Abstract
Background:
Targeted therapies offer novel opportunities to explore biomarkers based on their mode of action. Taking this into consideration, we evaluated six angiogenesis-related proteins as potential predictive biomarkers, which expression might predict the benefit of bevacizumab treatment in patients with metastatic colorectal cancer (mCRC).
Methods:
This was a phase II multicenter, two-armed, randomized study, in which patients with mCRC were treated with XELIRI (capecitabine and irinotecan) plus bevacizumab followed by XELOX (capecitabine and oxaliplatin) plus bevacizumab (Arm A) or the reverse sequence (Arm B). Tissue expression level of six prespecified candidates [microvessel density assessed by CD31, PTEN, αV integrin, CD98hc, uPAR and NRP-1] was analyzed via immunohistochemistry. The prognostic impact on survival was quantified using the Cox regression model. The predictive potential for benefit from Arm A versus Arm B treatment was investigated by fitting an interaction between the biomarkers and treatment assignment within a multivariable Cox model.
Results:
In total, 74 out of 126 patients were included in the analysis. The expression of PTEN, αV integrin, uPAR and NRP-1 was not associated with progression-free survival (PFS) or overall survival (OS). For the first time, we identified that patients with tumors expressing CD98hc had a longer PFS than patients without CD98hc-expression (p = 0.032). More importantly, and in accordance with previous studies, low microvessel density was found to be associated with a reduced PFS [adjusted HR per doubling of CD31-expression (p = 0.53, 95% confidence interval: 0.30–0.95, p = 0.034)].
Conclusions:
These results can contribute to the development of a personalized strategy for the treatment of mCRC with bevacizumab.
Keywords: angiogenesis, bevacizumab, biomarker, CD31, colorectal cancer
Introduction
Despite being the third most common cancer type and the second leading cause of cancer related deaths worldwide, colorectal cancer (CRC) might be one of the best examples of scientific progress in terms of improvement on cancer patient’s outcome.1 Incidence and death rates for CRC are decreasing at least in the USA and the European Union, partly because of improved public awareness of smoking associated risks, early cancer detection, and new cancer treatments.2,3 These developments are also reflected in an improved clinical outcome in patients with metastatic CRC (mCRC), whose overall survival (OS) has increased from an initial median OS of 12 months three decades ago to a median OS of around 30 months at present, especially due to the introduction of targeted therapies such as bevacizumab [an anti-vascular endothelial growth factor (VEGF) monoclonal antibody] and the establishment of tumor molecular profiling as the new standard-of-care for this disease.4,5
Back in 2004, bevacizumab was the first antiangiogenic compound approved by the US Food and Drug Administration (FDA) as an anticancer treatment and its approval was granted based on the results of a phase III clinical trial which showed that this monoclonal antibody prolonged median OS by about 5 months when given in combination with a standard backbone chemotherapy in patients with untreated mCRC.6 Preclinical models have evinced that this compound is able to bind to extracellular VEGF-A, the major pro-angiogenic growth factor; thus, leading to tumor vessel growth inhibition by hindering the interaction between VEGF-A and its receptor.7 Since its approval in 2004, bevacizumab has been approved for the treatment of several other types of cancers in combination with different cytotoxic chemotherapeutic therapies.8 However, despite these medical advances, there are primordial issues that still have to be elucidated in order to further improve the outcome of mCRC, like the identification of a preferred backbone of targeted therapies, optimized therapy sequences and biomarkers for targeted-treatments.
In this context, several clinical trials aiming to address these issues have been conducted.9–12 Due to the proposed mode of action of bevacizumab, angiogenesis-related molecules may be useful as predictors of response to bevacizumab. Thus, in the present study, our objective was to analyze if the tumor expression of six prespecified angiogenic-related proteins could predict the efficacy of bevacizumab in a phase II prospective clinical trial that aimed to determine the efficacy of modified XELIRI (capecitabine and irinotecan) plus bevacizumab followed by XELOX (capecitabine and oxaliplatin) plus bevacizumab at progression in comparison with the reverse sequence, based on the duration of disease control in patients with mCRC. The targets analyzed by immunohistochemistry (IHC) were: vascular density based on the expression of CD31 and expression of neuropilin-1 (NRP1), urokinase receptor (uPAR), αV integrin, CD98 heavy chain (CD98hc), and phosphatase and tensin homolog (PTEN). These biomarker candidates were selected on the proposed mode of action of bevacizumab and on previously published research works. With the exception of αV integrin, we hypothesized that the higher the expression of the candidate biomarker, the greater a patient could benefit from the treatment with bevacizumab.
Methods
Characteristics of study population
The PASSION study was a phase II multicenter, open label, two-armed, randomized pilot study (ML25153, EUDRACT Number: 2011-002191-16, Supplemental List 1), in which the primary endpoint was to assess the efficacy and safety of XELIRI plus bevacizumab followed by XELOX plus bevacizumab or the reverse sequence in patients with mCRC. A total of 126 patients were randomized into one of two treatment groups: Arm A (XELIRI plus bevacizumab followed by XELOX plus bevacizumab) or Arm B (XELOX plus bevacizumab followed by XELIRI plus bevacizumab). The length of the study was 64 months. All patients were older than 18 years and did not receive any prior systemic treatment for mCRC. Patients had to provide an expressed informed consent to be included in the study. The informed consent for the translational study included the immunostaining of the tumor tissue presented herein. The protocols of the clinical and translational study were approved by the ethics committees of each treating institution (Supplemental List 1) and were carried out in accordance with the Declaration of Helsinki. The present study was performed according to the REMARK guidelines. Patients had to sign both informed consents to participate in the present study. Treatment details and primary results regarding OS and PFS have not been published yet.
