Abstract
PURPOSE
Although MET exon 14 (METex14)–altered lung cancers were first identified more than a decade and a half ago, the frequency of CNS metastatic disease remains poorly defined. Furthermore, the seminal trial of crizotinib in these patients (PROFILE 1001) did not report patterns of CNS response or progression.
PATIENTS AND METHODS
Patients with pathologically confirmed, advanced non–small-cell lung cancers (NSCLC) harboring a METex14 alteration by targeted DNA/RNA sequencing were studied. The incidence of brain metastases and the outcomes of MET inhibition with crizotinib were analyzed.
RESULTS
Eighty-three patients with METex14-altered metastatic NSCLC were identified. The incidence of CNS metastases at diagnosis was 17% (95% CI, 10% to 27%). The lifetime incidence was 36% (95% CI, 26% to 47%); 83% of patients had parenchymal disease, and 17% had leptomeningeal disease. The probability of having brain metastasis at 1, 2, and 3 years was 24%, 35%, and 38%, respectively. Fifty-four patients received crizotinib. The median time to radiologic CNS progression was 5.8 months (range, 3.7-20.0 months). Patterns of crizotinib progression were as follows: intracranial only in 10% of patients, intracranial and extracranial in 12%, and extracranial only in 78%. In patients with brain metastases before treatment, the median time on crizotinib was 7.5 months (range, 7.2-11.7 months).
CONCLUSION
CNS metastases, including leptomeningeal disease, occurred in more than a third of patients with METex14-altered lung cancers. In crizotinib-treated patients with or without CNS metastases, CNS failure was seen in less than a quarter of patients on progression.
INTRODUCTION
MET exon 14 (METex14) alterations are oncogenic drivers that are found in 3%-4% of patients with non–small-cell lung cancers (NSCLCs).1-3 Although CNS metastases are common (20%-50%) in metastatic lung cancers,4 the frequency of brain metastases in METex14-altered lung cancers has not been reported. In addition, the PROFILE 1001 clinical trial of crizotinib that demonstrated an objective response rate (ORR) of 32% and a median progression-free survival of 7 months5 only enrolled patients without active baseline brain metastases. The CNS outcomes and patterns of disease progression with crizotinib therapy have not been described.
CONTEXT
Key Objective
In patients with MET exon 14–altered lung cancers, what is the frequency of CNS metastasis, and what are the CNS outcomes with the type Ia MET inhibitor crizotinib?
Knowledge Generated
Brain metastasis and leptomeningeal disease were identified in about a third of patients with advanced MET exon 14–altered lung cancers. Intracranial response was observed with crizotinib therapy, and eventual CNS progression was seen in less than a quarter of cases.
Relevance
Given the frequency of CNS metastases in MET exon 14–altered lung cancers, serial CNS imaging should be performed in patients who receive systemic therapy on clinical trials or in practice. Long-term comparative data are needed on CNS responses to crizotinib relative to type Ib selective MET inhibitors.
PATIENTS AND METHODS
This study was approved by the Memorial Sloan Kettering Cancer Center (MSK; New York, NY) Institutional Review Board and conducted in accordance with US Common Rule and the Federalwide Assurance for the Protection of Human Subjects (FW00004998). Patients with METex14 alterations diagnosed between January 2014 and March 2018 at MSK were identified. Eligible patients had pathologically confirmed stage IV or recurrent metastatic NSCLC that harbored a METex14 alteration identified using next-generation sequencing of DNA (MSK-IMPACT/FoundationOne [Foundation Medicine, Cambridge, MA]) or RNA (anchored multiplex polymerase chain reaction; MSK-Solid Fusion Panel).6-8 All patients had computed tomography or magnetic resonance imaging of the brain at the time of diagnosis of metastatic disease. Imaging after diagnosis was performed as per standard of care without predefined intervals; the 5 patients with known brain metastases who received crizotinib underwent CNS imaging approximately every 2-3 months, corresponding with their extracranial imaging.
