Abstract
Our purpose is to assess the role of deep medullary veins in pathogenesis of lacunes in patients with cerebral small vessel disease (cSVD). We included patients with baseline and 2.5-year follow-up MRI in CIRCLE study. Susceptibility Weighted Imaging-Phase images were used to evaluate deep medullary veins based on a brain region-based visual score, and T2-Fluid-Attenuated-Inversion-Recovery images were used to evaluate lacunes. Cerebral blood flow and microstructural parameters in white matter hyperintensities and normal appearing white matter were also analyzed. A total of 203 cSVD patients were analyzed and 101 (49.8%) patients had baseline lacunes. Among them, 64 patients had follow-up MRI, including 16 (25.0%) with new lacunes. The patients’ deep medullary veins median score was 9 (7–12). At baseline, high deep medullary veins score was independently associated with the presence of lacunes after adjusting for age, diabetes mellitus, white matter hyperintensities volume and cerebral blood flow or white matter microstructural parameters (all p < 0.001). Longitudinally, high deep medullary veins score was independently associated with new lacunes after adjusting for gender (p < 0.001). The association was also independent of white matter hyperintensities volumes, cerebral blood flow or white matter microstructural parameters (all p < 0.05). Our results suggest that deep medullary veins disruption might be involved in pathogenesis of lacunes.
Keywords: Cerebral small vessel disease, deep medullary veins, new lacunes, susceptibility weighted imaging, diffusion tensor imaging
Introduction
Regarded as an image characteristic of cerebral small vessel disease (cSVD), lacunes are usually round or ovoid, fluid-filled cavities of 3–15 mm in diameter with a signal intensity similar to cerebrospinal fluid (CSF).1 They may be evolved from acute lacunar infarcts, which are related to typical lacunar syndromes and have been associated with subtle cognitive dysfunction and a higher risk of future stroke.2,3
Traditionally, lacunes are considered resulting from the occlusion of a single perforating artery.4 However, increasing evidences demonstrated that other pathogenetic mechanisms were involved in the development of lacunes. Recently, a pathological study reported that venous collagenosis played an important role in the development of white matter hyperintensities (WMHs) and periventricular infarction.5 Moreover, close association between WMHs volume and increased visibility of deep medullary veins (DMVs) was also found on magnetic resonance imaging (MRI).6 As lacunes and WMHs are both important imaging biomarkers of cSVD and have similar pathogenesis, to some extent, it is thus rational to assume that venous disruption may also be involved in the pathogenesis of lacunes. Nevertheless, few studies have investigated the relationship between venous disruption and lacunes, yet.
Previous studies proposed a region-based 18-scale visual DMVs scoring system to assess venous disruption, which could monitor the course of DMVs disruption. They found that increased DMVs score was independently associated with higher WMHs volume.7,8 In this longitudinal study, we used this 18-scale visual DMVs scoring system to assess venous disruption, and explored the relationship between venous disruption and the presence and progress of lacunes in cSVD patients, and further investigated whether this relationship was independent of cerebral blood flow (CBF) and cerebral microstructure damage.
Methods
Study subjects
In this study, we retrospectively reviewed the data of consecutive patients recruited in the CIRCLE study (ClinicalTrials.gov ID: NCT03542734) between January 2010 and June 2018. The CIRCLE study is a single-center prospective observational study that enrolls adults (age >40) with and without cSVD and free of known dementia or stroke, who will then undergo neuropsychological test, retinal digital images and multimodal MRI scan. Patients admitted in our hospital were included if they met the inclusion and exclusion criteria. The inclusion criteria in this study included: (1) age >40; (2) cSVD imaging markers (WMHs with Fazekas score 1–3 in periventricular or deep white matter, lacunes, microbleeds) visible on MRI; (3) at least six months after the onset in patients with acute lacunar stroke; (4) had written informed consent. We excluded patients with: (1) secondary causes of white matter lesions, such as demyelinating, metabolic, immunological, toxic, infectious, and other causes; (2) abnormal brain MRI findings such as head trauma, hemorrhage, non-lacunar infarction and other space-occupying lesions; (3) definitive peripheral neuropathy and spinal cord disease; (4) evidence of calcification on CT scans or encephalomalacia in the deep gray matter structures since it may influence the observation of DMVs.
