Abstract
Internal carotid artery stenosis is a risk factor for ischemic stroke. Even in the absence of visible structural brain changes, patients with asymptomatic stenosis are prone to cognitive impairment. On a neuronal level, it was suggested that stenosis may lead to disturbed functional brain connectivity. If so, carotid revascularization should have an effect on hypothesized brain network disturbances. We studied functional connectivity in a motor network by resting-state electroencephalography in 12 patients with high grade asymptomatic carotid stenosis before and after interventional or surgical revascularization as compared to 23 controls. In patients with stenosis, functional connectivity of neural oscillations was significantly decreased prior and improved returning to normal connectivity after revascularization. In a subgroup of patients, also studied by contrast perfusion magnetic resonance imaging, reduced connectivity was associated with decreased regional brain perfusion reflected by increased mean transit time in the middle cerebral artery borderzone. Cognitive testing revealed only minor differences between patients and controls. In summary, we identified oscillatory connectivity changes in patients with asymptomatic carotid stenosis correlating with regional hypoperfusion, which both normalized after revascularization. Hence, electrophysiological changes might be a reversible precursor preceding macroscopic structural brain damage and behavioral impairment in patients with asymptomatic carotid stenosis.
Keywords: Asymptomatic carotid artery stenosis, carotid revascularization, EEG, functional connectivity, perfusion MRI
Introduction
Carotid artery stenosis is a well-known risk factor for ischemic stroke,1 and surgical or interventional treatment of carotid stenosis reduces the risk of stroke in symptomatic carotid stenosis.2 Patients with internal carotid artery (ICA) stenosis who have not suffered a stroke have been shown to be prone to cognitive impairment,3,4 suggesting that the labeling as “asymptomatic” might be misleading for carotid stenosis even in the absence of acute stroke symptoms. Supporting that notion, studies have shown, that even in the absence of visible structural brain changes on magnetic resonance imaging (MRI) such as silent brain infarctions, patients with carotid stenosis show signs of altered micro-structural integrity and brain network disturbances.5 The best treatment option for carotid stenosis without incident stroke is under debate, comprising best medical treatment and interventional revascularization via carotid artery stenting (CAS) or carotid endarterectomy (CEA).6 Carotid revascularization of asymptomatic stenosis has been shown to improve cognitive function in some studies, but there is contradictory data, and other studies reported even a decline of cognitive performances along with an increased risk of periprocedural stroke.7 Hence, it is of great importance to determine which patients are most likely to benefit from carotid revascularization procedures. Information on disruption of functional brain networks as well as on cognitive dysfunction might inform treatment decision in asymptomatic carotid stenosis.
Pathological alterations of neuronal interactions can be quantified by assessing the functional connectivity between multiple brain regions. Although structural and functional networks are often altered in parallel, functional connectivity can be modified without any structural damage.8 Recent functional-MRI (fMRI) studies indicate a subtle decrease of functional connectivity in patients with asymptomatic ICA stenosis.5,9–12 Only few studies investigated functional connectivity changes after revascularization.5,13,14 Previous studies, however, have mainly been conducted using fMRI, assessing functional connectivity from the resting-state blood-oxygen-level-dependent (BOLD) signal. This introduces confounding factors due to the hemodynamical dependency of the BOLD signal that is affected by cerebrovascular disease such as chronic hypoperfusion.15,16 One EEG pilot study provided first findings of alterations in cortical activity in patients with severe carotid stenosis,17 data analysis, however, was restricted to spectral power analysis in low-density EEG, hence not investigating brain connectivity. Furthermore, it is not known whether treatment of carotid stenosis has an impact on oscillatory functional connectivity, and it has not been studied yet if alterations of brain oscillations hypothesized to come along with carotid stenosis are reversible after revascularization.
In this study, we aimed at assessing functional connectivity changes in a brain network located in the carotid territory in patients with high-grade asymptomatic ICA stenosis. Moreover, we evaluated the impact of interventional revascularization by CAS or CEA on network connectivity. For this aim, we compared functional connectivity assessed by EEG before and after revascularization, and to a group of controls. Connectivity was assessed within a well-described motor network located within the internal carotid artery territory which has been shown to map relevant differences in patients with cerebrovascular disease.18,19 We further studied brain perfusion by MRI in order to relate changes in functional connectivity to regional brain hypoperfusion resulting from carotid stenosis. Cognitive function was evaluated in order to assess whether patients already showed clinical deficits.
Material and methods
Participants and neuropsychological testing
Fifteen participants with high grade (≥70% according to the North American Symptomatic Carotid Endarterectomy Trial NASCET criteria) asymptomatic internal carotid artery stenosis undergoing interventional revascularization (carotid endarterectomy or carotid artery stenting) were enrolled at the Department of Neurology, University Medical Center Hamburg-Eppendorf. Indication for treatment was discussed in an interdisciplinary board meeting.6 All patients underwent EEG recording, MR-imaging, ultrasound examination, neurologic examination and clinical history taking. EEG recordings were performed one day prior to revascularization as well as six to eight weeks after intervention. Out of the 15 participants, 3 participants had to be excluded due to extensive artifacts during EEG recordings. Moreover, 23 age-eligible controls were recruited. Inclusion criteria comprised (1) unilateral internal carotid artery stenosis ≥70% according to NASCET criteria, (2) no history of stroke or transient ischemic attack, (3) no acute ischemic infarctions, severe leukoaraiosis, or any other pathological findings on MR images as evaluated by a board-certified neuroradiologist, and (4) no history of dementia or depression or significant neurological symptoms. All participants were above 50 years of age. Exclusion criteria comprised (1) contraindications against MRI, (2) contraindications against Gadolinium application, (3) severe neuropsychiatric disease, and (4) history of cognitive impairment.
