Abstract
OBJECTIVES:
To decrease the average length of stay (LOS) of opioid-exposed newborns (OENs) by 20% from baseline from April 2017 to December 2019.
METHODS:
The Colorado Hospitals Substance Exposed Newborn Quality Improvement Collaborative is a consortium of neonatal providers, public health experts, and legislative experts that provides infrastructure and resources for Colorado birthing hospitals to undertake initiatives focused on improving the care of OENs. The Colorado Hospitals Substance Exposed Newborn Quality Improvement Collaborative was started in September 2017 and includes 19 birthing hospitals in Colorado, with 12 contributing data to the centralized database. The interventions were focused on (1) hospital engagement and (2) increasing nonpharmacologic care (by using the Eat, Sleep, Console assessment tool; developing guidelines for breastfeeding eligibility; employing comfort measures before pharmacologic therapy; and administering opiate therapy on an as-needed basis).
RESULTS:
From April 2017 to December 2019, 787 OENs were identified. Among infants ≥35 weeks’ gestational age without other medical diagnoses (n = 647), statistical process control charts revealed significant reduction in the primary outcome of interest, average hospital LOS, from 14.8 to 5.9 days. For all OENs, receipt of pharmacologic therapy declined from 61% to 23%. Among OENs who received pharmacologic therapy (and were ≥35 weeks’ gestational age without other medical diagnoses), average LOS also declined from 21.9 to 8.0 days.
CONCLUSIONS:
Through standardization of OEN care focused on family engagement and nonpharmacologic care, this statewide collaborative reduced average LOS, the percentage of OENs requiring opiate therapy, and average LOS for OENs requiring opiate therapy.
One in 5 pregnant women insured by Medicaid were prescribed and filled an opioid prescription in the United States between 2002 and 2007.1 The incidence of opioid use disorders among women of reproductive age has increased significantly.2,3 Subsequently, the population of opioid-exposed newborns (OENs) has increased from 1.20 to 3.39 per 1000 hospital births per year nationally.4,5 In Colorado, although the incidence of neonatal abstinence syndrome (NAS), at 2.9 per 1000 live births in 2013, is less than that in other parts of the United States,6 opioid overdose is the leading cause of maternal mortality.7 In our state, we recognized that the care of OENs and their families varied across and within birthing hospitals. With little data collected and no platform for sharing clinical practices, it was unclear whether certain care approaches by some providers or hospitals led to better outcomes. Hospital variations in OEN care have been associated with more pharmacologic treatment, longer lengths of stay (LOSs) and higher health care costs.8 Moreover, recent publications by Grossman et al9 and Wachman et al10 provided evidence for novel approaches that could be implemented within a quality improvement (QI) framework. Given the rising rate of maternal opioid use disorders and prenatal opioid exposure, the Colorado Hospitals Substance Exposed Newborn Quality Improvement Collaborative (CHoSEN QIC) was formed in September 2017 to provide the infrastructure for sharing of best practices, data collection, analysis and reporting, and provision of QI education, all focused on improving OEN care. Although the collaborative is multipronged with efforts related to hospital-based neonatal care, prenatal family engagement, and safe transitions home, the initial phase of this effort was focused on standardizing the hospital-based care of OENs.
Our aims for this first initiative were to (1) decrease average LOS by 20% from baseline for all OENs, (2) decrease the percentage of OENs receiving opiate therapy by 20% from baseline, and (3) decrease LOS by 20% from baseline for OENs requiring opiate treatment from April 2017 to December 2019.
Methods
Clinical, Public Health, and Policy Collaboration
The CHoSEN QIC is a collaboration among the following organizations: (1) a university-based academic group of researchers and clinicians; (2)the Colorado Perinatal Care Quality Collaborative (CPCQC),11 a statewide network of hospitals, health care facilities, clinicians, and public health professional focused on improving the health of women and infants through continuous QI; and (3) Illuminate Colorado, a nonprofit organization dedicated to preventing child maltreatment and building brighter childhoods through education, advocacy, and family support, and the convening entity of the Colorado Substance Exposed Newborns Steering Committee.12 The Breakthrough Series model for collaborative improvement from the Institute for Healthcare Improvement provided the framework on which this effort was developed.13 Participating hospitals were asked to organize local improvement teams focused on care of OENs and their families. CHoSEN QIC provided education on QI methods and intervention tool kits that were developed from a review of the literature and created a data system to help teams measure their practices. Teams were supported through a series of webinars and in-person summits and received quarterly progress reports of individual hospital and collaborative-level performance.
Within each hospital, improvement teams recruited multidisciplinary stakeholders, including nurses, physicians, social workers, therapists, pharmacists, and case managers. At the state level, key partners included the Colorado Department of Public Health and Environment, the Colorado Attorney General’s Office, the Colorado Department of Human Services (which includes the State Substance Abuse Agency and the Division of Child Welfare), the Colorado Department of Health Care Policy and Financing, maternal child health advocates, and behavioral health advocates.
