Abstract
Purpose
Microsatellite instable-high (MSI-H) colorectal cancers (CRCs) are known to carry better survival in the local disease stage even without treatment. The influence of types of treatment on survival of MSI-H metastatic CRCs (mCRCs) is still unclear and is evaluated in this study.
Materials and Methods
Patients with MSI-H mCRC treated with first-line chemotherapy, with or without bevacizumab, identified in the Israeli population-based Molecular Epidemiology of Colorectal Cancer (MECC) study, were diagnosed between 1998 and 2013 and followed up until May 2017; MSI status was determined by comparing 10 markers in tumor and normal tissue. Dates of metastases and death and treatment details were extracted from oncology records.
Results
Among 590 patients treated for mCRC, 106 (18%) had MSI-H tumors. Patients with MSI-H had a median overall survival (OS, from start of first-line treatment) of 1.6 years. The presence of a somatic B-Raf proto-oncogene (BRAF) mutation was a significant adverse prognostic factor in the MSI-H group (hazard ratio [HR], 1.8; 95% CI, 1.1 to 3.0; P = .026). MSI-H tumors without BRAF mutation (n = 87) had similar OS benefit from fluorouracil (FU) only as from any combination protocols (HR, 0.93; P = .78), whereas microsatellite-stable (MSS) tumors without BRAF mutation (n = 456) showed improved OS over FU-only regimens when combination chemotherapy with or without bevacizumab was used (HR, 0.58; P < .01; P value for interaction = .07). Patients with MSI-H/BRAF wild type (WT) had survival advantage over patients with MSS disease (adjusted HR, 0.58; 95% CI, 0.35 to 0.98) when treated with FU-only protocols.
Conclusion
Clinical outcomes differ substantially between patients with MSS/BRAF-WT mCRC and MSI-H/BRAF-WT mCRC, with measurable differences between chemotherapy regimens. MSI-H mCRCs are a clinically distinct subset of colorectal cancers. Their current poor outcome suggests that new clinical trials are needed to identify therapeutic options, potentially taking advantage of the new developments in the field of immunotherapy.
INTRODUCTION
Metastatic colorectal cancer (mCRC) is a challenging disease. Although treatment advances with modern protocols including anti–vascular endothelial growth factor and anti–epidermal growth factor receptor (EGFR) monoclonal antibodies1-3 prolonged median overall survival (OS) of patients with CRC to > 2 years, mCRC, unfortunately, is still an incurable disease in most cases.
Molecular heterogeneity in metastatic CRCs influences treatment and outcome. Anti-EGFR agents are less effective in the presence of a K-Ras proto-oncogene (KRAS) or N-Ras proto-oncogene (NRAS) mutation; the presence of a BRAF V600E mutation has a detrimental effect on overall survival; the presence of human epidermal growth factor receptor 2 (HER2) in mCRC tumors suggests the possibility of benefit from anti-HER2 biologic treatment; and, more recently, microsatellite instable (MSI)–high tumors have been identified as candidates for immunotherapy.4-7
Loss of genomic stability is identified as an early step in colon cancer tumorigenesis and has pathologic, phenotypic, and clinical consequences.8-11 Molecular analysis enables better understanding and separation of colorectal tumors now identified as carrying differences in clinical outcome.12,13 Under this classification, tumors defined as consensus molecular subtype-1 (CMS1) are characterized as microsatellite instable (MSI-H) and hypermutated tumors.14
The clinical importance and exclusiveness of MSI-H CRCs have formerly been established.15-18 MSI-H tumors have significantly better prognosis at early local stages of disease and rarely metastasize. Approximately 15% of all CRC cases are MSI-H. A small proportion of them (approximately 5% of all tumors) carry germline mutations in the mismatch repair (MMR) genes, corresponding with Lynch syndrome, whereas others acquire microsatellite instability and deficient mismatch repair on the basis of either methylation downregulation of mutL homolog-1 gene (MLH1) or two somatically acquired inactivating mutations corresponding to the recently defined double-somatic CRC.19 In addition to having a better prognosis of local tumor not related to treatment, MSI-H tumors have also been shown to have an inferior response to adjuvant chemotherapy with fluorouracil (FU).20-25
Although the inherent better prognosis of early MSI-H CRC and lack of effect of FU reduce the need for adjuvant chemotherapy treatment in many cases, patients with metastatic MSI-H (met/MSI-H) CRC have until recently been treated with standard chemotherapy protocols, with or without biologic agents, offered to all patients with metastatic disease with adequate performance status. The low incidence of MSI-H mCRCs (< 5% of all cases) did not allow analyzing them separately in subgroup analyses of prospective randomized trials evaluating effectiveness of chemotherapy, anti–vascular endothelial growth factor, or anti-EGFR.16,26-28 Thus, few data exploring the effect of MSI-H on metastatic disease progression during chemotherapy29,30 are available, resulting in a failure of a meta-analysis of published chemotherapy trial findings to analyze progression-free survival and OS in patients with met/MSI-H because of a lack of data.29,31 The current study evaluates the effect of microsatellite instability (MSI-H) on the overall survival of a large, prospectively collected cohort of treated patients with mCRC.