Neuropsychological testing was obtained in all participants, including the Mini-Mental State Examination (MMSE), the DemTect, a cognitive screening test for mild cognitive impairment and early dementia, and tests of executive function (trail-making test A/B and Stroop color-word test). Testing was performed within 10 days prior to revascularization as well as 6–10 weeks after intervention.
Participants gave written informed consent according to the Declaration of Helsinki. The study was approved by the local ethics committee of the Medical Association of Hamburg.
Recording and preprocessing
EEG
Ten minutes of task-free EEG data were recorded in participants seated in front of a screen. Participants were asked to visually fixate a cross presented on the screen in order to avoid drowsiness and ensure wakefulness. Data were sampled at 1000 Hz using a 63-channel EEG system positioned according to the 10–10 System of the American Electroencephalographic Society (using actiCAP®, Brain Products GmbH, Germany, Gilching; Electro-Cap International, Inc., Eaton, OH, USA) and referenced to the Cz electrode. The impedance of the EEG electrodes was kept below 25 kΩ. Data were segmented into 4 s epochs for further analysis, filtered from 2 to 256 Hz with a bandpass-filter of fourth order and a bandstop filter at 49–51 Hz, 99–101 Hz, and 149–151 Hz and resampled to 125 Hz. Epochs with artifactual amplitude steps were dismissed from the analysis. Eye-movement artifacts were removed employing an independent component analysis.20 Subsequently, data were re-referenced to a common average reference. Artifact rejection resulted in an overall number of µ = 115.3/120.0/101.3, SD = 46.2/59.2/54.5 trials (controls/ patients pre-intervention/ patients post-intervention). The Fieldtrip toolbox21 as well as custom written software using MATLAB Version 8.2.0 (R2013b, Mathworks Inc., MA) were used for EEG data processing.
Brain imaging and perfusion
High-resolution T1-weighted anatomical images data were acquired using a 3T Siemens Skyra MRI scanner (Siemens, Erlangen, Germany). The MRI protocol included among others T1 MPRage (flip angle = 9°, TR = 2500 ms, TE = 2.12 ms, slice thickness = 0.9 mm, inversion time 1100 ms, matrix = 232 × 288, FOV = 193 × 293 mm), T2 FLAIR (flip angle = 150°, TR = 9000 ms, TE = 90 ms, slice thickness = 5 mm, inversion time = 2500 ms, matrix = 320 × 270, FOV = 194 × 230 mm), CE-perfusion weighted imaging (PWI) (flip angle = 90°, TR = 1920 ms, TE = 30 ms, slice thickness = 4 mm, matrix = 128 × 128, FOV = 240 × 240 mm), during dynamic acquisition, a single dose of 0.1 mmol/kg of gadolinium contrast agent (Dotarem, Guerbet, France) was injected using an automatic injection pump.
Data analysis
Source reconstruction and coherence analysis
To resolve time-frequency dynamics in source space, we reconstructed activity at pre-defined coordinates of interest (COI) using spatial filtering. COIs were chosen based on previous studies investigating network differences in patients with cerebrovascular disease.18,19 Coordinates for the primary motor cortex (M1), ventral premotor cortex (PMv), supplementary motor area (SMA), anterior part of the intraparietal sulcus (aIPS) and the caudal part of the intraparietal sulcus (cIPS) were derived from a previous fMRI experiment involving a hand grip task in healthy elderly participants and stroke patients (please refer to Supplementary figure 1).19 Moreover, this motor network of brain regions is located within middle cerebral and anterior cerebral artery territory. We investigated EEG data for each hemisphere separately. As a forward model we computed a Boundary Element Method volume conduction model22 using the source space modeling functions of the Statistical Parametric Mapping software (SPM12b, Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm). These forward models were based on individual T1-weighted structural MRIs and individual electrode positions registered with an ultrasound localization system (CMS20, Zebris, Isny, Germany). Leadfields for the dipolar sources at the COIs were computed and in junction with the channel covariance matrix used to calculate a linearly constrained minimum variance (LCMV) beamformer23 for each location. Channel time series were then projected to source space with the filter oriented along the maximal signal variance. Spectral analysis was calculated from 1 to 30 Hz in steps of 2 Hz applying a fast Fourier transformation using one Hanning taper. Subsequently, the imaginary part of the coherence was calculated between all COIs resulting in a 10 × 10 connectivity matrix. We chose the imaginary part of the coherence to reduce the false connectivity arising from volume conduction.24 In participants with stenosis on the left hemisphere (n = 8/12) data were flipped to the right in order to allow a comparison of affected (stenosis side) and unaffected (non-stenosis side) activations across individuals. To avoid effects of lateralization in group comparison, data of randomly assigned healthy controls were flipped from left to right in the same ratio (n = 15/23). Subsequently, data were averaged within the alpha (8–13 Hz) and beta (14–25 Hz) frequency band, as these frequency bands have been shown to contain a high information content in elderly and stroke patients during tasks18,25–27 as well as in resting-state studies.28,29
Analysis of perfusion data