Immunohistochemistry
Immunostaining was performed according to standard protocols on paraffin-embedded tissue as we have described before.13 Tumor tissue was immunostained using antibodies against PTEN, αV integrin, CD98hc, uPAR, and NRP-1. Further details on the antibodies used and method are described in Supplemental Methods. Staining was scored by adding the distribution score (0 = no staining; 1+ = staining of <33% of cells; 2+ = between 33% and 66% of cells; and 3+ = staining of >66% of cells) to the intensity score (0 = no staining; 1+ = weak; 2+ = moderate; 3 = strong). The average number of microvessels was assessed in five higher-power fields (HPF) per sections stained for CD31. All immunostainings were evaluated by an expert pathologist who was blind to the clinical and treatment data. Representative stained samples were scanned using the Pannoramic 250 Scanner (3DHISTECH) at 20× objective magnification.
Statistics
All statistical analyses were performed with Stata 15.0 (Stata Corp., Houston, TX, USA) by FP. Continuous variables were reported as medians (25th–75th percentile), whereas count data were summarized as absolute frequencies (%). Missing data were reported as absolute counts (%), and a complete case analysis was performed. The distribution of baseline covariables between the two treatment arms was compared with χ2-tests, Fisher’s exact tests, and rank-sum tests, respectively. Co-primary endpoints were first-line PFS and OS. PFS and OS were defined as the time from randomization to (a) the date of disease progression, death-from-any-cause, or censoring alive, whatever came first, and (b) the date of death-from-any-cause or censoring alive, whatever came first, respectively. PFS and OS functions were estimated with Kaplan–Meier estimators and compared between groups using log-rank tests. The prognostic impact of IHC biomarker variables on PFS and OS was quantified with univariable and multivariable Cox regression. Treatment assignment to Arm A versus Arm B was pre-specified as a fixed covariable in all multivariable analyses. Moreover, we selected each non-biomarker covariable with a p-value for association with the outcome of <0.10 for multivariable analysis (Table 2, relaxed threshold due to low sample size). Multivariable analyses were only performed in case a biomarker was associated with the outcome in the univariable setting. Thus, multivariable Cox modeling included (a) the biomarker under study, (b) treatment assignment to Arm A versus Arm B, and (c) all covariables with a p of association with the outcome <0.10. To gauge whether biomarker expression may modulate the benefit from a certain treatment sequence, we also fitted interactions between the biomarkers and treatment assignment within these multivariable Cox models (thus additionally including an interaction term between biomarker expression and treatment assignment to Arm A versus Arm B). One patient had a PFS event at the day of study inclusion and was thus not assessable for the PFS analysis.
Table 2.
Predictors of first-line PFS and OS in the study cohort. Univariable Cox models.
| Endpoint |
PFS |
OS |
||||
|---|---|---|---|---|---|---|
| Variable | Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p |
| Demographic variables | ||||||
| Age (per 5 years increase) | 0.95 | 0.83–1.09 | 0.496 | 1.02 | 0.88–1.17 | 0.826 |
| Female sex | 1.05 | 0.59–1.86 | 0.876 | 1.33 | 0.72–2.44 | 0.360 |
| ECOG ⩾1 point | 0.85 | 0.46–1.57 | 0.595 | 1.77 | 0.96–3.27 | 0.070 |
| Caucasian ethnicity | N/A | N/A | N/A | N/A | N/A | N/A |
| Cancer variables | ||||||
| Stage IV at time of tumor diagnosis | 1.96 | 0.90–4.26 | 0.089 | 1.82 | 0.78–4.29 | 0.168 |
| Liver metastasis | 1.16 | 0.52–2.57 | 0.720 | 1.42 | 0.60–3.33 | 0.421 |
| Lung metastasis | 1.44 | 0.86–2.41 | 0.164 | 0.98 | 0.56–1.72 | 0.947 |
| KRAS mutation | 0.87 | 0.51–1.50 | 0.628 | 1.34 | 0.75–2.41 | 0.326 |
| IHC variables | ||||||
| CD31 (microvessels/HPF, per doubling) | 0.52 | 0.29–0.92 | 0.024 | 1.21 | 0.70–2.10 | 0.491 |
| PTEN IHC positive | N/A | N/A | N/A | N/A | N/A | N/A |
| Any uPAR expression | 0.71 | 0.42–1.20 | 0.204 | 0.76 | 0.43–1.34 | 0.339 |
| NRP-1 expression (per 1-point increase) | 0.99 | 0.86–1.14 | 0.869 | 1.07 | 0.92–1.25 | 0.380 |
| αV-integrin expression (per 1-point increase) | 0.97 | 0.85–1.12 | 0.697 | 1.01 | 0.86–1.18 | 0.949 |
| CD98he positivity | 0.55 | 0.31–0.96 | 0.035 | 0.68 | 0.37–1.22 | 0.192 |
| Treatment variables | ||||||
| Randomization to Arm B | 0.66 | 0.40–1.09 | 0.102 | 1.29 | 0.75–2.22 | 0.360 |
ECOG, ; HPF, higher-power fields; IHC, ; OS, overall survival; PFS, progression-free survival; PTEN, phosphatase and tensin homolog; uPAR, urokinase receptor.
