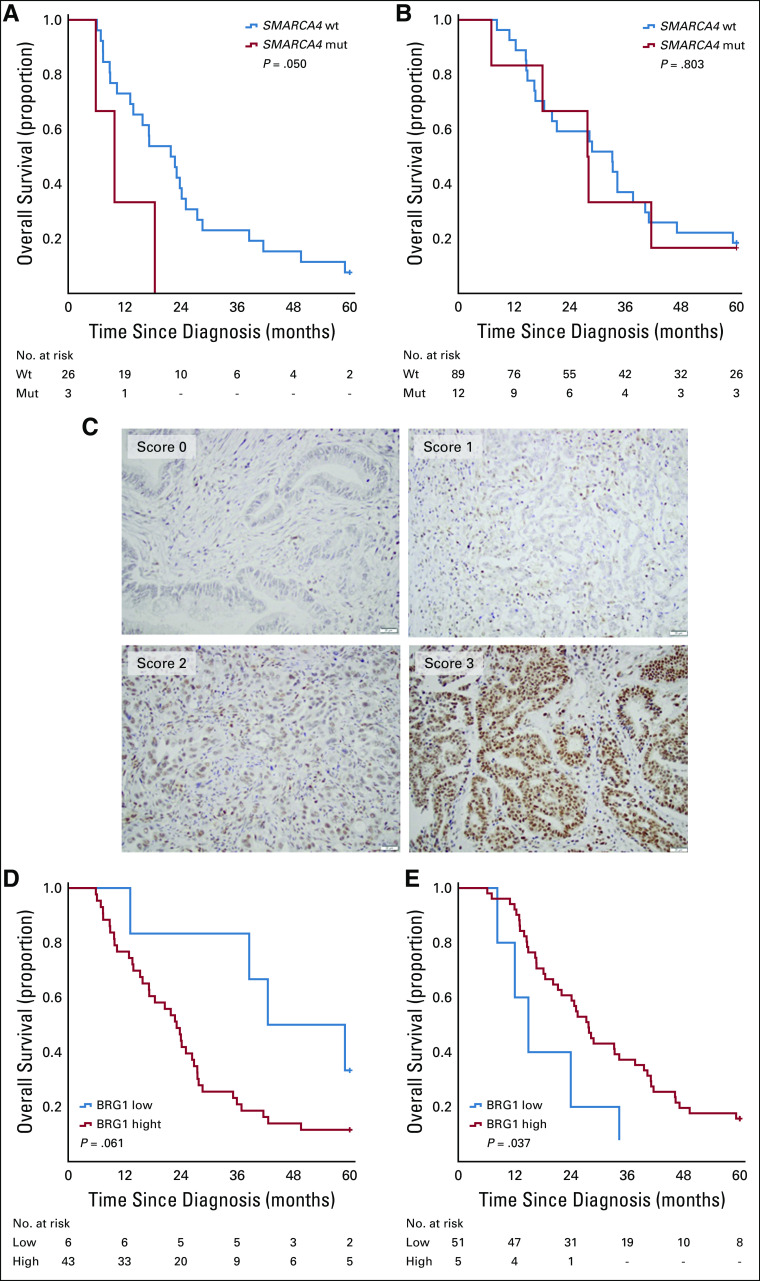
SMARCA4 mutation was not prognostic in the entire cohort or in strata according to morphology (data not shown). However, as shown in Figure 3, SMARCA4 mutation was significantly associated with a shorter OS in patients with PB-type tumors who did not receive adjuvant chemotherapy (P = .050; Fig 3A), but there was no significant interaction between SMARCA4 mutation and adjuvant treatment. SMARCA4 mutation was not prognostic in patients with PB-type tumors who received adjuvant chemotherapy (Fig 3B), and the prognostic value of SMARCA4 mutation did not differ according to adjuvant chemotherapy in I-type tumors (data not shown). Given the potential relationship of SMARCA4 mutation with chemotherapy seen in PB-type tumors, we proceeded to examine the prognostic value of immunohistochemical expression of BRG1, the protein encoded by the SMARCA4 gene, in tissue microarrays with samples from primary tumors (n = 175) from the original cohort.9 In all, BRG1 expression could be evaluated in 170 (97.1%) cases. Sample immunohistochemical images are shown in Figure 3C. In the entire cohort, high BRG1 expression was an adverse prognostic factor in patients not receiving adjuvant chemotherapy but was not prognostic in patients receiving adjuvant chemotherapy (data not shown). Subgroup analysis revealed that BRG1 was only prognostic in PB-type tumors, depending on adjuvant chemotherapy (Figs 3D and 3E), and there was a significant interaction between BRG1 expression (0 or 1 v > 1) and adjuvant treatment in PB-type tumors (P for interaction = .007). BRG1 was not prognostic in I-type tumors overall or in strata according to chemotherapy (data not shown). BRG1 expression was significantly higher in PB-type, but not in I-type, tumors harboring SMARCA4 mutations compared with wild type. The mutational burden of the analyzed genes did not differ significantly between I-type and PB-type tumors (P = .577) and was not prognostic in the whole cohort or stratified for morphologic type.
