Abstract
In vivo dopamine D2-receptor availability is frequently assessed with 11C-raclopride and positron emission tomography. Due to low signal-to-noise ratios for 11C-raclopride in areas with low D2 receptor densities, the ligand has been considered unreliable for measurements outside the dopamine-dense striatum. Intriguingly, recent studies show that extrastriatal 11C-raclopride binding potential (BPND) values are (i) reliably higher than in the cerebellum (where D2-receptor levels are negligible), (ii) correlate with behavior in the expected direction, and (iii) showed good test–retest reliability in a sample of younger adults. The present work demonstrates high seven-month test–retest reliability of striatal and extrastriatal 11C-raclopride BPND values in healthy, older adults (n = 27, age: 64–78 years). Mean 11C-raclopride BPND values were stable between test sessions in subcortical nuclei, and in frontal and temporal cortices (p > 0.05). Across all structures analyzed, intraclass correlation coefficients were high (0.85–0.96), absolute variability was low (mean: 4–8%), and coefficients of variance ranged between 9 and 25%. Furthermore, regional 11C-raclopride BPND values correlated with previously determined 18F-fallypride BPND values (ρ = 0.97 and 0.92 in correlations with and without striatal values, respectively, p < 0.01) and postmortem determined D2-receptor densities (including striatum: ρ = 0.92; p < 0.001; excluding striatum: ρ = 0.75; p = 0.067). These observations suggest that extrastriatal 11C-raclopride measurements represent a true D2 signal.
Keywords: 11C-raclopride, binding potential, positron emission tomography, extrastriatal, reliability
Introduction
The dopamine (DA) system is implicated in a broad range of functions, including motor control, cognition, and reward.1–3 It is a target of investigation in several disciplines, such as neuroscience, neurology, and psychiatry. Ever since its discovery in the 1980s,4–6 the DA D2 receptor (D2DR) antagonist 11C-raclopride has been a widely used radioligand in positron emission tomography (PET) studies of the DA system, e.g.7-17. Its wide use is due to its pharmacological profile and high specificity for D2DRs,18–21 its high reproducibility in measurements of D2DR availability at rest,22 and its high reliability in measurement of changes in synaptic DA levels via ligand displacement upon tasks and interventions.23–27
11C-raclopride is a moderate affinity ligand with low signal-to-noise ratios in areas with low D2DR levels. Seminal work demonstrated that raclopride binding is several-folds higher in the striatum compared to cortical regions28 and only a few percent higher in frontal and temporal cortices compared to its inactive enantiomer, FLB472.29 Therefore, it has not been considered reliable for D2DR assessments outside the DA-dense striatal compartment.5,29,30 However, cortical raclopride binding depicted a rostral-caudal gradient, which is consistent with DA levels in these brain areas and was displaceable by D2-antagonists.31 More recent studies show that extrastriatal 11C-raclopride binding potential (BPND), even though being low, is reliably above zero and within expected ranges32,33 when comparing with D2 receptor levels quantified postmortem.28,34 Additional support for the reliability of extrastriatal 11C-raclopride BPND comes from recent findings showing that striatal, hippocampal, and cortical 11C-raclopride BPND were interrelated, similarly linked to brain activation during a memory task, and predictive of episodic memory performance.32,33,35 Furthermore, individuals with lower working memory performance had lower frontal cortical 11C-raclopride BPND along with less beneficial brain activation patterns at rest and task.36 In patient groups with DA disorders characterized by frontostriatal dysfunction (Parkinson's and Huntington's diseases), parallel reductions of striatal and cortical 11C-raclopride binding have been reported.37,38 Moreover, striatal as well as extrastriatal 11C-raclopride displacement was observed in DA release paradigms in healthy individuals.10,39–43 Taken together, these observations suggest that extrastriatal 11C-raclopride may represent a meaningful D2DR signal, rather than noise.
Measurement reliability can be assessed by consecutive testing within a group of individuals. High test–retest reliability has been shown for striatal 11C-raclopride measurements,44–46 but also for extrastriatal 11C-raclopride binding in cortical regions and thalamus.47,48 Alakurtti and colleagues performed their investigations with a high-resolution brain-designated PET scanner in a small sample of younger adults. Consequently, it remains unclear whether their findings can be generalized to other settings, such as when using a scanner with lower resolution or when examining samples of older ages with varying degrees of age-induced DA decline.49 For this reason, the present work examined ≥6-month test–retest reliability of extrastriatal 11C-raclopride BPND in a sample of healthy, older adults (n = 27, ages: 64–78 years, 10 men) belonging to the Physical Influences on Brain in Aging (PHIBRA) study.50 To elucidate the validity of our extrastriatal 11C-raclopride signal, we compared regional 11C-raclopride BPND values with values determined with the high-affinity ligand 18F-fallypride,51 and also, with D2DR density values (Bmax) determined with 3H-raclopride in postmortem autoradiography assessments.28
Material and methods
The study was approved by the Swedish Ethical Review Authority (Umeå, Sweden; registration number: 2013-238-31M) and was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Written informed consent was obtained from all participants prior to any testing was done.