Ethics statement
All subjects had been given written informed consent prior to the study, and the protocols had been approved by human ethics committee of The Second Affiliated Hospital of Zhejiang University. All clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki.
MRI protocol
All subjects underwent multi-model MRI by a 3.0 T MR (MR750, GE Healthcare, United States) scanner using an 8-channel brain phased array coil, including T1, T2-Fluid-Attenuated-Inversion-Recovery (FLAIR), diffusion-tensor imaging (DTI), 3D-arterial spin labeling (ASL) and Susceptibility Weighted Imaging (SWI) sequence. In order to minimize head motion, foam pads were inserted into the space between the subject’s head and the MRI head coil. The parameters of Axial T2-FLAIR sequence was: repetition time = 8400 ms, echo time = 150 ms, FOV = 24 cm, matrix size = 256 × 256, inversion time = 2100 ms, slice thickness = 4 mm with no slice gap, and in-plane spatial resolution = 0.4688 mm/pixel. For DTI, we performed a single shot, diffusion-weighted spin echo echo-planar imaging sequence. Maximum b-value was 1000 s/mm2 in 30 non-collinear directions; 1 volume was acquired without diffusion weighting (b-value = 0 s/mm2). Other parameters of DTI were as follows: repetition time/echo time = 8000/80.8 ms, flip angle = 90°, slice thickness = 2 mm with no slice gap, matrix size = 128 × 128, FOV = 25.6 cm. 3D-ASL was acquired using spin-echo pulse sequence with repetition time/echo time = 4611/10.5 ms, inversion time = 152 ms, flip angle = 111°, slice thickness = 4 mm, matrix = 128 × 128, FOV = 24 cm. The SWI sequence was in an axial orientation parallel to the anterior commissure to posterior commissure line and covered the whole lateral ventricles, using a three-dimension multi-echo gradient-echo sequence with eight equally spaced echoes: echo time = 4.5 ms (first echo), inter-echo spacing = 3.7 ms, repetition time = 34 ms, FOV= 24 cm, matrix size = 416 × 384, flip angle = 20°, slice thickness = 2 mm with no slice gap and in-plane spatial resolution = 0.4688 mm/pixel. Flow compensation was applied. Parameters of 3D-T1 were repetition time = 7.3 ms, echo time = 3.0 ms, flip angle = 15°, slice thickness = 1 mm, matrix size = 250 × 250, and FOV = 25 cm. The total scan time was 23 min 18 s.
Measurement of DMVs
The raw data were transferred to a separate workstation (ADW4.4, GE), and a custom-built program was used to reconstruct the Magnitude and Phase images. As described previously, we assessed DMVs on five consecutive periventricular slices (10 mm thick) of SWI phase images from the level of the ventricles immediately above the basal ganglia to the level of the ventricles immediately disappeared for each patient, considering that these slices cover most of the DMVs. According to medullary venous anatomy, six regions including frontal region, parietal region and occipital region (bilateral, respectively) were separated on the above five slices and the characteristics of the DMVs were then evaluated in each region, respectively. The following four-point score was used for the evaluation of DMVs: Grade 0—each vein was continuous and had homogeneous signal; Grade 1—each vein was continuous, but one or more than one vein had inhomogeneous signal; Grade 2—one or more than one vein were not continuous, presented with spot-like hypointensity; Grade 3—no observed vein was found continuous. The final DMVs score was the sum of the six regions ranging from 0 to 18.7 The higher score reflects more severe disruption of DWVs. Two neurologists (YZ and QL), blinded to the subjects’ clinical data, visually assessed the vein changes.
Assessment of lacunes
Lacunes were defined on MRI as cavities with a diameter of 3 to 15 mm with signal intensities similar to cerebrospinal fluid in all performed scan sequences in the whole brain. They were different from the enlarged Virchow–Robin spaces by the size, shape, and rim.9
Image analysis
DTI images were post-processed using FSL (http://www.fmrib.ox.ac.uk/fsl) to extract brain, remove bulk motion, and eddy current-induced distortions. Then we calculated microstructural parameters including fractional anisotropy (FA) and mean diffusivity (MD) with DTIfit command in FSL. 3D-ASL images were post-processed to generate CBF images on the separate workstation (ADW4.4, GE).