Results
Cohort characteristics
A total of 126 patients were enrolled in the clinical study and underwent random assignment to a treatment group. Twenty of these patients underwent surgery in external hospitals and therefore tumor tissue was not available for the proposed analysis. In six cases, there was not enough tumor tissue material to perform the immunostainings. A total of 26 patients did not sign the additional informed consent for the present study. Thus, 74 (59%) out of these 126 patients were included in the current translational study of whom n = 36 patients (49%) and n = 38 patients (51%) were randomized to treatment arms A and B, respectively. Except for the presence of lung and liver metastases, baseline characteristics were well balanced between the two treatment groups (Table 1). During a median follow-up of 37.2 months, we observed 64 PFS events during first-line therapy, and 53 patients eventually died. This corresponded to median first-line PFS and OS estimates of 7.8 months [95% confidence interval (CI): 6.96–11.28, Supplemental Figure S1] and 19.44 months (16.08–29.88, Supplemental Figure S2), respectively. Univariable predictors of PFS and OS are reported in Table 2. Both PFS and OS did not differ by treatment assignment (Supplemental Figure S3).
Table 1.
Baseline characteristics of the study cohort (n = 74).
| Variable | n (% miss.) | Overall (n = 74) | Arm A (n = 36) | Arm B (n = 38) | p |
|---|---|---|---|---|---|
| Demographic variables | |||||
| Age (years) | 74 (0%) | 66 (58–72) | 66 (58–71) | 66 (56–73) | 0.871 |
| Female sex | 74 (0%) | 20 (27%) | 9 (25%) | 11 (29%) | 0.702 |
| ECOG ⩾1 point | 73 (1%) | 17 (23%) | 9 (25%) | 8 (22%) | 0.733 |
| Caucasian ethnicity | 74 (0%) | 74 (100%) | 36 (100%) | 38 (100%) | 0.999 |
| Cancer variables | |||||
| Stage IV at time of tumor diagnosis | 74 (0%) | 61 (82%) | 30 (83%) | 31 (82%) | 0.843 |
| Liver metastasis | 74 (0%) | 66 (89%) | 29 (81%) | 37 (97%) | 0.020 |
| Lung metastasis | 70 (5%) | 32 (46%) | 21 (62%) | 11 (31%) | 0.009 |
| KRAS mutation | 66 (11%) | 32 (48%) | 15 (44%) | 17 (53%) | 0.464 |
| IHC variables | |||||
| Origin of tissue: Primary tumor | 74 (0%) | 59 (80%) | 27 (75%) | 32 (84%) | 0.325 |
| CD31 (microvessels/HPF) | 73 (1%) | 11.2 (9.0–13.0) | 11.2 (9.2–13.0) | 11.1 (9.0–13.4) | 0.925 |
| PTEN IHC positive | 72 (3%) | 2 (3%) | 2 (6%) | 0 (0%) | 0.493 |
| uPAR IHC positive | 71 (4%) | 30 (42%) | 18 (50%) | 12 (34%) | 0.180 |
| NRP-1 expression | 72 (3%) | 2 (0–4) | 2 (0–4) | 2 (0–4) | 0.866 |
| αV-integrin expression | 67 (9%) | 4 (2–5) | 4 (2–5) | 4 (2–4) | 0.832 |
| CD98he IHC positive | 70 (5%) | 28 (40%) | 15 (44%) | 13 (36%) | 0.494 |
ECOG, Eastern Cooperative Oncology Group Performance Status; HPF, higher-power fields; IHC, immunohistochemistry; PTEN, phosphatase and tensin homolog; uPAR, urokinase receptor.