MATERIALS AND METHODS
The Molecular Epidemiology of Colorectal Cancer (MECC) study32 is a prospective cohort identifying all newly detected (incident) CRC cases in a defined geographical area in northern Israel between 1998 and 2016. The study includes population-based, randomly chosen, matched controls without CRC, which are irrelevant to this report. Cases identified in the MECC study were approached for risk-factors interview and contributed a venous blood sample after signing a consent form approved by Carmel Medical Center in Israel and the University of Southern California in the United States. Formalin-fixed paraffin-embedded tissue blocks were collected from diagnostic colonoscopy or surgical resection. DNA was extracted from both the peripheral blood and the tissue sample and was routinely studied for MSI status, KRAS and BRAF mutations, as well as known founder mutations in MMR genes, mostly in Ashkenazi Jews. Clinical data were collected at diagnosis, including TNM staging, tumor grade, and histology. Clinical follow-up included identification of treatment modalities used during the whole follow-up period and identification of changes in disease status, including identification of new metastases. Details of treatments, as well as date of identification of metastases (at diagnosis or during disease progression), were extracted from the medical files of the patients, which were extracted by experienced senior physicians.
For this study, MECC cases that presented with metastatic disease at diagnosis or developed metastases during follow-up were sought. In addition to cases from the MECC population-based series, we recruited into the study other mCRC cases that were evaluated in our laboratory on the basis of their clinical presentation or suspicion of Lynch syndrome to increase the size of the case series. We included all patients with first-line therapy for metastatic disease. Detailed treatment options were grouped into three categories: FU or capecitabine only, combination chemotherapy (oxaliplatin-based or irinotecan-based in combination with fluoropyrimidine), and combination chemotherapy with bevacizumab. Time of death of cases was defined using the Israeli population register. The vital status was assessed at the end of April 2017. To be eligible for analysis, a case had to have mCRC, have been treated for the metastases with information on treatment details, and have available tissue and germline DNA to analyze MSI and BRAF status.
Laboratory Assay
DNA was extracted using a commercial DNA extraction kit. Analysis was performed on samples of tumor tissue and normal tissue of the same patient, and microsatellite status was compared between the two. Ten microsatellite loci were analyzed for differences in the length of the microsatellite sequence between DNA from normal tissue and DNA extracted from paraffin block of the tumor tissue. Five loci of mononucleotide repeats (BAT25, BAT26, Bat40, β-catenin, and TGFBRII) and five of dinucleotide repeats (D2S123, D5S346, D10S197, D17S250, and D18S58) were amplified and tested. Those include the five original Bethesda panel markers and five additional mono- and dinucleotide markers.33 Results for each marker were registered as stable, unstable, equivocal, or suspected as loss of heterozygosity. MSI-H was called when at least 30% of the informative markers were found to be instable. Of all MSI-H cases, only 10% did not qualify if only the original five markers were used. Most MSI-H cases were also validated by immunohistochemistry tests of the MMR genes.
Mutations in codon 600 of BRAF were identified by direct sequencing of exon 15 of the BRAF gene. Purified amplicons were submitted to direct sequencing in the automated fluorescent sequencer 3130 XL Genetic Analyzer (Applied Biosystems, Foster City, CA). Similarly, KRAS mutations in exon 12, 13, and 61 were identified using a Taqman-based single-nucleotide polymorphism genotyping assay on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems) in a 96-well format.
Statistical Methods
Baseline characteristics were analyzed using descriptive statistics and compared using χ2 tests (exact test when appropriate) for categorical variables and two-sample t test or nonparametric Mann-Whitney test, as appropriate, for continuous variables. Overall survival was calculated from the start date of first treatment of the first identified metastasis (either at time of CRC diagnosis or at time of disease progression) and until the recorded date of death (of any cause) or the last date of available follow-up. Overall survival was estimated with the use of Kaplan-Meier method and presented using medians and 2-year and 5-year survival probabilities.