Sample
The analyses were performed using the participants randomized to the control group of the PHIBRA study, a six-month aerobic exercise intervention (see Jonasson et al. (2017) for details).50 Between test sessions, individuals included in the present work engaged in exercises of stretching and toning to improve muscle strength, flexibility, and balance. Participants underwent brain assessments, including magnetic resonance imaging (MRI) and PET with 11C-raclopride, at two occasions separated by approximately seven months (mean: 7.3 months; min: 6 months; max: 8 months). Exclusion criteria included factors that affect brain and cognition, such as diabetes; medication known to influence the DA system; neurological, psychiatric, and motor diseases; head trauma; and Mini Mental State Examination scores below 27. Further exclusion criteria consisted of MR-incompatible factors, e.g. claustrophobia and metal implants. All structural MRI images were inspected by a radiologist to screen for structural abnormalities.
BPND values were excluded for two individuals (from of the original 29), as the percent change between test session was found extreme according to the outlier labeling rule with 2.2 interquartile ranges.52 Thus, the effective sample consisted of 27 healthy older adults (age: 64–78 years, 10 men; Table 1).
Table 1.
Sample descriptives.
| Frequency or mean (SD) | min, max | |
|---|---|---|
| Men | 10 | 64, 78 |
| Women | 17 | 7, 25 |
| Age | 69.0 (2.9) | 19.4, 37.3 |
| Years of education | 13.7 (4.7) | 28, 30 |
| Years of education | 26.7 (3.5) | |
| MMSE | 29.5 (0.6) |
BMI: body mass index; max: maximum; min: minimum; MMSE: Mini Mental State Examination; SD: standard deviation.
Brain imaging
MRI
MRI was performed with a 3T Discovery MR 750 scanner (General Electric, WI, US), equipped with a 32-channel phased-array head coil. A 3D fast spoiled gradient-echo sequence was used to obtain high-resolution anatomical T1-weighted images. Imaging parameters were 176 slices with 1 mm thickness, TR = 8.2 ms, TE = 3.2 ms, flip angle = 12°, and field of view = 25 × 25 cm.
The longitudinal image processing pipeline in Freesurfer, version 653 was used to process T1 images and achieve subcortical54 and cortical gray-matter55 segmentations of regions-of-interest (ROIs) for the two time points. Following cortical parcellation, subregions were merged to represent the following ROIs: orbitofrontal (lateral and medial orbitofrontal), anterior cingulate cortex (ACC; rostral and caudal anterior cingulate), superior frontal, middle frontal (rostral and caudal middle frontal), inferior frontal (pars opercularis, pars triangularis, and pars orbitalis), and temporal (superior, middle, and inferior temporal) cortices (Table 2 and Supplementary Table 1).
Table 2.
Test–retest statistics for 11C-raclopride BPND measurements performed approximately seven months apart.
| Baseline | Follow-up | VAR (%) | min; max | ICC | r | |||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | CV (%) | Mean (SD) | CV (%) | mean (SD) | ||||
| Caudate | 2.276 (0.306) | 13.4 | 2.215 (0.283) | 12.8 | 6.4 (4.6) | 0.4; 16.7 | 0.91 | 0.82 |
| Putamen | 3.384 (0.336) | 9.9 | 3.326 (0.290) | 8.7 | 4.1 (3.4) | 0.3; 12.9 | 0.91 | 0.82 |
| Hippocampus | 0.267 (0.052) | 19.5 | 0.264 (0.048) | 18.2 | 7.7 (5.3) | 0.0; 27.4 | 0.95 | 0.91 |
| Amygdala | 0.393 (0.049) | 12.5 | 0.393 (0.051) | 13.0 | 6.7 (6.1) | 0.0; 21.7 | 0.86 | 0.74 |
| Pallidus | 1.384 (0.149) | 10.8 | 1.352 (0.142) | 10.5 | 6.0 (4.7) | 0.7; 18.4 | 0.85 | 0.75 |
| Thalamus | 0.486 (0.059) | 12.1 | 0.487 (0.054) | 11.1 | 4.7 (3.4) | 0.0; 11.5 | 0.94 | 0.90 |
| Orbitofrontal | 0.249 (0.046) | 18.5 | 0.255 (0.044) | 17.3 | 7.0 (6.0) | 0.0; 20.5 | 0.93 | 0.88 |
| ACC | 0.256 (0.047) | 18.4 | 0.261 (0.038) | 14.6 | 7.0 (4.7) | 0.0; 16.0 | 0.93 | 0.83 |
| Sup. frontal | 0.193 (0.045) | 23.3 | 0.192 (0.036) | 18.8 | 8.2 (6.5) | 0.0; 24.5 | 0.93 | 0.88 |
| Mid. frontal | 0.227 (0.042) | 18.5 | 0.226 (0.037) | 16.4 | 5.3 (3.9) | 0.0; 13.4 | 0.96 | 0.93 |
| Inf. frontal | 0.208 (0.051) | 24.5 | 0.207 (0.050) | 24.2 | 7.9 (8.4) | 0.0; 29.7 | 0.95 | 0.92 |
| Temporal | 0.277 (0.038) | 13.7 | 0.283 (0.038) | 13.4 | 4.9 (3.8) | 0.0; 16.3 | 0.95 | 0.91 |
CV: coefficient of variance; ICC: intraclass correlation coefficient; max: maximum; min: minimum; r: Pearson's correlations coefficient (adjusted for gray-matter volume); SD: standard deviation; VAR: absolute variability.
Note: p > 0.05 for comparisons of mean values between sessions.
