INTRODUCTION
Glioblastoma (GBM) is the most common malignant primary brain tumor.1 Despite aggressive multimodality treatment, median overall survival (OS) is 14 to 18 months.2 One potential novel therapeutic target is V-raf murine sarcoma viral oncogene homolog B1 (BRAF), which is mutated in 1% to 2% of GBM,3-6 as well as other primary brain tumors including pleomorphic xanthoastrocytoma (PXA) and ganglioglioma. BRAF is among the most commonly mutated kinases in human cancer, particularly in melanoma.7
Typically regulated by extracellular factors, mutant BRAF results in uncontrolled cellular growth via the MEK-ERK extracellular signal-regulated kinase pathway.6 Although oral inhibitors of the oncogenic BRAFv600 kinase have demonstrated some efficacy in gliomas, several mechanisms mediating resistance to BRAF inhibitors (BRAFis) are described. Moreover, this therapy is associated with an increased risk of hyperproliferative skin lesions such as squamous cell carcinoma.8 Combining BRAF with MEK inhibitors mitigates these effects and improves progression-free survival and OS compared with BRAFi monotherapy in metastatic melanoma.9-12 Furthermore, studies demonstrate that the use of BRAFi in combination with MEK inhibitors prevents the development of resistance because of the reactivation of the MAPK pathway while simultaneously decreasing hyperproliferative skin lesions.8
Although there is strong evidence for the use of BRAF and MEK inhibitors in other malignancies, there are few cases in the literature of primary brain tumors treated with this combination, mainly in pediatric glioma.13-15 We present a case of a patient with a recurrent BRAF-mutant GBM, who had a clinical and radiographic response when treated with BRAFi dabrafenib in combination with MEK inhibitor trametinib (D+T), after failing treatment with BRAFi monotherapy.
CLINICAL CASE
The patient is a 44-year-old man who initially presented with headaches and seizures. Brain magnetic resonance imaging (MRI) showed a left temporal, peripherally enhancing lesion (Fig 1). The patient underwent gross total resection, and pathology showed MGMT promoter unmethylated, IDH wild-type, ATRX retained, WHO grade 4 GBM. Tumor histology displayed a cellular astrocytic neoplasm, nuclear atypia, mitoses, and vascular proliferation (Fig 2). No epithelioid or rhabdoid morphology were observed. A next-generation sequence–based assay (FoundationOne) showed that the tumor cells were positive for BRAF V600E mutation, CDKN2A/B loss, and CHEK2 T367fs*15 mutation. He had radiation therapy with concurrent temozolomide (75 mg/m2 once per day) for a total of 59.4 Gy in 33 fractions, followed by 9 adjuvant temozolomide cycles (first cycle at 150 mg/m2 followed by 8 cycles at 200 mg/m2 once per day on a 5-days-on-23-days-off schedule). He developed asymptomatic radiographic progression on temozolomide and subsequently failed carboplatin (area under the curve, 5) after 2 cycles.
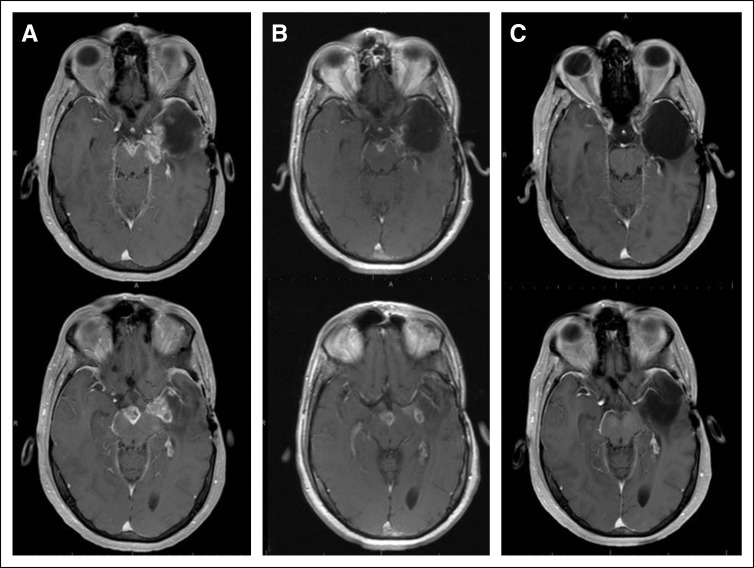
FIG 1.
