Abstract
PURPOSE
Alterations in DNA damage repair (DDR) genes occur in up to 25% of patients with metastatic castration-resistant prostate cancer (mCRPC) and may sensitize to platinum chemotherapy. We aimed to evaluate the efficacy of platinum-based chemotherapy in DDR-mutant (DDRmut) mCRPC.
METHODS
We assessed response to platinum chemotherapy based on DDR gene alteration status in men with mCRPC who underwent tumor and germline genomic profiling. Patients with deleterious alterations in a gene panel that included BRCA2, BRCA1, ATM, PALB2, FANCA, and CDK12 were considered DDRmut.
RESULTS
A total of 109 patients with mCRPC received platinum-based chemotherapy between October 2013 and July 2018. Sixty-four of 109 patients were taxane refractory and poly (ADP-ribose) polymerase inhibitor (PARPi) naïve. Within this subset, DDRmut was found in 16/64 patients (25%) and was associated with an increased likelihood of achieving a prostate-specific antigen (PSA) decline of 50% or more from baseline (PSA50; odds ratio, 7.0; 95% CI, 1.9 to 29.2). Time on platinum chemotherapy tended to be longer in the DDRmut group (median, 3.0 v 1.6 months; hazard ratio, 0.55, 95% CI, 0.29 to 1.24). No difference in survival was detected. Of 8 patients with DDRmut disease who received platinum-based therapy after a PARPi, 3/7 evaluable patients had radiographic partial response or stable disease, and 2/7 had a PSA50 response. None of 4 patients with ATM mutations had platinum responses regardless of prior PARPi exposure.
CONCLUSION
Patients with DDRmut disease had better response to platinum-based chemotherapy, suggesting that DDR status warrants prospective validation as a potential biomarker for patient selection. Responses to platinum chemotherapy were observed in BRCA-altered prostate cancer after PARPi progression. Additional studies are needed to determine the predictive role of individual genes on platinum sensitivity in the context of other clinical and genomic factors.
INTRODUCTION
Platinum-based chemotherapy has been shown to confer palliative benefit, objective responses, and longer progression-free survival in phase II studies of metastatic castration-resistant prostate cancer (mCRPC), although improved overall survival (OS) has not been demonstrated.1-4 Specific patient subpopulations may derive more meaningful benefit, including patients with aggressive variants of prostate cancer5 or patients with genomic defects in DNA damage repair (DDR) pathways.6,7
Up to 25% of men with mCRPC harbor tumor somatic or germline alterations in DDR.8-11 Deleterious genomic alterations in these genes, including BRCA2, BRCA1, ATM, PALB2, and FANCA, are associated with deficiency in DNA damage sensing or repair and may sensitize tumors to platinum chemotherapy6,7,12 or to poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi).13,14 Several studies are ongoing to confirm the role of BRCA alterations in predicting response to PARPi and to explore the role of less frequently altered genes in this context (ClinicalTrials.gov identifiers: NCT02952534, NCT02975934, NCT02987543, and NCT02854436).
We leveraged a prospective, institution-wide, tumor somatic and germline molecular profiling initiative to examine the association between somatic and germline mutations in DDR and response to platinum chemotherapy. We also asked whether patients with DDR gene alterations could respond to platinum therapy after progression on a PARPi.
METHODS
Study Design and Patients
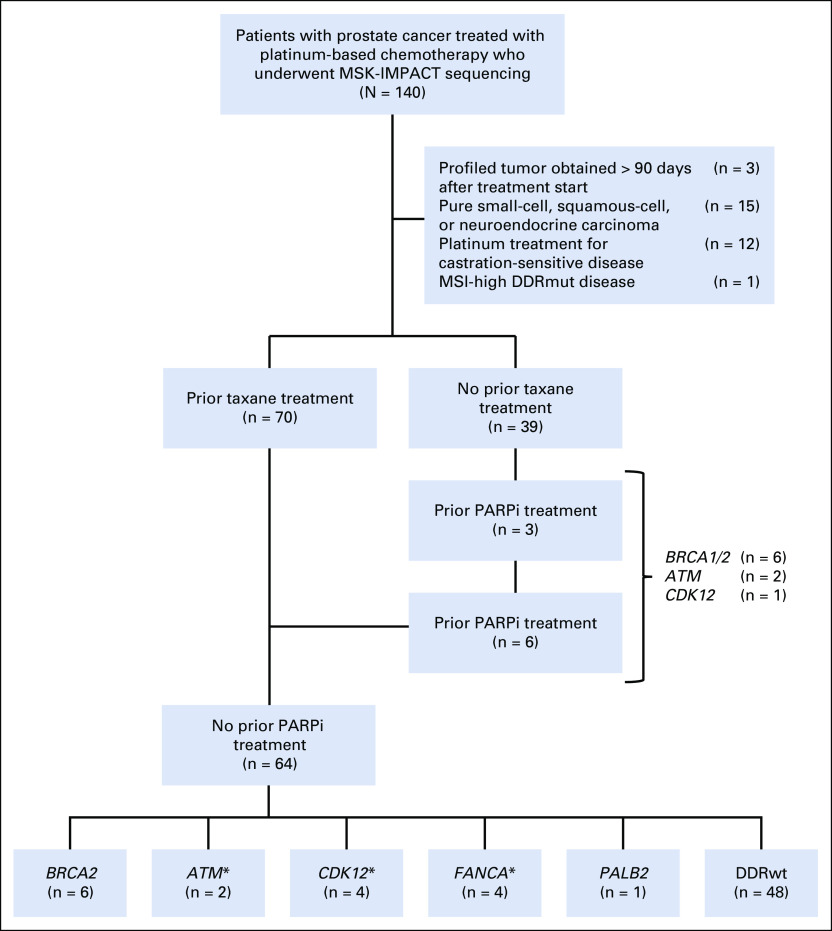
We searched the Memorial Sloan Kettering Cancer Center (MSKCC) clinical database to identify patients with prostate cancer who underwent tumor and germline genomic sequencing and received platinum-based chemotherapy between October 1, 2013, and July 30, 2018, as part of the prospective Memorial Sloan Kettering Integrated Molecular Profiling of Actionable Cancer Targets (MSK-IMPACT) initiative (ClinicalTrials.gov identifier: NCT01775072). Eligible patients had histologically confirmed mCRPC and received at least 1 cycle of carboplatin or cisplatin as monotherapy or in combination with a taxane or etoposide. We excluded patients with pure nonadenocarcinoma histology (eg, pure small-cell carcinoma); patients who received platinum chemotherapy for non-mCRPC, DDR mutant (DDRmut) disease with microsatellite instability (MSI)-high prostate cancer; and patients whose profiled tumor was acquired more than 90 days after receiving platinum chemotherapy (Fig 1). Chart review was performed to extract clinical and pathologic data. MSKCC Institutional Review Board approval was obtained prior to any study-related procedures.