A review of clinicopathologic characteristics and treatment history was performed. MET amplification, a concurrent alteration that occurs in a subset of METex14-altered lung cancers, was defined based on local assay cutoffs.9 Patients treated with crizotinib were evaluated for systemic (extracranial and intracranial) or intracranial-only ORR and time to treatment discontinuation (TTD) using RECIST version 1.1. TTD was measured from crizotinib initiation to discontinuation for any reason. Overall survival (OS) was measured from the date of diagnosis of metastatic disease to death. TTD and OS were evaluated using Kaplan-Meier estimates. The log-rank test was used to compare OS in patients with or without brain metastasis. The probability of CNS metastasis over time was evaluated using the cumulative incidence function. The risk of developing brain metastases on treatment was computed using kernel smoothing.
RESULTS
Patients
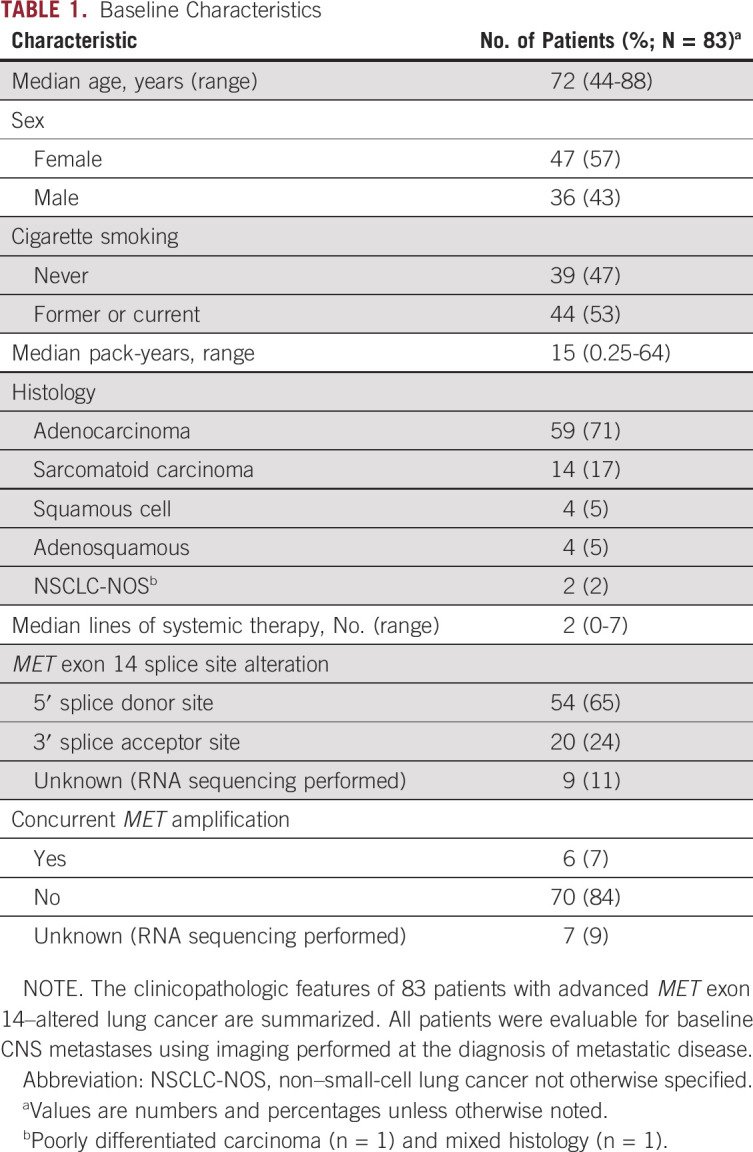
Of 3,841 patients with NSCLC who underwent prospective next-generation sequencing, 114 (3%) had METex14 alterations; 83 patients (73%) had metastatic disease and were eligible for analysis. Clinicopathologic features (Table 1) were consistent with historic cohorts of METex14-altered lung cancers.1,3 Most (65%) had a 5′ splice donor site alteration. and a minority (7%) had concurrent MET amplification.9 The median follow-up time from the date of diagnosis of metastatic disease was 3.3 years (range, 0.4-10.2 years).