Key Drivers
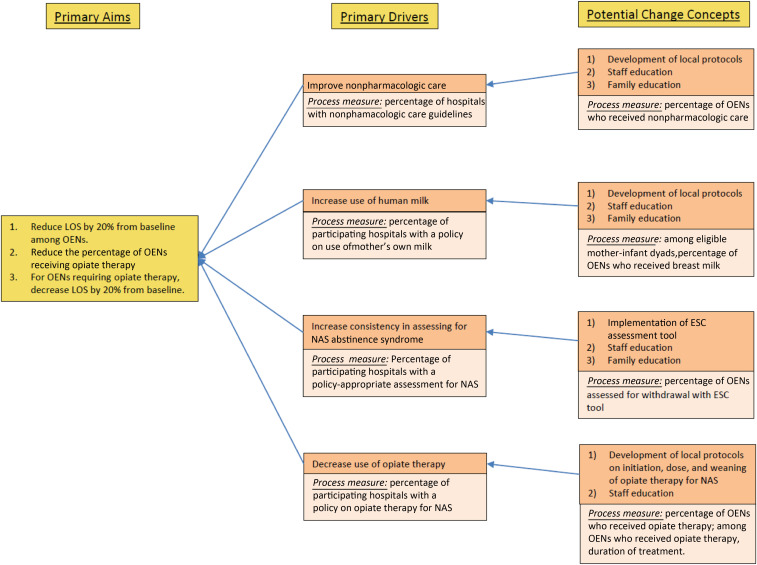
Shown in Fig 1 is our collaborative’s key driver diagram, in which the outcome and process measures of the project are outlined. It was developed through an iterative process with input from an array of stakeholders, including the CHoSEN QIC Steering Committee, clinical providers, public health and policy experts, and QI specialists.
FIGURE 1.
CHoSEN QIC key driver diagram.
On the basis of the published QI initiatives by Grossman et al9 and Wachman et al,10 the following 4 key drivers, along with associated change concepts, were developed:
1. Improve nonpharmacologic care: All participating hospital teams implemented protocols to standardize nonpharmacologic care before initiating opiate therapy, if needed. Interventions included rooming-in to promote ad lib breastfeeding (for eligible mothers), using volunteer cuddlers to provide comfort to OENs, and minimizing external stimulation, such as noise and light.
2. Increase use of human milk: Significant inter- and intrahospital variability existed in the use of the mother’s own milk for OENs. Local experts on breastfeeding and maternal substance use developed a set of guidelines with eligibility criteria for use of the mother’s own milk. Once infectious risk factors were assessed, the guideline created 3 categories of breastfeeding eligibility based on types of maternal opiate use: (a) mothers with isolated opioid use within a treatment program demonstrating adherence, (b) mothers with prescribed opioid use under the care of a physician, and (c) mothers with polysubstance use or illicit opioid use.14–16 Mothers under the care of a physician or in a drug treatment program are encouraged to breastfeed. Safe breastfeeding is prioritized, and referral to substance abuse treatment programs is recommended for mothers with polysubstance use or active illicit opioid use.
3. Increase consistency in assessing for NAS: Given the tremendous variability in NAS scoring with the Finnegan assessment tool, the collaborative replaced this tool with the Eat, Sleep, Console (ESC) approach, first published by pediatricians at Yale.9 The ESC assessment tool allows for a more standardized, objective assessment of 3 normal newborn behaviors: the ability to eat expected amounts for gestational age, sleep for at least one hour uninterrupted, and be consoled within a reasonable amount of time. On the basis of the ESC tool created by pediatric hospitalists at Yale, an electronic version was integrated into the Epic electronic medical record system. The tool has clear nursing documentation of nonpharmacologic interventions initiated or optimized. It also includes definitions of each component of newborn behavior for ease of use. One of our CHoSEN QIC Steering Committee members was designated as the ESC expert in our state, and they provided in-person and virtual educational training sessions to hospital staff on how to implement ESC, including real-life clinical cases for team members to work through and outline next steps.
4. Decrease use of opiate therapy: Although the aforementioned 3 key drivers indirectly decreased opiate use, we sought to directly decrease the initiation and cumulative administration of opiate therapy by replacing the scheduled administration approach with an as-needed, or pro re nata (PRN), approach. Participating hospitals first screened for withdrawal with the ESC tool and optimized nonpharmacologic therapies, and, if medication was indicated, morphine or methadone was given as a 1-time dose. Only if subsequent consecutive doses were required was a scheduled regimen started.
Data Collection and Analysis
Participating hospitals were asked to collect data on OENs admitted to their nursery or NICU and enter patient-level data into the central Research Electronic Data Capture database.17 Data elements included limited sociodemographic information, such as race and/or ethnicity and insurance status, and clinical data, such as infant gestational age, location of care, and prenatal exposures beyond opiates. Hospitals also collected data on whether OENs had additional medical diagnoses that required hospitalization beyond prenatal opioid exposure. For process measures, we collected data on whether nonpharmacologic and pharmacologic therapies were used as well as data on whether mothers were eligible to provide breast milk and if so, whether it occurred. Hospital teams were also asked whether the ESC assessment tool was used for their OENs.