FIG 1.
Description of patients with prostate cancer treated with platinum. A total of 140 patients with prostate cancer who underwent tumor genomic profiling received platinum chemotherapy. Thirty-nine patients were excluded from downstream analysis for the reasons listed. A total of 64 patients with metastatic castration-resistant prostate cancer received platinum-based chemotherapy after a taxane and were poly (ADP-ribose) polymerase inhibitor (PARPi) naïve. Six patients with BRCA and 2 with ATM alterations received platinum chemotherapy after progression on a PARPi. (*) One patient had concurrent ATM and RAD51 alterations, and another had concurrent FANCA and CDK12 alterations. DDRmut, DNA damage repair mutant; DDRwt, DNA damage repair wild type; MSI, microsatellite instability; MSK-IMPACT, Memorial Sloan-Kettering Integrated Molecular Profiling of Actionable Cancer Targets.
CONTEXT
Key Objective
Do genomic alterations in DNA damage repair (DDR) genes sensitize to platinum chemotherapy in metastatic castration-resistant prostate cancer (mCRPC)?
Knowledge Generated
In a single-center dataset of patients with mCRPC who received platinum therapy and underwent somatic and germline DNA profiling, we found that DDR gene alterations were associated with better response to platinum-based chemotherapy after taxane therapy. Responses were observed among patients with BRCA mutations after poly (ADP-ribose) polymerase (PARP) inhibitor progression, although no responses were observed among 4 patients with ATM mutations, regardless of previous exposure to PARP inhibitors.
Relevance
Genomic DDR gene deficiency is associated with response to platinum chemotherapy in mCRPC and, in conjunction with clinical factors, may be useful for patient selection.
Tumor Sequencing and DDR Gene Status
Tumor sequencing was performed using the MSK-IMPACT clinical sequencing assay, a hybridization capture-based, next-generation sequencing platform.15,16 Sixty-six percent of patients also consented to matched germline analysis.17 Patients were defined as DDRmut if they harbored a deleterious somatic alteration18 or pathogenic germline alteration17 in a gene associated with DNA repair pathway as previously described.10 CHEK2,19 NBN,20 RAD50,21 RAD51,22 and RAD51C23 were added to this panel based on a literature search showing these genes are also implicated in homologous recombination/DNA damage recognition and repair. Therefore, our DDR panel consisted of a total of 13 genes: ATM, ATR, BRCA1, BRCA2, CDK12, CHEK2, FANCA, MRE11, NBN, PALB2, RAD50, RAD51, and RAD51C. Tumors with deleterious alterations in at least 1 of these genes, predicted to result in loss of function of at least 1 allele per OncoKB annotation,17 were considered DDRmut. Tumors harboring no alterations or only variants of unknown significance (per OncoKB)18 in these genes were categorized as DDR wild type (DDRwt). Patients with known MSI-high prostate cancer were excluded from analysis if they had a mutation in a DDR gene because of the high likelihood that these mutations represented passenger alterations in patients whose tumors exhibited a high tumor mutation burden.24 Zygosity for patients with DDRmut was determined using FACETS.25 Biallelic loss was defined as loss of wild-type alleles through mutation, deletion, or chromosomal rearrangement, or a combination of these events, including a deleterious alteration with loss of heterozygosity. Two distinct deleterious alterations in a single tumor were assumed to result in biallelic loss. Monoallelic loss indicates a deleterious alteration with retention of the wild-type allele.
Outcomes and Statistics
The association between DDR mutations and a 50% prostate-specific antigen (PSA) decline from baseline while receiving platinum therapy (PSA50) response was evaluated using a logistic regression model. Patients were considered evaluable for PSA50 response if they had a baseline PSA (ie, PSA within 3 weeks prior to chemotherapy start) of at least 2.0 ng/mL and at least 1 PSA value beginning 30 days after the start of platinum-based chemotherapy. The relationship between DDR mutations and time on treatment (ToT), defined as the time from start of platinum-based chemotherapy to the last day of treatment or OS from start of platinum-based chemotherapy was evaluated using the Cox proportional hazards model. All outcomes were adjusted for pretreatment PSA, Gleason score, and the presence of visceral metastasis. Radiologic responses were determined by RECIST 1.1, as assessed by an experienced radiologist (A.W.).
RESULTS
Cohort Characteristics
A total of 140 patients with prostate cancer were identified as having received platinum-based chemotherapy, either as monotherapy or in combination, and having undergone genomic profiling. Of these, 31/140 (22%) were excluded from analysis because of pure nonadenocarcinoma histology (eg, small cell), platinum therapy given for non–castration-resistant disease, tumor profiling performed on a sample acquired after platinum therapy, or MSI-high status (Fig 1). Of the remaining 109 patients, we initially focused on 64 patients who were PARPi naïve and taxane refractory prior to starting platinum-based chemotherapy, where response was less likely to be attributed to the platinum-combination agent if it was a taxane (Fig 1).
