Abstract
PURPOSE
Previous studies have shown EndoPredict (EPclin), a test that integrates 12-gene expression data with nodal status and tumor size, to be predictive for risk of distant recurrence in women with estrogen receptor–positive, human epidermal growth factor receptor 2–negative early-stage breast cancer. Here, we modeled expected absolute chemotherapy benefit on the basis of EPclin test results.
METHODS
The effect of chemotherapy was modeled using previously validated 10-year risk of distant recurrence as a function of EPclin score for patients treated without chemotherapy. Average relative chemotherapy benefit to reduce breast cancer distant recurrence was evaluated using a published meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group. Absolute chemotherapy benefit differences were estimated across a range of interaction strengths between relative chemotherapy benefit and EPclin score. The average absolute benefit was calculated for patients with high and low EPclin scores using the distribution of scores in 2,185 samples tested by Myriad Genetics.
RESULTS
The average expected absolute benefit of chemotherapy treatment for patients with a low EPclin score was 1.8% in the absence of interaction and 1.5% for maximal interaction. Conversely, the expected average absolute chemotherapy benefit for patients with a high EPclin score was 5.3% and 7.3% for no interaction and maximal interaction, respectively.
CONCLUSION
For women with estrogen receptor–positive, human epidermal growth factor receptor 2–negative early-stage breast cancer, a high EPclin score identified which patients would benefit most from adjuvant chemotherapy in terms of absolute reduction of distant recurrence, regardless of the amount of interaction between EPclin and relative chemotherapy benefit. A high degree of prognostic discrimination for distant recurrence is more important for identifying patients likely to benefit most from chemotherapy than an interaction between EPclin and treatment-relative benefit.
INTRODUCTION
One in eight women in the United States will develop invasive breast cancer during her lifetime, and more than 40,000 women were expected to die as a result of the disease in 2018.1 Approximately 80% of primary breast cancers are estrogen receptor (ER) positive,2 such that adjuvant endocrine therapy (ET) after surgery is associated with improved outcomes and is now standard practice.3-5 In addition to ET, a subset of patients benefit from the addition of chemotherapy (ET + C). The Early Breast Cancer Trialists’ Collaborative Group performed a meta-analysis of more than 100 clinical trials in more than 100,000 women to evaluate the benefit of adjuvant chemotherapy.6 Although the benefit in individual trials varied considerably,3 this meta-analysis demonstrated an average 30% relative reduction in distant recurrence among women who received chemotherapy compared with those who did not.6
CONTEXT
Key Objective
Can an integrated, 12-gene, clinicomolecular assay predict absolute benefit from chemotherapy for women with estrogen receptor (ER)–positive, human epidermal growth factor receptor 2 (HER2)–negative breast cancer?
Knowledge Generated
In this model, patients with low 12-gene scores showed no difference in recurrence-free survival with or without chemotherapy, whereas those with high 12-gene scores showed a marked increase in recurrence-free survival with the addition of chemotherapy. Overall, this study suggests that the 12-gene clinicomolecular score is able to predict absolute benefit of chemotherapy for women with ER-positive, HER2-negative breast cancer.
Relevance
Accurate risk assessment is critical for determining appropriate treatment of women with breast cancer. Breast cancer prognostic assays like the 12-gene clinicomolecular score have been shown to provide more information about risk than standard clinical information alone in predicting recurrence-free survival in the absence of treatment. These new data support the ability of the 12-gene clinicomolecular score to also predict absolute benefit from chemotherapy to help to guide chemotherapy decisions for women with ER-positive, HER2-negative breast cancer.
For many patients with ER-positive disease, the risk of distant recurrence is sufficiently low that ET alone is adequate. In these patients, the adverse effects associated with chemotherapy may outweigh any benefit provided by a reduced risk of disease recurrence. Historically, identification of these low-risk patients has relied on clinicopathologic parameters, such as nodal status, tumor size, and tumor grade. However, studies have shown that clinicopathologic features alone are inadequate to guide adjuvant chemotherapy decisions reliably.7 Thus, when risk is defined using clinicopathologic features alone, a subset of truly low-risk patients receives unnecessary chemotherapy, whereas others at high risk forgo chemotherapy and experience avoidable disease recurrence.
To address this clinical dilemma, multigene expression prognostic assays have been developed for patients with ER-positive, human epidermal growth factor receptor 2 (HER2)–negative early-stage breast cancer. Several such assays are clinically available, with variable performance according to nodal status, and early (0 to 5 years) versus late (5 or more years) recurrence.8-11 These assays better quantify residual risk of recurrence after surgery and ET than clinicopathologic features alone, which enables physicians to tailor the use of adjuvant therapies (ET alone v ET + C) more accurately. To this end, evidence-based practice guidelines now indicate that prognostic assays are appropriate tools to help guide decision making.12-14
One such test is EndoPredict (Myriad Genetics, Salt Lake City, UT), which integrates a 12-gene molecular score with nodal status and tumor size into a combined clinicomolecular score (EPclin). Previous studies have validated that EPclin accurately predicts the risk of distant metastases in patients with ER-positive, HER2-negative breast cancers.11,15-18 Although this information can reliably identify patients at sufficiently low risk so that chemotherapy may be avoided safely, the ability of EPclin to predict benefit from the addition of chemotherapy has yet to be fully defined. Ideal approaches to evaluate this are limited because archival samples from previous randomized trials of ET ± C in appropriate patients largely have been exhausted.3,5 A prospective trial of EPclin that includes an arm randomized to receive no chemotherapy would now be unethical, given the established benefit of chemotherapy for patients with high-risk disease. Alternative study designs, therefore, are required to explore the scope of EPclin’s ability to predict adjuvant chemotherapy benefit for patients with ER-positive, HER2-negative early-stage breast cancer.