On a quarterly basis, the collaborative’s data and QI analyst generated hospital-specific and collaborative-level data reports, including process control charts for primary outcomes and selected process measures. For each infant entered into the database, the quarter and year of birth was entered instead of the date of birth so that patients would remain deidentified, particularly for units with few OENs admitted per month. In addition, for hospitals who requested additional analyses, more specific data reports were also made available. The goal of data collection was to produce comparative data reports that provided benchmarking of performance driving ongoing local improvement. Importantly, participating teams agreed to open sharing of practices and data; at webinars and summits, comparative reports were shown transparently, with each hospital able to see the performance of the other hospitals in the collaborative.
The primary outcome measures were (1) average hospital LOS for all OENs, (2) the percentage of OENs who received opiate therapy, and (3) average hospital LOS for OENs who received opiate therapy. Statistical process control charts (X-bar and S charts) were used to analyze variation and performance over time for outcome and process measures. Standard rules were used to identify special cause variation.18 When sustained special cause variation was noted, a process change was introduced. Because this was a QI initiative across multiple institutions, with interventions happening frequently and at various times, process changes were identified only by sustained special cause variation on the control charts. We also used a folded F equality of variance test to assess homogeneity of hospital-level variation in average LOS for OENs in the previous year compared with current year. We used SAS version 9.4 (SAS Institute, Inc, Cary, NC) software to summarize the collaborative data by quarter and QI Macros 230 for Excel (KnowWare International, Inc, Denver, CO) to create the control charts. When statistical testing was performed, a P value significance was set at P < .001.
The institutional review board at the home institution for CHoSEN QIC (university-based institution) determined this project to be QI and not human subjects research; thus, it was not subject to institutional review board review.
Time Line
The project was launched in September 2017 in NICUs where the university-based neonatologists provided clinical care. Some hospitals collected retrospective data starting from April 2017. Within a few months, several additional hospitals joined the effort. By 2018, 15 hospitals were actively participating, and by 2019, 19 hospitals were implementing CHoSEN QIC tool kits and attending forums, with 12 sites entering data.
Results
From April 2017 to December 2019, 787 OENs were identified in participating hospitals. The descriptive characteristics of the cohort are listed in Table 1. A majority of mothers were white, non-Hispanic, and publicly insured. The mean gestational age of included infants was 37.3 weeks, with a mean birth weight of 2856 g. Among these 787 OENs, 654 were ≥35 weeks and did not have other medical diagnoses. Among hospitals collecting data for this initiative, the rate of prenatal opioid exposure was calculated to be 9.5 per 1000 live births. For the primary outcome measure of average LOS, statistical process control analysis by using X-bar and S charts revealed significant improvement (Fig 2A); over the course of the initiative, average LOS declined from 14.8 to 5.9 days, a reduction of 8.9 days or 60% (P < .001). For receipt of opiate therapy, at the start of the collaborative, 61% of all OENs received therapy compared with 23% in quarter 4 of 2019 (Fig 3). For OENs who required opiate therapy, their average LOS also declined significantly from 21.9 to 8.0 days (P < .001; Fig 4). Of note, variability in LOS for OENs ≥35 weeks who did not have other medical diagnoses across participating hospitals in October 2017 to September 2018 was significantly less than variability across sites in October 2018 to December 2019 (P < .001; Fig 2B).
TABLE 1.
Infant and Maternal Demographics of the CHoSEN QIC Cohort
| Results | |
|---|---|
| Infant characteristics | |
| Weeks’ gestation, mean (SD) | 37.7 (2.2) |
| Birth wt, mean (SD), g | 2856.1 (539.5) |
| Infant sex, n (%) | |
| Female | 337 (42.8) |
| Male | 450 (57.2) |
| Maternal characteristics, n (%) | |
| Maternal race | |
| White | 512 (65.0) |
| Black | 24 (3.1) |
| Asian American | 1 (0.12) |
| American Indian or Alaskan native | 28 (3.7) |
| Native Hawaiian or Pacific Islander | 5 (0.6) |
| Other | 46 (5.9) |
| Missing or unknown | 170 (21.6) |
| Maternal ethnicity | |
| Hispanic | 205 (26.0) |
| Non-Hispanic | 502 (63.8) |
| Missing or unknown | 73 (9.3) |
| Maternal insurance | |
| Public | 693 (88.1) |
| Private | 59 (7.5) |
| Combination | 4 (0.5) |
| Self-pay or none | 15 (1.9) |
| Missing or unknown | 16 (2.0) |
| Maternal primary language | |
| English | 449 (57.0) |
| Spanish | 3 (0.4) |
| Other | 3 (0.4) |
| Missing or unknown | 334 (42.4) |
| Total, N | 787 |
FIGURE 2.