Differences in OS were summarized using hazard ratios (HRs), estimated using Cox proportional hazard modeling. The model was adjusted to age at treatment onset and indicator for surgery for metastatic disease. The predictive effect of MSI status on treatment effects was assessed using Cox proportional hazard models with terms for treatment, MSI status, and their interaction
RESULTS
Patient Population
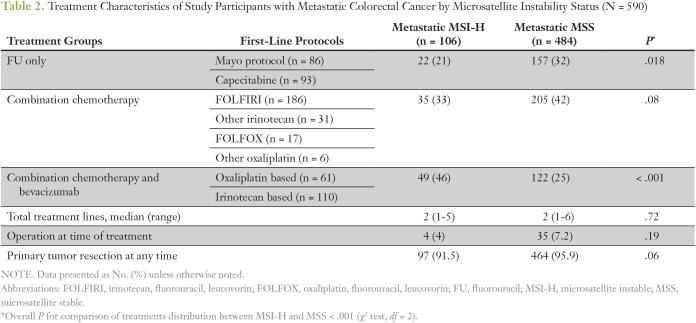
The study population consisted of 106 MSI-H mCRC (met/MSI-H) cases for which first-line chemotherapy-based treatment was identified. A group of 484 metastatic MSS cases (met/MSS) with available information on their treatment was identified as control group. Demographic and clinical characteristics of the total 590 study participants by their MSI status are listed in Table 1. The metastatic MSI-H group had mean age at treatment of 63 ± 15 years, 54% female, 86% with Jewish ethnicity, and 48% presenting at stage IV at diagnosis. Generally, the met/MSS group had comparable sex and ethnicity (Jewish/non-Jewish) distribution, as well as frequency of presenting at stage IV at diagnosis. However, met/MSI-H cases tended to have lower age at treatment onset (23% age < 50 years v 9% among met/MSS). This distribution reflects that fact that the patients with met/MSI-H were younger at diagnosis (proportion of patients younger than 50 years: 25% in met/MSI-H group and only 10% in met/MSS group). BRAF mutation presence was higher among patients with metastatic MSI-H than metastatic MSS (18% v 6% only in met/MSS group, P < .001).
Table 1.
Demographic and Disease Characteristics of Study Participants With Metastatic Colorectal Cancer by Microsatellite Instability Status (N = 590)
Treatments for Metastatic Disease
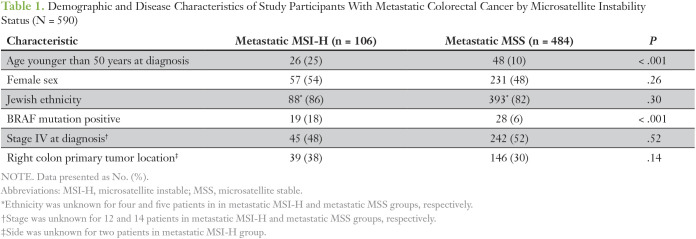
Common first-line treatment chemotherapy for the metastatic disease in the MSI-H group included FU + leucovorin (LCV) or capecitabine only in 21% (n = 22), combination chemotherapy protocols (irinotecan based or oxaliplatin based) in 33% (n = 35), and combination chemotherapy (irinotecan based or oxaliplatin based) and bevacizumab in 46% (n = 49) of cases. Treatment protocols significantly differed between met/MSI-H and met/MSS groups, where fewer patients in the met/MSS group received bevacizumab-containing regimens (25% v 46%) and more patients received FU or capecitabine only (overall P < .001; Table 2).
Table 2.
Treatment Characteristics of Study Participants with Metastatic Colorectal Cancer by Microsatellite Instability Status (N = 590)
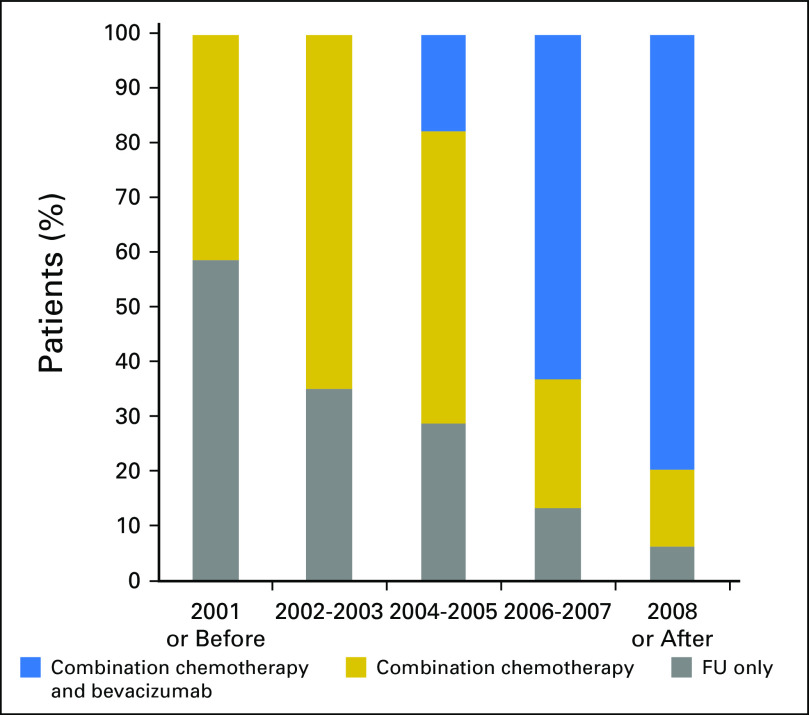
Treatment protocols have changed during the study period and represent the natural evolution of chemotherapy for patients with mCRC. Figure 1 presents frequency of the three classes across period years.
Fig 1.
Year of treatment onset by treatment protocol group in patients with metastatic colorectal cancer (n = 590). FU, fluorouracil.
Second-line treatment was given to 55% of the patients treated before 2003, 63% of those treated in 2003 to 2006, and 68% of patients treated in 2007 and after. No difference in number of treatment lines was noticed between cases with MSI-H and cases with MSS tumors, probably reflecting the fact that in those years the treating oncologists were unaware of the MSI status at time of treatment.