So far, classic factors that influence breast cancer prognosis have not shown an interaction between the relative effect of adjuvant chemotherapy on absolute risk of recurrence.19 Furthermore, the absolute benefit of chemotherapy depends on the patient’s baseline risk of developing recurrent disease.19 As such, as we evaluate a biomarker, it is helpful to understand the principal drivers of its chemopredictive ability. One possibility is that chemopredictive ability is driven by high prognostic accuracy, that is, the ability of the biomarker to differentiate those patients at highest versus lowest absolute risk of recurrence and, thus, those latter patients for whom chemotherapy cannot yield any clinically meaningful benefit. Alternatively, it is possible that a biomarker’s chemopredictive ability is driven by differences in relative chemotherapy benefit between higher versus lower biomarker-defined patient groups (ie, interaction between the biomarker score and relative chemotherapy benefit).
In the current study, we describe a modeling study that uses EPclin score, absolute risk of recurrence, and reduction of this risk associated with the addition of chemotherapy to estimate the differences in absolute risk for distant recurrence across EPclin scores. We demonstrate that for a molecular marker such as EPclin, patients with the highest baseline absolute risk for recurrence experience the greatest absolute benefit from chemotherapy. Furthermore, we establish that the key factor in any biomarker’s chemopredictive capacity is the ability to predict accurately the baseline absolute risk of recurrence. Any interaction of the biomarker with relative chemotherapy benefit will play only a small role in the absolute risk of recurrence. Through this modeling, we estimate the differences in absolute risk of distant recurrence across EPclin scores and examine the impact of different amounts of statistical interaction between EPclin and chemotherapy on these estimates.
METHODS
Gene Expression Assay
The 12-gene mRNA expression assay has been previously described in detail.20-22 In brief, the expression of three proliferation-related target genes (BIRC5, DHCR7, UBE2C), five hormone receptor–related target genes (AZGP1, IL6ST, MGP, RBBP8, STC2), and three normalization genes (CALM2, OAZ1, RPL37A) were measured by reverse-transcription quantitative polymerase chain reaction. A 12-gene molecular score was calculated as the linear combination of the normalized target gene expression.11,22 The clinicomolecular score EPclin was calculated by combining the 12-gene molecular score with tumor size and the number of positive lymph nodes and was reported as a numerical score from 1 to 6.11,22 Tumors with an EPclin score of less than 3.3 are considered low risk for distant recurrence and tumors with scores of 3.3 or greater are considered high risk for distant recurrence.11,17,18
Statistical Methods
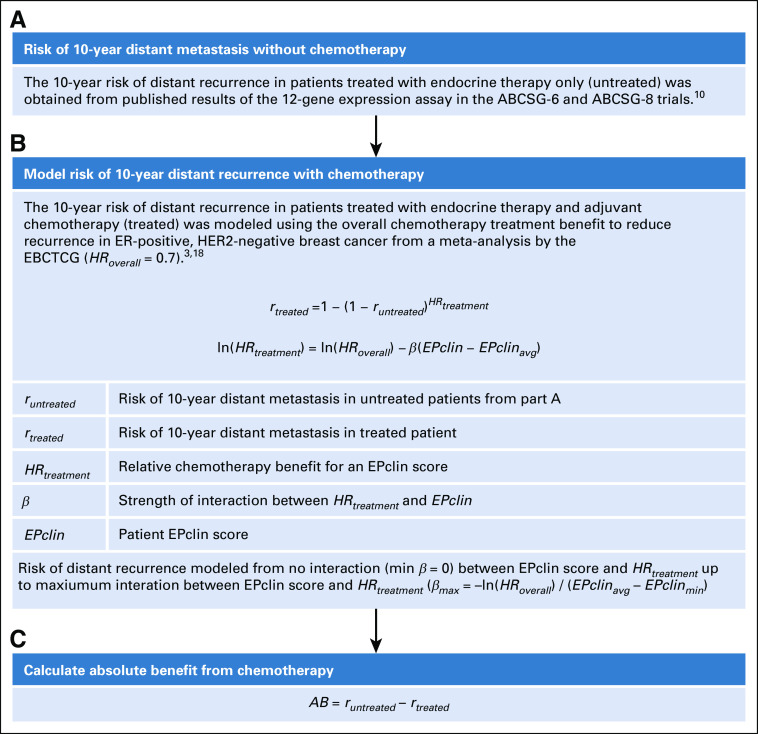
The ability of EPclin to provide an estimate of expected chemotherapy benefit was modeled, as shown in Figure 1. The primary component was the prognostic value of EPclin to predict the rate of distant recurrence in the first 10 years of follow-up. First, risk of 10-year distant recurrence in patients treated with ET only (untreated) as a function of EPclin score, runtreated(EPclin), was obtained from a previously published study of two large randomized phase III trials from the Austrian Breast and Colorectal Cancer Study Group (ABCSG-6 and ABCSG-8).11 Second, the risk of 10-year distant recurrence in patients treated with ET + C (treated) was estimated by introducing a relative chemotherapy benefit for a particular EPclin score, HRtreatment, to the untreated risk according to a proportional hazards model (Fig 1B), as in Equation 1:
FIG 1.