PET
PET was performed with a Discovery PET/CT 690 (General Electric, WI, US) and 11C-raclopride. Head movements were minimized with individually fitted thermoplastic masks that were attached to the bed surface. A 55 min, 18-frame dynamic PET scan was acquired during resting-state conditions following intravenous bolus injection of approximately 250 MBq 11C-raclopride (baseline: 276.00 ± 12.22 MBq; follow-up: 269.10 ± 10.91 MBq; p > 0.05). Mass of injected raclopride was lower at baseline (0.13 ± 0.06 µg) than at follow-up (0.48 ± 0.15 µg; t(19) = −12.28, p < 0.001), due to differences in specific activity of batches (baseline: 771.78 ± 341.70; follow-up: 190.96 ± 70.64 GBq/µmol; t(19)=7.70, p < 0.001). A CT scan (20 mA, 120 kV, 0.8 s/revolution) preceded ligand injection for attenuation-correction purposes.
Attenuation- and decay-corrected images (47 slices, field of view = 25 cm, 256 × 256-pixel transaxial images, voxel size = 0.977 × 0.977 × 3.27 mm3) were reconstructed with the iterative point-spread function ordered subset maximization (PSF-OSEM) algorithm VUE Point HD-SharpIR (GE56; six iterations, 24 subsets, 3.0 mm post filtering), yielding full width at half maximum (FWHM) of 3.2 mm.57
The following procedures were performed to determine 11C-raclopride BPND in striatal and extrastriatal regions. PET images were motion corrected and co-registered to the structural T1-weighted images from the corresponding session (baseline and follow-up) using the Statistical Parametric Mapping software (SPM8). Within T1-segmented subcortical and cortical structures BPND was calculated with orthogonal regression reference Logan analysis58 and time–activity curves with the median of ROI voxel values from each time frame. Logan regression was based on frame 10–18 (18–55 min). Cerebellar gray matter served as the reference area, due to its negligible D2DR expression.5,34
Statistical analyses
Median 11C-raclopride BPND was extracted for each ROI and subject, averaged over hemispheres, and entered into analyses. Results for the sample are presented with frequencies, means, standard deviations (SDs), and minimum and maximum values. Differences in mean values for 11C-raclopride BPND, gray-matter volumes, injected radioactivity dose (MBq), and specific radioactivity (GBq/µmol) were compared between sessions with paired sample-tests.
Reliability was assessed with the intraclass correlation coefficient (ICC), and also, partial correlations (covariate: regional gray-matter volumes) with the Pearson's correlation coefficient (r). Test–retest absolute variability (VAR, %) between sessions was calculated by entering BPND values from the two sessions in the equation below
Furthermore, the coefficient of variance (CV), defined as the ratio of SD to the mean for the sample (in %), was calculated to compare dispersion of values around the mean for the regions analyzed.
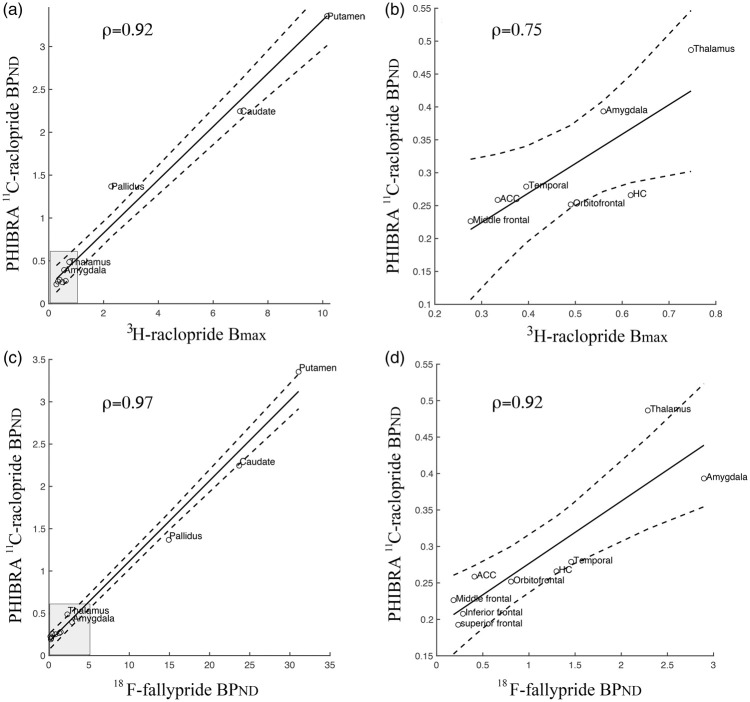
For indications of validity, our regional 11C-raclopride BPND values were compared with previously determined regional values of 3H-raclopride Bmax28 and 18F-fallypride BPND (available at https://osf.io/h67k4/). Whereas 18F-fallypride values were found for all 12 brain regions reported in Table 2 of the present work, 3H-raclopride Bmax for the superior frontal cortex was an outlier (>3 SD from the other cortical values) and the inferior frontal cortex was not reported by Hall et al.,28 and thus, these data points are missing (Figure 1(a) and (b)). Associations among these values are described with the Spearman's rank correlation coefficient (ρ). As DA constituents decline in aging,49 18F-fallypride data were attained for a subsample aged >60 years (mean: 69.4, SD: 6.8, n = 29) for approximate age-matching with the PHIBRA data. Furthermore, 18F-fallypride BPND values were averaged over subregions and hemispheres to represent the ROIs. Values for Bmax based on 3H-raclopride from Hall et al.28 (Figure 1(b) in the original publication) were digitized with the PlotDigitizer software (http://plotdigitizer.sourceforge.net).