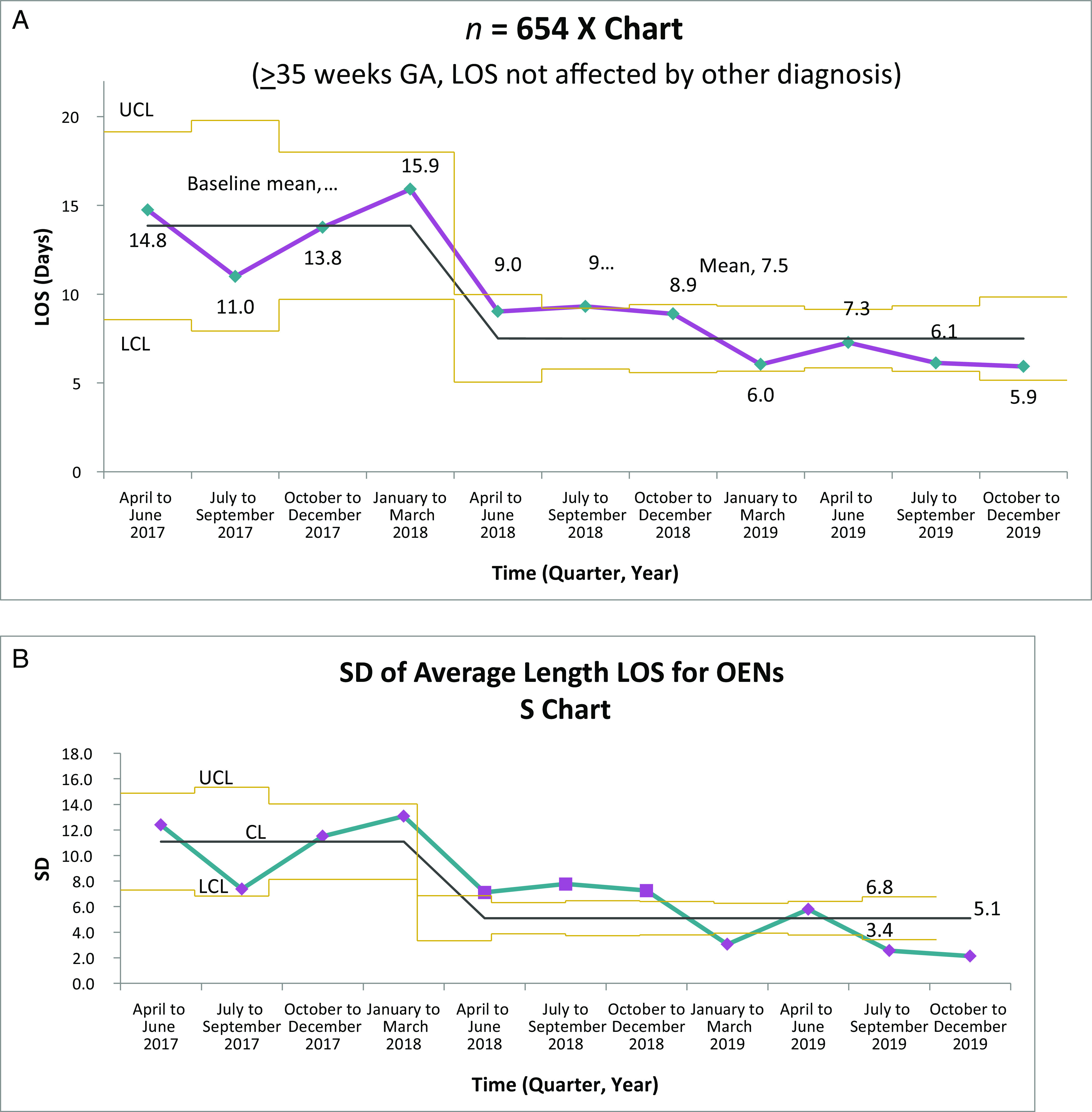
A, Average LOS for OENs. B, Variation in average LOS for OENs. CL, control limit; GA, gestational age; LCL, lower control limit; UCL, upper control limit.
FIGURE 3.