High CD31 expression predicts a better PFS, independently of the sequence treatment
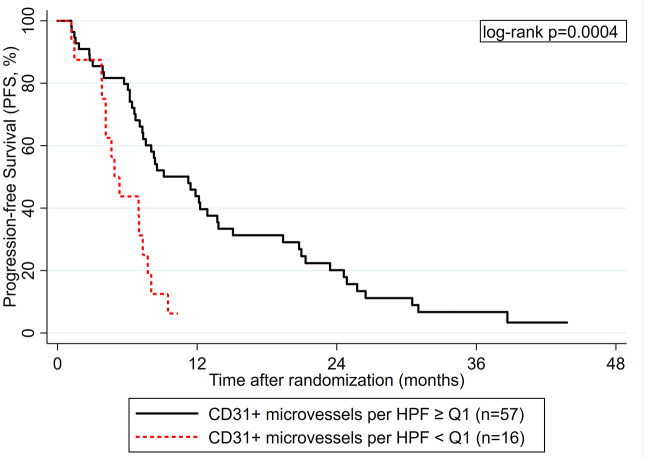
A total of 73 tissue samples were available for examination. CD31 immuno-reactive vascular structures were found in all tumor samples with a median of 11.2 microvessels per HPF (range: 1.8–21.4). Representative immunostainings are shown in Figure 1. A higher number of CD31+ microvessels per HPF was significantly associated with a better PFS experience [Hazard ratio (HR) per doubling of CD31+ microvessels/HPF = 0.52, 95% CI: 0.29–0.92, p = 0.024]. In detail, median PFS estimates were 11.28 and 4.92 months in patients with CD31+ microvessels per HPF ⩾ and < the 25th percentile of this variable’s distribution, respectively (HR = 3.07, 1.60–5.87, p = 0.001; log-rank p = 0.0004, Figure 2). The association between low CD31+ expression and worse PFS prevailed in multivariable analysis adjusting for stage IV at initial diagnosis and treatment assignment (adjusted HR per doubling of CD31 expression = 0.53, 95% CI: 0.30–0.95, p = 0.034). However, low CD31 expression did not emerge as a predictive biomarker for benefit from a certain treatment sequence (Interaction p-value between CD31 expression and treatment arms A and B = 0.814), confirming it as a prognostic but not predictive biomarker (Supplemental Figure S4). Moreover, low CD31 expression appeared to be associated with worse OS (log-rank p = 0.038, Supplemental Figure S5), but this was not the case when considering CD31 expression as a continuous variable (Table 2).
Figure 1.
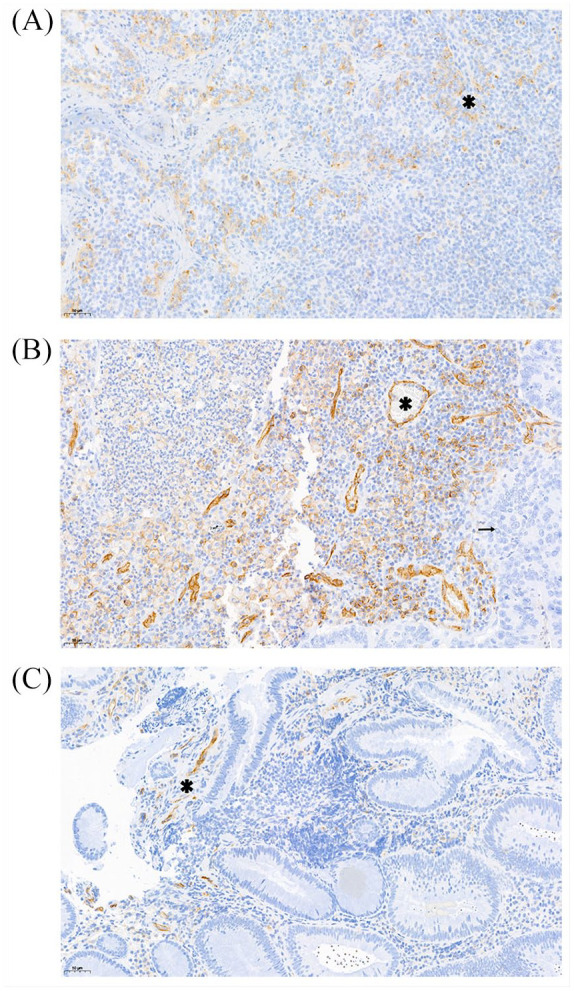
Representative immunostaining of CD31. (a) CD31 positive vascular proliferates (asterisk) in palatine tonsil tissue (positive control). (b) High number (>5/HPF) of CD31 positive vascular proliferates (asterisk) between tumor cells of primary colonic cancer (arrow). (c) Low number (<5/HPF) of CD31 positive vascular proliferates (asterisk) between tumor cells of primary colonic cancer.
HPF, higher-power field.
Figure 2.
Progression-free survival (PFS) experience according to CD31+ microvessel density.
HPF, higher-power field.
uPAR, αV integrin, NRP1, and PTEN expression did not predict survival outcome
Protein expressions of uPAR, NRP1, αV integrin, and PTEN were examined by immunohistochemistry in all available samples (Table 1). Representative micrographs of the immunostainings are shown in Supplemental Figure S6. Cytoplasmic uPAR expression was observed in tumor cells in 30 samples. Of note, immunopositivity for uPAR was also detected in goblet cells surrounding the tumor in 13 samples (Supplemental Figure S6). This was considered as an unspecific staining. Membrane and cytoplasm staining of αV integrin and cytoplasm expression of NRP1 were detected in tumor cells and in some cases, immunostaining was also observed in stroma cells (Supplemental Figure S6). PTEN expression in tumor cells was only observed in two cases, whereas 18 cases showed stromal expression of PTEN (Supplemental Figure S6). We did not observe any evidence for an association between uPAR, NRP1, and αV integrin expression and any of the survival variables under study (Table 2).