FIG 3.
Relationship of SMARCA4 mutation status and Brahma-related gene 1 (BRG1) protein expression with overall survival according to adjuvant chemotherapy in patients with pancreatobiliary (PB)-type tumors. (A, B) Kaplan-Meier curves visualizing differences in 5-year overall survival in patients with PB-type tumors according to SMARCA4 mutation status (A) without adjuvant chemotherapy, and (B) with adjuvant chemotherapy. (C) Sample immunohistochemical images of BRG-1 expression. (D, E) Kaplan-Meier analyses visualizing differences in 5-year overall survival in patients with PB-type tumors according to low and high BRG1 expression (D) without adjuvant chemotherapy, and (E) with adjuvant chemotherapy. mut, mutated; wt, wild type.
DISCUSSION
Morphology is emerging as an important prognostic factor in periampullary adenocarcinoma,1,2 and to our knowledge, this is the first study to comprehensively map common cancer-associated mutations in the full range of periampullary adenocarcinoma, with particular reference to morphology. Herein, we describe that the pattern of the mutational status, such as CDKN2A and APC mutations, of periampullary adenocarcinomas differs by morphologic subtype. These findings are in line with a study on 112 cancers of ampullary origin, demonstrating that the mutational spectrum in I-type tumors resembles that of colorectal cancer, and the mutational spectrum in PB-type tumors resembles that of pancreatic cancer.12 In the current study, mutations in APC but not in CDKN2A were found to be prognostic. Given that no PB-type tumors harbored APC mutations, it is plausible to assume that the link between these mutations and a favorable outcome in the entire cohort is mainly due to their association with I-type morphology. The same line of reasoning applies to the association between ERBB3 mutations, being more prevalent in I-type tumors, and a prolonged survival in the entire cohort. Adding to this, there was no significant association between HER3 expression and ERBB3 mutational status, and the clinical utility of assessment of ERBB3 mutation status for prognostication purposes needs additional validation.
KRAS mutation was found to signify a significantly shorter survival in patients with I-type tumors, also when adjusted for established clinical factors, whereas patients with KRAS wild-type I-type tumors could indeed be classified as long-term survivors. Two other studies have demonstrated KRAS mutations to be associated with poor prognosis in ampullary cancer;13,14 however, none of these considered tumor morphology. As of yet, epidermal growth factor receptor (EGFR) inhibition has not shown clinical efficacy in pancreatic cancer,15 but no study has investigated the efficacy of EGFR inhibitors in periampullary adenocarcinoma in relation to morphology. Although none of the patients in this study had received EGFR-inhibiting treatment, the findings indicate that some RAS wild-type periampullary cancers of I-type may indeed benefit from such treatment. Furthermore, the findings highlight a pressing need to identify optimized and individualized treatment protocols for patients with these tumors according to morphology and mutational status and that molecular profiling of these tumors may be of clinical benefit.
The only gene mutation for which the prognostic significance was found to differ by adjuvant treatment was SMARCA4, where a significant association was observed between SMARCA4 mutation and reduced survival in patients with PB-type tumors not having received adjuvant treatment. Despite the lack of a significant interaction between SMARCA4 mutation and adjuvant treatment, this observation led us to further explore the expression and prognostic value of BRG1, the protein encoded by SMARCA4, in tumors from the full study cohort. BRG1 was found to be an adverse factor in patients who did not receive adjuvant chemotherapy and, in addition, there was a significant interaction with adjuvant chemotherapy in PB-type tumors. BRG1 is an ATP-dependent chromatin remodeling protein16 that has been sparsely studied in periampullary cancer. One study on pancreatic cancer (n = 68) failed to demonstrate an association between BRG1 expression and gemcitabine response or survival,17 but the finding of a potential predictive role for SMARCA4/BRG1 in the current study merits further validation in additional patient cohorts.