Overview of statistical methods to model chemotherapy benefit as a function of EPclin score. (A) Risk of 10-year distant metastasis without chemotherapy. (B) Model risk of 10-year distance recurrence with chemotherapy. (C) Calculate absolute benefit from chemotherapy. ABCSG, Austrian Breast and Colorectal Cancer Study Group; EBCTCG, Early Breast Cancer Trialists’ Collaborative Group; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
| (1) |
Mathematically, the dependence of the relative chemotherapy benefit on the risk of distant recurrence was modeled using a main effect for chemotherapy and an interaction between treatment status and the prognostic signature.23 Interactions were modeled as a linear dependence of the logarithm of HR for treatment on EPclin score according to Equation 2:
| (2) |
In this equation, β represents the interaction strength and defines how strongly the relative chemotherapy benefit for a patient depends on EPclin score, EPclinavg is the mean EPclin score in the population, and HRoverall is the hazard ratio for the chemotherapy benefit in the population. The term ln(HRoverall) ensures that the overall benefit of chemotherapy in the population does not depend on the interaction strength and matches the results from meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group overview of more than 100 clinical trials that evaluated the benefit of adjuvant chemotherapy in more than 100,000 women with breast cancer.6 On the basis of this meta-analysis, the overall reduction in distant recurrence rates for treatment was set at 30%, but 40% and 20% also were explored, corresponding to an HRoverall rate of 0.7, 0.6, and 0.8, respectively.
Maximal interaction was defined such that a patient’s relative chemotherapy benefit would be 0 at the minimum EPclin score (EPclinmin = 1; Fig 1B. In this scenario, β was equal to βmax = −ln(HRoverall) / (EPclinavg − EPclinmin). With the maximum value of β determined for an interaction giving no chemotherapy benefit at EPclinmin, weaker interactions were modeled using a percentage of the maximum value of β (Fig 1B).
Finally, the absolute benefit AB from chemotherapy for treated patients was determined according to Equation 3 (Fig 1C):
| (3) |
Absolute chemotherapy benefit for the low-risk category was calculated as a mean absolute chemotherapy benefit for all low-risk patients, and the same went for the high-risk category. For this calculation, EPclin scores were obtained from all patients tested with the 12-gene molecular assay at Myriad Genetics between March 31 and December 11, 2017, or at Myriad International (Munich, Germany) between October 13, 2014, and December 8, 2017, who received valid test results. Formalin-fixed paraffin-embedded breast resections of treatment-naïve ER-positive, HER2-negative breast tissue were tested. Biopsy samples were excluded. All data were collected in the course of normal health care operations. All patients provided consent for clinical testing at the time of testing, and all patient data were de-identified for analysis. These scores also were used to calculate EPclinavg.
RESULTS
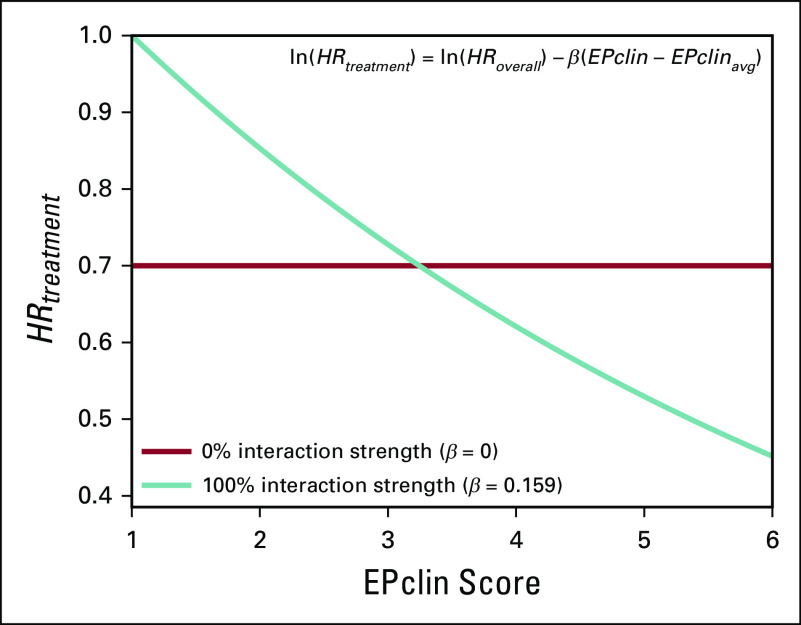
We examined the impact of different magnitudes of treatment effect and interaction strengths on the estimated 10-year risk of distant recurrence (Table 1). Maximal interaction strength (βmax = 0.159) was first determined by assuming that a patient’s relative chemotherapy benefit is 0 at the lowest possible EPclin score and maintaining the HRoverall of treatment at 0.7. The corresponding HRtreatment as a function of EPclin score is shown in Figure 2. HRtreatment is 1 for a patient with an EPclin score of 1 and 0.45 for a patient with the maximum observed EPclin score of 6. The risk of distant recurrence in the treated arm also was evaluated in a conservative scenario that assumed no interaction between chemotherapy benefit and EPclin score (ie, 0% interaction strength, constant HRtreatment). In this scenario, the relative chemotherapy benefit was equal to the overall chemotherapy benefit (β = 0; HRtreatment = 0.7; Fig 2).