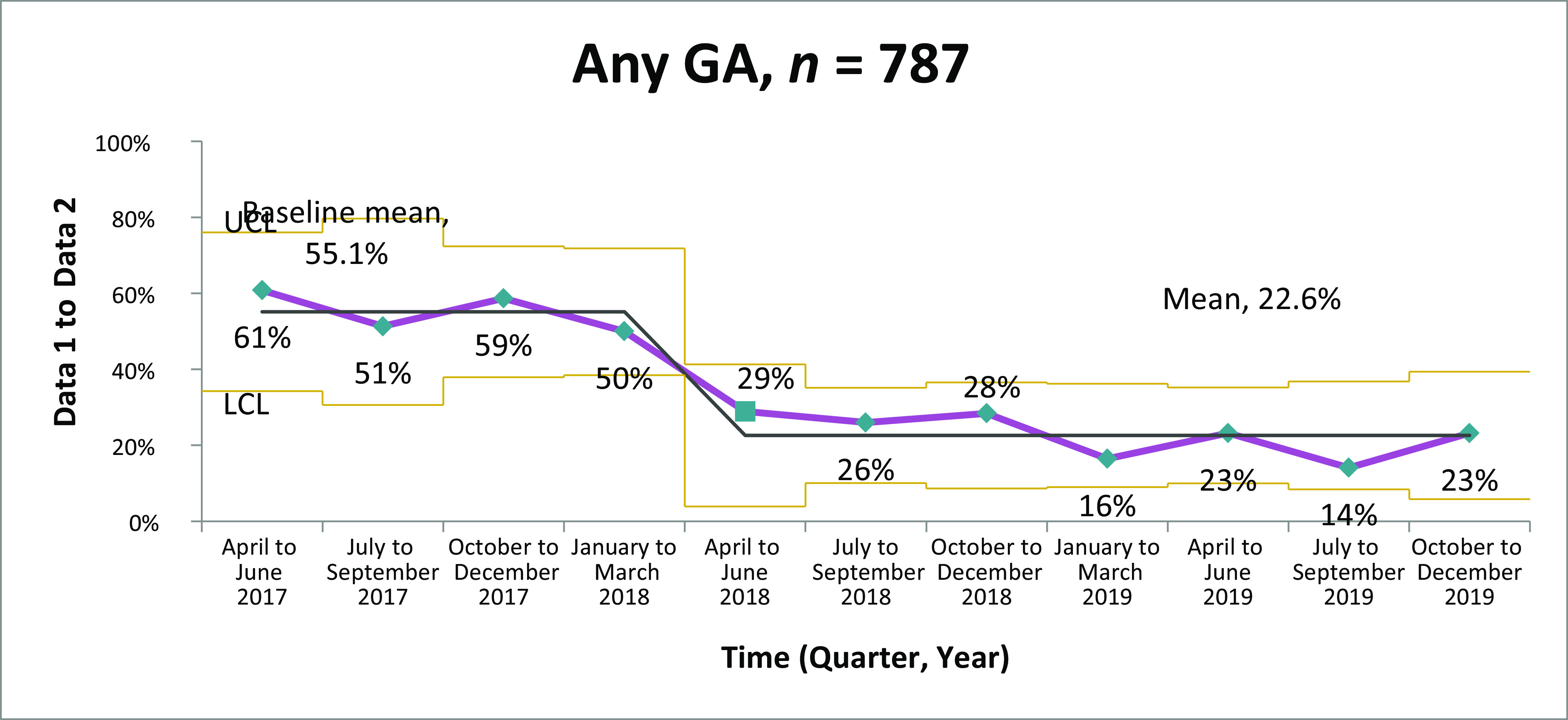
Percentage of OENs receiving pharmacology therapy. GA, gestational age; LCL, lower control limit; UCL, upper control limit.
FIGURE 4.
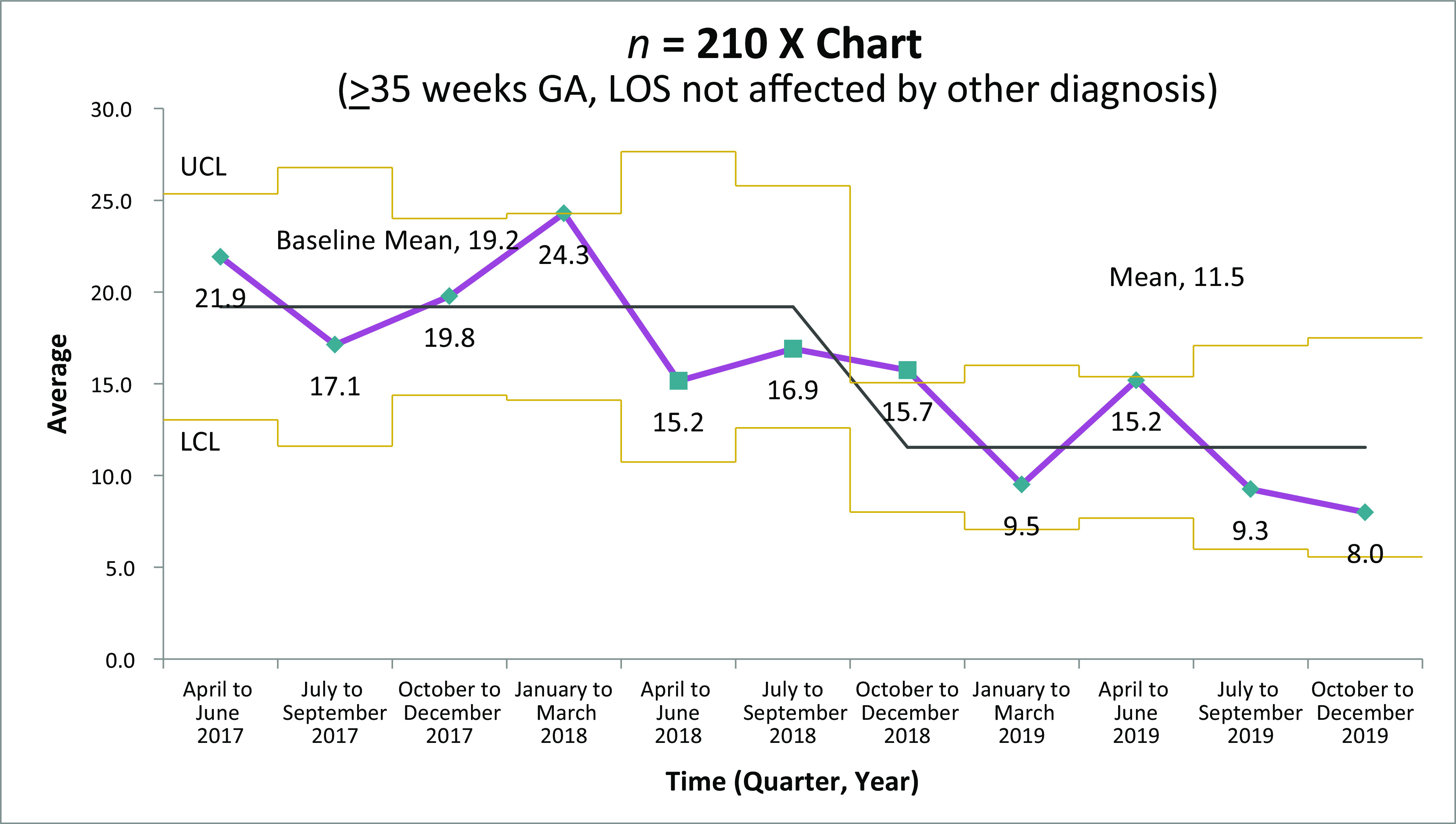
Average LOS for OEN receiving pharmacologic therapy. GA, gestational age; LCL, lower control limit; UCL, upper control limit.
Discussion
Through collaboration among academic, public health, and policy organizations, CHoSEN QIC provided the leadership and infrastructure to standardize the care of OENs across Colorado. We demonstrated significant reductions in LOS and in the percentage of OENs receiving opiate therapy. Although other recently published studies have also revealed similar outcomes, particularly after implementation of the ESC care approach, this QI effort is the first, to our knowledge, to reveal significant reductions in LOS and infant receipt of postnatal opiate therapy in a multisite statewide collaborative effort.
There were several critical elements that contributed to the success of this initiative.
ESC and Opiate Therapy
The implementation of the ESC care approach has been shown to decrease LOS and the number of OENs requiring medication treatment.9 Combining an emphasis on nonpharmacologic care and use of ESC assessments, Grossman et al9 reported a reduction in average LOS by 74% in infants exposed to methadone. Grossman et al9 also initiated the novel approach of PRN morphine dosing. The CHoSEN collaborative adopted this standardized approach, and participating hospitals used the ESC approach combined with treatment algorithms for PRN morphine dosing. Treatment protocols and medication algorithms were shared among participating hospitals.
Our results add to the current body of evidence suggesting that standardized treatment protocols, including nonpharmacologic care, and PRN dosing algorithms lead to shorter LOS and fewer OENs needing medication treatment. OENs receiving medication treatment are treated for fewer days and discharged from the hospital sooner than they were with previous scheduled treatment regimens. The ESC approach has also empowered parents to be a critical component of their infant’s care.