Patients with CD98hc positive tumor cells exhibited a better PFS, independently of treatment arm
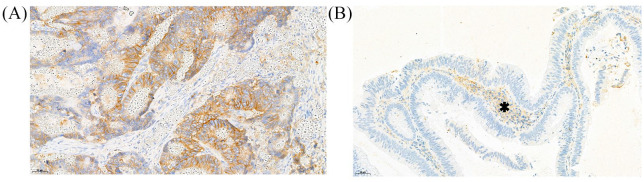
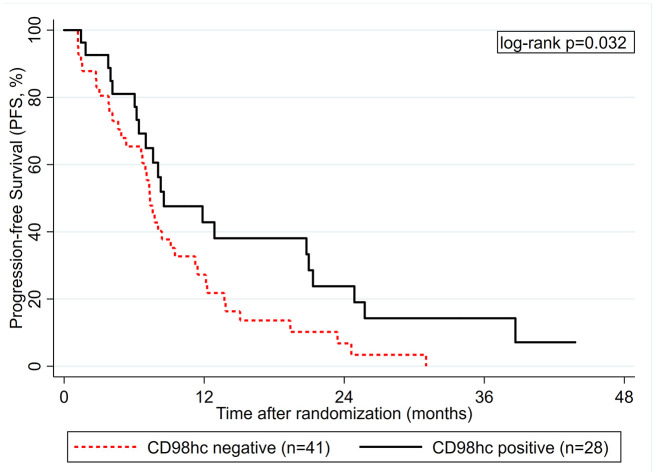
CD98hc expression was positive in 28 cases, with positivity defined as any CD98hc expression (Figure 3a). In some cases, CD98hc was observed in stroma cells (Figure 3b) but this was not considered as positivity. Patients with CD98hc+ tumors experienced a more favorable PFS experience than patients with CD98hc- tumors, respectively (log-rank p = 0.032, Table 2, Figure 4). This association prevailed in multivariable analysis adjusting for stage IV at initial diagnosis (adjusted HR for CD98hc positivity = 0.49, 0.27–0.91, p = 0.023). Moreover, CD98hc positivity was associated with numerically better OS (Supplemental Figure S7), although this did not reach statistical significance with the number of patients we had (Table 2). Although the beneficial association between CD98hc positivity and a more favorable PFS experienced appeared to be stronger in treatment arm A, CD98hc positivity did not statistically significantly emerge as a predictive biomarker for treatment sequence with the numbers of patients and events we had (p-value for interaction between CD98hc status and treat-ment assignment within a PFS analysis = 0.363, Supplemental Figure S8).
Figure 3.
(a) Moderate to high expression of CD98hc in primary colonic cancer. (b) Expression of CD98hc in in tumor surrounding stromal cells (asterisk). Tumor cells showed no expression of CD98hc.
Figure 4.
Progression-free survival (PFS) experience according to CD98hc status (n = 69).
Discussion
The proposed mode of action of bevacizumab has opened a myriad of possibilities to investigate putative biomarkers that could predict response to this anti-angiogenic monoclonal antibody (reviewed in14). Several studies with focuses on different types of candidates have been conducted, such as circulating and tissue proteins, tissue mRNAs, or genetic variants. However, none of these candidates have reached clinical use yet. In the present study, we aimed to assess predictive tissue markers in patients treated with XELIRI and XELOX in combination with bevacizumab. The six candidates for this prospective study were pre-selected based on previous research. NRP1 is a transmembrane protein expressed by endothelial and tumor cells which promotes angiogenesis by interacting with VEGF.15 It was shown that NRP1 expression could predict the responsiveness of treatments in different types of cancers, including treatment with bevacizumab.16 For instance, the AVAGAST trial has shown that there was an inverse correlation between NRP1 protein expression and OS benefit upon bevacizumab treatment in gastric cancer.9 Up to now, the correlation between NRP1 expression level and treatment benefit in patients with mCRC was only assessed in the BOND-2 study by Saltz et al. and in the present study by our group. The BOND-2 study has shown via RT-PCR that NRP1-mRNA expression level in tumor tissue was associated with a longer OS for the treatment of cetuximab plus bevacizumab with or without irinotecan.17 The discrepancy between both studies might be grounded in the fact that one study has focused on mRNA, whereas our study has focused on the protein level. This discordance between protein and mRNA level is widely accepted and has been reported before.13 Furthermore, the findings of the BOND-2 study were based on patients with CRC who received a different chemotherapy setting than our patients. Therefore, this could also explain the discrepancies in the correlation between NRP1 and treatment outcome.
αV integrins are expressed by different types of cells, including tumor-associated endothelial cells and neoplastic cells, and are involved in different pathophysiological processes, such as tumor angiogenesis.18 Studies have shown that some integrins might be involved in bevacizumab resistance and therefore, we hypothesized that high tumor expression levels of αV integrin might predict a poor clinical benefit of bevacizumab treatment, which has not been previously evaluated.19 However, our study has demonstrated that αV integrin expression was not associated with PFS or OS in patients with mCRC. We could not confirm our hypothesis in the case of uPAR tissue expression either. The urokinase-type plasminogen activation (uPA)/uPAR system is involved in physiological and tumor angiogenesis, tumor cell migration, and invasion.20,21 In CRC, uPAR tissue expression was shown to inversely correlate with OS and soluble levels of uPAR were shown to inversely correlate with bevacizumab-based first-line treatment response.22,23 However, in the present study, uPAR tissue expression did not correlate with any patient’s outcome.