In line with a recent study of the same cohort30 focusing on brain perfusion in patients with ICA stenosis, CE-PWI maps of cerebral blood flow (CBF), cerebral blood volume (CBV) and mean transit time (MTT) were generated using an in-house developed software tool for automated perfusion analysis (ANTONIA).31 Voxels for detection of the arterial input function were selected manually contralaterally to the ICA stenosis in distal ICA or proximal M1 segments. For quantitative analysis cortical masks of arterial flow territories in the anterior circulation, (i.e. branches of carotid arteries) including middle (MCA) and anterior cerebral arteries (ACA) were created in Montreal Neurological Institute (MNI) space based on an available atlas.32 Additionally, based on these masks an “MCA borderzone” mask including the marginal areas of MCA-PCA and MCA-ACA territories was created (refer to Supplementary figure 2 for MCA borderzone maps). The media borderzone has been shown to be most vulnerable for perfusion changes in patients with ICA stenosis30,33 for which reason we focused our analysis on the borderzone. Territory masks were registered to individual space and the respective cortical perfusion values were recorded using tools from the “Functional MRI of the Brain Software Library” (FMRIB Software Library; http://www.fmrib.ox.ac.uk/fsl). As previously suggested we calculated relative perfusion values for each arterial territory and perfusion measure (relative perfusion = perfusionipsilateral/perfusioncontralateral) to facilitate longitudinal comparisons.34
Statistical analysis
We used the R statistical package 3.2.3 to perform linear mixed model analyses (package lme4, maximum likelihood) of the relationship between the connectivity (imaginary coherence) and groups. In total, we employed three models, with (I) controls vs. patients pre-intervention, (II) patients pre-intervention vs. patients post-intervention and (III) controls vs. patients post-intervention, respectively. The models contained the fixed effects “group” (controls (CON), patients pre-intervention (PRE), patients post-intervention (POST) and “side” (intrahemispheric connectivity ipsilateral to the stenosis, intrahemispheric connectivity contralateral to the stenosis, and interhemispheric connectivity) with interaction term into the model. As additional random effects, we included intercepts for frequency band (alpha, beta), COI, and by-patient variability. The imaginary coherence was transformed by the natural logarithm to obtain a better distribution. Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. Model p values were obtained by analysis of variance. Estimates are reported with 95% confidence intervals. Post-hoc testing was performed by calculating the least square means for interaction of group and side. Similarly, to assess the relationship between connectivity and perfusion, we also performed linear mixed model analyses in a model containing the fixed effect “perfusion” as well as additional random effects with intercepts for frequency band (alpha, beta), COI, by-patient variability, and group.
Results
Patient characteristics and results of cognitive testing
Baseline characteristics and clinical data are shown in Table 1. There was no significant difference in age, gender or level of education between 12 patients and 23 control subjects. In 50% of patients (n = 6), degree of ICA stenosis was 70–89%, and in 50% of patients (n = 6), stenosis was ≥90%, with a stenosis on the left in eight and a stenosis on the right in four patients. Nine patients underwent CEA whereas three patients were treated with CAS. In tests of cognitive function, patients revealed a worse performance than controls in the Stroop color word test (p = 0.0004, unpaired Wilcoxon test, see Table 2). Revascularization did not improve performance in the Stroop test in patients (p = 1, paired Wilcoxon test). For all other tests (MMSE, DemTect, trail-making test A and B), no significant differences were observed between patients and controls, nor in patients before and after revascularization.
Table 1.
Baseline characteristics and clinical data.
| Patients (n = 12) | Controls (n = 23) | p value | |
|---|---|---|---|
| Demographics | |||
| Age, year | 65.3 (6.9) | 65.0 (8.4) | 0.90 |
| Male:female | 6:6 | 14:9 | 0.55 |
| Education, year Characteristics of stenosis | 14.1 (3.7) | 16 (3.1) | 0.12 |
| Left:right | 8:4 | NA | NA |
| Degree of stenosis | |||
| 70–89% | 6 | NA | NA |
| ≥90% | 6 | ||
| CEA:CAS | 9:3 | NA | NA |
Values expressed as the mean (standard deviation); p values were obtained from an unpaired t-test.
CAS: carotid artery stenting; CEA: carotid endarterectomy.
Table 2.
Neuropsychological testing.
| CON (n = 23) | p value CON vs. PRE | PRE (n = 12) | p value PRE vs. POST | POST (n = 12) | |
|---|---|---|---|---|---|
| MMSE | 28.4 (1.5) | 0.48 | 27.9 (1.8) | 0.71 | 27.8 (1.9) |
| DemTecT | 17.5 (0.90) | 0.02 | 15.7 (2.9) | 0.86 | 15.8 (2.9) |
| TMT A (s) | 37.2 (10.6) | 0.20 | 42.3 (11.0) | 0.81 | 41.6 (14.3) |
| TMT B (s) | 85.0 (50.1) | 0.02 | 108.4 (41.7) | 0.42 | 116.2 (64.8) |
| Stroop (s) | 36.6 (8.7) | 0.0004*** | 54.9 (17.0) | 1 | 55.4 (20.9) |
Values expressed as the mean (standard deviation).
CON: control group; PRE: patients pre-intervention; POST: patients post-intervention; MMSE: Mini-Mental State Examination; DemTecT: cognitive screening test for mild cognitive impairment and early dementia; TMT A: trail-making test Part A; TMT B: trail-making test part B; Stroop: Stroop color-word test.