Parental Engagement
Parental engagement in the care of newborns with substance exposure is of critical importance and spans prenatal counseling, labor and delivery, the well-baby nursery or NICU admission, and transition to home. Increased parental presence during the birth hospitalization and initiation of breastfeeding, when appropriate, are associated with decreased LOS and need for opiate therapy, with notable differences even before implementation of ESC scoring techniques.19–21 Other described advantages of rooming-in include increased odds of discharge to maternal custody and increased initiation of breastfeeding.19,20 In this collaborative, engaging parents in the care of their infants included education surrounding the implementation of the ESC tool, which relies heavily on parental recognition and response to hunger cues and prompts infant calming when irritable. Teaching parents how to best console their newborn, increasing skin-to-skin time and, when appropriate, encouraging rooming-in were key factors. Decreasing NICU admissions and increasing nursing, social work, and other ancillary supports in the well-baby nurseries enabled for increased rates of rooming-in, allowing parents direct and constant access to their newborns.
Collaboration With Public Health Organizations
Through collaboration with the CPCQC and Illuminate Colorado, CHoSEN QIC has been afforded invaluable resources, including grant support for program management, hospital site visits, and outreach. CHoSEN QIC fits within the state’s Substance Exposed Newborns Steering Committee, a multidisciplinary entity convened by Illuminate Colorado and tasked with identifying and implementing strategies for preventing prenatal substance exposure and ensuring that impacted families are appropriately identified and supported. The Substance Exposed Newborns Steering Committee was established in 2008 and is a subcommittee of the Colorado Substance Abuse Trend and Response Task Force, which was created in a Colorado statute and is chaired by the state’s attorney general.