PTEN is a tumor suppressor gene which is involved in angiogenesis and its loss of expression in neoplastic cells has already been reported in CRC.24 Furthermore, it has been suggested that expression of PTEN might be associated with a beneficial response to cetuximab.25 In our study, only two patients retained PTEN expression and therefore, due to the small sample size, we could not assess any association between PTEN expression and PFS or OS.
CD98hc is a surface protein which is involved in crucial pathological and physiological processes, such as modulation of integrin signaling and adaptive immunity, amino acid transport, angiogenesis, and tumor growth.26–28 Previously, we have shown that CD98hc expression was expressed in renal cancer and that its expression correlated with the grade of malignancy.29 Moreover, we have observed that patients with no expression of CD98hc in tumor tissue or stroma cells surrounding pancreatic tumors exhibited a longer OS in comparison with patients with CD98hc immunopositivity (data unpublished). Our findings were confirmed by Ying et al., who showed that CD98hc was upregulated in up to ten different types of cancers, including CRC.30 In line with our previous findings, this group has demonstrated that CD98hc expression predicted a poor outcome in patients with resectable CRC.30 Although our present study has not confirmed these findings, there is no inconsistency between the results presented herein and those mentioned above. Here, we have demonstrated that expression of CD98hc predicted a better PFS in mCRC when patients were treated with bevacizumab, independently of the treatment arm. As initially hypothesized, CD98hc expression might predict a clinical benefit of a bevacizumab treatment.
Different studies have shown that the assessment of microvessel density measured by immunostaining might predict OS or disease recurrence in primary CRC.31–33 A meta-analysis reported that high microvessel density assessed by CD31, CD34 and/or factor VIII predicted poor disease recurrence and OS.34,35 The present study revealed that high microvessel density, assessed by CD31 staining, predicted a longer PFS for the treatment of XELOX or XELIRI plus bevacizumab. These findings are in line with a retrospective study performed by Bais et al., which included 980 patients with ovarian cancer treated with chemotherapy plus bevacizumab.36 In mCRC, Jubb et al. have shown that microvessel density measured by CD34 staining was not associated with clinical benefit upon bevacizumab treatment.37 No correlation was found either by Zygoń et al. when microvessel density was measured using CD34 antibodies.38 Thus, the discrepancy might lie in the selected protein for microvessel density assessment. Of note, this year, the FDA approved some bevacizumab biosimilars for the treatment of five cancer types and some are being studied for the treatment of mCRC.39,40 As the name implies, biosimilars are highly similar (but not identical) to the approved reference products and, notwithstanding of some minor differences, they do not have clinically meaningful differences from the originator molecules in terms of safety and effectiveness.41 However, some structural differences, such as glycosylation patterns, do exist and this can have an impact in several properties as reviewed here.42 Therefore, these and previous findings should also be re-evaluated when patients are treated with these biosimilars.
In conclusion, our analysis has shown that microvessel density measured by CD31 staining predicted a longer PFS in patients with mCRC treated with XELIRI or XELOX plus bevacizumab. As previously described, this finding is in line with previous reports and might pave the way for a tailored treatment for patients with mCRC. In addition, we have demonstrated for the first time that CD98hc expression in tumor tissue might predict a better PFS in patients with mCRC treated with the evaluated chemotherapy strategy. Additional studies are urgently needed to confirm our findings and ensure that they can be translated into the clinical use as rapidly as possible.
Supplemental Material
Supplemental material, PassionATE_Supplementary_Figures_rev1 for Microvascular density assessed by CD31 predicts clinical benefit upon bevacizumab treatment in metastatic colorectal cancer: results of the PassionATE study, a translational prospective Phase II study of capecitabine and irinotecan plus bevacizumab followed by capecitabine and oxaliplatin plus bevacizumab or the reverse sequence in patients in mCRC by Daniela Bianconi, Merima Herac, Florian Posch, Margit Schmeidl, Matthias Unseld, Markus Kieler, Robert Brettner, Leonhard Müllauer, Jakob Riedl, Armin Gerger, Werner Scheithauer and Gerald Prager in Therapeutic Advances in Medical Oncology
Supplemental material, PassionATE_Supplementary_Methods for Microvascular density assessed by CD31 predicts clinical benefit upon bevacizumab treatment in metastatic colorectal cancer: results of the PassionATE study, a translational prospective Phase II study of capecitabine and irinotecan plus bevacizumab followed by capecitabine and oxaliplatin plus bevacizumab or the reverse sequence in patients in mCRC by Daniela Bianconi, Merima Herac, Florian Posch, Margit Schmeidl, Matthias Unseld, Markus Kieler, Robert Brettner, Leonhard Müllauer, Jakob Riedl, Armin Gerger, Werner Scheithauer and Gerald Prager in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_List_1 for Microvascular density assessed by CD31 predicts clinical benefit upon bevacizumab treatment in metastatic colorectal cancer: results of the PassionATE study, a translational prospective Phase II study of capecitabine and irinotecan plus bevacizumab followed by capecitabine and oxaliplatin plus bevacizumab or the reverse sequence in patients in mCRC by Daniela Bianconi, Merima Herac, Florian Posch, Margit Schmeidl, Matthias Unseld, Markus Kieler, Robert Brettner, Leonhard Müllauer, Jakob Riedl, Armin Gerger, Werner Scheithauer and Gerald Prager in Therapeutic Advances in Medical Oncology
Acknowledgments
We are grateful to Adam Calija for his technical support with the digital slide scanner and to Florencia Citrino for carefully reading the manuscript.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest: MK received travel support from Merck, Bayer, Bristol-Myers Squibb, and Roche and has participated in advisory board meetings from Bayer. All other authors declare no conflict of interest.