TABLE 1.
Baseline Characteristics
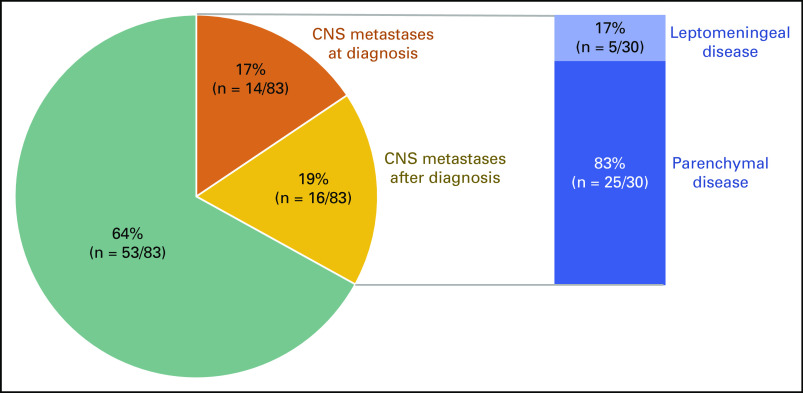
CNS Metastases
The incidence of CNS metastases is shown in Figure 1. Eighty-three patients had CNS imaging at the time of diagnosis of metastatic disease. Of these patients, 17% (95% CI, 10% to 27%; 14 of 83 patients) had brain metastasis. This included 1 patient with leptomeningeal disease as well. An additional 19% of patients (95% CI, 11% to 29%; 16 of 83 patients) developed brain metastasis later in their disease course. In total, CNS metastases were identified in 36% of patients (95% CI, 10% to 27%; 30 of 83 patients) with METex14-altered lung cancers. Among these individuals, 83% (25 of 30 patients) had parenchymal metastases and 17% (5 of 30 patients) had leptomeningeal disease. Sixty-five percent of patients with CNS disease received CNS-directed therapy; 52% (17 of 30 patients) received radiation and 13% (4 of 30 patients) underwent surgery.
FIG 1.
Brain metastases frequency. The frequency of CNS metastases at the diagnosis of metastatic disease or acquired during the course of treatment are depicted in 83 patients with advanced disease. The rates of parenchymal metastases and leptomeningeal disease are also shown.
The probability of developing brain metastases at 1, 2, and 3 years was 24%, 35%, and 38%, respectively (Fig 2A). Compared with not having brain metastasis, having brain metastasis at 6, 12, and 24 months increased the risk of death by 2.61-, 2.73-, and 2.94-fold (Fig 2B). The survival of patients who had brain metastases at diagnosis (median OS, 26.7 months) compared with those who did not (median OS, 27.2 months) was similar (hazard ratio, 0.70; 95% CI, 0.28 to 1.75; P = .3; Data Supplement).