***p < 0.001, Wilcoxon test, FDR corrected.
Analysis of brain connectivity
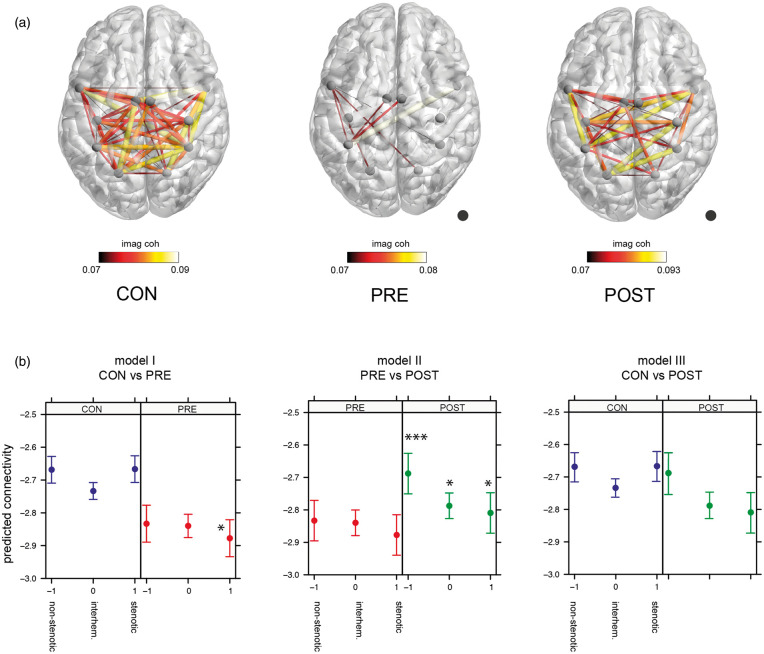
Figure 1(a) displays the mean imaginary coherence as a surrogate marker for brain connectivity across participants for all three groups respectively (controls (CON), patients pre-intervention (PRE), patients post-intervention (POST)). In the following, “connectivity” describes the imaginary coherence. Controls showed a greater overall connectivity compared to patients with an ICA stenosis. After revascularization, connectivity in patients increased markedly, with a connectivity pattern comparable to that in controls.
Figure 1.
(a) Imaginary coherence of all three groups. Imaginary coherence is plotted as an edge between regions, projected on a template brain. The mean alpha imaginary coherence across participants above a threshold of 0.07 is plotted for each group. Size and color of the edges mark the value of the imaginary coherence. A higher imaginary coherence value represents a greater connectivity between regions. Lighter colors and larger size of connections represent a greater imaginary coherence, whereas darker colors and smaller size represent less imaginary coherence. Dot marks the side of stenosis. (b) Linear mixed model analysis. Effect plot of group × side interaction for each model. Dots mark the predicted connectivity with 95% confidence intervals. Blue: controls (CON); red: patients pre-intervention (PRE); green: patients post-intervention (POST). Side −1 = connectivity on non-stenotic side, side 0 = interhemispheric connections, side 1 = connectivity on stenotic side. Asterixis depict significant post-hoc testing using lsmeans of group × side interaction between groups within the same side. *p < 0.05, ***p < 0.001. CON: controls; PRE: patients pre-intervention; POST: patients post-intervention.
We employed three different linear mixed models to evaluate differences in brain connectivity in three network compartments, i.e., ipsi- and contralateral to upstream stenosis and interhemispherical (“side”), between patients pre- and post-revascularization and controls (“group”) with the following results (see Figure 1(b)):
Model I—“Controls vs. patients pre-intervention”
The model revealed a significant interaction of “group” × “side” (F(2, 3070.98) = 6.31, p = 0.0018) and post-hoc testing confirmed a lower connectivity in patients pre-intervention compared to controls ipsilateral to carotid stenosis (t (38.62) = 2.043, p = 0.048). Neither the fixed effect “side” nor “group” was significant.
Model II—“Patients pre-intervention vs. patients post-intervention”
There was a significant difference between the groups with higher connectivity in patients post-intervention as compared to pre-intervention (F(1, 2102.97) = 25.02, p = 6.137e-07). Post-hoc tests confirmed higher connectivity in all tested compartments, i.e., ipsilateral to stenosis (t (2102.97) = −1.98, p = 0.048), interhemispherical (t (2102.97) = −2.43, p = 0.015), and contralateral to stenosis (t (2102.97) = −4.23, p = <.0001). The interaction “group” × “side” showed a non-significant trend ((F(2,2102.97) = 2.64, p = 0.071), the fixed effect “side” was not significant.
Model III—“Controls vs. patients post-intervention”
Model III did not show a significant “group” effect and post-hoc testing confirmed no significant difference between controls and patients post-intervention. Model results showed a significant interaction of “group” × “side” (F(2, 3070.98) = 6.35, p = 0.0018), elicited by significant differences within the patient post-intervention group, however there was no significant differences of connectivity between controls and patients.
Additionally, we employed a whole-brain connectivity analysis using the AAL atlas to parcellate the brain in 90 regions of interest. The whole-brain connectivity approach supports our finding that connectivity is reduced in patients with severe stenosis which increases again after revascularization (for further details on methods and results, please refer to Supplementary material).
Changes in perfusion after revascularization
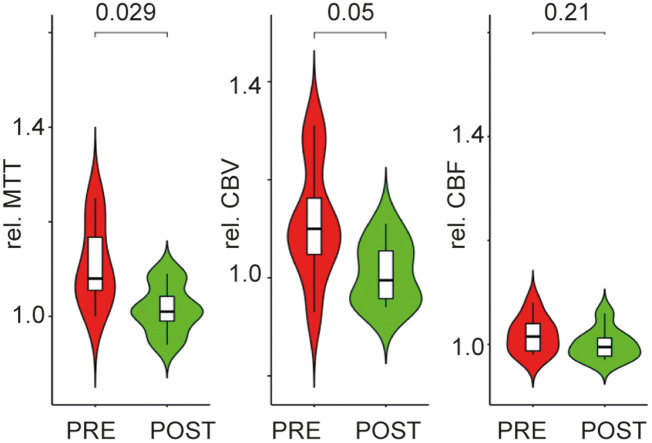
In a recent study of the same cohort30 focusing on brain perfusion in patients with ICA stenosis, we identified a significant hypoperfusion in patients that normalized after revascularization. In a subgroup of eight patients from our study population who were studied by both EEG and CE- perfusion MRI, we repeated the analysis and found significant alterations in relative perfusion ipsilateral to stenosis as compared to the contralateral brain regions. In the MCA borderzone, there was a prolonged mean transit time (MTT) before revascularization as well as a higher cerebral blood volume (CBV) (see Table 3). After revascularization, both MTT and CBV normalized and did not show any significant difference to the contralateral hemisphere any longer (see Figure 2). CBF was not significantly different between ipsi- and contralateral MCA borderzone before and after revascularization.