TABLE 1.
Absolute Chemotherapy Benefit for Patients With Low and High EPclin Scores Assuming Different Overall Relative Chemotherapy Benefit (20%, 30%, or 40%) for Different Relative Interaction Strengths (0%, 50%, or 100%)
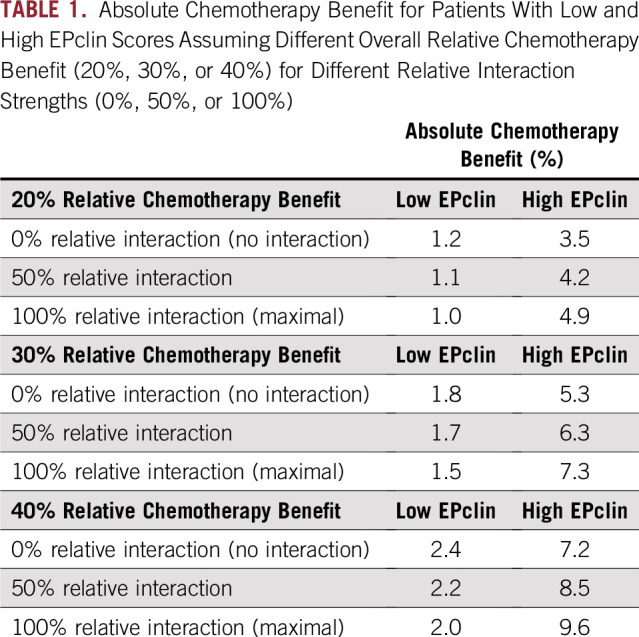
FIG 2.
Patient benefit from chemotherapy (HRtreatment) according to EPclin score at no interaction (0%) and maximal interaction (100%).
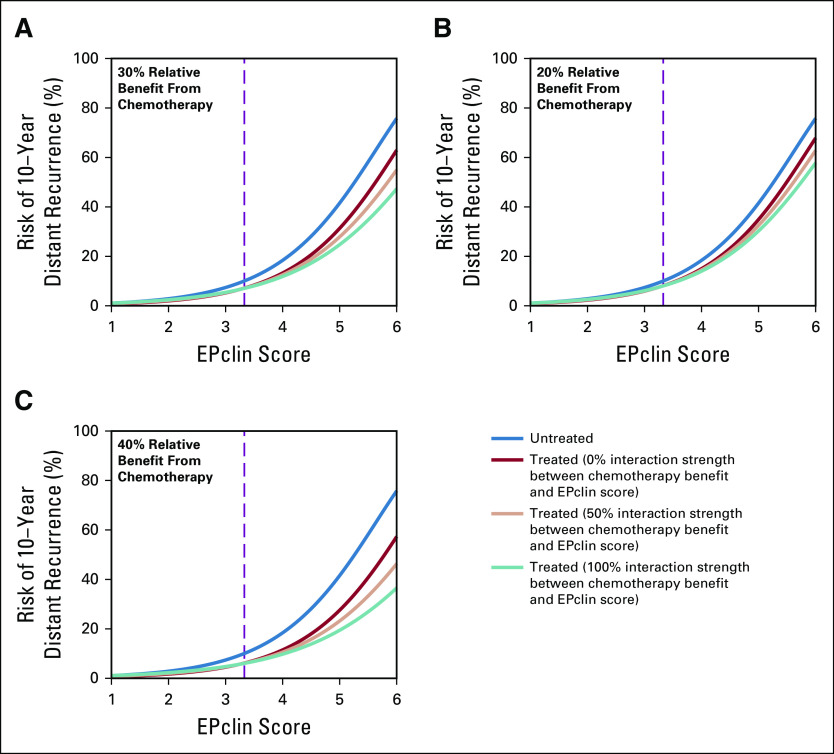
After βmax was determined, risk of distant recurrence was calculated for scenarios with a variety of interaction strengths between EPclin and chemotherapy. Figure 3A shows the relationship between EPclin score and predicted risk of distant recurrence by 10 years for untreated and treated patients under three scenarios of interaction strength (0%, 50%, and 100%). In all cases, the risk of recurrence is low for low EPclin scores, which results in small absolute chemotherapy benefit. For example, the expected absolute benefit of chemotherapy treatment for a patient with low EPclin scores ranged from 0.3% (EPclin = 1) to 2.9% (EPclin = 3.3) for 0% interaction strength and from 0% to 3.0% for 100% interaction strength. In comparison, there was much more separation between the treated and untreated risk curves for patients with high EPclin scores (Fig 3A). When there was no interaction, the expected absolute benefit of chemotherapy for high EPclin scores ranged from 2.9% (EPclin = 3.3) to 12.8% (EPclin = 6). This increased to a range of 3.0% to 28.5% for 100% interaction strength. Similar results were obtained when overall relative chemotherapy benefit was modeled to be lower (20%; Fig 3B) or higher (40%; Table 1; Fig 3C).