The CPCQC works with providers, public health professionals, families, and community-based organizations in an inclusive and transparent environment. The CPCQC promotes continuous learning and builds capacity for QI work by providing access to materials, best practices, expert support through in-person summits, virtual coaching calls, hospital site visits. The collaborative also provides access to timely and accurate statewide and national comparative data, with ongoing analysis and rapid feedback. In this way, the CPCQC provided CHoSEN QIC hospital teams with robust QI education and data analysis, allowing hospital teams to not only assess their improvements in outcomes over time but also compare themselves with other sites and the overall collaborative.
The collaboration among the University of Colorado School of Medicine Section of Neonatology, the CPCQC, and Illuminate Colorado enabled rapid expansion of CHoSEN QIC because each organization focused on their area of expertise, with leaders from each organization meeting regularly to discuss successes, obstacles, and next steps. Academic medicine team members from the Section of Neonatology developed clinical care guidelines, workflow protocols, and the centralized data system on the basis of available literature on the care of infants and mothers affected by substance use disorders. The CPCQC participated in all participating hospital site visits to provide QI education and help local team members understand their processes and procedures related to OEN care. Finally, Illuminate Colorado shared the work and outcomes of CHoSEN QIC with a broad array of clinical providers and legislative and social service agencies, leading to rapid expansion of CHoSEN QIC to underserved areas of our state, in particular, the rural communities in the western slope.
As with all hospital-based QI initiatives, sustainment over time requires continued effort. A critical part of continued engagement was growing interest from the legislative and public health communities in Colorado, which recognized the value of CHoSEN QIC and supported the effort with financial resources, such as grants, and also included CHoSEN QIC hospitals in other opioid-related work in the state. For instance, Colorado was awarded 1 of the 10 grants from the Centers for Medicare and Medicaid Services Maternal Opioid Misuse Model program in 2019, and CHoSEN QIC involvement will be essential in this program’s development and implementation across the state.
Limitations
There are several limitations to this work. First, our results are presented as process control charts with only 4 data points in the most recent period, and thus there may be concern that a stable system has not been reached. However, given that each data point includes a 3-month interval and the entire initiative duration includes >650 infants ≥35 weeks’ gestational age, we are reassured about the stability of trends in primary outcomes. Second, although nearly half of all Colorado births occurred in a CHoSEN QIC hospital, clearly not all OENs were touched by this broad effort. Anecdotally, providers from some non–CHoSEN QIC sites have shared with us that care practices have changed at these sites as well, including the adoption of the ESC tool and optimization of nonpharmacologic approaches. However, data from these other sites are lacking. Third, this effort was a hospital-based initiative, and outcomes were captured at the time of discharge. Thus, postdischarge outcomes and experiences of OENs, their families, primary care providers, and social service providers remain to be evaluated. Although we consider the reduction in LOS and percentage of OENs requiring opiate therapy a short-term success, it is unclear whether the burdens of caring for these infants have shifted to families and outpatient providers. Recent studies implementing ESC and enhancing nonpharmacologic care have revealed no increase in readmissions, but we have little data on whether other outcomes, such as parental stress, infant feeding and weight gain problems, and frequency of outpatient pediatric visits, have increased since adoption of these novel care approaches.
To address some of these deficits, CHoSEN QIC is creating additional partnerships with community and social service agencies to better understand the postdischarge experience of OENs, their families, and providers. From a population-level perspective, the Colorado Data Linkage Project was initiated in 2019 and is a state-legislated prospective data project focused on linkage of vital statistics, birth hospitalization, and postnatal hospital care records for mothers and infants affected by substance use disorders. In addition, these data will be linked to several data elements from various social service and child welfare agencies. This linked data system will allow for understanding of maternal and infant hospital readmissions, social service use, and interaction with child welfare services stratified by birth hospital. In collaboration with Illuminate Colorado, CHoSEN QIC will also conduct qualitative interviews and focus groups with mothers impacted by substance use disorders to understand the barriers and facilitators to receipt of health care and other social services encountered by mothers during the perinatal period.
Conclusions
CHoSEN QIC significantly reduced the average LOS and the percentage of OENs requiring opiate treatment of withdrawal. Critical next steps include better engagement of prenatal providers to better support mothers before and after delivery as well as working more closely with outpatient pediatric providers so that we can effectively operationalize the federal mandate to develop plans of safe care for all newborns with substance exposure.
Acknowledgments
We acknowledge the CHoSEN QIC teams who participated in this collaborative: Denver Health, Lutheran Medical Center, McKee Medical Center, Medical Center of the Rockies, Memorial Hospital, North Colorado Medical Center, North Suburban Medical Center, Parker Adventist Hospital, Parkview Medical Center, Platte Valley Medical Center, Poudre Valley Hospital, Saint Joseph Hospital. St Mary’s Medical Center, University of Colorado Hospital, and Valley View Hospital.