Magnetic resonance imaging (MRI), axial postgadolinium T1-weighted imaging. (A) Baseline brain MRI images, before starting dabrafenib and trametinib (D+T), demonstrated progression of disease into the surgical cavity, as well as new lesions in the left insular region and midbrain. (B) Brain MRI images at 1 month after initiation of D+T, confirming > 50% decrease of all measurable enhancing lesions. (C) Brain MRI images at 7 months of D+T, demonstrating disappearance of all enhancing disease.
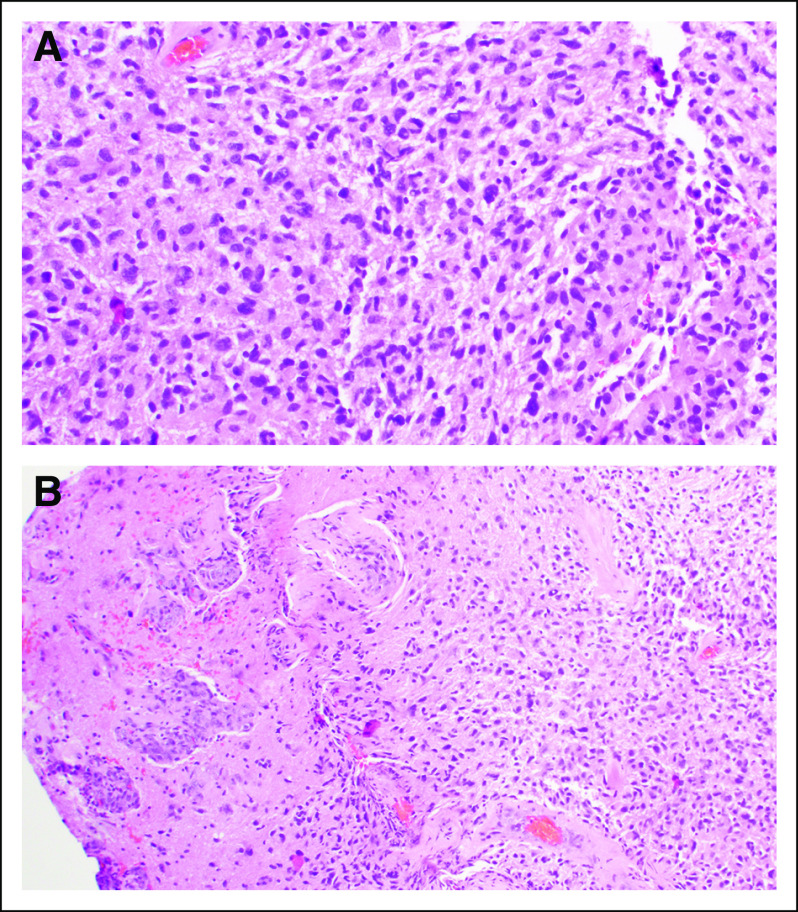
FIG 2.
Histologic features at diagnosis. (A) Hematoxylin and eosin stain (40× magnification). (B) Hematoxylin and eosin stain (20× magnification).Tumor sample demonstrating an infiltrative cellular astrocytic neoplasm with fibrillary background, nuclear atypia, mitoses, and vascular proliferation; consistent with glioblastoma, WHO grade 4.
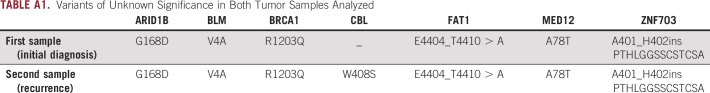
The patient had a second gross total resection 17 months after his diagnosis and was enrolled into clinical trial NCT01808820 of autologous dendritic cell vaccine for recurrent GBM. Pathology showed high cellular malignant astrocytoma with the same pathologic features as the initial tumor, together with extensive necrosis (Fig 3). The same next-generation sequence platform was used in this second sample, and the tumor cells again were positive for BRAF V600E mutation, CDKN2A/B loss, and CHEK2 T367fs*15 mutation. Variants of unknown significance are listed in Appendix Table A1. The patient completed treatment on trial protocol, and after 10 months, he presented with clinical and radiographic progression. He was enrolled into a clinical trial (ClinicalTrials.gov identifier: NCT02428712) of BRAFi (PLX8394) in combination with cobicistat, achieving radiographic partial response and complete resolution of his symptoms for 7 months. He then developed severe headaches and right-sided weakness, and MRI showed multifocal radiographic progression. The case was discussed at the Precision Medicine Tumor Board, and the patient was administered D+T (dabrafenib 150 mg 2 times a day and trametinib 2 mg once per day), achieving, on his first on-treatment assessment, complete resolution of symptoms and radiographic partial response. He tolerated treatment well, although after 2 months, he developed acute-onset right-sided weakness and had a left internal capsule ischemic stroke of unclear etiology. The patient was able to continue treatment with D+T. After 11 months of treatment, the patient exhibits complete response on MRI, and has no significant toxicities. His stroke-related right hemiparesis continues to improve as well.