FIG 2.
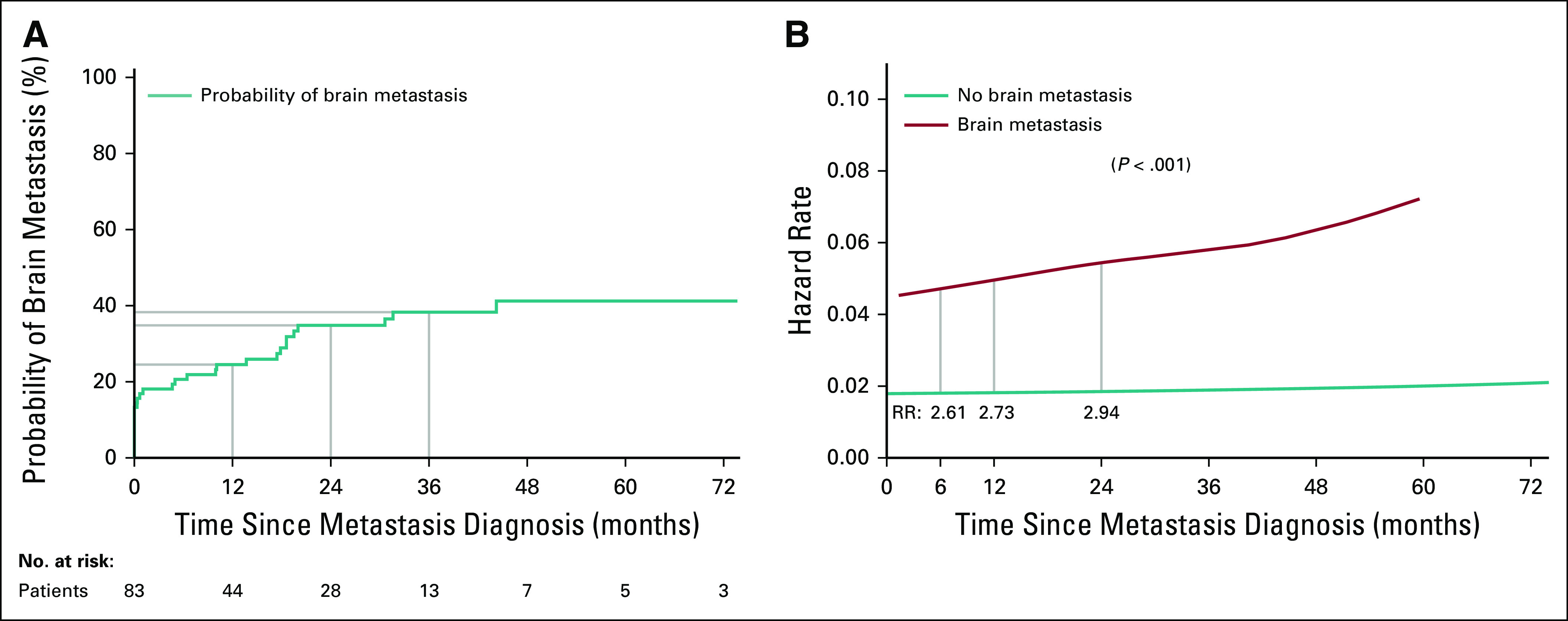
Cumulative brain metastases incidence and death. (A) The cumulative incidence function of the probability of developing brain metastasis in the 83 patients with MET exon 14–altered metastatic lung cancers is shown. The probabilities at 12, 24, and 36 months are 24%, 35%, and 38%, respectively. (B) Hazard functions of patients with MET exon 14–altered metastatic lung cancers who do and do not have brain metastases are depicted. The lines represent the likelihood a patient will experience death as a function of time depending on the presence of brain metastasis at baseline. The risk ratio (RR) comparing the 2 groups at a specific time is calculated by the division of hazard rates at that time point. Compared with not having brain metastasis, having brain metastasis at 6, 12, and 24 months increases the risk of death by 2.61-, 2.73-, and 2.94-fold, respectively.
Crizotinib Outcomes
Fifty-four patients with advanced METex14-altered lung cancers received crizotinib. The median line of systemic therapy at which crizotinib was administered was 2 (range, 1-7 lines). The ORR with crizotinib in all patients was 31% (95% CI, 18% to 45%). The median TTD was 7.8 months (95% CI, 5.5 to 13.0 months). The median OS was 13.7 months (95% CI, 10.8 to 22.9 months). Therapies administered after crizotinib are summarized in the Data Supplement.