Table 3.
Perfusion ratio in MCA borderzone.
| MCA borderzone | PRE Mean perfusion ratio (SD) | PRE p value | POST Mean perfusion ratio (SD) | POST p value |
|---|---|---|---|---|
| Perfusion ratio | ||||
| MTT | 1.110 (0.088) | 0.009775** | 1.015 (0.052) | 0.4423 |
| CBV | 1.115 (0.122) | 0.03207* | 1.009 (0.064) | 0.7124 |
| CBF | 1.018 (0.035) | 0.1996 | 1.0 (0.030) | 1 |
Mean perfusion ratio, standard deviation (SD) and statistical results comparing the ratio of perfusion against 1. p values obtained with a t-test against 1. MTT and CBV show a significant increase in patients with stenosis before revascularization. After revascularization MTT and CBV are normalized. CBF ratio does not differ, neither pre- nor post-intervention.
CBF: cerebral blood flow; CBV: cerebral blood volume; MTT: mean transit time; PRE: pre-intervention; POST: post-intervention; MCA: middle cerebral arteries.
Figure 2.
Difference in relative brain perfusion in the MCA borderzone ipsilateral to upstream carotid stenosis pre- and post-revascularization. Box plots and violin plot for each group (red: patients pre-intervention (PRE); green: patients post-intervention (POST)). p values are depicted for the difference between groups in a paired t-test.
Changes of connectivity depending on brain perfusion
Finally, we aimed to explore the relationship of connectivity changes assessed by EEG and brain perfusion captured by perfusion MRI in the subgroup of eight patients in whom perfusion was acquired before and after revascularization. In the mixed linear model, relative MTT ipsilateral to stenosis and global brain connectivity showed a significant inverse relation, with an association of prolonged relative MTT with reduced brain connectivity (F(1, 79.5) = 10.739, p = 0.001556). When looking separately at connectivity in the different compartments studied, i.e. ipsilateral to stenosis, contralateral to stenosis, and interhemispherical, only the model assessing relative MTT and connectivity ipsilateral to stenosis was significant (F(1, 309.52) = 18.654, p = 2.113e-05, Figure 3). The model assessing connectivity contralateral to stenosis (F(1, 85.974) = 3.5226, p = 0.06393) and interhemispherical (F(1, 4.316) = 5.077, p = 0.08082), however, showed a trend in the same direction. The same pattern was observed in the linear mixed model of relative CBV and brain connectivity (data not shown).
Figure 3.
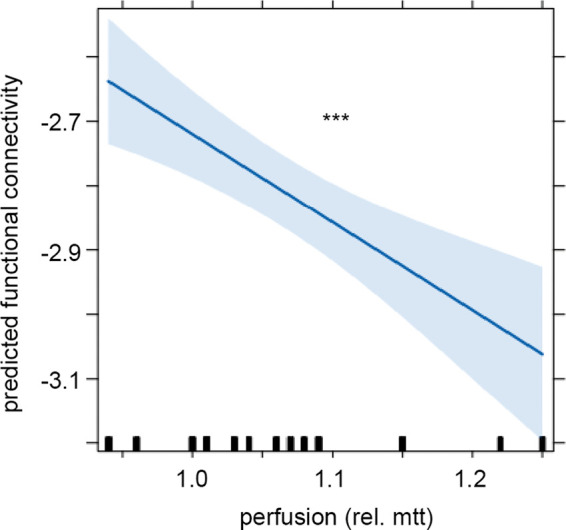
Linear mixed model analysis of connectivity on the stenotic side and brain perfusion ratio. Effect plot of factor “perfusion”. With a prolonged relative MTT, connectivity on the stenotic side decreases. Line displays the prediction line, shaded area the 95% confidence interval. ***p < 0.001. The rug plot at the bottom of the graph shows the location of perfusion (mtt) values.
Discussion
In our study of patients with asymptomatic high-grade carotid stenosis without global cognitive impairment, we found a significant decrease of functional brain connectivity measured by resting-state EEG that was reversible after carotid revascularization. Changes in connectivity were most pronounced in the hemisphere ipsilateral to carotid stenosis. Combined analysis of EEG and perfusion MRI revealed an association of decreased brain connectivity with regional hypoperfusion in the carotid territory downstream the stenosis.
In patients with carotid artery stenosis, “asymptomatic” is referred to a stenosis in the absence of recent stroke or TIA. Patients, however may exhibit subtle alterations of brain structure such as a reduced microstructural white matter integrity5 and alterations of brain function, i.e. decrease of functional connectivity in resting-state fMRI compared to controls.9–12,5 In line with these reports, we observed alterations of functional brain connectivity in the cortical motor network of brain regions located in the vascular brain territory supplied by the internal carotid artery, which were most pronounced ipsilateral to carotid stenosis.