Figure 1.
Regional coherence between our 11C-raclopride BPND measures and previously determined 3H-raclopride Bmax28 and 18F-fallypride BPND51. Scatter plots illustrate associations when including striatum and globus pallidus (a), and low uptake-regions (b), which are visualized in the gray box in (a). Similarly, high correlations were found between our 11C-raclopride BPND measures and 18F-fallypride BPND over regions when including striatum and globus pallidus (d), and only the low uptake-regions (d).
Results
High seven-month test–retest reliability for striatal and extrastriatal 11C-raclopride binding
Test–retest statistics demonstrated high reliability for 11C-raclopride BPND measurements in both striatal and extrastriatal regions (Table 2), with minor differences between hemispheres (Supplementary Table 1). No between-scan differences were observed for mean values in any of the regions (p > 0.05). CV indicated that the extent of variability in relation to the means was similar for cortical and subcortical regions, albeit lowest for the putamen (∼9%) and slightly elevated for frontal cortical regions (∼20%). Mean VAR was similar among regions, ranging between approximately 4 and 8%, and with comparable minimum and maximum values. ICCs were all >0.85, and thus in the good-to-excellent range.59,60 Adjusting for gray-matter volumes, first-order correlations indicated high coherence for within-subject BPND values from the two sessions (rs: 0.74–0.93). Notably, similar values were achieved in zero-order correlations, i.e. without adjustment for volumes (rs for putamen: 0.85, caudate: 0.85, hippocampus: 0.91, amygdala: 0.74, globus pallidus: 0.75, thalamus: 0.89, orbitofrontal: 0.87, ACC: 0.89, superior: 0.89, middle: 0.93, inferior frontal: 0.91, and temporal: 0.91 cortices). Aside from the hippocampus,50 volumes for all other structures remained similar between test sessions (p > 0.10).
Due to the significant differences between mass of injected raclopride at the baseline and follow-up sessions, we performed zero-order correlations among the percent change in injected raclopride and (1) percent change in 11C-raclopride BPND values and (2) VAR in 11C-raclopride BPND values. No significant associations were observed for any of the ROIs in Table 2.
Relation between 11C-raclopride BPND and previously determined D2-receptor levels
11C-raclopride BPND values from our study were compared with previously determined 3H-raclopride Bmax28 and 18F-fallypride BPND (https://osf.io/h67k4/) in the corresponding regions. High correlations were found between our 11C-raclopride BPND measures and 3H-raclopride Bmax over regions when including striatal and pallidal ROIs (ρ = 0.92, p < 0.001; Figure 1(a)), but also, among low D2DR density-regions (ρ = 0.75, p = 0.067; Figure 1(b)). In addition, high correlations were found between our 11C-raclopride BPND measures and 18F-fallypride BPND values over regions, with small differences in the strength of the correlation when excluding high-density regions (ρ = 0.97; p < 0.001 and ρ = 0.92; p < 0.01 with and without striatal and pallidal ROIs, respectively; Figure 1(c) and (d)).
Discussion
The use of high-affinity ligands such as 11C-FLB45761 and fallypride62 has been recommended for extrastriatal D2DR assessments. Notably, high-affinity ligands come with their own limitations, including unreliable striatal BPND estimations with 11C-FLB45763 and unreliable measurements of DA release with 18F-fallypride displacement.64 Hence, it is well-motivated to further investigate the feasibility of extrastriatal 11C-raclopride assessments. Here, we demonstrate high test–retest reliability for striatal as well as extrastriatal measurements performed seven months apart in older adults. Striatal and extrastriatal values were comparable in terms of degree of reliability, absolute variance, and dispersion of BPND values around the mean, with small differences between hemispheres. Importantly, 11C-raclopride BPND values throughout frontal, temporal, and subcortical regions were ranked according to previously determined 18F-fallypride BPND,51 as well as 3H-raclopride Bmax from postmortem autoradiography.28 As such, these data add evidence for feasibility and validity of extrastriatal 11C-raclopride assessment.
Test–retest reliability measures the consistency over measurement occasions. Test scores can vary due to variability in true scores and measurement errors. With the estimated striatal D2DR decline being around 5–10% per decade,3,49 D2DR levels were not expected to change considerably over seven months in this group of healthy, older adults. Any observation of variability would then likely arise from measurement error. In line with previous reports, we noted high test–retest reliability for striatum,44–46,65 but also for extrastriatal regions.47,48,66 This stability may relate to factors such as high camera resolution and sensitivity, minimal movement artifacts (achieved via masks and movement correction of images), and small differences in blood flow between sessions.67 A recent finding shows, however, that effects from changes in blood flow are negligible.68 When it comes to data modeling, BPND values estimated with Logan analysis have been shown to be reliable and comparable to those estimated with simplified reference tissue modeling.69,70 The underlying reasons for the large variability in specific activity between batches at the baseline and follow-up sessions remain unknown but could be a consequence of a service of the system that took place between the two test sessions. At other PET sites, fluctuations in specific activity over time have been related to, e.g. changes in the flow of the carrier gas from the target.71 That said, the levels of injected mass of raclopride were at tracer doses, not exceeding those of previous reports, e.g. 5,48,72-73, and thus, the number of available receptors should have remained largely unaffected throughout the experiments. This is supported by the stable mean values for 11C-raclopride BPND values between test sessions.