In addition, in the MSI-H population, the baseline characteristics of patients in the different treatment regimen were comparable (data not shown). Because of the modest sample size of therapeutic subgroups, we pooled all regimens other than FU or capecitabine only into an any-combination group to aid interpretability of results.
OS in Metastatic Cases With MSI-H
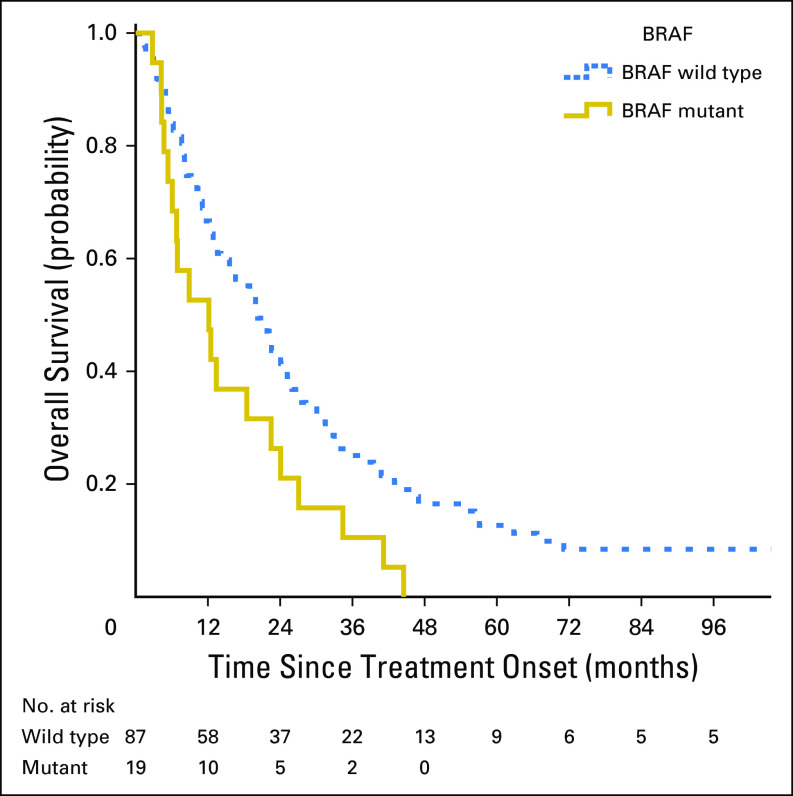
At the end of follow-up, a total of six patients were alive in the met/MSI-H group (median follow-up time for these six patients was 5.9 years, with range of 2.6 to 16.9 years). Overall, the median OS in the met/MSI-H group was 1.6 years. Assessing the effect of BRAF status on OS in this group showed that BRAF mutation had negative prognostic effect, with a univariate HR of 1.8 (95% CI, 1.1 to 3.0; P = .026). Kaplan-Meier estimate of OS by BRAF status is presented in Figure 2.
Fig 2.
Overall survival according to tumor BRAF mutation status in treated patients with microsatellite-instable metastatic colorectal cancer in the Molecular Epidemiology of Colorectal Cancer study, northern Israel (n = 106).
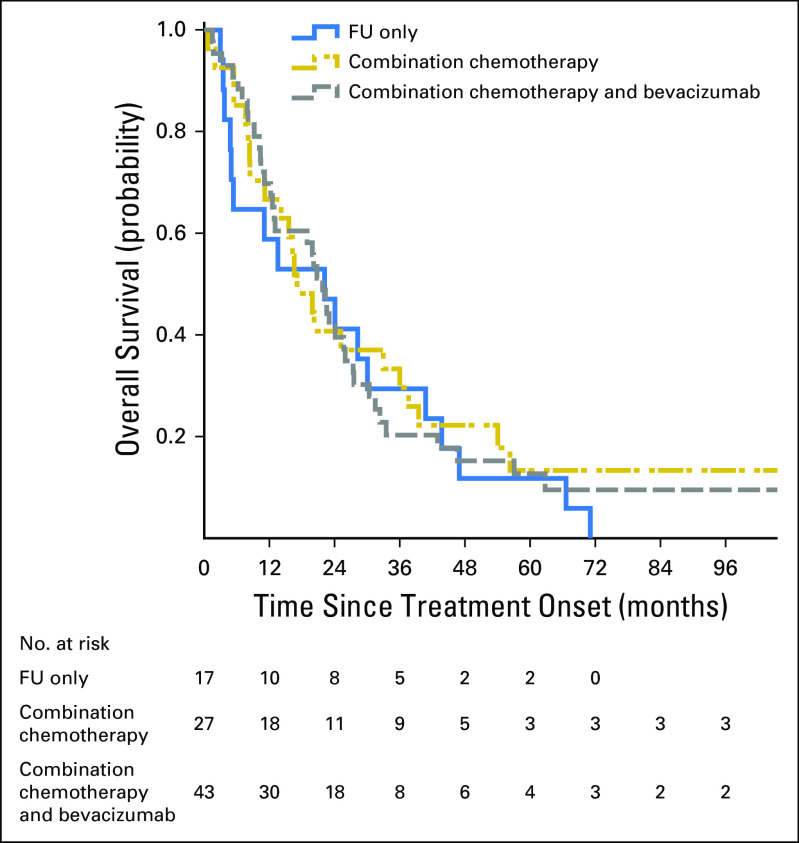
In the general population, the median OS in groups treated with FU only, combination chemotherapy, and combination treatments with bevacizumab was 1.1 years, 1.4 years, and 1.7 years, respectively. The results of these treatments in met/MSI-H BRAF-WT only patients was 1.8 year, 1.4 year, and 1.8 year, respectively. Kaplan-Mayer plot of OS in the BRAF-WT population is presented in Figure 3 (log-rank P value for treatment comparison = .81).