FIG 3.
Risk of distant recurrence as a function of EPclin score for different interaction strengths under different assumptions about overall relative chemotherapy benefit: (A) 30%, (B) 20%, and (C) 40%. The threshold between high (3.3 or greater) and low (less than 3.3) EPclin scores is shown by the dashed line.
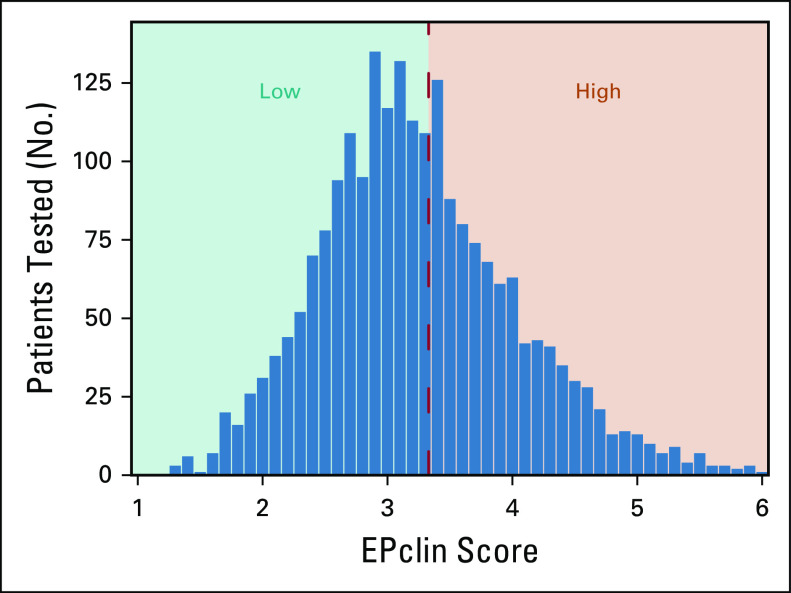
The various risk estimates across all scenarios of interaction strength were applied to a clinical cohort of patients with ER-positive, HER2-negative breast resections and representative EPclin scores (n = 2,185). EPclin ranged from 1.3 to 6, with a mean EPclin score of 3.2 (standard deviation, 0.79). The full EPclin distribution is shown in Figure 4. Overall, 1,275 samples (58%) were low risk, and 910 (42%) were high risk. The mean age at testing was 54.3 years, with the majority of patients having T1c or greater tumors and node-negative disease (82.3% and 77.6%, respectively; Table A1).
FIG 4.
EPclin scores in pretreatment estrogen receptor–positive, human epidermal growth factor receptor 2–negative breast resection tissue (n = 2,185).
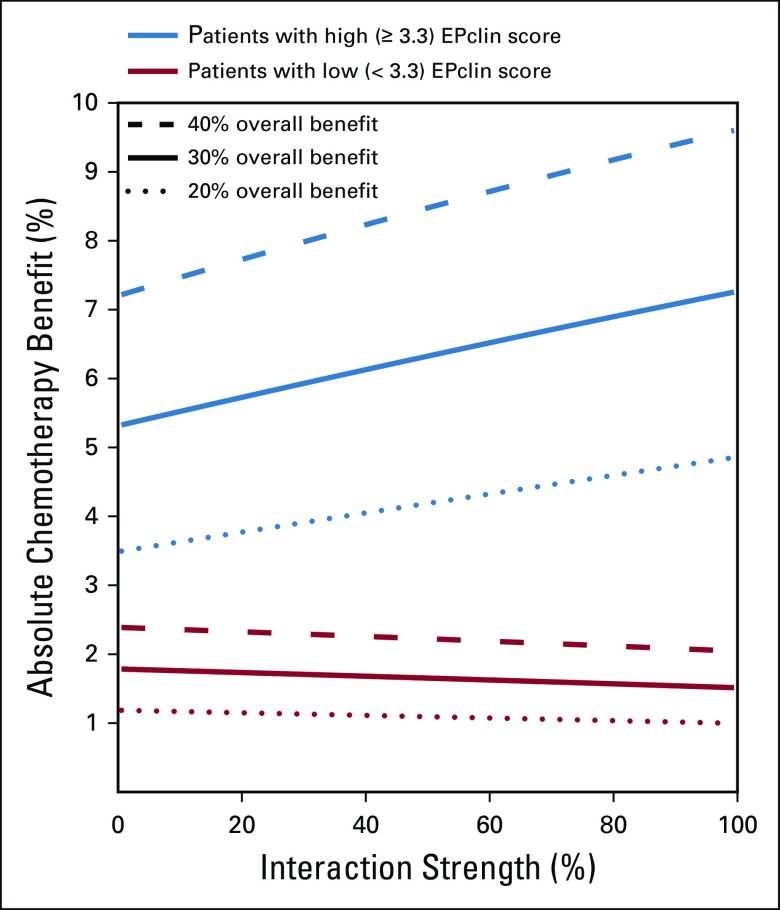
The absolute chemotherapy benefit in low- and high-risk patients was calculated as a function of interaction strength (Fig 5). Absolute chemotherapy benefit has an almost linear dependence on interaction strength. The benefit in high-risk patients increases with an increase in the interaction strength, whereas the benefit in low-risk patients decreases slightly. Of note, the effect of interaction strength is moderate. Indeed, even with the strongest interaction, the absolute chemotherapy benefit for a 30% relative benefit in high-risk patients is 7.3%, which is not dramatically different from the 5.3% benefit modeled in the case of no interaction (Table 1). Similarly, in low-risk patients, the absolute benefit is 1.5% when we assume the strongest interaction, which is close to a 1.8% benefit in the case of no interaction.