To ensure minimal variability of volumes between sessions, the longitudinal pipeline of the Freesurfer software was used. It is well known that the volume of ROIs may cause under- and overestimations of BP values.74 Partial volume effects, caused by limited spatial resolution of the scanner and reconstruction algorithms, give rise to varying recovery of the signal in different regions, where structures having high surface-to-volume ratios are most vulnerable.75,76 FWHM of the PSF-OSEM algorithm has been estimated to be 3.2 mm on the scanner used in the present study.57 Furthermore, higher signal recovery was found for the reconstruction algorithm used here, PSF-OSEM, when compared to filtered back-projection, and did not induce a bias in the estimation of BP depending on the level of radioactivity.76 Despite the varying morphology of the structures analyzed, reliability was constant over regions and correlations between 11C-raclopride BPND values from the consecutive trials did not change when controlling for gray-matter volume.
When scrutinizing the statistics presented here, 11C-raclopride BPND values for subcortical, temporal, and frontal cortical regions were coherent between test sessions at the group level. ICC values ranged from 0.85 to 0.96 and were thus in the range of excellent59,60 and even higher than in the reports by Alakurtti et al.47,48 The high ICC values indicate that variance arise from inter- rather than intra-individual variation. Furthermore, the VAR of BPND values as well as the dispersion of values around the mean was similar among regions with high and low D2DR expression. The coefficient of variation was, however, slightly elevated for frontal cortical ROIs (∼20%) when comparing with striatal, thalamic, and temporal regions (∼10%), which is on a par with previous reports.47 In further comparisons among studies, the values for VAR for subcortical and cortical regions were similar to previous reports47,65,66 and comparable to test–retest experiments with high-affinity D2DR ligands.77,78 Consequently, it seems that 11C-raclopride measurements are robust and consistent in regions with varying numbers of D2DRs. Since the present work replicates previous findings in young adults47,48,66 reliability is not compromised by the well-known age-related DA decline.49
It is critical to point out that high reliability does not imply validity of measurements, which in this context would translate to D2DR levels. With its moderate affinity to D2DRs, relatively low signal-to-noise ratio, and only slightly elevated binding when compared with its inactive enantiomer in areas with low D2DR levels,29 11C-raclopride has been considered excellent for striatal and unfit for extrastriatal D2DR measurements. Early work showed minor accumulation of 11C-raclopride in cortical areas30,79 that was only a few percent higher than binding of its inactive enantiomer.29 Low binding would, then again, be reasonable given that cortical D2DR levels constitute only a few percent of the striatal levels.28,34,80 Our 11C-raclopride BPND measures in striatal as well as extrastriatal regions were ranked according to previously determined 18F-fallypride BPND and 3H-raclopride Bmax measures, thereby indicating that extrastriatal 11C-raclopride BPND reflects the presence of D2DRs. Noteworthy, the BPND values were higher in several subcortical nuclei, but not cortical regions, when determined with fallypride as compared to raclopride. Consequently, the striatal-to-extrastriatal BPND ratios differed several-folds between the two D2DR ligands. This may relate to ligand characteristics; however, it remains for future research to understand the source of such observations. Using data from a multiligand study,81 Egerton et al.67 did not observe similar coherence between extrastriatal 11C-raclopride and 11C-FLB457 binding. As pointed out by the authors, the lack of association may relate to the small sample size (n = 10). Small sample sizes are a limitation of most PET studies, and possibly, even more troublesome for the use of 11C-raclopride in extrastriatal regions with low D2DR levels.
Other indications of validity come from studies showing 11C-raclopride displacement during tasks in theoretically expected regions, including in prefrontal regions during working memory,10 in ACC and substantia nigra during a planning task,67 and in motor regions upon motor actions.42 Furthermore, in-scanner interventions have resulted in extrastriatal 11C-raclopride displacement during reward-settings,39,82,83 but also upon pharmacological DA interventions.40,41,43,66,84 Findings from a well-powered 11C-raclopride study have shown that both striatal and extrastriatal D2DR correlate and predict cognitive performance,32,33,36 with observed allelic group differences when stratifying the sample according to a D2DR polymorphism.85 Finally, 11C-raclopride binding was found to be organized according to anatomical and functional DA pathways, which further supports the validity of extrastriatal 11C-raclopride measurements.86
Concluding remarks
The findings presented here and elsewhere open up the possibility of a reliable extrastriatal 11C-raclopride signal that is displaceable by interventions.67 For definite conclusions of the nature of the extrastriatal signal, further observations of extrastriatal 11C-raclopride displacement upon D2DR manipulation are needed in well-powered settings.
Supplemental Material
Supplemental Material for High long-term test–retest reliability for extrastriatal 11C-raclopride binding in healthy older adults by Nina Karalija, Lars Jonassson, Jarkko Johansson, Goran Papenberg, Alireza Salami, Micael Andersson, Katrine Riklund, Lars Nyberg and Carl-Johan Boraxbekk in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by grants from the Swedish Research Council (2012-00530), Västerbotten County Council and Umeå University, the Swedish Research Council for Sport Science and Umeå School of Sport Sciences, the Kamprad Family Foundation, and the Swedish Brain Foundation. The Freesurfer analyses were performed on resources provided by the Swedish National Infrastructure for Computing (SNIC) at HPC2N in Umeå.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contribution
NK and LJ performed research. C-JB, KR, LJ, and LN designed and funded the research; NK, LJ, JJ, GP, AS, and MA analyzed data. NK wrote the manuscript, which was edited by all authors.