Fig 3.
Overall survival of patients with microsatellite-instable, BRAF–wild type metastatic colorectal cancer by treatment groups in the in the Molecular Epidemiology of Colorectal Cancer study, northern Israel (n = 87). FU, fluorouracil.
Effect of Treatment Onset Period on OS in the BRAF-WT Population
No significant treatment-period effect was seen in BRAF-WT met/MSI-H cases (median OS of 22, 17, and 20 months for study periods of 2002 or earlier, 2003 to 2006, and 2007 or later, respectively). In contrast, among the BRAF-WT met/MSS cases, a significant improvement in OS was observed over the same time periods (median OS of 14, 18, and 25 months, respectively; log-rank P = .002).
Predictive Effect of MSI on OS in the BRAF-WT Population
To evaluate possible differences in response to metastatic disease treatment between the patients with met/MSI-H and those with met/MSS, all treatment protocols were pooled to represent any combination treatment as opposed to FU-only regimens that consisted of FU + LCV and capecitabine. Figure 4 presents OS by treatment group, separately for patients with met/MSS (Fig 4A) and patients with met/MSI-H (Fig 4B), in the homogenous population of BRAF-WT cases. Although there is no significant benefit observed from any combination treatment in the met/MSI-H group (adjusted HR, 0.93; 95% CI, 0.54 to 1.60; P = .78), in the larger met/MSS tumor group, a significant improvement of OS with an any combination treatment was observed (adjusted HR, 0.58; 95% CI, 0.47 to 0.71; P < .001). The P value of the interaction was .07, suggesting meaningful differences in the behavior of metastatic disease by treatment approach. A sensitivity analysis adjusting for study period revealed similar results.
Fig 4.
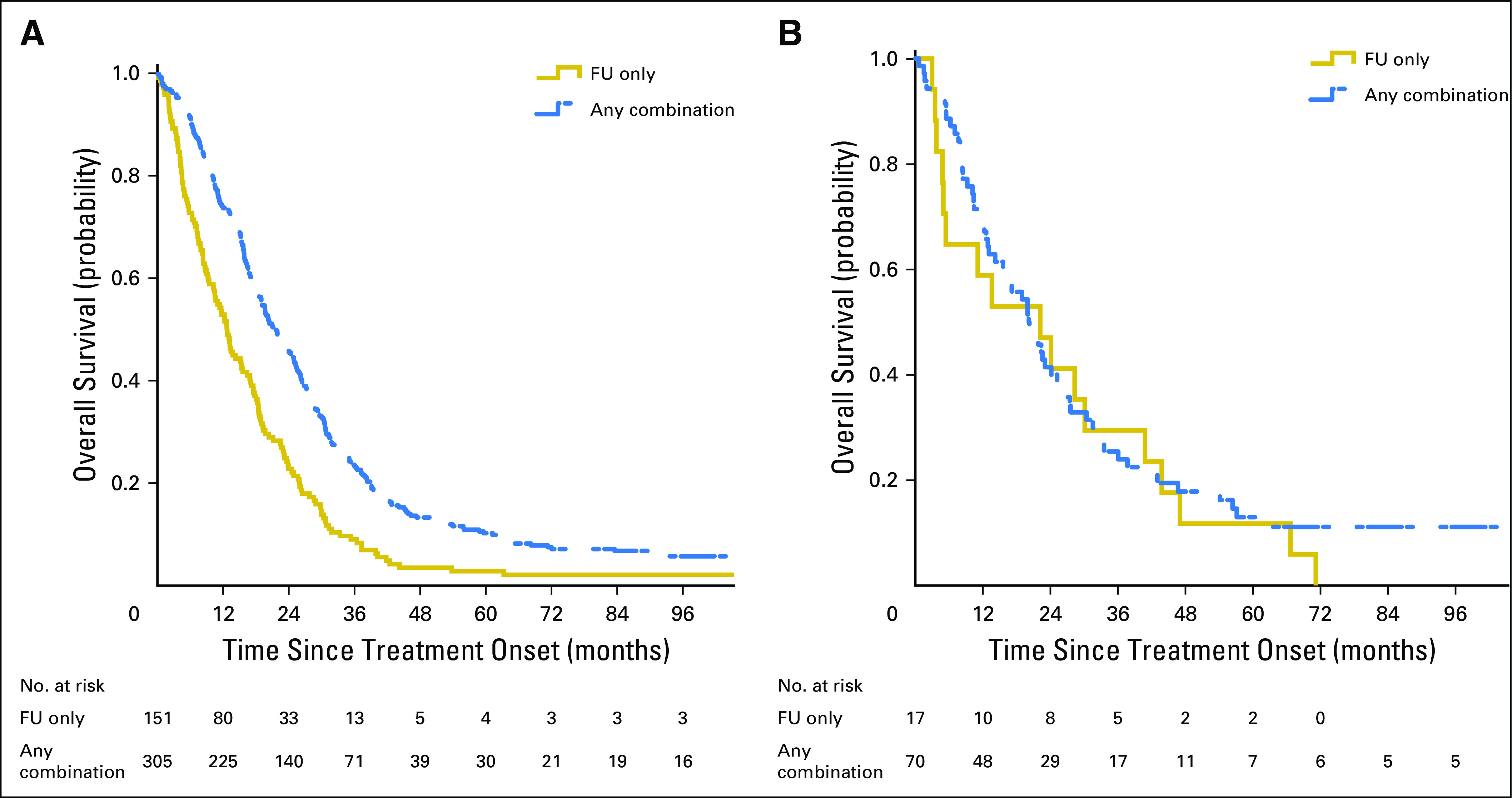
Effect of combination treatment on overall survival of patients with metastatic, BRAF–wild type colorectal cancer by microsatellite instability status in the Molecular Epidemiology of Colorectal Cancer study, northern Israel. (A) Microsatellite stable (n = 456). (B) Microsatellite instable (n = 87).
Prognostic Effect of MSI Status in the BRAF-WT Population
The prognostic role of MSI status in the metastatic disease setting was assessed in the population of patients who were indicated to receive an FU-only regimen as having the smallest or even no treatment effect, according to the literature. The adjusted HR comparing met/MSI-H versus met/MSS in this population is 0.58, with 95% CI of 0.35 to 0.98 (P = .042).
DISCUSSION
Data on chemotherapy treatment response of MSI-H mCRC are sparse. The MECC study reported here has recruited and collected materials over a time span of 17 years from close to 6,000 cases of CRC and served as the source for the 590 cases who were diagnosed with, or later developed, metastases and for whom detailed treatment information was available. All cases participating in the final analysis were evaluated for their MSI status, which was performed in only approximately two thirds of all cases recruited into MECC, with enrichment for suggestive phenotypes.