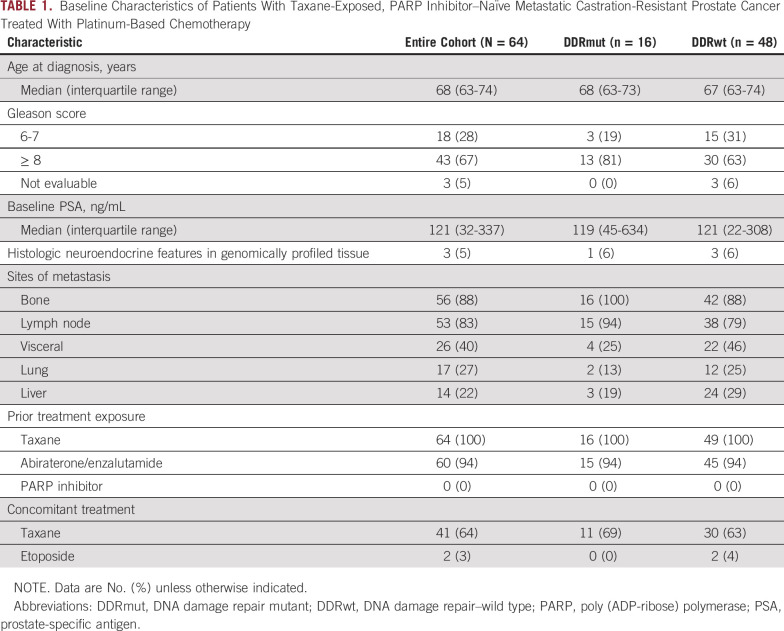
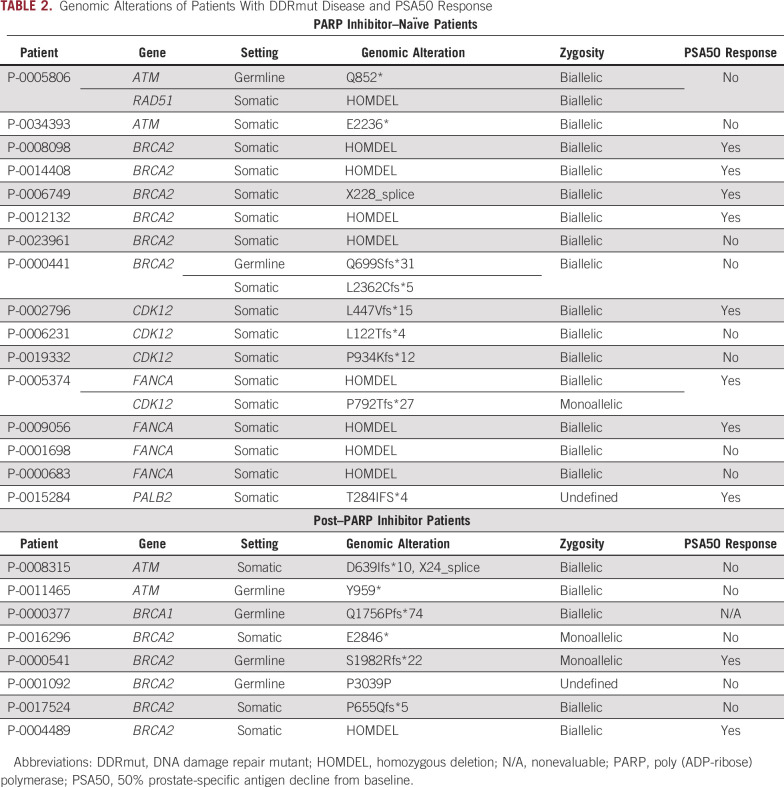
Of these 64 patients, 16 (25%) were DDRmut and 48 (75%) were DDRwt, in line with frequencies identified in larger datasets.8,9 The most frequently altered DDR gene was BRCA2 in 6 patients (9% of total and 37% of the DDRmut population), with deleterious alterations also observed in ATM, FANCA, CDK12, PALB2, and RAD51 (Fig 1). One patient had concurrent germline ATM and somatic RAD51 alterations, and another patient had concurrent CDK12 and FANCA alterations. Patient clinical characteristics are summarized in Table 1. Median age and the proportion of patients with neuroendocrine features on histopathologic reviews were similar in the DDRmut and DDRwt groups. Ninety-four percent of patients in both groups had prior treatment with a next-generation androgen receptor targeted agent (enzalutamide or abiraterone acetate). Visceral metastases at baseline were more common in the DDRwt (22/64; 46%) than the DDRmut group (4/16; 25%). The DDRmut and DDRwt groups were balanced for concomitant treatment with a taxane (11/16 [69%] v 30/64 [63%], respectively). Details of the specific mutations identified in the DDRmut group are shown in Table 2.
TABLE 1.
Baseline Characteristics of Patients With Taxane-Exposed, PARP Inhibitor–Naïve Metastatic Castration-Resistant Prostate Cancer Treated With Platinum-Based Chemotherapy
TABLE 2.