Supplemental material
Supplemental material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology 1992; 107: 160–174. [DOI] [PubMed] [Google Scholar]
- 2.Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, et al. Dopaminergic reward system: a short integrative review. Int Arch Med 2010; 3: 24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäckman L, Nyberg L, Lindenberger U, et al. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev 2006; 30: 791–807. [DOI] [PubMed] [Google Scholar]
- 4.Farde L, Ehrin E, Eriksson L, et al. Substituted benzamides as ligands for visualization of dopamine receptor binding in the human brain by positron emission tomography. Proc Natl Acad Sci USA 1985; 82: 3863–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farde L, Hall H, Ehrin E, et al. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 1986; 231: 258–261. [DOI] [PubMed] [Google Scholar]
- 6.Ehrin E, Farde L, de Paulis T, et al. Preparation of 11C-labelled raclopride, a new potent dopamine receptor antagonist: preliminary PET studies of cerebral dopamine receptors in the monkey. Int J Appl Radiat Isot 1985; 36: 269–273. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155: 344–349. [DOI] [PubMed] [Google Scholar]
- 8.Lou HC, Rosa P, Pryds O, et al. ADHD: increased dopamine receptor availability linked to attention deficit and low neonatal cerebral blood flow. Dev Med Child Neurol 2004; 46: 179–183. [DOI] [PubMed] [Google Scholar]
- 9.Rajji TK, Mulsant BH, Nakajima S, et al. Cognition and dopamine D2 receptor availability in the striatum in older patients with schizophrenia. Am J Geriatr Psychiatry 2017; 25: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawamoto N, Piccini P, Hotton G, et al. Cognitive deficits and striato-frontal dopamine release in Parkinson's disease. Brain 2008; 131: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 11.Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev 2006; 30: 1–23. [DOI] [PubMed] [Google Scholar]
- 12.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III – the final common pathway. Schizophr Bull 2009; 35: 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res 2002; 130: 65–71. [DOI] [PubMed] [Google Scholar]
- 14.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007; 64: 327–337. [DOI] [PubMed] [Google Scholar]
- 15.Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 2011; 69: e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol 1998; 42: 707–711. [DOI] [PubMed] [Google Scholar]
- 17.Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol 1997; 11: 151–162. [DOI] [PubMed] [Google Scholar]
- 18.Hall H, Kohler C, Gawell L, et al. Raclopride, a new selective ligand for the dopamine-D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry 1988; 12: 559–568. [DOI] [PubMed] [Google Scholar]
- 19.Seeman P, Ulpian C. Dopamine D1 and D2 receptor selectivities of agonists and antagonists. Adv Exp Med Biol 1988; 235: 55–63. [DOI] [PubMed] [Google Scholar]
- 20.Ögren SO, Hall H, Köhler C, et al. The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology 1986; 90: 287–294. [DOI] [PubMed] [Google Scholar]
- 21.Farde L, Wiesel FA, Jansson P, et al. An open label trial of raclopride in acute schizophrenia. Confirmation of D2-dopamine receptor occupancy by PET. Psychopharmacology 1988; 94: 1–7. [DOI] [PubMed] [Google Scholar]
- 22.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 2000; 20: 423–451. [DOI] [PubMed] [Google Scholar]
- 23.Koepp MJ, Gunn RN, Lawrence AD, et al. Evidence for striatal dopamine release during a video game. Nature 1998; 393: 266–268. [DOI] [PubMed] [Google Scholar]
- 24.Wang GJ, Volkow ND, Fowler JS, et al. Reproducibility of repeated measures of endogenous dopamine competition with [11C]raclopride in the human brain in response to methylphenidate. J Nucl Med 1999; 40: 1285–1291. [PubMed] [Google Scholar]
- 25.Bäckman L, Nyberg L, Soveri A, et al. Effects of working-memory training on striatal dopamine release. Science 2011; 333: 718. [DOI] [PubMed] [Google Scholar]
- 26.Jonasson LS, Axelsson J, Riklund K, et al. Dopamine release in nucleus accumbens during rewarded task switching measured by [(1)(1)C]raclopride. Neuroimage 2014; 99: 357–364. [DOI] [PubMed] [Google Scholar]
- 27.Martinez D, Kim JH, Krystal J, et al. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am 2007; 17: 539–555. [DOI] [PubMed] [Google Scholar]
- 28.Hall H, Sedvall G, Magnusson O, et al. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology 1994; 11: 245–256. [DOI] [PubMed] [Google Scholar]
- 29.Farde L, Pauli S, Hall H, et al. Stereoselective binding of 11C-raclopride in living human brain – a search for extrastriatal central D2-dopamine receptors by PET. Psychopharmacology 1988; 94: 471–478. [DOI] [PubMed] [Google Scholar]
- 30.Farde L, Halldin C, Stone-Elander S, et al. PET analysis of human dopamine receptor subtypes using 11C-SCH 23390 and 11C-raclopride. Psychopharmacology 1987; 92: 278–284. [DOI] [PubMed] [Google Scholar]