In accordance with other published literature, cases with MSI-high mCRC in our study were younger at diagnosis and had a higher proportion of BRAF mutations,33,34 with a similar proportion of right-sided tumors. Although MSI-H tumors are described as having a much better prognosis than MSS tumors for early-stage disease, the proportion of tumors diagnosed with metastases at the time of diagnosis was similar between MSI-H cases and MSS cases. The leading randomized controlled trials evaluating the effect of various chemotherapy regimens in mCRC were not randomized by MSI status, and most have not provided subgroup analyses of the results according to their MSI status.
Our study has three major findings. We reinforced the formerly reported poor prognosis associated with BRAF mutations and demonstrated that this also holds for met/MSI-H BRAF-mutated cases. We verified that the survival of patients with mCRC indeed improved with the introduction of new chemotherapies but showed that the improvement in survival was limited to the met/MSS, BRAF-WT cases and was not evident in the met/MSI-H, BRAF-WT group of patients. We were unable to have a comparison with an observation-only group, and no future studies are likely to include such an arm. Our observational data found no difference in OS among metastatic MSI-H cases between cases treated with a basic FU + LCV regimen only, compared with cases treated with any (oxaliplatin or irinotecan) combination chemotherapy with or without bevacizumab, whereas a significant OS improvement was found with advanced chemotherapy in met/MSS cases. We could not evaluate the role of anti-EGFR treatment because of the low number of years of follow-up of patients receiving this recently developed treatment. Similarly, we did not have any cases that were treated with immune checkpoint inhibitors at the time interval of the current analysis.
Few reports evaluated the effect of chemotherapy in met/MSI-H cases. Although early studies reported a positive effect of high-dose FU + LCV in patients with met/MSI-H in comparison with patients with met/MSS,35,36 more recent studies of FU, in combination with irinotecan or oxaliplatin, did not show benefit.16,31,34,37
One study31 showed a trend toward benefit of oxaliplatin-based treatment in cases of MSI-H mCRC. Our data do not have enough power to evaluate specific therapeutic regimens.
We chose to exclude from our study all metastatic cases that were not treated at all, because of the inability to assess the causes for no treatment and biases that could stem from it. Therefore, we could not compare treatment effects with supportive care only. Comparison of OS results in met/MSS and met/MSI-H groups supports biologic differences, with a baseline survival advantage for the MSI-H group. Because our observation period spread over many years, with changes in treatment guidelines, we studied the effects of time periods on OS and found a significant improvement in MSS tumors but not in MSI-H tumors.