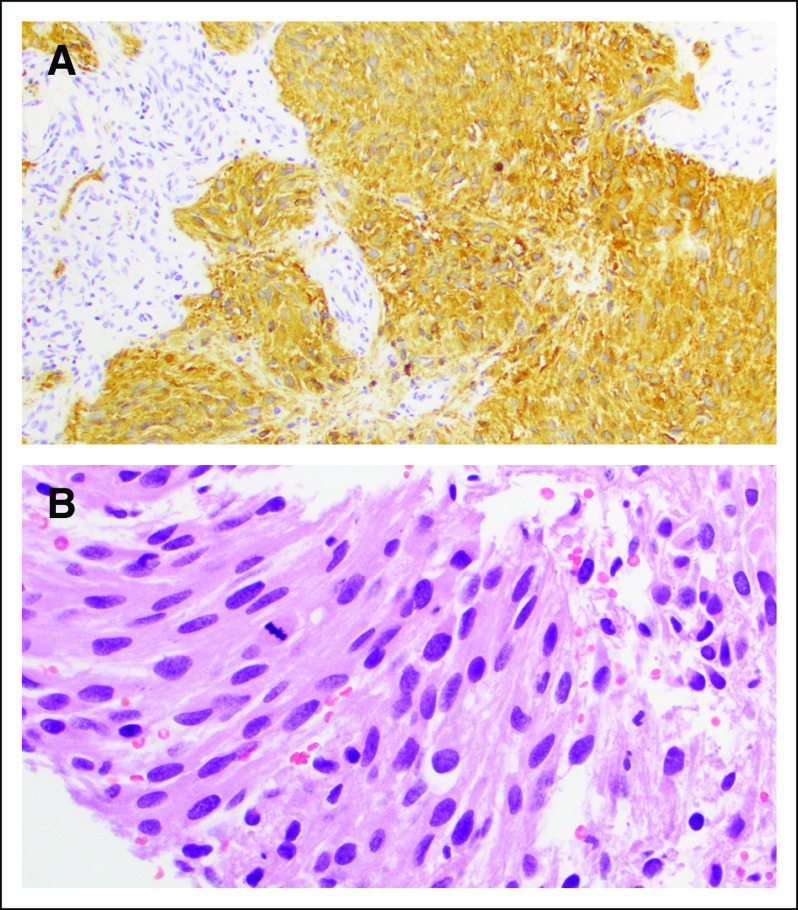
FIG 3.
Histologic features at tumor recurrence (second resection). (A) Glial fibrillary acidic protein immunohistochemical stain (20× magnification). (B) Hematoxylin and eosin image (40× magnification). Second resection shows a similarly high cellular malignant astrocytoma with nuclear atypia, focal gemistocytic morphology, abundant fibrillary processes, vascular proliferation, and mitoses.
DISCUSSION
The BRAF protein is an intermediary in the RAS-RAF pathway. After a ligand-mediated receptor tyrosine kinase is triggered by extracellular growth factors, it activates RAS, which initiates BRAF-mediated activation of MEK and ERK, causing transcription of factors for cell proliferation.6,7 The BRAFv600 mutation results in constitutive activation of the MEK-ERK pathway and uncontrolled cell division. BRAF mutations are drivers of oncogenesis in approximately 6% of human cancer, including melanoma (40%-80% BRAF mutation prevalence4), thyroid cancers (up to 35%, depending on histology16), colorectal cancers (7%-10%17), and non-small cell lung cancer (3%-5%18).