The segmentation of gray matter, normal appearing white matter (NAWM) and WMHs tissue masks was automatically processed in the native space using 3D-T1 and T2-FLAIR images by lesion segmentation tool (LST) toolbox in Statistical Parametric Mapping Version 12 (SPM12).10 Then the automatically segmented NAWM and WMHs were manually checked and corrected on mricron (http://www.nitrc.org/projects/mricron) by two experienced neuro-radiologists (MZ and SW) who were blinded to all other images and clinical data after NAWM and WMHs were coregistered to the 3D-T1 images through SPM12. The manual correction process included: (1) correction of non-white matter area being labeled as WMHs; (2) WMHs area not adequately labeled as WMHs or normal-appearing white matter falsely labeled as WMHs.
Afterward, the volume of WMHs was measured automatically on mricron. The masks of WMHs and NAWM were used to obtain averaged FA, MD and CBF of corresponding tissues in each participant, including the FA value in WMH (WMH-FA) and in NAWM (NAWM-FA), the MD value in WMH (WMH-MD) and in NAWM (NAWM-MD), and the CBF value in the whole brain (Brain-CBF), WMH (WMH-CBF) and NAWM (NAWM-CBF).
Statistical analysis
Comparison between groups were assessed by using Student’s t test for data that followed normal distribution, Mann–Whitney U test for data that did not follow normal distribution, and Fisher’s Exact test for categorical data. We also conducted binary logistic regression to provide an odds ratio statistic, thus facilitating comparison with other known risk factors. All analyses were performed blinded to the participant identifying information. Statistical significance was set at a p value of < 0.05. All statistical analysis was performed with SPSS package (21st for Windows, IBM).
Results
Patients characteristics
Totally, 203 cSVD patients were enrolled in this study (97 female; mean age, 56.3 ± 20.8 years). The main reasons for admission of those patients were transient ischemic attack or lacunar stroke (included at least six months after the acute onset, n = 110, 54.2%), dizziness (n = 41, 20.2%), cognitive impairment (n = 17, 8.4%), gait disturbance (n = 12, 5.9%), anxiety or depression (n = 11, 5.4%), incidental findings of cSVDs on MRI without symptoms (n = 23, 11.3%). At baseline, 101 (49.8%) patients presented with lacunes with a median number of 2 (1–5) and mean WMHs volume was 35.5 ± 38.0 mL. Follow-up MRI was accomplished among 64 patients at 19.6 ± 10.0 months after the baseline MRI. Among them, 16 (25.0%) patients presented with new lacunes. The patients’ DMVs score ranged from 0 to 18, with a median score of 9 (7–12).
Univariate and multivariate regression analysis for the presence of baseline lacunes
As Supplementary Table 1 shows, patients with baseline lacunes had a higher proportion of diabetes mellitus, higher WMHs volume and higher DMVs scores (all p < 0.05), and a trend of a higher proportion of smoking (p = 0.176), compared with patients without lacunes. Decreased WMH-CBF (22.9 ± 6.4 vs. 26.4 ± 6.1 mL/100 g-tissue/min, p < 0.001), NAWM-CBF (36.6 ± 9.6 vs. 39.6 ± 9.2 mL/100 g-tissue/min, p = 0.026) and Brain-CBF (38.5 ± 10.6 vs. 42.5 ± 10.4 mL/100 g-tissue/min, p = 0.007) were observed in patients with lacunes than those without lacunes (all p < 0.05). Patients with lacunes also had lower WMH-FA (0.30 ± 0.05 vs. 0.32 ± 0.06, p = 0.010) and NAWM-FA (0.33 ± 0.05 vs. 0.36 ± 0.04, p < 0.001) and higher WMH-MD (1.21 ± 0.15 vs. 1.14 ± 0.14, p = 0.002) and NAWM-MD (0.97 ± 0.15 vs. 0.90 ± 0.09, p < 0.001) than those without lacunes.