Published data on the effect of BRAF mutation on prognosis in patients with met/MSI-H were published on a group of CRC stages II to IV,38 potentially underrepresenting the few cases with stage IV disease. Extremely poor outcomes in this patient group support the use of the FOLFOXIRI (oxaliplatin, irinotecan, fluorouracil, leucovorin) protocol in the unselected group of BRAF-mutated cases. Given the much higher prevalence of BRAF mutations in the MSI-H cases,11,26,39 it is important to study the outcomes of FOLFOXIRI in this subgroup of patients with met/MSI-H.
Our study is limited by the relatively modest sample size of patients with MSI-H mCRC. Advantages of our population-based sampling of incident cases with long-term follow-up include generalizability to community practice in a large and well-defined population. The representative, observational nature of the data from a large health system, in combination with molecular evaluation of all tumor tissues, offers a framework for understanding clinical practice over nearly two decades in a universally insured group of patients in a single country.
MSI status is becoming a cornerstone of individualized decision making with regard to a variety of potential oncological interventions, and this trend is likely to continue with the introduction of immunotherapy.40,41 Routine mutational profiling of mCRC and specifically MSI testing and other assays that designate deficient mismatch repair have already been recommended.39,42,43 The failure of conventional advanced treatments to improve survival in the meaningful subset of met/MSI-H cases emphasizes the need to evaluate the role of the newly introduced immunotherapy as a first-line treatment in these cases with MSI-H/BRAF-WT mCRC.
In conclusion, therapeutic approaches to mCRC have changed dramatically during the past two decades, with increasing reliance on the molecular features of tumors to inform treatment decisions. Microsatellite instability and its molecular subtypes are likely to continue to be a target for development of new treatment options for patients with CRC. Ongoing and future clinical trials evaluating the effect of biologic and immunologic agents should be designed to take into account the MSI status of the tumors.
Footnotes
The Molecular Epidemiology of Colorectal Cancer study was funded by National Institutes of Health Grant No. R01 CA81488.
AUTHOR CONTRIBUTIONS
Conception and design: Katerina Shulman, Ofra Barnett-Griness, Stephen B. Gruber, Gad Rennert
Financial support: Stephen B. Gruber
Administrative support: Stephen B. Gruber
Provision of study material or patients: Flavio Lejbkowicz
Collection and assembly of data: All authors
Data analysis and interpretation: Katerina Shulman, Ofra Barnett-Griness, Joel K. Greenson, Gad Rennert
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Katerina Shulman
No relationship to disclose
Ofra Barnett-Griness
Employment: Teva Pharmaceuticals
Stock and Other Ownership Interests: Teva Pharmaceuticals
Vered Friedman
No relationship to disclose
Joel K. Greenson
Consulting or Advisory Role: Genentech
Stephen B. Gruber
Employment: Brogent Technologies
Leadership: Brogent Technologies
Stock and Other Ownership Interests: Brogent Technologies, Fulgent Therapeutics
Consulting or Advisory Role: Myriad Genetics, Fulgent Therapeutics
Research Funding: Myriad Genetics (Inst)
Flavio Lejbkowicz
No relationship to disclose
Gad Rennert
No relationship to disclose
REFERENCES
- 1.Tol J, Punt CJA. Monoclonal antibodies in the treatment of metastatic colorectal cancer: A review. Clin Ther. 2010;32:437–453. doi: 10.1016/j.clinthera.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Zhou SW, Huang YY, Wei Y, et al. No survival benefit from adding cetuximab or panitumumab to oxaliplatin-based chemotherapy in the first-line treatment of metastatic colorectal cancer in KRAS wild type patients: A meta-analysis. PLoS One. 2012;7:e50925. doi: 10.1371/journal.pone.0050925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan DL, Pavlakis N, Shapiro J, et al. Does the chemotherapy backbone impact on the efficacy of targeted agents in metastatic colorectal cancer? A systematic review and meta-analysis of the literature. PLoS One. 2015;10:e0135599. doi: 10.1371/journal.pone.0135599. Erratum: PLoS One 10:e0138916, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritchard CC, Grady WM. Colorectal cancer molecular biology moves into clinical practice. Gut. 2011;60:116–129. doi: 10.1136/gut.2009.206250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siena S, Sartore-Bianchi A, Di Nicolantonio F, et al. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101:1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tol J, Dijkstra JR, Klomp M, et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer. 2010;46:1997–2009. doi: 10.1016/j.ejca.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Kavuri SM, Jain N, Galimi F, et al. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov. 2015;5:832–841. doi: 10.1158/2159-8290.CD-14-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenson JK, Huang S-C, Herron C, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol. 2009;33:126–133. doi: 10.1097/PAS.0b013e31817ec2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016;108:1–8. doi: 10.1093/jnci/djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev. 2004;23:11–27. doi: 10.1023/a:1025861527711. [DOI] [PubMed] [Google Scholar]