- 31.Lidow MS, Goldman-Rakic PS, Rakic P, et al. Dopamine D2 receptors in the cerebral cortex: distribution and pharmacological characterization with [3H]raclopride. Proc Natl Acad Sci USA 1989; 86: 6412–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyberg L, Karalija N, Salami A, et al. Dopamine D2 receptor availability is linked to hippocampal-caudate functional connectivity and episodic memory. Proc Natl Acad Sci USA 2016; 113: 7918–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lövden M, Karalija N, Andersson M, et al. Latent-profile analysis reveals behavioral and brain correlates of dopamine-cognition associations. Cereb Cortex 2018, pp. 28: 3894–3907. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camps M, Cortés R, Gueye B, et al. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience 1989; 28: 275–290. [DOI] [PubMed] [Google Scholar]
- 35.Salami A, Garrett DD, Wahlin A, et al. Dopamine D2/3 binding potential modulates neural signatures of working memory in a load-dependent fashion. J Neurosci 2019; 39: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salami A, Rieckmann A, Karalija N, et al. Neurocognitive profiles of older adults with working-memory dysfunction. Cereb Cortex 2018; 28: 2525–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribeiro MJ, Thobois S, Lohmann E, et al. A multitracer dopaminergic PET study of young-onset parkinsonian patients with and without parkin gene mutations. J Nucl Med 2009; 50: 1244–1250. [DOI] [PubMed] [Google Scholar]
- 38.Politis M, Pavese N, Tai YF, et al. Microglial activation in regions related to cognitive function predicts disease onset in Huntington's disease: a multimodal imaging study. Hum Brain Mapp 2011; 32: 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaasinen V, Aalto S, Nagren K, et al. Expectation of caffeine induces dopaminergic responses in humans. Eur J Neurosci 2004; 19: 2352–2356. [DOI] [PubMed] [Google Scholar]
- 40.Piccini P, Pavese N, Brooks DJ. Endogenous dopamine release after pharmacological challenges in Parkinson's disease. Ann Neurol 2003; 53: 647–653. [DOI] [PubMed] [Google Scholar]
- 41.Stokes PR, Egerton A, Watson B, et al. Significant decreases in frontal and temporal [11C]-raclopride binding after THC challenge. Neuroimage 2010; 52: 1521–1527. [DOI] [PubMed] [Google Scholar]
- 42.Garraux G, Peigneux P, Carson RE, et al. Task-related interaction between basal ganglia and cortical dopamine release. J Neurosci 2007; 27: 14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berry AS, White RL, 3rd, Furman DJ, et al. Dopaminergic mechanisms underlying normal variation in trait anxiety. J Neurosci 2019; 39: 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordström A-L, Farde L, Pauli S, et al. PET analysis of central [11 C]raclopride binding in healthy young adults and schizophrenic patients – reliability and age effects. Hum Psychopharm Clin Exp 1992; 7: 157–165. [Google Scholar]
- 45.Nyberg S, Farde L, Halldin C. Test-retest reliability of central [11C]raclopride binding at high D2 receptor occupancy. A PET study in haloperidol-treated patients. Psychiatry Res 1996; 67: 163–171. [DOI] [PubMed] [Google Scholar]
- 46.Yoder KK, Albrecht DS, Kareken DA, et al. Test-retest variability of [(1)(1)C]raclopride-binding potential in nontreatment-seeking alcoholics. Synapse 2011; 65: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alakurtti K, Johansson JJ, Joutsa J, et al. Long-term test-retest reliability of striatal and extrastriatal dopamine D2/3 receptor binding: study with [(11)C]raclopride and high-resolution PET. J Cereb Blood Flow Metab 2015; 35: 1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alakurtti K, Aalto S, Johansson JJ, et al. Reproducibility of striatal and thalamic dopamine D2 receptor binding using [11C]raclopride with high-resolution positron emission tomography. J Cereb Blood Flow Metab 2011; 31: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karrer TM, Josef AK, Mata R, et al. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: a meta-analysis. Neurobiol Aging 2017; 57: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonasson LS, Nyberg L, Kramer AF, et al. Aerobic exercise intervention, cognitive performance, and brain structure: results from the Physical Influences on Brain in Aging (PHIBRA) Study. Front Aging Neurosci 2017; 8: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seaman KL, Smith CT, Juarez EJ, et al. Differential regional decline in dopamine receptor availability across adulthood: linear and nonlinear effects of age. Hum Brain Mapp 2019; 40: 3125–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. J Am Stat Assoc 1987; 82: 1147–1149. [Google Scholar]
- 53.Reuter M, Schmansky NJ, Rosas HD, et al. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012; 61: 1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 55.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–980. [DOI] [PubMed] [Google Scholar]
- 56.Bettinardi V, Presotto L, Rapisarda E, et al. Physical performance of the new hybrid PETCT Discovery-690. Med Phys 2011; 38: 5394–5411. [DOI] [PubMed] [Google Scholar]
- 57.Wallsten E, Axelsson J, Sundstrom T, et al. Subcentimeter tumor lesion delineation for high-resolution 18F-FDG PET images: optimizing correction for partial-volume effects. J Nucl Med Technol 2013; 41: 85–91. [DOI] [PubMed] [Google Scholar]
- 58.Logan J, Fowler JS, Volkow ND, et al. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996; 16: 834–840. [DOI] [PubMed] [Google Scholar]
- 59.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assessment 1994; 6: 284–290. [Google Scholar]
- 60.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016; 15: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Halldin C, Farde L, Hogberg T, et al. Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med 1995; 36: 1275–1281. [PubMed] [Google Scholar]