Previous MRI studies have investigated the impact of revascularization on brain structure and function in patients with asymptomatic stenosis. After revascularization, an increase of functional connectivity mainly in the default mode network compared to the patients pre-intervention was reported, suggesting that connectivity changes due to chronic hypoperfusion might be reversible.13,35,5 To our knowledge, there is only one study so far that investigated functional connectivity of patients pre- and post-revascularization as well as controls, but functional connectivity post revascularization was only assessed in a subgroup of patients, limiting the comparison across all three groups.5 No study so far has investigated whether patients after revascularization reach an overall level of functional brain connectivity similar to that of control subjects again. Thus, our study provides novel findings showing a significant overall increase of functional connectivity after reperfusion ipsilateral to carotid stenosis, but also of interhemispheric connectivity, and connectivity contralateral to the stenosis, pointing to a global network effect. Finally, after successful revascularization, connectivity normalized and was no longer significantly different from controls. An additional whole-brain connectivity approach confirmed our finding that patients with severe stenosis show a global decrease of connectivity compared to controls, whereas connectivity increases again after revascularization resembling the pattern observed in controls (Supplementary figure 3).
Former studies of functional connectivity in carotid stenosis relied on functional MRI. Even though neural oscillations are assumed to be the basis of functional connectivity measured by the BOLD signal, the link between the hemodynamic BOLD signal and the underlying neural signal is only indirect and the communication through coupling and synchrony is not accessible to fMRI.36,37 Alterations in the cerebrovascular dynamics due to vascular pathology might affect neurovascular coupling.15 Especially in patients with impaired perfusion, the BOLD signal might fail to depict activation patterns as they can be seen in neurophysiological recordings.38 In our study, functional connectivity was obtained from oscillatory coherence measured with EEG. In contrast to fMRI measurements, electrophysiological recordings offer a more direct measure of neural activity and thus might be able to detect more subtle changes of functional connectivity. Even more important, assessment of functional connectivity by EEG is not at risk of being spoiled by changes of brain perfusion in carotid stenosis. In this first study investigating functional connectivity from electrophysiological recordings in patients with carotid artery stenosis we confirm that connectivity changes, as reported before, are also evident using a method that bypasses the impaired hemodynamic response. By this, our study indicates that the observed alterations of functional connectivity in carotid stenosis are present beyond potential artifacts present in functional MRI measurements.
In the subgroup of patients that were also studied by perfusion MRI, we were able to reproduce findings of regional hypoperfusion downstream carotid stenosis, as previously reported.30,39 The most substantial effect due to hemodynamic changes has been reported for the mean transit time, as it directly relates to cerebral perfusion pressure,40 whereas the overall cerebral blood flow is still relatively well preserved.39 Accordingly, when assessing the ratio of perfusion, we found the most pronounced alterations in relative MTT that normalized after revascularization. Moreover, relative CBV was increased in our patients, and also normalized after intervention, whereas the relative CBF was not significantly different from 1. This pattern, i.e., increased CBV together with unchanged CBF is assumed to indicate sufficient compensatory vasodilation.41 Linking brain perfusion and functional connectivity, a study by Liang et al.42 demonstrated an interaction between functional connectivity architecture obtained from fMRI and regional cerebral blood flow. Studies looking at the relationship of brain perfusion and neural oscillatory activity are sparse43,44 and the neurophysiological basis between the two remains to be investigated further. Here, we identified an association of a prolonged mean transit time in the MCA borderzone with a reduced global connectivity within our ICA network, again most pronounced in the hemisphere ipsilateral to stenosis. Hence, both pathologies seem to be coupled, the causal nature, however, cannot be addressed in this cross-sectional study.
Neurocognitive functions in patients with asymptomatic stenosis and without structural damage have been shown to be impaired3,4; however, the degree of decline varies based on patient population. Especially when relating cognitive function and functional connectivity in patients with stenosis and comparing them to controls, baseline cognition in patients considerably varies among studies hampering general conclusions.9–12 In our study, global cognition assessed via MMSE and DemTect was comparable between patients with stenosis and controls. Only in the Stroop test patients performed worse than controls. Hence, patients pre-intervention showed good overall global cognition and were not highly impaired. A status of sufficient compensation of regionally decreased perfusion would also explain the lack of global cognitive impairment in our patients, as well as the reversibility of decreased functional connectivity, as observed in our population after revascularization. Thus, functional connectivity levels measured from neural oscillatory activity might be a reversible neuronal marker of chronic hypoperfusion preceding global cognitive changes.
Compared to patients with a symptomatic stenosis, the need for interventional revascularization in patients with an asymptomatic stenosis is controversially discussed.6 The annual stroke risk in patients with asymptomatic stenosis varies from 0.5 to 2.5% in patients with a stenosis >50%.45 In order to decide which patients are likely to benefit from interventional revascularization, it is of great importance to improve risk stratification strategies. Hence, further markers that contribute predictive information and help to identify patients likely to benefit from interventional revascularization would be of great value. Our results, together with previous reports, suggest that neuronal markers, such as a diminished brain connectivity, might be pre-symptomatic markers indicating insufficient perfusion due to stenosis that is reversible after revascularization. It would be of great interest to address this further in longitudinal studies.
There a limitations to our study. The extensive study protocol comprising multiple pre-interventional and follow-up test, EEG and imaging protocols in combination with a limited number of patients in whom interventional revascularization was chosen over best medical treatment, resulted in a small number of patients included, which limits the generalizability of our results and carries the risk of a selection bias. Moreover, neurocognitive testing was focused on global cognition, processing speed and cognitive flexibility, hence we cannot infer on other domains such as memory and visuospatial perception to evaluate the baseline level of cognition in patients in more details.