To our knowledge, this is the first study to map the prevalence and prognostic significance of mutations in common cancer-associated genes in the full spectrum of periampullary adenocarcinoma. The findings demonstrate that the distribution and prognostic significance of some of the most commonly mutated genes differ by morphologic type. In particular, KRAS mutation status may be a suitable biomarker for prognostication in patients with I-type tumors, and SMARCA4/BRG1 expression may add value in the prediction of response to adjuvant chemotherapy treatment in patients with PB-type tumors. Moreover, the study supports that the use of molecular profiling could be of clinical benefit for patients with these types of tumors.
Footnotes
Preprint version available on bioRxiv.
Presented in part at the European Society for Medical Oncology annual meeting, Madrid, Spain, September 8-12, 2017.
Supported by the Swedish Cancer Society (Grant No. 2015-03598), the Swedish Research Council (Grant No. CAN 2018/418), the Mrs Berta Kamprad Foundation (Grant No. FBKS-2016-21), Governmental Funding of Clinical Research within the National Health Service (ALF; Grant No. 2014/354), and Lund University Faculty of Medicine and Skåne University Hospital funds and donations.
AUTHOR CONTRIBUTIONS
Conception and design: Sebastian Lundgren, Sofie Olsson Hau, Karin Jirström
Financial support: Karin Jirström
Collection and assembly of data: Sebastian Lundgren, Sofie Olsson Hau, Jacob Elebro, Margareta Heby, Björn Nodin, Johan Staaf
Data analysis and interpretation: Sebastian Lundgren, Sofie Olsson Hau, Emelie Karnevi, Jakob Eberhard, Karolina Holm, Johan Staaf, Göran B. Jönsson
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Margareta Heby
Consulting or Advisory Role: Bristol-Myers Squibb
Karolina Holm
Employment: SAGA Diagnostics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Westgaard A, Tafjord S, Farstad IN, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. doi: 10.1186/1471-2407-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronsert P, Kohler I, Werner M, et al. Intestinal-type of differentiation predicts favourable overall survival: Confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin. BMC Cancer. 2013;13:428. doi: 10.1186/1471-2407-13-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura W, Futakawa N, Zhao B. Neoplastic diseases of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:223–231. doi: 10.1007/s00534-004-0894-7. [DOI] [PubMed] [Google Scholar]
- 4.Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 6.Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elebro J, Jirström K. Use of a standardized diagnostic approach improves the prognostic information of histopathologic factors in pancreatic and periampullary adenocarcinoma. Diagn Pathol. 2014;9:80. doi: 10.1186/1746-1596-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elebro J, Heby M, Warfvinge CF, et al. Expression and prognostic significance of human epidermal growth factor receptors 1, 2 and 3 in periampullary adenocarcinoma. PLoS One. 2016;11:e0153533. doi: 10.1371/journal.pone.0153533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yachida S, Wood LD, Suzuki M, et al. Genomic sequencing identifies ELF3 as a driver of ampullary carcinoma. Cancer Cell. 2016;29:229–240. doi: 10.1016/j.ccell.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultz NA, Roslind A, Christensen IJ, et al. Frequencies and prognostic role of KRAS and BRAF mutations in patients with localized pancreatic and ampullary adenocarcinomas. Pancreas. 2012;41:759–766. doi: 10.1097/MPA.0b013e31823cd9df. [DOI] [PubMed] [Google Scholar]
- 14.Valsangkar NP, Ingkakul T, Correa-Gallego C, et al. Survival in ampullary cancer: Potential role of different KRAS mutations. Surgery. 2015;157:260–268. doi: 10.1016/j.surg.2014.08.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 17.Numata M, Morinaga S, Watanabe T, et al. The clinical significance of SWI/SNF complex in pancreatic cancer. Int J Oncol. 2013;42:403–410. doi: 10.3892/ijo.2012.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]