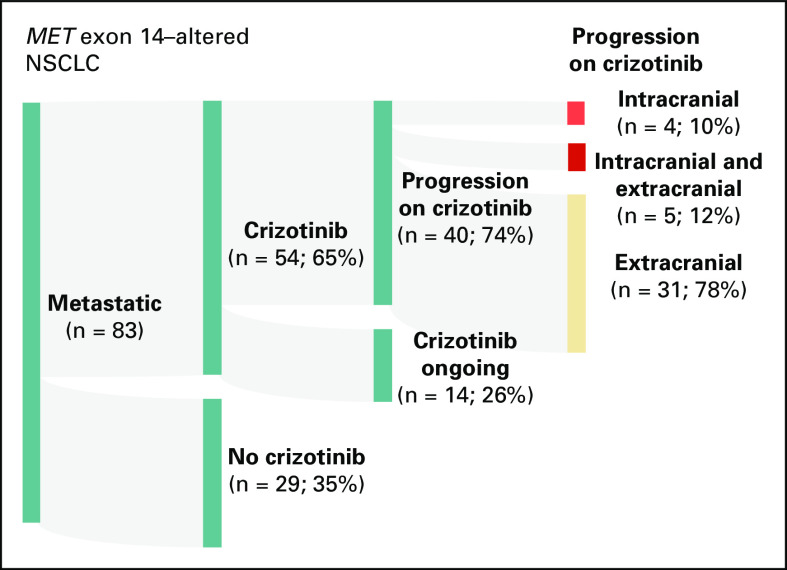
Forty of the 54 patients developed progressive disease on crizotinib (Fig 3). The rates of intracranial-only, intracranial and extracranial, and extracranial-only progression were 10% (4 of 40 patients), 12% (5 of 40 patients), and 78% (31 of 40 patients), respectively. In total, the rate of CNS progression on crizotinib was 22% (9 of 40 patients). The median time to radiologic CNS progression was 5.8 months (range, 3.7-20.0 months). Of these patients, 44% (4 of 9 patients) developed new leptomeningeal disease (the rest had parenchymal metastases only). The median time to death after the diagnosis of leptomeningeal disease was 6 weeks (range, 2-62 weeks).
FIG 3.
Crizotinib progression patterns. An alluvial plot of patients with MET exon 14–altered non–small-cell lung cancer (NSCLC) including 54 patients who were treated with crizotinib is shown. Patterns of disease progression including intracranial-only progression, intracranial and extracranial progression, and extracranial-only progression are depicted.
Only 5 of the 54 patients had known brain metastasis before the initiation of crizotinib. A partial response was observed in 1 patient, stable disease in 3 patients, and progressive disease in the remaining patient (Data Supplement). The median TTD was 7.5 months (range, 7.2-11.7 months). Of the 5 patients with preexisting brain metastasis, 3 had RECIST-measurable intracranial disease. The best intracranial response was a partial response in 1 patient and stable disease in the remaining 2 patients.
DISCUSSION
We report the largest evaluation of the incidence of CNS metastasis in patients with METex14-altered lung cancers to date. CNS metastases at the time of diagnosis were observed in 17% of patients, and 19% developed CNS metastases during the course of their disease. Importantly, a substantial proportion of patients with METex14-altered lung cancers were found to have leptomeningeal disease, many of whom died quickly thereafter. The 36% lifetime incidence of CNS metastases observed in METex14-altered lung cancers was comparable to that of KRAS-mutant lung cancers (32%) and less than that seen in EGFR-mutant (47%), HER2-mutant (47%), and RET fusion–positive (46%) lung cancers (Data Supplement).10
MET kinase inhibition with crizotinib was effective in this series. Both overall and intracranial responses were achieved. The median time to crizotinib discontinuation (7.5 months) and time to CNS progression (5.8 months) were meaningful. These outcomes highlight the utility of crizotinib in patients with CNS metastases and complement the published activity of the drug in PROFILE 1001.5 Crizotinib is widely available, has a well-recognized safety profile, and is currently listed in the National Comprehensive Cancer Network guidelines as a therapy for patients with advanced METex14-altered lung cancers.
In ALK fusion–positive lung cancers, CNS outcomes with crizotinib are poorer than those with other tyrosine kinase inhibitors (TKIs; ie, alectinib or brigatinib), and approximately half of patients have intracranial progression on crizotinib.11,12 A similar rate of intracranial progression on crizotinib was seen in a small cohort of patients with ROS1 fusion–positive NSCLC. The rate of intracranial progression on crizotinib was 22% in this series of patients with METex14-altered lung cancers. Although this rate seems lower compared with that observed in ALK fusion– and ROS1 fusion–positive lung cancers, the observed rate may be secondary to subclinical intracranial progression in asymptomatic patients who did not undergo CNS imaging after crizotinib progression, and the true frequency may be higher.