Genomic Alterations of Patients With DDRmut Disease and PSA50 Response
Response to Platinum-Based Chemotherapy for DDRmut Versus DDRwt mCRPC
We assessed the proportion of PSA50 in the 64 patients who received platinum-based chemotherapy after receiving a taxane. Of these, 56 patients (DDRmut, n = 16; DDRwt, n = 40) were evaluable for a PSA50 response. In total, 13/56 evaluable patients (23%) achieved a PSA50 response (Fig 2A; Table 3). A PSA50 response was more likely in DDRmut (8/16; 50%) compared with DDRwt (5/40; 13%) patients (unadjusted odds ratio [OR], 7.0; 95% CI, 1.9 to 29.2; P = .005; adjusted OR, 8.0; 95% CI, 1.9 to 39.9; P = .006). Notably, 4/6 patients with BRCA2 mutations (67%) achieved a PSA50 response (unadjusted OR, 9.1; 95% CI, 1.5 to 73.6; P = .019; adjusted OR, 9.5; 95% CI, 1.5 to 82.9; P = .022; compared with DDRwt), consistent with the reported sensitivity of BRCA-deficient tumors of several lineages to platinum-based chemotherapy.6,7,26,27 Other DDR gene alterations in the PSA50 responder group included PALB2, FANCA, and CDK12. We found no clear association of other genomic characteristics, including alterations in TP53 and RB1, with PSA50 response (Fig 2A). Of the 8 patients with DDRwt disease who were not evaluable for PSA50 response, 4 had a baseline PSA < 2.0 ng/mL, and 4 had no PSA measurement after the start of platinum-based chemotherapy. All 8 patients received a limited duration of platinum chemotherapy (≤ 2.1 months), with the exception of 1 patient who received treatment for 8.6 months at the time of the data freeze, with ongoing clinical benefit. This patient had a baseline PSA < 2.0 ng/mL and no evidence of visceral metastasis or histologic neuroendocrine differentiation. We also evaluated ToT as a surrogate of clinical benefit in the overall population of 64 patients (Fig 2B). Median ToT for the DDRwt group was 1.6 months, compared with 3.0 months for DDRmut (hazard ratio [HR], 0.55; 95% CI, 0.29 to 1.04; P = .064) and 3.9 months for patients with BRCA2 mutations (HR, 0.63; 95% CI, 0.26 to 1.52; P = .300). OS from the start of platinum-based therapy was not significantly different in the DDRmut and DDRwt groups (HR, 0.79; 95% CI, 0.41 to 1.52; P = .480; Fig 2C).
FIG 2.
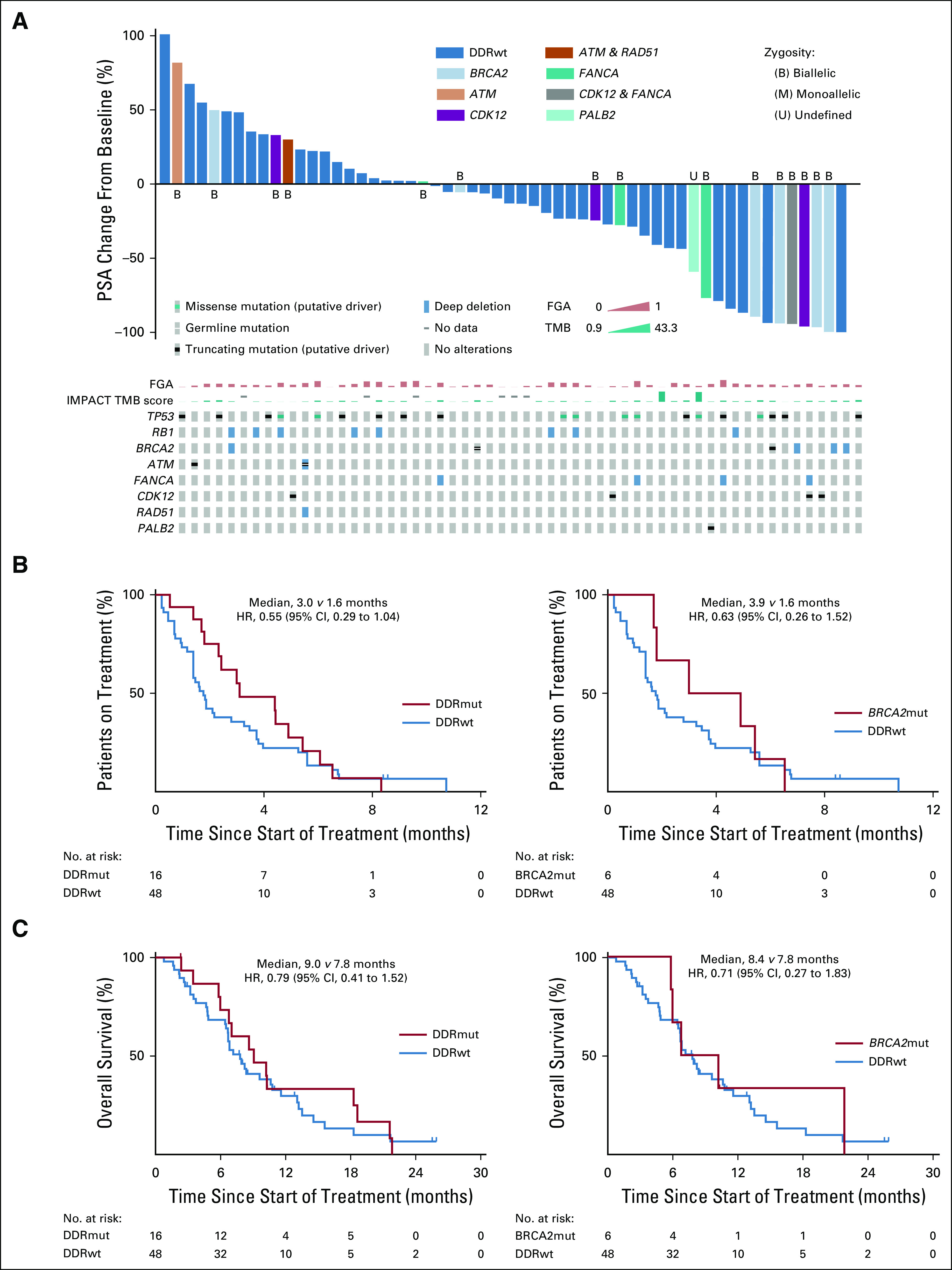
Response to platinum-based chemotherapy in patients with DNA damage repair mutant (DDRmut) and DNA damage repair–wild type (DDRwt) disease. A total of 64 patients received platinum-based chemotherapy after a taxane. (A) Waterfall plot showing best prostate-specific antigen (PSA) change from baseline for patients with DDRmut and DDRwt disease among 56 patients evaluable for PSA response (top panel). The oncoprint (bottom panel) shows details of the types of alterations in DNA damage repair (DDR) genes, as well as alterations in TP53, RB1, tumor mutation burden (TMB; in mutations per megabase), and fraction of the genome altered (FGA). Zygosity status for the relevant DDR genes is indicated. (B) Time on treatment and (C) overall survival with platinum chemotherapy for DDRmut (left) and the BRCA2-mutated (BRCA2mut) subset (right) compared with patients with DDRwt disease. HR, hazard ratio.