- 11.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3:502–511. doi: 10.1158/2159-8290.CD-12-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Einem JC, Heinemann V, von Weikersthal LF, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: An analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140:1607–1614. doi: 10.1007/s00432-014-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang GH. Four molecular subtypes of colorectal cancer and their precursor lesions. Arch Pathol Lab Med. 2011;135:698–703. doi: 10.5858/2010-0523-RA.1. [DOI] [PubMed] [Google Scholar]
- 14.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haddad R, Ogilvie RT, Croitoru M, et al. Microsatellite instability as a prognostic factor in resected colorectal cancer liver metastases. Ann Surg Oncol. 2004;11:977–982. doi: 10.1245/ASO.2004.03.585. [DOI] [PubMed] [Google Scholar]
- 16.Müller CI, Schulmann K, Reinacher-Schick A, et al. Predictive and prognostic value of microsatellite instability in patients with advanced colorectal cancer treated with a fluoropyrimidine and oxaliplatin containing first-line chemotherapy. A report of the AIO Colorectal Study Group. Int J Colorectal Dis. 2008;23:1033–1039. doi: 10.1007/s00384-008-0504-2. [DOI] [PubMed] [Google Scholar]
- 17.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weitzel JN, Blazer KR, MacDonald DJ, et al. Genetics, genomics, and cancer risk assessment: State of the art and future directions in the era of personalized medicine. CA Cancer J Clin. 2011;61:327–359. doi: 10.3322/caac.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinicrope FA, Sargent DJ. Molecular pathways: Microsatellite instability in colorectal cancer: Prognostic, predictive, and therapeutic implications. Clin Cancer Res. 2012;18:1506–1512. doi: 10.1158/1078-0432.CCR-11-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webber EM, Kauffman TL, O’Connor E, et al. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer. 2015;15:156. doi: 10.1186/s12885-015-1093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Des Guetz G, Schischmanoff O, Nicolas P, et al. Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer. 2009;45:1890–1896. doi: 10.1016/j.ejca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 23.Warusavitarne J, Schnitzler M. The role of chemotherapy in microsatellite unstable (MSI-H) colorectal cancer. Int J Colorectal Dis. 2007;22:739–748. doi: 10.1007/s00384-006-0228-0. [DOI] [PubMed] [Google Scholar]
- 24.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16:30. doi: 10.1007/s11864-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cremolini C, Loupakis F, Masi G, et al. FOLFOXIRI or FOLFOXIRI plus bevacizumab as first-line treatment of metastatic colorectal cancer: A propensity score-adjusted analysis from two randomized clinical trials. Ann Oncol. 2016;27:843–849. doi: 10.1093/annonc/mdw052. [DOI] [PubMed] [Google Scholar]
- 27.Van Cutsem E, Köhne C-H, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 28.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–4713. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 29.Des Guetz G, Uzzan B, Nicolas P, et al. Microsatellite instability does not predict the efficacy of chemotherapy in metastatic colorectal cancer. A systematic review and meta-analysis. Anticancer Res. 2009;29:1615–1620. [PubMed] [Google Scholar]
- 30.Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.des Guetz G, Mariani P, Cucherousset J, et al. Microsatellite instability and sensitivitiy to FOLFOX treatment in metastatic colorectal cancer. Anticancer Res. 2007;27:2715–2719. [PubMed] [Google Scholar]
- 32.Poynter JN, Gruber SB, Higgins PDR, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 33.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann Oncol. 2014;25:1032–1038. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang J-T, Huang K-C, Lai H-S, et al. High-frequency microsatellite instability predicts better chemosensitivity to high-dose 5-fluorouracil plus leucovorin chemotherapy for stage IV sporadic colorectal cancer after palliative bowel resection. Int J Cancer. 2002;101:519–525. doi: 10.1002/ijc.10643. [DOI] [PubMed] [Google Scholar]
- 36.Brueckl WM, Moesch C, Brabletz T, et al. Relationship between microsatellite instability, response and survival in palliative patients with colorectal cancer undergoing first-line chemotherapy. Anticancer Res. 2003;23:1773–1777. [PubMed] [Google Scholar]
- 37.Kim JE, Hong YS, Ryu M-H, et al. Association between deficient mismatch repair system and efficacy to irinotecan-containing chemotherapy in metastatic colon cancer. Cancer Sci. 2011;102:1706–1711. doi: 10.1111/j.1349-7006.2011.02009.x. [DOI] [PubMed] [Google Scholar]
- 38.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 39.Lin EI, Tseng LH, Gocke CD, et al. Mutational profiling of colorectal cancers with microsatellite instability. Oncotarget. 2015;6:42334–42344. doi: 10.18632/oncotarget.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunne PD, O’Reilly PG, Coleman HG, et al. Stratified analysis reveals chemokine-like factor (CKLF) as a potential prognostic marker in the MSI-immune consensus molecular subtype CMS1 of colorectal cancer. Oncotarget. 2016;7:36632–36644. doi: 10.18632/oncotarget.9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen R, Svrcek M, Dreyer C, et al. New therapeutic opportunities based on DNA mismatch repair and BRAF status in metastatic colorectal cancer. Curr Oncol Rep. 2016;18:18. doi: 10.1007/s11912-016-0504-2. [DOI] [PubMed] [Google Scholar]