FIG 5.
Absolute chemotherapy benefit in patients with low and high EPclin scores as a function of average treatment effect and relative interaction strength.
DISCUSSION
Previous studies have demonstrated that EPclin accurately identifies patients at low risk for distant recurrence 10 years after surgery, with improved prognostic performance compared with clinical features alone or other molecular tests.11,15-18 For these low-risk patients, it is clear that the addition of adjuvant chemotherapy likely will not yield any clinically meaningful risk reduction. However, the ability of EPclin to predict chemotherapy benefit has not been fully explored. Here, we evaluated the capacity of EPclin to identify patients with ER-positive, HER2-negative early-stage breast cancer who may benefit most from adding adjuvant chemotherapy to ET. In addition, we examined whether this chemopredictive capacity is driven by high proficiency as a prognostic assay or as an interaction between EPclin score and chemotherapy. Thus, we evaluated the impact by modeling across a range of potential chemotherapy effects sizes and interaction strengths with EPclin.
To minimize potential error associated with individual trial values, the absolute benefit of chemotherapy in women with various EPclin scores was modeled using well-established estimates of the average relative benefit of adjuvant chemotherapy. The risk of over- or underestimating risk can never be avoided entirely, despite best efforts in trial design. This risk is particularly noteworthy in studies of adjuvant interventions in early-stage breast cancer because event rates may be low and distant recurrence may manifest many years or even decades after the initial diagnosis and treatment. In recognition of this, investigators try to increase sample size, enrich for events, and include the longest possible follow-up. Still, chemotherapy benefit estimated from a single trial may be significantly inaccurate. To address potential single-study error, we used the predicted chemotherapy benefit determined from a meta-analysis of data from 123 randomized trials, which demonstrated that combination chemotherapy reduced breast cancer distant recurrence by approximately 30%.6 Using this information, as well as estimates around this number (20% and 40%), we were able to generate a reliable estimate of the benefit of chemotherapy in patients with breast cancer.
In all scenarios, there was little separation between the risk curves for treated and untreated patients with low EPclin scores (less than 3.3). As a result, the absolute predicted chemotherapy benefit in low-risk patients was small and largely unaffected by average chemotherapy effect or interaction strength, whereas the toxicity of therapy remains the same. However, substantial separation existed between the treated and untreated risk curves at all interaction strengths for high EPclin scores (3.3 or greater). This separation increased at higher interaction strengths, and the absolute benefit in the high EPclin group ranged from 5.3% to 7.3% for a 30% average relative predicted chemotherapy benefit, which is substantially higher than differences associated with different degrees of interaction. Overall, these data demonstrate that high EPclin scores are associated with maximal predicted chemotherapy benefit, and low EPclin scores are associated with no clinically meaningful benefit. This association is irrespective of interaction strength between EPclin and predicted chemotherapy benefit, and the impact of any interaction on absolute benefit is much smaller than the ability to accurately estimate absolute risk.
As is typical of modeling studies, this study is not without limitations. For example, this is not an analysis of samples obtained from patients randomly assigned prospectively to ET + C or ET alone. No such samples or data sets were identified for retrospective analysis. Given current accepted standards of care for patients with high-risk disease, future studies that withhold chemotherapy for any of these patients are unlikely to occur. In addition, the overall predicted chemotherapy benefit used here was based on a meta-analysis of 123 clinical studies, which included patients treated with multiple different regimens. Inevitably, such heterogeneity in chemotherapy regimens introduces variability in predicted chemotherapy benefit. We sought to offset such factors by modeling at a range of benefit values (20%, 30%, 40%). Of note, the approximately 30% reduction in distant recurrence was selected on the basis of its association with chemotherapy regimens that are more consistent with modern patterns of care in contrast to more historic regimens.
Other aspects of the statistical model are also limitations of the study. It is assumed that the relative benefit of chemotherapy linearly depends on EPclin score (Eq 2), which is the simplest mathematical implementation of dependence. Linear dependence also was used to demonstrate interaction between chemotherapy and a prognostic score.8 In statistical analysis, the most common implementation of interaction between variables has the same functional form. In addition, only positive interaction between chemotherapy benefit and EPclin score was included in this analysis because it is expected that high expression of the proliferation-related genes in EPclin is associated with higher rather than lower relative chemotherapy benefit. Conversely, high expression of the hormone receptor–related genes is expected to be associated with smaller relative chemotherapy benefit because ER-negative tumors have a much stronger response to chemotherapy than ER-positive tumors. This again corresponds to a positive interaction because the hormone receptor–related genes contribute negatively to the EPclin score.