TABLE 3.
PSA50 Response Rate by DDR Gene Status
Platinum-Based Chemotherapy After PARPi Treatment
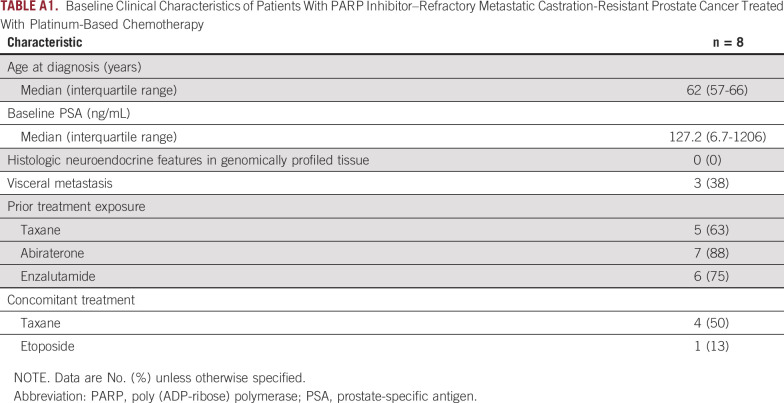
We evaluated response to platinum chemotherapy in patients with DDRmut disease who received platinum chemotherapy after experiencing progression on a PARPi. We focused on the 8 patients with an alteration in either BRCA2 (n = 5), BRCA1 (n = 1), or ATM (n = 2), given the previously reported sensitivity of these tumors to PARP inhibition,13 with US Food and Drug Administration (FDA) breakthrough therapy designation having been granted for olaparib for BRCA1/2 and ATM-mutated mCRPC. Baseline clinical characteristics are summarized in Appendix Table A1. Median time on the prior PARPi for all 8 patients was 4.6 months (range, 1.0-13.1 months). Best radiographic response on the prior PARPi was partial response (PR) in 2/8, stable disease (SD) in 3/8, and progression of disease in 3/8, although all ultimately experienced progression (Fig 3A).
FIG 3.
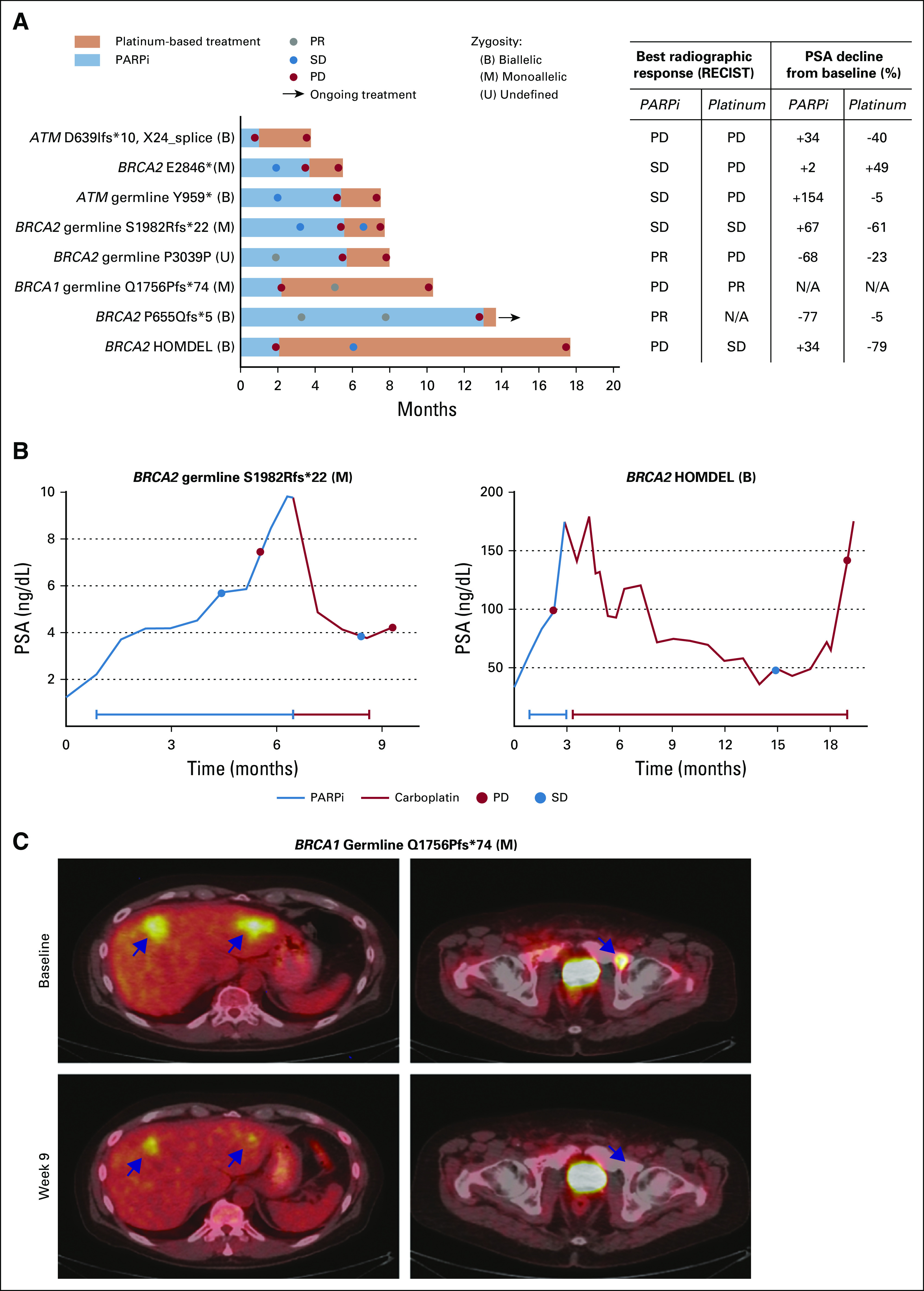
Platinum-based chemotherapy after poly (ADP-ribose) polymerase inhibitor (PARPi) progression. (A) Time on treatment on PARPi (blue) and subsequent platinum-based chemotherapy (orange), which did not necessarily occur immediately after PARPi therapy. Best radiographic and prostate-specific antigen (PSA) responses are summarized in the table. Zygosity status for the DNA damage repair gene is indicated. Three of 7 evaluable patients had RECIST 1.1 stable disease (SD) or partial response (PR) on platinum-based chemotherapy. (B) Six of 7 evaluable patients had PSA decrease on platinum therapy, with 2 BRCA2-altered patients achieving a 50% prostate-specific antigen decline from baseline response. (C) One patient with a BRCA1 germline mutation had a RECIST PR on platinum chemotherapy after progression on a PARPi, with representative 18F-labeled fluorodeoxyglucose–positron emission tomography/computed tomography images. This patient was taxane naïve and received carboplatin with docetaxel; therefore, his response cannot be definitely attributed to the platinum agent alone. HOMDEL, homozygous deletion; N/A, not evaluable; PD, progression of disease; PR, partial response; SD, stable disease.