We also acknowledge Munish Gupta, MD, and the Massachusetts Neonatal QI Collaborative, who shared their experience, wisdom, and resources to help Colorado start CHoSEN QIC. Finally, we acknowledge Chief of Neonatology Randall Wilkening, MD, Department of Pediatrics, University of Colorado School of Medicine, who first proposed this collaborative approach to the care of OENs in Colorado and invested resources into the effort.
Footnotes
Dr Hwang conceptualized and designed the study, analyzed and interpreted data, and drafted the initial manuscript; Ms Weikel conducted the analysis, interpreted the data, and critically reviewed the manuscript; Ms Adams, Dr Bourque, Ms Cabrera, Dr Hall, Ms Scott, Dr Smith, Ms Wheeler, Ms Woodard, and Dr Wymore conceptualized and designed the study, analyzed and interpreted data, and critically reviewed the manuscript; Ms Griffith conducted the analysis, interpreted the data, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the University of Colorado School of Medicine Upper Payment Limit Program, by custodial funds from the Colorado Attorney General’s Office, by the COPIC Medical Foundation, by the Caring for Colorado Foundation, and by National Institutes of Health National Center for Research Resources Colorado Clinical and Translational Science Institute grant UL1 RR025780. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hand DJ, Short VL, Abatemarco DJ. Substance use, treatment, and demographic characteristics of pregnant women entering treatment for opioid use disorder differ by United States census region. J Subst Abuse Treat. 2017;76:58–63 [DOI] [PubMed] [Google Scholar]
- 3.Kozhimannil KB, Graves AJ, Jarlenski M, Kennedy-Hendricks A, Gollust S, Barry CL. Non-medical opioid use and sources of opioids among pregnant and non-pregnant reproductive-aged women. Drug Alcohol Depend. 2017;174:201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012 [published correction appears in J Perinatol. 2015;35(8):667]. J Perinatol. 2015;35(8):650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934–1940 [DOI] [PubMed] [Google Scholar]
- 6.Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD. Incidence of neonatal abstinence syndrome - 28 states, 1999-2013. MMWR Morb Mortal Wkly Rep. 2016;65(31):799–802 [DOI] [PubMed] [Google Scholar]
- 7.Metz TD, Rovner P, Hoffman MC, Allshouse AA, Beckwith KM, Binswanger IA. Maternal deaths from suicide and overdose in Colorado, 2004-2012. Obstet Gynecol. 2016;128(6):1233–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milliren CE, Gupta M, Graham DA, Melvin P, Jorina M, Ozonoff A. Hospital variation in neonatal abstinence syndrome incidence, treatment modalities, resource use, and costs across pediatric hospitals in the United States, 2013 to 2016. Hosp Pediatr. 2018;8(1):15–20 [DOI] [PubMed] [Google Scholar]
- 9.Grossman MR, Berkwitt AK, Osborn RR, et al. An initiative to improve the quality of care of infants with neonatal abstinence syndrome. Pediatrics. 2017;139(6):e20163360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wachman EM, Grossman M, Schiff DM, et al. Quality improvement initiative to improve inpatient outcomes for neonatal abstinence syndrome. J Perinatol. 2018;38(8):1114–1122 [DOI] [PubMed] [Google Scholar]
- 11.Colorado Perinatal Care Quality Collaborative. Our Approach. Available at: https://cpcqc.org/our-approach/. Accessed October 1, 2019
- 12.Illuminate Colorado. About Us. Available at: https://www.illuminatecolorado.org/aboutus. Accessed October 1, 2019
- 13.Institute for Healthcare Improvement. The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement. Boston, MA: Institute for Healthcare Improvement; 2003 [Google Scholar]
- 14.Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3). Available at: www.pediatrics.org/cgi/content/full/129/3/e827 [DOI] [PubMed] [Google Scholar]
- 16.Reddy UM, Davis JM, Ren Z, Greene MF; Opioid Use in Pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes Workshop Invited Speakers. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet Gynecol. 2017;130(1):10–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Provost LP, Murray SK. The Health Care Data Guide: Learning From Data for Improvement. 1st ed. San Francisco, CA: Jossey-Bass; 2011 [Google Scholar]
- 19.Howard MB, Schiff DM, Penwill N, et al. Impact of parental presence at infants’ bedside on neonatal abstinence syndrome. Hosp Pediatr. 2017;7(2):63–69 [DOI] [PubMed] [Google Scholar]
- 20.Abrahams RR, Kelly SA, Payne S, Thiessen PN, Mackintosh J, Janssen PA. Rooming-in compared with standard care for newborns of mothers using methadone or heroin. Can Fam Physician. 2007;53(10):1722–1730 [PMC free article] [PubMed] [Google Scholar]
- 21.Newman A, Davies GA, Dow K, et al. Rooming-in care for infants of opioid-dependent mothers: implementation and evaluation at a tertiary care hospital. Can Fam Physician. 2015;61(12):e555–e561 [PMC free article] [PubMed] [Google Scholar]