In summary, this modeling analysis demonstrates that EPclin is able to predict which patients will gain maximum absolute benefit from chemotherapy. In addition, these data indicate that the predicted absolute impact of chemotherapy on distant recurrence depends much more on the prognostic ability of the biomarker to predict an individual woman’s risk of recurrence accurately. Therefore, within a reasonable range, any interaction between EPclin and relative predicted chemotherapy benefit is a secondary factor. EndoPredict has been shown in multiple studies to predict risk of distant recurrence accurately in women with ER-positive, HER2-negative early-stage breast cancer who receive 5 years of ET, with an accuracy similar to the other best breast prognostic tests and substantially better than the 21-gene recurrence score (Oncotype DX; Genomic Health, Redwood City, CA).18,24 This modeling indicates that EndoPredict identified a substantial proportion of women whose risk of breast cancer recurrence was so low that the addition of adjuvant chemotherapy would not produce a clinically meaningful benefit on recurrence risk. Conversely, patients with high EPclin scores were predicted to experience the greatest benefit from chemotherapy regardless of the simulated interaction strength between EPclin and predicted chemotherapy benefit. Overall, this demonstrates that EndoPredict provides guidance on the expected absolute benefit from adjuvant chemotherapy in addition to prognostic information for patients with ER-positive, HER2-negative early-stage breast cancer. Therefore, EndoPredict can identify patients likely to benefit sufficiently from adjuvant chemotherapy to justify associated toxicities.
APPENDIX
TABLE A1.
Clinical Characteristics of the Tested Cohort
Footnotes
Presented at the American Society of Clinical Oncology 2018 Annual Meeting, Chicago, IL, June 1-5, 2018.
AUTHOR CONTRIBUTIONS
Conception and design: Darl D. Flake, Anthony Magliocco, Mark Robson, Lee Schwartzberg, Priyanka Sharma, Ralf Kronenwett, Alexander Gutin, Johnathan Lancaster, Jack Cuzick, William Gradishar
Collection and assembly of data: Darl D. Flake, Lee Schwartzberg, Saskia Wehnelt
Data analysis and interpretation: Hatem Soliman, Darl D. Flake, Anthony Magliocco, Mark Robson, Lee Schwartzberg, Priyanka Sharma, Krystal Brown, Ralf Kronenwett, Alexander Gutin, Jack Cuzick, William Gradishar
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Hatem Soliman
Consulting or Advisory Role: Celgene, AstraZeneca, Eli Lilly, Novartis, Pfizer, Puma Biotechnology
Research Funding: Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Eli Lilly
Darl D. Flake
Employment: Myriad Genetics
Stock and Other Ownership Interests: Myriad Genetics
Patents, Royalties, Other Intellectual Property: Multiple patents related to the development of tests at Myriad Genetics
Anthony Magliocco
Stock and Other Ownership Interests: Protean Biodiagnostics, The Genomic Cancer Institute, Proscia
Honoraria: Bristol-Myers Squibb, Merck, Illumina, Leica
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Proscia, Roche, Illumina
Speakers’ Bureau: Bristol-Myers Squibb
Research Funding: bioTheranostics, Roche, Genentech
Patents, Royalties, Other Intellectual Property: Various patents pending as co-inventor through Moffitt Cancer Center
Travel, Accommodations, Expenses: Menarini Silicon Biosystems, Illumina, Bristol-Myers Squibb, Merck, Ventana Medical Systems
Mark Robson
Honoraria: AstraZeneca
Consulting or Advisory Role: McKesson, AstraZeneca, Merck, Pfizer, Daiichi Sankyo
Research Funding: AstraZeneca (Inst), Myriad Genetics (Inst), Invitae (Inst), AbbVie (Inst), Tesaro (Inst), Medivation (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Pfizer
Lee Schwartzberg
Stock and Other Ownership Interests: Vector Oncology
Honoraria: Pfizer, Amgen, NanoString Technologies, Novartis, Napo, Taiho Pharmaceutical, Genentech, E.R. Squibb and Sons, Helsinn Therapeutics, Genomic Health, Myriad Genetics, AstraZeneca
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Helsinn Therapeutics, Caris Life Sciences, Spectrum Pharmaceuticals, Tesaro, Genomic Health, AstraZeneca, E.R. Squibb and Sons, Amgen, F. Hoffmann-La Roche, Genentech, Eli Lilly, Novartis, Merck, Biocept, AbbVie, bioTheranostics, Athenex, Coherus Biosciences
Speakers’ Bureau: Coherus Biosciences, Puma Biotechnology
Research Funding: Amgen (Inst), GlaxoSmithKline (Inst), Spectrum Pharmaceuticals (Inst), Medivation (Inst), Bayer AG (Inst), Genentech (Inst), Pfizer (Inst), Tesaro (Inst), Sanofi (Inst), E.R. Squibb and Sons (Inst), Novartis (Inst), MedImmune (Inst), Helsinn Therapeutics
Priyanka Sharma
Consulting or Advisory Role: TapImmune, Almac Diagnostics, AstraZeneca, Novartis, Pfizer, Myriad Genetics, Puma Biotechnology
Research Funding: Novartis (Inst), Celgene (Inst), Bristol-Myers Squibb (Inst), Cosmo Biosciences (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Almac Diagnostics
Krystal Brown