It is important to recognize that the true incidence of CNS metastases in METex14-altered lung cancers may have been underestimated in this series given that imaging was performed per standard of care without predefined intervals. Thus, it is critical that systemic therapy trials require CNS imaging before therapy initiation and serially even in patients without baseline CNS disease. This will allow more careful characterization of the frequency of CNS metastases and the profile of treatment response.
Next-generation MET-directed therapies, many of which are selective type Ib MET TKIs, are either approved or currently under study in the clinic. These include capmatinib,13 tepotinib,14 savolitinib,15 glumetinib (ClinicalTrials.gov identifier: NCT04270591), and APL-101 (ClinicalTrials.gov identifier: NCT03175224). Preliminary data from the GEOMETRY phase II study of capmatinib showed that intracranial response was achieved in 7 (54%) of 13 patients, a rate higher than that observed with crizotinib in this series.13 Intracranial responses were also observed in a phase II trial of savolitinib.15 The VISION phase II study of tepotinib had 11 patients with brain metastases; however, intracranial response could not be determined because none of these patients had measurable intracranial disease.14 Capmatinib and tepotinib have been granted accelerated approval and breakthrough designation, respectively, by the US Food and Drug Administration (FDA) for the treatment of METex14-altered lung cancers. Additional response data and long-term outcomes will elucidate the relative activity of these agents compared with crizotinib.
In conclusion, brain metastases and leptomeningeal disease occur in one third of METex14-altered lung cancers at diagnosis and during the course of therapy. This series demonstrates that crizotinib can achieve disease control in patients with METex14-altered lung cancers and intracranial metastases. However, selective MET inhibitors such as the FDA-approved type Ib inhibitor capmatinib may provide better intracranial disease control.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants (P01 CA129243 and P30 CA008748). We thank Clare Wilhelm for his assistance with manuscript preparation.
SUPPORT
Supported by National Institutes of Health, National Cancer Institute Grant No. P30-CA008748.
AUTHOR CONTRIBUTIONS
Conception and design: Michael Offin, Jia Luo, Maria E. Arcila, Mark G. Kris, Alexander Drilon
Financial support: Mark G. Kris
Provision of study materials or patients: Charles M. Rudin, Gregory Riely, Natasha Rekhtman, Marc Ladanyi, Bob T. Li, Mark G. Kris
Collection and assembly of data: Michael Offin, Jia Luo, Robin Guo, John K. Lyo, Christina Falcon, Jordan Dienstag, Olivia Wilkins, Jason Chang, Charles M. Rudin, Natasha Rekhtman, Maria E. Arcila, Marc Ladanyi, Mark G. Kris, Paul Paik, Alexander Drilon
Data analysis and interpretation: Michael Offin, Jia Luo, Gregory Riely, Natasha Rekhtman, Maria E. Arcila, Glenn Heller, Marc Ladanyi, Bob T. Li, Mark G. Kris, Alexander Drilon
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael Offin
Honoraria: OncLive
Consulting or Advisory Role: PharmaMar, Novartis, Targeted Oncology
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck Sharp & Dohme
Jia Luo
Honoraria: Targeted Oncology
Jordan Dienstag
Stock and Other Ownership Interests: AbbVie, Bristol Myers Squibb, Thermo Fisher Scientific, Allergan
Charles M. Rudin
Consulting or Advisory Role: AbbVie, Harpoon Therapeutics, Genentech, AstraZeneca, Ascentage Pharma, Bicycle Therapeutics, Celgene, Daiichi Sankyo, Ipsen, Loxo, PharmaMar, Bridge Medicines, Amgen, Jansen, Syros Pharmaceuticals
Research Funding: AbbVie/Stemcentrx (Inst), Viralytics (Inst), Merck (Inst), Daiichi Sankyo (Inst), Genentech (Inst)
Gregory Riely
Research Funding: Novartis (Inst), Genentech (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Infinity Pharmaceuticals (Inst), Mirati Therapeutics (Inst), Merck (Inst), Takeda (Inst)