Disclaimer: This study was funded in part by Roche. The sponsor had no role in the study design, analysis of results or manuscript writing or submission.
ORCID iDs: Daniela Bianconi  https://orcid.org/0000-0002-8102-4199
https://orcid.org/0000-0002-8102-4199
Markus Kieler  https://orcid.org/0000-0002-4082-5731
https://orcid.org/0000-0002-4082-5731
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Daniela Bianconi, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Vienna, Austria.
Merima Herac, Department of Pathology, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Florian Posch, Division of Clinical Oncology, Comprehensive Cancer Center Graz, Medical University of Graz, Graz, Austria.
Margit Schmeidl, Department of Pathology, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Matthias Unseld, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Vienna, Austria.
Markus Kieler, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Vienna, Austria.
Robert Brettner, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Vienna, Austria.
Leonhard Müllauer, Department of Pathology, Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Jakob Riedl, Division of Clinical Oncology, Comprehensive Cancer Center Graz, Medical University of Graz, Graz, Austria.
Armin Gerger, Division of Clinical Oncology, Comprehensive Cancer Center Graz, Medical University of Graz, Graz, Austria.
Werner Scheithauer, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Vienna, Austria.
Gerald Prager, Department of Medicine I, Division of Oncology, Comprehensive Cancer Center, Medical University Vienna, Waehringer Guertel 18-20, 1090, Vienna, Austria.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst 2017; 109: djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2019 with focus on breast cancer. Ann Oncol 2019; 30: 781–787. [DOI] [PubMed] [Google Scholar]
- 4. Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med 2015; 66: 83–95. [DOI] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 6. Goodman L. Persistence–luck–Avastin. J Clin Invest 2004; 113: 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Avastin® (bevacizumab). About Avastin: proposed mechanism of action. Genentech, Inc.: South San Francisco, CA, https://www.avastin.com/hcp/mcrc/proposed-moa.html (Accessed 04 April 2019). [Google Scholar]
- 8. Avastin® (bevacizumab). Prescribing information. Genentech, Inc, South San Francisco, CA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125085s317lbl.pdf. (accessed December 2016). [Google Scholar]
- 9. Van Cutsem E, de Haas S, Kang YK, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 2012; 30: 2119–2127. [DOI] [PubMed] [Google Scholar]
- 10. Parikh AR, Lee FC, Yau L, et al. MAVERICC, a randomized, biomarker-stratified, phase II study of mFOLFOX6-bevacizumab versus FOLFIRI-bevacizumab as first-line chemotherapy in metastatic colorectal cancer. Clin Cancer Res 2019; 25: 2988–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stintzing S, Fischer von Weikersthal L, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol 2012; 23: 1693–1699. [DOI] [PubMed] [Google Scholar]
- 12. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 1065–1075. [DOI] [PubMed] [Google Scholar]
- 13. Bianconi D, Herac M, Spies D, et al. SERPINB7 expression predicts poor pancreatic cancer survival upon gemcitabine treatment. Transl Oncol 2019; 12: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez-Pascual J, Cubillo A. Dynamic biomarkers of response to antiangiogenic therapies in colorectal cancer: a review. Curr Pharmacogenomics Person Med 2017; 15: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soker S, Takashima S, Miao HQ, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998; 92: 735–745. [DOI] [PubMed] [Google Scholar]
- 16. Napolitano V, Tamagnone L. Neuropilins controlling cancer therapy responsiveness. Int J Mol Sci 2019; 20: 2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang W, Azuma M, Lurje G, et al. Molecular predictors of combination targeted therapies (cetuximab, bevacizumab) in irinotecan-refractory colorectal cancer (BOND-2 study). Anticancer Res 2010; 30: 4209–4217. [PubMed] [Google Scholar]
- 18. Weis SM, Cheresh DA. αV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med 2011; 1: a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cruz da, Silva E, Dontenwill M, Choulier L, et al. Role of integrins in resistance to therapies targeting growth factor receptors in cancer. Cancers (Basel) 2019; 11: 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prager GW, Poettler M, Unseld M, et al. Angiogenesis in cancer: anti-VEGF escape mechanisms. Transl Lung Cancer Res 2012; 1: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Margheri F, Luciani C, Taddei ML, et al. The receptor for urokinase-plasminogen activator (uPAR) controls plasticity of cancer cell movement in mesenchymal and amoeboid migration style. Oncotarget 2014; 5: 1538–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Unseld M, Kornek G, Gleiss A, et al. Soluble CD87 (s-uPAR) predicts bevacizumab-based first line treatment of metastatic colorectal cancer (mCRC): results from a prospective multi-center study. J Clin Oncol 2017; 35(Suppl. 4): Abstract 604. [Google Scholar]