Employment: Myriad Genetics
Saskia Wehnelt
Employment: Crescendo Bioscience, Myriad International
Stock and Other Ownership Interests: Myriad Genetics
Ralf Kronenwett
Employment: Myriad International
Stock and Other Ownership Interests: Siemens
Patents, Royalties, Other Intellectual Property: Pending patent application with EndoPredict, previous shareholder of Sividon Diagnostics (Myriad International) with milestone payment
Alexander Gutin
Employment: Myriad Genetics, Myriad Genetics (I)
Stock and Other Ownership Interests: Myriad Genetics, Myriad Genetics (I), Gilead
Johnathan Lancaster
Employment: Myriad Genetics
Leadership: Myriad Genetics
Stock and Other Ownership Interests: Myriad Genetics
Consulting or Advisory Role: Protean Biodiagnostics
Travel, Accommodations, Expenses: Myriad Genetics
Jack Cuzick
Honoraria: Merck, Roche, QIAGEN
Consulting or Advisory Role: Merck, Roche, QIAGEN, Myriad Genetics
Speakers’ Bureau: Becton Dickinson
Research Funding: QIAGEN (Inst), Becton Dickinson (Inst), Genera Biosystems (Inst), Myriad Genetics (Inst), AstraZeneca (Inst), Aventis Pharma (Inst), GeneFirst (Inst), Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Myriad Genetics to institution of which I receive a share for development of cell cycle progression score (Prolaris)
Travel, Accommodations, Expenses: GE Healthcare
William Gradishar
Consulting or Advisory Role: Genentech, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1. American Cancer Society: Cancer Facts & Figures 2018. Atlanta, GA, American Cancer Society, 2018. [Google Scholar]
- 2.Keen JC, Davidson NE. The biology of breast carcinoma. Cancer. 2003;97:825–833. doi: 10.1002/cncr.11126. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681–1692. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group Tamoxifen for early breast cancer: An overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 6.Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobain EF, Hayes DF. Indications for prognostic gene expression profiling in early breast cancer. Curr Treat Options Oncol. 2015;16:23. doi: 10.1007/s11864-015-0340-x. [DOI] [PubMed] [Google Scholar]
- 8.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 9.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 10.Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: Using the PAM50 risk of recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol. 2014;25:339–345. doi: 10.1093/annonc/mdt494. [DOI] [PubMed] [Google Scholar]
- 11.Filipits M, Rudas M, Jakesz R, et al. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res. 2011;17:6012–6020. doi: 10.1158/1078-0432.CCR-11-0926. [DOI] [PubMed] [Google Scholar]
- 12.Coates AS, Winer EP, Goldhirsch A, et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 15.Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;109:2959–2964. doi: 10.1038/bjc.2013.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubsky P, Filipits M, Jakesz R, et al. EndoPredict improves the prognostic classification derived from common clinical guidelines in ER-positive, HER2-negative early breast cancer. Ann Oncol. 2013;24:640–647. doi: 10.1093/annonc/mds334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin M, Brase JC, Calvo L, et al. Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2- breast cancer patients: Results from the GEICAM 9906 trial. Breast Cancer Res. 2014;16:R38. doi: 10.1186/bcr3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buus R, Sestak I, Kronenwett R, et al. Comparison of EndoPredict and EPclin with Oncotype DX recurrence score for prediction of risk of distant recurrence after endocrine therapy. J Natl Cancer Inst. 2016;108:djw149. doi: 10.1093/jnci/djw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuzick J. Prognosis vs treatment interaction. JNCI Cancer Spectrum. 2018;2:pky006. doi: 10.1093/jncics/pky006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warf MB, Rajamani S, Krappmann K, et al. Analytical validation of a 12-gene molecular test for the prediction of distant recurrence in breast cancer. Future Sci OA. 2017;3:FSO221. doi: 10.4155/fsoa-2017-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronenwett R, Bohmann K, Prinzler J, et al. Decentral gene expression analysis: Analytical validation of the EndoPredict genomic multianalyte breast cancer prognosis test. BMC Cancer. 2012;12:456. doi: 10.1186/1471-2407-12-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myriad Genetic Laboratories: Myriad EndoPredict Technical Specifications. Salt Lake City, UT, Myriad Genetics, 2017. [Google Scholar]
- 23.Michiels S, Ternès N, Rotolo F. Statistical controversies in clinical research: Prognostic gene signatures are not (yet) useful in clinical practice. Ann Oncol. 2016;27:2160–2167. doi: 10.1093/annonc/mdw307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sestak I, Buus R, Cuzick J, et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:545–553. doi: 10.1001/jamaoncol.2017.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]