Patents, Royalties, Other Intellectual Property: Patent application submitted covering pulsatile use of erlotinib to treat or prevent brain metastases (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Other Relationship: Pfizer, Genentech, Takeda
Natasha Rekhtman
Honoraria: Physicians’ Education Resource
Maria E. Arcila
Honoraria: Invivoscribe, Biocartis
Consulting or Advisory Role: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Invivoscribe, Raindance Technologies
Glenn Heller
Research Funding: Janssen Diagnostics
Marc Ladanyi
Consulting or Advisory Role: Bristol Myers Squibb, Bayer
Research Funding: Loxo (Inst), Helsinn Therapeutics, Merus NV (Inst), Elevation Oncology (Inst)
Bob T. Li
Consulting or Advisory Role: Guardant Health, Hengrui Therapeutics, Eli Lilly (Inst), Genentech (Inst)
Research Funding: AstraZeneca (Inst), GRAIL (Inst), Daiichi Sankyo (Inst), Hengrui Therapeutics (Inst), Guardant Health (Inst), Amgen (Inst), Eli Lilly (Inst), MORE Health (Inst)
Patents, Royalties, Other Intellectual Property: US62/514,661 (Inst), US62/685,057 (Inst)
Mark G. Kris
Consulting or Advisory Role: AstraZeneca, Regeneron, Pfizer, Daiichi Sankyo
Travel, Accommodations, Expenses: AstraZeneca, Pfizer
Other Relationship: Memorial Sloan Kettering Cancer Center, Genentech
Open Payments Link: https://openpaymentsdata.cms.gov/physician/markgkris/summary
Paul Paik
Honoraria: Celgene, Takeda, Calithera Biosciences, Boehringer Ingelheim, EMD Serono, AstraZeneca
Consulting or Advisory Role: Celgene, EMD Serono, Takeda, Calithera Biosciences, AstraZeneca, Boehringer Ingelheim
Research Funding: Celgene, EMD Serono
Travel, Accommodations, Expenses: EMD Serono
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, Peerview
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Ariad/Millennium, BerGenBio, MORE Health, Eli Lilly, Verastem, AbbVie, 14ner/Elevation Oncology, Remedica, Archer Biosciences, Monopteros
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Drilon A, Cappuzzo F, Ou SI, et al. Targeting MET in lung cancer: Will expectations finally be MET? J Thorac Oncol. 2017;12:15–26. doi: 10.1016/j.jtho.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5:850–859. doi: 10.1158/2159-8290.CD-15-0285. [DOI] [PubMed] [Google Scholar]
- 3.Schrock AB, Frampton GM, Suh J, et al. Characterization of 298 patients with lung cancer harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11:1493–1502. doi: 10.1016/j.jtho.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Sørensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: Frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 5.Drilon A, Clark JW, Weiss J, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26:47–51. doi: 10.1038/s41591-019-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benayed R, Offin M, Mullaney K, et al. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res. 2019;25:4712–4722. doi: 10.1158/1078-0432.CCR-19-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 9.Guo R, Berry LD, Aisner DL, et al. MET IHC is a poor screen for MET amplification or MET exon 14 mutations in lung adenocarcinomas: Data from a tri-institutional cohort of the lung cancer mutation consortium. J Thorac Oncol. 2019;14:1666–1671. doi: 10.1016/j.jtho.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Offin M, Feldman D, Ni A, et al. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer. 2019;125:4380–4387. doi: 10.1002/cncr.32461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol. 2015;16:e510–e521. doi: 10.1016/S1470-2045(15)00013-3. [DOI] [PubMed] [Google Scholar]
- 13.Wolf J, Seto T, Han J-Y, et al. Capmatinib (INC280) in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): Efficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol. 2019;37(suppl 15; abstr 9004) [Google Scholar]
- 14.Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020:NEJMoa2004407. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu S, Fang J, Cao L, et al: Preliminary efficacy and safety results of savolitinib treating patients with pulmonary sarcomatoid carcinoma (PSC) and other types of non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping mutations. Cancer Res 79:CT031, 2019 (abstr) [Google Scholar]