- 62.Mukherjee J, Shi B, Christian BT, et al. 11C-Fallypride: radiosynthesis and preliminary evaluation of a novel dopamine D2/D3 receptor PET radiotracer in non-human primate brain. Bioorg Med Chem 2004; 12: 95–102. [DOI] [PubMed] [Google Scholar]
- 63.Olsson H, Halldin C, Swahn C-G, et al. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab 1999; 19: 1164–1173. [DOI] [PubMed] [Google Scholar]
- 64.Slifstein M, Kegeles LS, Xu X, et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse 2010; 64: 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hietala J, Nagren K, Lehikoinen P, et al. Measurement of striatal D2 dopamine receptor density and affinity with [11C]-raclopride in vivo: a test-retest analysis. J Cereb Blood Flow Metab 1999; 19: 210–217. [DOI] [PubMed] [Google Scholar]
- 66.Hirvonen J, Aalto S, Lumme V, et al. Measurement of striatal and thalamic dopamine D2 receptor binding with 11C-raclopride. Nucl Med Commun 2003; 24: 1207–1214. [DOI] [PubMed] [Google Scholar]
- 67.Egerton A, Mehta MA, Montgomery AJ, et al. The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev 2009; 33: 1109–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sander CY, Mandeville JB, Wey HY, et al. Effects of flow changes on radiotracer binding: simultaneous measurement of neuroreceptor binding and cerebral blood flow modulation. J Cereb Blood Flow Metab 2019; 39: 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer K, Sossi V, Schmid A, et al. Noninvasive nuclear imaging enables the in vivo quantification of striatal dopamine receptor expression and raclopride affinity in mice. J Nucl Med 2011; 52: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 70.Logan J, Fowler JS, Volkow ND, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(-)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990; 10: 740–747. [DOI] [PubMed] [Google Scholar]
- 71.Andersson J, Truong P, Halldin C. In-target produced [11C]methane: increased specific radioactivity. Appl Radiat Isot 2009; 67: 106–110. [DOI] [PubMed] [Google Scholar]
- 72.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry 2010; 67: 231–239. [DOI] [PubMed] [Google Scholar]
- 73.Lammertsma AA, Bench CJ, Hume SP, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 1996; 16: 42–52. [DOI] [PubMed] [Google Scholar]
- 74.Smith CT, Crawford JL, Dang LC, et al. Partial-volume correction increases estimated dopamine D2-like receptor binding potential and reduces adult age differences. J Cereb Blood Flow Metab 2019; 39: 822–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dewaraja YK, Ljungberg M, Koral KF. Monte Carlo evaluation of object shape effects in iodine-131 SPET tumor activity quantification. Eur J Nucl Med 2001; 28: 900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jonasson LS, Axelsson J, Riklund K, et al. Simulating effects of brain atrophy in longitudinal PET imaging with an anthropomorphic brain phantom. Phys Med Biol 2017; 62: 5213–5227. [DOI] [PubMed] [Google Scholar]
- 77.Mukherjee J, Christian BT, Dunigan KA, et al. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse 2002; 46: 170–188. [DOI] [PubMed] [Google Scholar]
- 78.Vilkman H, Kajander J, Nagren K, et al. Measurement of extrastriatal D2-like receptor binding with [11C]FLB 457 – a test-retest analysis. Eur J Nucl Med 2000; 27: 1666–1673. [DOI] [PubMed] [Google Scholar]
- 79.Hall H, Farde L, Sedvall G. Human dopamine receptor subtypes – in vitro binding analysis using 3H-SCH 23390 and 3H-raclopride. J Neural Transm 1988; 73: 7–21. [DOI] [PubMed] [Google Scholar]
- 80.Suhara T, Sudo Y, Okauchi T, et al. Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmacol 1999; 2: 73–82. [DOI] [PubMed] [Google Scholar]
- 81.Ito H, Takahashi H, Arakawa R, et al. Normal database of dopaminergic neurotransmission system in human brain measured by positron emission tomography. Neuroimage 2008; 39: 555–565. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi K, Mizuno K, Sasaki AT, et al. Imaging the passionate stage of romantic love by dopamine dynamics. Front Hum Neurosci 2015; 9: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lippert RN, Cremer AL, Edwin Thanarajah S, et al. Time-dependent assessment of stimulus-evoked regional dopamine release. Nat Commun 2019; 10: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mehta MA, Montgomery AJ, Kitamura Y, et al. Dopamine D2 receptor occupancy levels of acute sulpiride challenges that produce working memory and learning impairments in healthy volunteers. Psychopharmacology 2008; 196: 157–165. [DOI] [PubMed] [Google Scholar]
- 85.Karalija N, Papenberg G, Wahlin A, et al. C957T-mediated variation in ligand affinity affects the association between (11)C-raclopride binding potential and cognition. J Cogn Neurosci 2019; 31: 314–325. [DOI] [PubMed] [Google Scholar]
- 86.Papenberg G, Jonasson L, Karalija N, et al. Mapping the landscape of human dopamine D2/3 receptors with [(11)C]raclopride. Brain structure & function. Epub ahead of print 23 August 2019. DOI: 10.1007/s00429-019-01938-1. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for High long-term test–retest reliability for extrastriatal 11C-raclopride binding in healthy older adults by Nina Karalija, Lars Jonassson, Jarkko Johansson, Goran Papenberg, Alireza Salami, Micael Andersson, Katrine Riklund, Lars Nyberg and Carl-Johan Boraxbekk in Journal of Cerebral Blood Flow & Metabolism