The median time on platinum-based chemotherapy for these 8 patients after progression on PARPi was 2.1 months (range, 1.8 to 15.6 months). Six of 7 PSA-evaluable patients (86%) had a decline in PSA from baseline on platinum therapy, 2 of whom achieved a PSA50 response (Fig 3A). Two of 7 evaluable patients, both with BRCA2 mutations, achieved SD, with PSA declines of 61% and 79% (Fig 3B). Both of these patients received platinum chemotherapy as monotherapy when their PSA responses could not be attributed to another agent. One patient with a BRCA1 germline mutation and nonmeasurable PSA achieved a radiographic PR on carboplatin plus docetaxel after progression on a PARPi (Fig 3C). However, this patient was taxane naïve at baseline; therefore, his response could not be definitely attributed to the platinum agent. Of note, none of the 4 patients with deleterious mutations in ATM, regardless of prior PARPi or taxane exposure (Fig 1), had a PSA or radiographic response to platinum-based chemotherapy (Figs 2A and 3A; Table 2).
DISCUSSION
In this study, we retrospectively assessed response to platinum-based chemotherapy in patients with mCRPC who underwent clinical tumor and germline genomic sequencing at a tertiary referral center. We found that PSA responses occurred more frequently in patients who harbored genomic alterations in DDR genes. There was a trend toward longer ToT in the DDR-mutant group, but we could not detect a difference in OS. Importantly, we limited the analysis to patients who received platinum-based chemotherapy after progression on a taxane, where response was more likely attributable to the platinum agent, because many patients with mCRPC receive platinum chemotherapy in combination with a taxane.2,28
Our findings are consistent with prior reports of improved response of BRCA-altered tumors to platinum-based chemotherapy6,7,12 and identify responses in tumors with non-BRCA DDR gene alterations, including PALB2, FANCA, and CDK12,8,9 suggesting that a broader DDR gene panel encompassing nearly 25% of patients with mCRPC could be used to identify patients who are more likely to derive benefit from platinum chemotherapy, either administered alone or concurrently with a taxane. The limited sample size of our study likely made it difficult to reliably detect differences in ToT and OS. Importantly, other clinical disease subsets, sometimes described as “aggressive variants” of prostate cancer, including those with low PSA expression, visceral metastasis, or histologic neuroendocrine differentiation, may also derive particular benefit from platinum chemotherapy,5 and the presence of a genomic alteration in a DDR gene is only 1 variable that may aid in patient selection for this type of therapy.
We also examined responses to platinum chemotherapy after progression on a PARPi for patients with BRCA and ATM mutations. The PARPi olaparib and rucaparib were recently granted FDA breakthrough therapy designation for BRCA-mutated mCRPC, based on phase II studies showing a high rate of objective responses in this setting.14,29 However, it remains unknown whether tumors that acquire resistance to PARPi30 can still respond to other DNA damage-targeting agents, including platinum chemotherapy. We found that 3/ 8 patients with DDR mutations (37%) derived some clinical benefit from platinum-based chemotherapy after progression on a PARPi, with 1 patient achieving a radiographic PR, although outcomes in this advanced patient population were generally poor.
Of note, our study included 4 patients with deleterious alterations in ATM who received platinum-based chemotherapy either before or after receiving a PARPi. None of these patients achieved a PSA50 response, and all experienced rapid disease progression. This finding is from a limited sample size and will need to be confirmed in larger studies, but it reinforces the need for novel therapeutic approaches for the approximately 4% of patients with mCRPC who harbor deleterious alterations in ATM.31
In summary, our study suggests that a subset of patients with DDR gene alterations detected by tumor or germline sequencing may derive benefit from platinum-based chemotherapy, including patients with BRCA mutations who have progressed after treatment with a PARPi, a group with particular clinical relevance. Our study differs from prior studies in that it represents a single-institution experience using a single panel sequencing assay that includes DDR genes beyond BRCA and includes both somatic and germline alterations in DDR genes. We recognize that our study is limited by its retrospective nature, the incorporation of distinct DDR genes with varying functions into a single panel, and sample size; thus, our findings will need to be validated in larger prospective studies, which are currently ongoing. We also recognize that other clinical factors linked to aggressive variants of prostate cancer are associated with response to platinum chemotherapy and that a combination of genomic and clinical characteristics may ultimately aid in patient selection.