In conclusion, we investigated functional brain connectivity from electrophysiological recordings in patients with asymptomatic carotid artery stenosis. We observed an overall decrease of connectivity in patients, most pronounced ipsilateral to stenosis that was evident despite no significant global cognitive impairment. Patients showed altered regional perfusion with a pattern of perfusion changes indicating a state of compensated hypoperfusion. The extent of perfusion changes was correlated with decreased functional connectivity. Finally, functional connectivity levels normalized after carotid revascularization to a level similar to that of healthy subjects. Our study adds to the understanding of pathophysiological alterations in brain function in asymptomatic patients with carotid stenosis by the detection of a pre-symptomatic and reversible adaption of neural activity to chronic hypoperfusion.
Supplemental Material
Supplemental Material for Normalization of reduced functional connectivity after revascularization of asymptomatic carotid stenosis by Fanny Quandt, Felix Fischer, Julian Schröder, Marlene Heinze, Simon S Kessner, Caroline Malherbe, Robert Schulz, Bastian Cheng, Jens Fiehler, Christian Gerloff and Götz Thomalla in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) Sonderforschungsbereich (SFB) 936, Project C1 (CG) and C2 (GT)
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
FQ: analysis and interpretation of data, drafted the article; FF: acquisition of data, critical revision of manuscript; JS: acquisition and analysis of data, critical revision of manuscript; MH: acquisition of data, critical revision of manuscript; SK: acquisition of data, critical revision of manuscript; CM: analysis of data, critical revision of manuscript; RS: interpretation of data, critical revision of manuscript; BC: substantial contribution to the concept and design, critical revision of manuscript; JF: substantial contribution to the concept and design, critical revision of manuscript; CG: substantial contribution to the concept and design, critical revision of manuscript; GT: substantial contribution to the concept and design, interpretation of data, drafting and critical revision of manuscript. All authors approved the version to be published.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000; 342: 1693–1700. [DOI] [PubMed] [Google Scholar]
- 2.North American Symptomatic Carotid Endarterectomy Trial Collaborators Barnett HJM, Taylor DW, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991; 325: 445–453. [DOI] [PubMed] [Google Scholar]
- 3.Mathiesen EB, Waterloo K, Joakimsen O, et al. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the Tromsø Study. Neurology 2004; 62: 695–701. [DOI] [PubMed] [Google Scholar]
- 4.Silvestrini M, Paolino I, Vernieri F, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology 2009; 72: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H-L, Lin C-J, Soong B-W, et al. Impairments in cognitive function and brain connectivity in severe asymptomatic carotid stenosis. Stroke J Cereb Circ 2012; 43: 2567–2573. [DOI] [PubMed] [Google Scholar]
- 6.Rimmele DL, Larena-Avellaneda A, Alegiani AC, et al. Real-world experience of treatment decision-making in carotid stenosis in a neurovascular board. Neurology 2017; 89: 399–407. [DOI] [PubMed] [Google Scholar]
- 7.Plessers M, Van Herzeele I, Vermassen F, et al. Neurocognitive functioning after carotid revascularization: a systematic review. Cerebrovasc Dis Extra 2014; 4: 132–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sporns O. The human connectome: a complex network. Ann NY Acad Sci 2011; 1224: 109–125. [DOI] [PubMed] [Google Scholar]
- 9.Avirame K, Lesemann A, List J, et al. Cerebral autoregulation and brain networks in occlusive processes of the internal carotid artery. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2015; 35: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang T-Y, Huang K-L, Ho M-Y, et al. Graph theoretical analysis of functional networks and its relationship to cognitive decline in patients with carotid stenosis. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2016; 36: 808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin C-J, Tu P-C, Chern C-M, et al. Connectivity features for identifying cognitive impairment in presymptomatic carotid stenosis. PloS One 2014; 9: e85441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T, Xiao F, Wu G, et al. Impairments in brain perfusion, metabolites, functional connectivity, and cognition in severe asymptomatic carotid stenosis patients: an integrated MRI study. Neural Plast 2017; 2017: 8738714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin C-J, Chang F-C, Chou K-H, et al. Intervention versus aggressive medical therapy for cognition in severe asymptomatic carotid stenosis. Am J Neuroradiol 2016; 37: 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang T, Sun D, Liu Y, et al. The impact of carotid artery stenting on cerebral perfusion, functional connectivity, and cognition in severe asymptomatic carotid stenosis patients. Front Neurol 2017; 8: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci 2003; 4: 863–872. [DOI] [PubMed] [Google Scholar]
- 16.Röther J, Knab R, Hamzei F, et al. Negative dip in BOLD fMRI is caused by blood flow–oxygen consumption uncoupling in humans. NeuroImage 2002; 15: 98–102. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao F-J, Hsieh F-Y, Chen W-T, et al. Altered resting-state cortical EEG oscillations in patients with severe asymptomatic carotid stenosis. Clin EEG Neurosci 2016; 47: 142–149. [DOI] [PubMed] [Google Scholar]
- 18.Quandt F, Bönstrup M, Schulz R, et al. The functional role of beta-oscillations in the supplementary motor area during reaching and grasping after stroke: A question of structural damage to the corticospinal tract. Hum Brain Mapp 2019; 40: 3091–3101. [DOI] [PMC free article] [PubMed]