- 23. Boonstra MC, Verbeek FP, Mazar AP, et al. Expression of uPAR in tumor-associated stromal cells is associated with colorectal cancer patient prognosis: a TMA study. BMC cancer 2014; 14: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Negri FV, Bozzetti C, Lagrasta CA, et al. PTEN status in advanced colorectal cancer treated with cetuximab. Br J Cancer 2010; 102: 162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez S, Huynh-Do U. The role of PTEN in tumor angiogenesis. J Oncol 2012; 2012: 141236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fenczik CA, Zent R, Dellos M, et al. Distinct domains of CD98hc regulate integrins and amino acid transport. J Biol Chem 2001; 276: 8746–8752. [DOI] [PubMed] [Google Scholar]
- 27. Feral CC, Nishiya N, Fenczik CA, et al. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci U S A 2005; 102: 355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cantor J, Browne CD, Ruppert R, et al. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat Immunol 2009; 10: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prager GW, Poettler M, Schmidinger M, et al. CD98hc (SLC3A2), a novel marker in renal cell cancer. Eur J Clin Invest 2009; 39: 304–310. [DOI] [PubMed] [Google Scholar]
- 30. Ye Y, Wang M, Wang B, et al. CD98, a potential diagnostic cancer-related biomarker, and its prognostic impact in colorectal cancer patients. Int J Clin Exp Pathol 2017; 10: 5418–5429. [Google Scholar]
- 31. Rajaganeshan R, Prasad R, Guillou PJ, et al. The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br J Cancer 2007; 96: 1112–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohamed SY, Mohammed HL, Ibrahim HM, et al. Role of VEGF, CD105, and CD31 in the prognosis of colorectal cancer cases. J Gastrointest Cancer 2019; 50: 23–34. [DOI] [PubMed] [Google Scholar]
- 33. Vermeulen PB, Van den Eynden GG, Huget P, et al. Prospective study of intratumoral microvessel density, p53 expression and survival in colorectal cancer. Br J Cancer 1999; 79: 316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Des Guetz G, Uzzan B, Nicolas P, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 2006; 94: 1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst 2002; 94: 883–893. [DOI] [PubMed] [Google Scholar]
- 36. Bais C, Mueller B, Brady MF, et al. Tumor microvessel density as a potential predictive marker for bevacizumab benefit: GOG-0218 biomarker analyses. J Natl Cancer Inst 2017; 109: djx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 2006; 24: 217–227. [DOI] [PubMed] [Google Scholar]
- 38. Zygon J, Szajewski M, Kruszewski WJ, et al. VEGF, Flt-1, and microvessel density in primary tumors as predictive factors of colorectal cancer prognosis. Mol Clin Oncol 2017; 6: 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. The ASCO Post. FDA approves bevacizumab biosimilar for five cancer types, https://www.ascopost.com/News/60204 (2019, accessed 18 July 2019).
- 40. Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in colorectal cancer: current role in treatment and the potential of biosimilars. Target Oncol 2017; 12: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seo N, Polozova A, Zhang M, et al. Analytical and functional similarity of Amgen biosimilar ABP 215 to bevacizumab. MAbs 2018; 10: 678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang LC. The biosimilar pathway in the USA: an analysis of the innovator company and biosimilar company perspectives and beyond. J Food Drug Anal 2019; 27: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, PassionATE_Supplementary_Figures_rev1 for Microvascular density assessed by CD31 predicts clinical benefit upon bevacizumab treatment in metastatic colorectal cancer: results of the PassionATE study, a translational prospective Phase II study of capecitabine and irinotecan plus bevacizumab followed by capecitabine and oxaliplatin plus bevacizumab or the reverse sequence in patients in mCRC by Daniela Bianconi, Merima Herac, Florian Posch, Margit Schmeidl, Matthias Unseld, Markus Kieler, Robert Brettner, Leonhard Müllauer, Jakob Riedl, Armin Gerger, Werner Scheithauer and Gerald Prager in Therapeutic Advances in Medical Oncology
Supplemental material, PassionATE_Supplementary_Methods for Microvascular density assessed by CD31 predicts clinical benefit upon bevacizumab treatment in metastatic colorectal cancer: results of the PassionATE study, a translational prospective Phase II study of capecitabine and irinotecan plus bevacizumab followed by capecitabine and oxaliplatin plus bevacizumab or the reverse sequence in patients in mCRC by Daniela Bianconi, Merima Herac, Florian Posch, Margit Schmeidl, Matthias Unseld, Markus Kieler, Robert Brettner, Leonhard Müllauer, Jakob Riedl, Armin Gerger, Werner Scheithauer and Gerald Prager in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_List_1 for Microvascular density assessed by CD31 predicts clinical benefit upon bevacizumab treatment in metastatic colorectal cancer: results of the PassionATE study, a translational prospective Phase II study of capecitabine and irinotecan plus bevacizumab followed by capecitabine and oxaliplatin plus bevacizumab or the reverse sequence in patients in mCRC by Daniela Bianconi, Merima Herac, Florian Posch, Margit Schmeidl, Matthias Unseld, Markus Kieler, Robert Brettner, Leonhard Müllauer, Jakob Riedl, Armin Gerger, Werner Scheithauer and Gerald Prager in Therapeutic Advances in Medical Oncology