APPENDIX
TABLE A1.
Baseline Clinical Characteristics of Patients With PARP Inhibitor–Refractory Metastatic Castration-Resistant Prostate Cancer Treated With Platinum-Based Chemotherapy
Presented in part at the ASCO 2019 Annual Meeting, Chicago, IL, May 31 to June 4, 2019.
SUPPORT
Supported by Young Investigator Awards from the Prostate Cancer Foundation (W.A., K.H.S.); Department of Defense Prostate Cancer Research Program Physician Research Award W81XWH-17-1-0124 (W.A.) and Early Investigator Research Award W81XWH-18-1-0330 (K.H.S.); National Cancer Institute (NCI) cancer center support Grant No. P30CA008748; NCI prostate cancer Specialized Programs of Research Excellence (SPORE) Grant No. P50CA092629; and Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
AUTHOR CONTRIBUTIONS
Conception and design: Jose Mauricio Mota, Ethan Barnett, Jones T. Nauseef, Daniel C. Danila, Howard I. Scher, Michael J. Morris, David B. Solit, Wassim Abida
Financial support: David B. Solit, Wassim Abida
Administrative support: Ethan Barnett, David B. Solit, Wassim Abida
Provision of study materials or patients: Susan Slovin, Michael J. Morris, David B. Solit, Wassim Abida
Collection and assembly of data: Ethan Barnett, Jones T. Nauseef, Andreas Wibmer, Daniel C. Danila, Howard I. Scher, David B. Solit, Wassim Abida
Data analysis and interpretation: Jose Mauricio Mota, Ethan Barnett, Jones T. Nauseef, Bastien Nguyen, Konrad H. Stopsack, Glenn Heller, Daniel C. Danila, Dana Rathkopf, Susan Slovin, Philip W. Kantoff, Howard I. Scher, Michael J. Morris, Nikolaus Schultz, David B. Solit, Wassim Abida
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jose Mauricio Mota
Consulting or Advisory Role: QED Therapeutics, Janssen Oncology
Speakers' Bureau: Janssen Oncology, Amgen, Zodiac Pharma
Ethan Barnett
Stock and Other Ownership Interests: Gilead Sciences, Biondvax
Glenn Heller
Research Funding: Janssen Diagnostics
Daniel C. Danila
Honoraria: Angle, Bayer, ScreenCell, Janssen Oncology, Pfizer, AxImmune
Consulting or Advisory Role: Angle, Bayer, Sanador, AxImmune, Pfizer
Research Funding: Prostate Cancer Foundation, Genentech, Janssen Research & Development (Inst)
Patents, Royalties, Other Intellectual Property: Gene expression profile associated with prostate cancer
Travel, Accommodations, Expenses: Cambridge Healthtech Institute, Prostate Cancer Foundation, Angle, Bayer, ScreenCell, StopCancer, American Austrian Open Medical Institute
Dana Rathkopf
Consulting or Advisory Role: Janssen, Genentech, AstraZeneca, Bayer
Research Funding: Janssen Oncology (Inst), Medivation (Inst), Celgene (Inst), Takeda (Inst), Millennium (Inst), Ferring (Inst), Novartis (Inst), Taiho Pharmaceutical (Inst), AstraZeneca (Inst), Genentech (Inst), TRACON Pharma (Inst)
Susan Slovin
Consulting or Advisory Role: Bayer, Astellas/Pfizer, Clovis Oncology
Speakers' Bureau: OncLive, Prime Oncology, SITC, PER
Research Funding: AstraZeneca
Philip W. Kantoff
Leadership: Context Therapeutics
Stock and Other Ownership Interests: Placon, Druggablity Technologies, Context Therapeutics, Seer
Consulting or Advisory Role: Bavarian Nordic, Janssen, Merck, OncoCellMDX, Genentech/Roche, Tarveda Therapeutics, Druggablity Technologies, Progenity, Context Therapeutics, GE Healthcare, Seer
Patents, Royalties, Other Intellectual Property: Method for predicting the risk of prostate cancer morbidity and mortality, predicting and treating prostate cancer, methods for predicting likelihood of responding to treatment, chromosome copy number gain as a biomarker of urothelial carcinoma lethality, drug combinations to treat cancer, somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma (patent), Up-to-Date royalties, Wolters Kluwer Royalties, methods and kits for determining sensitivity to cancer treatment, composition and methods for screening and diagnosis of prostate cancer.