- 19.Schulz R, Buchholz A, Frey BM, et al. Enhanced effective connectivity between primary motor cortex and intraparietal sulcus in well-recovered stroke patients. Stroke 2016; 47: 482–489. [DOI] [PubMed]
- 20.Makeig S, Bell A, Jung T. Independent component analysis of electroencephalographic data. Adv Neural Inf Process Syst. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.7.7888&rep=rep1&type=pdf (1996, accessed 24 August 2019). [Google Scholar]
- 21.Oostenveld R, Fries P, Maris E, et al. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011; 2011: 156869. [DOI] [PMC free article] [PubMed]
- 22.Fuchs M, Kastner J, Wagner M, et al. A standardized boundary element method volume conductor model. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 2002; 113: 702–712. [DOI] [PubMed] [Google Scholar]
- 23.Van Veen BD, van Drongelen W, Yuchtman M, et al. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 1997; 44: 867–880. [DOI] [PubMed] [Google Scholar]
- 24.Nolte G, Bai O, Wheaton L, et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 2004; 115: 2292–2307. [DOI] [PubMed] [Google Scholar]
- 25.Bönstrup M, Schulz R, Cheng B, et al. Evolution of brain activation after stroke in a constant-effort versus constant-output motor task. Restor Neurol Neurosci 2015; 33: 845–864. [DOI] [PubMed] [Google Scholar]
- 26.Quandt F, Bönstrup M, Schulz R, et al. Spectral variability in the aged brain during fine motor control. Front Aging Neurosci 2016; 8: 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossiter HE, Boudrias M-H, Ward NS. Do movement-related beta oscillations change after stroke?. J Neurophysiol 2014; 112: 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubovik S, Pignat J-M, Ptak R, et al. The behavioral significance of coherent resting-state oscillations after stroke. NeuroImage 2012; 61: 249–257. [DOI] [PubMed] [Google Scholar]
- 29.Guggisberg AG, Rizk S, Ptak R, et al. Two intrinsic coupling types for resting-state integration in the human brain. Brain Topogr 2015; 28: 318–329. [DOI] [PubMed] [Google Scholar]
- 30.Schröder J, Heinze M, Günther M, et al. Dynamics of brain perfusion and cognitive performance in revascularization of carotid artery stenosis. NeuroImage Clin 2019; 22: 101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forkert ND, Cheng B, Kemmling A, et al. ANTONIA perfusion and stroke. A software tool for the multi-purpose analysis of MR perfusion-weighted datasets and quantitative ischemic stroke assessment. Methods Inf Med 2014; 53: 469–481. [DOI] [PubMed] [Google Scholar]
- 32.Tatu L, Moulin T, Bogousslavsky J, et al. Arterial territories of the human brain: cerebral hemispheres. Neurology 1998; 50: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 33.Cheng B, Golsari A, Fiehler J, et al. Dynamics of regional distribution of ischemic lesions in middle cerebral artery trunk occlusion relates to collateral circulation. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2011; 31: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin SZ, Madai VI, von Samson-Himmelstjerna FC, et al. 3D GRASE pulsed arterial spin labeling at multiple inflow times in patients with long arterial transit times: comparison with dynamic susceptibility-weighted contrast-enhanced MRI at 3 Tesla. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2015; 35: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porcu M, Craboledda D, Garofalo P, et al. Reorganization of brain networks following carotid endarterectomy: an exploratory study using resting state functional connectivity with a focus on the changes in Default Mode Network connectivity. Eur J Radiol 2019; 110: 233–241. [DOI] [PubMed] [Google Scholar]
- 36.Schölvinck ML, Leopold DA, Brookes MJ, et al. The contribution of electrophysiology to functional connectivity mapping. NeuroImage 2013; 80: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossini PM, Calautti C, Pauri F, et al. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol 2003; 2: 493–502. [DOI] [PubMed] [Google Scholar]
- 38.Rossini PM, Altamura C, Ferretti A, et al. Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics?. Brain J Neurol 2004; 127: 99–110. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y-F, Tang S-C, Wu W-C, et al. Alterations of cerebral perfusion in asymptomatic internal carotid artery steno-occlusive disease. Sci Rep 2017; 7: 1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soinne L, Helenius J, Tatlisumak T, et al. Cerebral hemodynamics in asymptomatic and symptomatic patients with high-grade carotid stenosis undergoing carotid endarterectomy. Stroke 2003; 34: 1655–1661. [DOI] [PubMed] [Google Scholar]
- 41.Lythgoe DJ, Ostergaard L, William SC, et al. Quantitative perfusion imaging in carotid artery stenosis using dynamic susceptibility contrast-enhanced magnetic resonance imaging. Magn Reson Imaging 2000; 18: 1–11. [DOI] [PubMed] [Google Scholar]
- 42.Liang X, Zou Q, He Y, et al. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci USA 2013; 110: 1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jann K, Federspiel A, Giezendanner S, et al. Linking brain connectivity across different time scales with electroencephalogram, functional magnetic resonance imaging, and diffusion tensor imaging. Brain Connect 2012; 2: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Gorman RL, Poil S-S, Brandeis D, et al. Coupling between resting cerebral perfusion and EEG. Brain Topogr 2013; 26: 442–457. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Kesavabhotla K, Baradaran H, et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke 2015; 46: 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Normalization of reduced functional connectivity after revascularization of asymptomatic carotid stenosis by Fanny Quandt, Felix Fischer, Julian Schröder, Marlene Heinze, Simon S Kessner, Caroline Malherbe, Robert Schulz, Bastian Cheng, Jens Fiehler, Christian Gerloff and Götz Thomalla in Journal of Cerebral Blood Flow & Metabolism