BRAFv600 mutations have been identified in a variety of primary brain tumors, but they are uncommon in GBM,19 with the exception of the epithelioid GBM; Korshunov et al20 found that 56% of epithelioid GBMs carry BRAF mutations. Indeed, anaplastic PXA and epithelioid GBM are postulated by some groups to be similar, if not the same entity, because there are no clear histopathologic or molecular defining features to differentiate them.21 Generally, epithelioid GBMs contain epithelioid or melanoma‐like cells with loose cohesion, abundant cytoplasm, and eccentric nuclei.20 Our patient, however, had no features of PXA or epithelioid GBM. Tissue from the patient’s first resection (Fig 2) shows a cellular astrocytic neoplasm, nuclear atypia, mitoses, and vascular proliferation. The second resection (Fig 3) shows a similarly high cellular malignant astrocytoma with the same features, together with extensive necrosis. In both resections, epithelioid or rhabdoid morphology is lacking. In addition, there are no features of a PXA, including Rosenthal fibers, eosinophilic granular bodies, collagen deposits, giant nuclei, or lipidized tumor cells.
The rarity of this mutation in adult primary brain tumors has limited the opportunity to run disease-specific studies. Patients are usually enrolled in “basket” clinical trials that include a variety of histologic subtypes within brain tumor cohorts, making it challenging to apply the results to 1 specific histologic subtype. Moreover, for radiographic assessment, these trials often use RECIST, which is designed for the assessment of solid tumors, rather than a dedicated brain tumor response criteria, such as RANO. A recently published study by Kaley et al22 reported on 24 patients with gliomas treated with vemurafenib monotherapy, including 6 patients with GBM. In the patients with GBM, the best response was stable disease (SD) in 3 patients, with 2 patients experiencing progression at 3.6 months and 3.7 months, and 1 patient with prolonged SD until 12.9 months. Our patient was treated at third recurrence with a novel RAF inhibitor, PLX8394. A recent publication suggested that this drug, unlike current RAF inhibitors that are monomer selective, can disrupt BRAF homo- and BRAF-CRAF heterodimers.23
In treating BRAF-mutant tumors, dual-targeted therapy with BRAF and MEK inhibition has several advantages over BRAFi monotherapy.11,24-26 First, resistance develops rapidly with BRAFi alone, resulting in progression in 6-8 months because of reactivation of the MAPK pathway.9,11,15 Second, BRAFi monotherapy causes significant skin toxicity. Peuvrel et al27 reported that up to 90% of patients in their study had cutaneous adverse events. The most serious toxicities, occurring in 25% of their patients, included drug rash with eosinophilia and systemic symptoms syndrome, Stevens-Johnson syndrome, and