Open Payments Link: https://openpaymentsdata.cms.gov/physician/55315/summary
Howard I. Scher
Leadership: Asterias Biotherapeutics
Stock and Other Ownership Interests: Asterias Biotherapeutics
Honoraria: Research to Practice
Consulting or Advisory Role: Janssen Biotech, Amgen, Janssen Research & Development, Menarini Silicon Biosystems, WIRB-Copernicus Group, ESSA, Sanofi Aventis, Ambry Genetics Corporation, Konica Minolta, Bayer
Research Funding: Janssen (Inst), Innocrin Pharma (Inst), Illumina (Inst), Epic Sciences (Inst), Menarini Silicon Biosystems (Inst), Thermo Fisher Scientific Biomarkers (Inst)
Travel, Accommodations, Expenses: Asterias Biotherapeutics, Menarini Silicon Biosystems, Amgen, WIRB-Copernicus Group, Konica Minolta, ESSA, Prostate Cancer Foundation, Sanofi Aventis, Bayer, Phosplatin Therapeutics
Michael J. Morris
Consulting or Advisory Role: Astellas Pharma, Bayer, Endocyte, Advanced Accelerator Applications, Blue Earth Diagnostics, Tokai Pharmaceuticals, Tolmar Pharmaceuticals, ORIC Pharmaceuticals, Johnson & Johnson
Research Funding: Bayer (Inst), Sanofi (Inst), Endocyte (Inst), Progenics (Inst), Corcept Therapeutics (Inst), Genentech (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Bayer, Endocyte, Fujifilm
David B. Solit
Stock and Other Ownership Interests: Loxo
Consulting or Advisory Role: Pfizer, Loxo, Illumina, Intezyne Technologies, Vivideon Therapeutics, Lilly Oncology, QED Therapeutics
Travel, Accommodations, Expenses: Merck
Wassim Abida
Honoraria: CARET
Consulting or Advisory Role: Clovis Oncology, Janssen, MORE Health, ORIC Pharmaceuticals, Daiichi Sankyo
Research Funding: AstraZeneca (Inst), Zenith Epigenetics (Inst), Clovis Oncology (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline, Clovis Oncology, ORIC Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sternberg CN, Petrylak DP, Sartor O, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: The SPARC trial. J Clin Oncol. 2009;27:5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 2.Ross RW, Beer TM, Jacobus S, et al. A phase 2 study of carboplatin plus docetaxel in men with metastatic hormone-refractory prostate cancer who are refractory to docetaxel. Cancer. 2008;112:521–526. doi: 10.1002/cncr.23195. [DOI] [PubMed] [Google Scholar]
- 3.Hager S, Ackermann CJ, Joerger M, et al. Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann Oncol. 2016;27:975–984. doi: 10.1093/annonc/mdw156. [DOI] [PubMed] [Google Scholar]
- 4.Corn PG, Heath EI, Zurita A, et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: A randomised, open-label, phase 1-2 trial. Lancet Oncol. 2019;20:1432–1443. doi: 10.1016/S1470-2045(19)30408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–3630. doi: 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng HH, Pritchard CC, Boyd T, et al. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol. 2016;69:992–995. doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pomerantz MM, Spisák S, Jia L, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer. 2017;123:3532–3539. doi: 10.1002/cncr.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson D, Van Allen EM, Wu Y-M, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;162:454. doi: 10.1016/j.cell.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 9. Abida W, Armenia J, Gopalan A, et al: Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol 10.1200/PO.17.00029. [DOI] [PMC free article] [PubMed]
- 10. doi: 10.1038/s41588-018-0078-z. Armenia J, Wankowicz SAM, Liu D, et al: The long tail of oncogenic drivers in prostate cancer. Nat Genet 50:645-651, 2018 [Erratum Nat Gen 51:1194, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375:443–453. doi: 10.1056/NEJMoa1603144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zafeiriou Z, Bianchini D, Chandler R, et al. Genomic analysis of three metastatic prostate cancer patients with exceptional responses to carboplatin indicating different types of DNA repair deficiency. Eur Urol. 2019;75:184–192. doi: 10.1016/j.eururo.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abida W, Bryce AH, Vogelzang NJ, et al: Preliminary results from TRITON2: A phase II study of rucaparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination repair (HRR) gene alterations. Ann Oncol 29, 2018 (suppl 8; abstr 793PD)
- 15.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. doi: 10.1038/nm.4333. Zehir A, Benayed R, Shah RH, et al: Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [Erratum: Nat Med 23:1004, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA. 2017;318:825–835. doi: 10.1001/jama.2017.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chakravarty D, Gao J, Phillips SM, et al: OncoKB: A precision oncology knowledge base. JCO Precis Oncol 10.1200/PO.17.00011. [DOI] [PMC free article] [PubMed]
- 19.Zannini L, Delia D, Buscemi G. CHK2 kinase in the DNA damage response and beyond. J Mol Cell Biol. 2014;6:442–457. doi: 10.1093/jmcb/mju045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varon R, Vissinga C, Platzer M, et al. Nibrin, a novel DNA double-strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell. 1998;93:467–476. doi: 10.1016/s0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 21.Hopfner KP, Karcher A, Shin DS, et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. doi: 10.1016/s0092-8674(00)80890-9. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharya S, Srinivasan K, Abdisalaam S, et al. RAD51 interconnects between DNA replication, DNA repair and immunity. Nucleic Acids Res. 2017;45:4590–4605. doi: 10.1093/nar/gkx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. doi: 10.1074/jbc.M111.311241. Somyajit K, Subramanya S, Nagaraju G: Distinct roles of FANCO/RAD51C protein in DNA damage signaling and repair: Implications for Fanconi anemia and breast cancer susceptibility. J Biol Chem 287:3366-3380, 2012 [Erratum: J Biol Chem 294:13198, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5:471–478. doi: 10.1001/jamaoncol.2018.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen R, Seshan VE. FACETS: Allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 2016;44:e131. doi: 10.1093/nar/gkw520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang J, D’Andrea AD, Kozono D. A DNA repair pathway-focused score for prediction of outcomes in ovarian cancer treated with platinum-based chemotherapy. J Natl Cancer Inst. 2012;104:670–681. doi: 10.1093/jnci/djs177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telli ML, Timms KM, Reid J, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22:3764–3773. doi: 10.1158/1078-0432.CCR-15-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regan MM, O’Donnell EK, Kelly WK, et al. Efficacy of carboplatin-taxane combinations in the management of castration-resistant prostate cancer: A pooled analysis of seven prospective clinical trials. Ann Oncol. 2010;21:312–318. doi: 10.1093/annonc/mdp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mateo J, Porta N, McGovern UB, et al. TOPARP-B: A phase II randomized trial of the poly(ADP)-ribose polymerase (PARP) inhibitor olaparib for metastatic castration resistant prostate cancers (mCRPC) with DNA damage repair (DDR) alterations. J Clin Oncol. 2019;37(suppl; abstr 5005) [Google Scholar]
- 30.Lin KK, Harrell MI, Oza AM, et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019;9:210–219. doi: 10.1158/2159-8290.CD-18-0715. [DOI] [PubMed] [Google Scholar]
- 31. doi: 10.1158/1078-0432.CCR-20-0394. Abida W, Campbell D, Patnaik A, et al: Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: Analysis from the phase 2 TRITON2 study. Clin Cancer Res . [epub ahead of print on February 21, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]