diffuse maculopapular rash.26 In addition, BRAFi alone was associated with keratosis pilaris, photosensitivity, and cutaneous carcinomas.27 Robinson et al28 describe the use of BRAFi monotherapy for the treatment of a 12-year-old patient with GBM, which resulted in complete response radiographically. Using a downstream MEK inhibitor such as trametinib mitigates the dermatologic effects of BRAFi and prevents the development of resistance.8,9,27 Trametinib, as a single agent, improves progression-free survival and OS in BRAFi-naïve patients with BRAF mutant metastatic melanoma.9 However, minimal clinical activity was observed with sequential trametinib monotherapy in patients treated previously with BRAFi.29
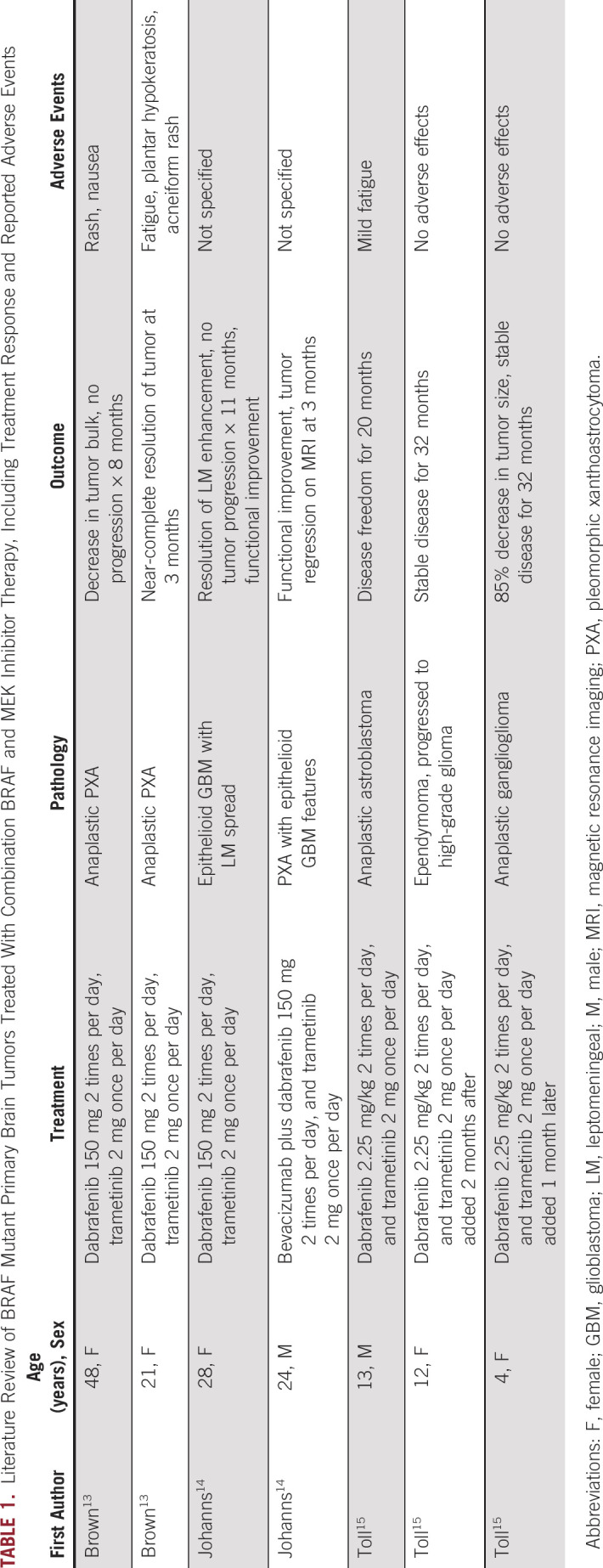
D+T for the treatment of primary brain tumors has been described in few case reports (Table 1). Brown et al13 report on 2 patients with anaplastic PXA. Notably, one of these patients was treated previously with dabrafenib single agent for 18 months, at which point the treatment was discontinued and the patient commenced radiographic surveillance. The patient had radiographic progression 2 months later and was administered D+T. Johanns et al14 describe 2 adults, both of whom had epithelioid/anaplastic PXA histologic subtypes. One patient was started on D+T, with clinical and radiographic response, but had progression 11 months after initiating treatment. The second patient was treated with D+T and bevacizumab, achieving clinical and radiographic response, but presented with tumor recurrence 3 months later because of medication nonadherence. Toll et al15 describe 3 pediatric gliomas. One anaplastic astroblastoma had complete radiographic response for 20 months, after which the patient developed disseminated disease and expired. Two others, with an ependymoma and an anaplastic ganglioglioma, had SD for 32 months and 23 months, respectively. Our patient lacks epithelioid/PXA histologic features, making him distinct from the described cases. However, these reports demonstrate efficacy of D+T in primary brain tumors. To our knowledge, ours is the first report of clinical and radiographic response to D+T after BRAFi failure in BRAF-mutated GBM. Several ongoing trials (ClinicalTrials.gov identifiers: NCT01677741, NCT01748149, NCT02124772, NCT02684058, and NCT02285439), will elucidate the precise role of these drugs as single agents and in combination in pediatric and adult brain tumors.
TABLE 1.
Literature Review of BRAF Mutant Primary Brain Tumors Treated With Combination BRAF and MEK Inhibitor Therapy, Including Treatment Response and Reported Adverse Events
APPENDIX
TABLE A1.
Variants of Unknown Significance in Both Tumor Samples Analyzed
Footnotes
Supported by grants from the American Cancer Society (M.I.d.l.F.) and the Sylvester Comprehensive Cancer Center (M.I.d.l.F.).
AUTHOR CONTRIBUTIONS
Conception and design: Marina Kushnirsky, Macarena I. de la Fuente
Collection and assembly of data: Marina Kushnirsky
Data analysis and interpretation: Lynn G. Feun, Sakir H. Gultekin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lynn G. Feun
Research Funding: Merck Sharp & Dohme (Inst)
Macarena I. de la Fuente
Consulting or Advisory Role: Agios, Puma Biotechnology, Foundation Medicine, FORMA Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro-oncol. 2018;20:iv1–iv86. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas AA, Brennan CW, DeAngelis LM, et al. Emerging therapies for glioblastoma. JAMA Neurol. 2014;71:1437–1444. doi: 10.1001/jamaneurol.2014.1701. [DOI] [PubMed] [Google Scholar]
- 3.Brennan CW, Verhaak RGW, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [Erratum: Cell 157:753, 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Maraka S, Janku F. BRAF alterations in primary brain tumors. Discov Med. 2018;26:51–60. [PubMed] [Google Scholar]
- 7.Dankner M, Rose AAN, Rajkumar S, et al. Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene. 2018;37:3183–3199. doi: 10.1038/s41388-018-0171-x. [DOI] [PubMed] [Google Scholar]
- 8.Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103–1109. doi: 10.1001/jamadermatol.2015.1745. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson DB, Flaherty KT, Weber JS, et al. Combined BRAF (dabrafenib) and MEK inhibition (trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697–3704. doi: 10.1200/JCO.2014.57.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631–1639. doi: 10.1093/annonc/mdx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 13.Brown NF, Carter T, Kitchen N, et al. Dabrafenib and trametinib in BRAFV600E mutated glioma. CNS Oncol. 2017;6:291–296. doi: 10.2217/cns-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johanns TM, Ferguson CJ, Grierson PM, et al. Rapid clinical and radiographic response with combined dabrafenib and trametinib in adults with BRAF-mutated high-grade glioma. J Natl Compr Canc Netw. 2018;16:4–10. doi: 10.6004/jnccn.2017.7032. [DOI] [PubMed] [Google Scholar]
- 15.Toll SA, Tran HN, Cotter J, et al. Sustained response of three pediatric BRAFV600E mutated high-grade gliomas to combined BRAF and MEK inhibitor therapy. Oncotarget. 2019;10:551–557. doi: 10.18632/oncotarget.26560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 17.Baldus SE, Schaefer K-L, Engers R, et al. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res. 2010;16:790–799. doi: 10.1158/1078-0432.CCR-09-2446. [DOI] [PubMed] [Google Scholar]
- 18.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- 19.Knobbe CB, Reifenberger J, Reifenberger G. Mutation analysis of the Ras pathway genes NRAS, HRAS, KRAS and BRAF in glioblastomas. Acta Neuropathol. 2004;108:467–470. doi: 10.1007/s00401-004-0929-9. [DOI] [PubMed] [Google Scholar]
- 20.Korshunov A, Chavez L, Sharma T, et al. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2018;28:656–662. doi: 10.1111/bpa.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexandrescu S, Korshunov A, Lai SH, et al. Epithelioid glioblastomas and anaplastic epithelioid pleomorphic xanthoastrocytomas–same entity or first cousins? Brain Pathol. 2016;26:215–223. doi: 10.1111/bpa.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaley T, Touat M, Subbiah V, et al. BRAF inhibition in BRAFV600-mutant gliomas: Results from the VE-BASKET study. J Clin Oncol. doi: 10.1200/JCO.2018.78.9990. [epub ahead of print on October 23, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Z, Gao Y, Su W, et al. RAF inhibitor PLX8394 selectively disrupts BRAF dimers and RAS-independent BRAF-mutant-driven signaling. Nat Med. 2019;25:284–291. doi: 10.1038/s41591-018-0274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. doi: 10.1016/S0140-6736(15)60898-4. [DOI] [PubMed] [Google Scholar]
- 25.Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 26.Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): Updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 27.Peuvrel L, Quéreux G, Saint-Jean M, et al. Profile of vemurafenib-induced severe skin toxicities. J Eur Acad Dermatol Venereol. 2016;30:250–257. doi: 10.1111/jdv.13443. [DOI] [PubMed] [Google Scholar]
- 28.Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer. 2014;14:258. doi: 10.1186/1471-2407-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31:482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]