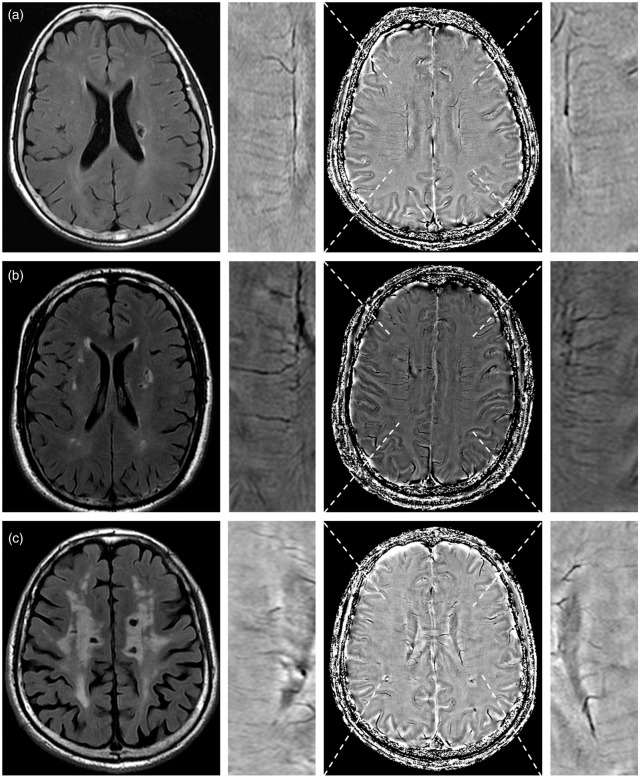
As Table 1 shows, DMVs score was independently associated with the presence of baseline lacunes after adjusting for age and diabetes mellitus, with or without WMHs volume (both p < 0.001 in model 1 and model 2). Moreover, DMVs score was still independently associated with the presence of baseline lacunes, even after additionally adjusting for white matter microstructural parameters or CBF (all p < 0.001, Table 2). Figure 1 shows representative images of the relationship between DMVs and the presence of lacunes.
Table 1.
Binary logistic regression analysis for the presence of baseline lacunes.
| Model 1, OR (p) | Model 2, OR (p) | Model 3, OR (p) | |
|---|---|---|---|
| DMVs score, ±SD | 1.299 (<0.001) | 1.248 (<0.001) | – |
| Brain-CBF, mL/100 g-tissue/min, ±SD | 0.965 (0.012) | 0.977 (0.110) | 0.973 (0.074) |
| WMH-CBF, mL/100 g-tissue/min, ±SD | 0.913 (<0.001) | 0.939 (0.014) | 0.925 (0.004) |
| NAWM-CBF, mL/100 g-tissue/min, ±SD | 0.968 (0.035) | 0.972 (0.086) | 0.965 (0.044) |
| WMH-FA, ×10−3 | 0.993 (0.008) | 0.997 (0.301) | 0.998 (0.505) |
| NAWM-FA, ×10−3 | 0.983 (<0.001) | 0.988 (0.028) | 0.992 (0.167) |
| WMH-MD, ×10−3 mm2/s, ±SD | 21.974 (0.002) | 3.860 (0.232) | 1.680 (0.665) |
| NAWM-MD, ×10−3 mm2/s, ±SD | 167.669 (<0.001) | 12.412 (0.112) | 7.275 (0.249) |
Model 1 included age and diabetes mellitus; Model 2 included age, diabetes mellitus and WMH volume; Model 3 included age, diabetes mellitus, WMH volume and DMVs score.
DMVs: deep medullary veins; CBF: cerebral blood fluid; WMH: white matter hyperintensity; NAWM: normal appearing white matter; FA: fractional anisotropy; MD: mean diffusivity; OR: odds ratio.
Table 2.
Binary logistic regression analysis for the presence of baseline lacunes.
| DMVs score, OR (p) | |
|---|---|
| Adjusted for age, diabetes mellitus, WMH volume (mL) and Brain-CBF (mL/100 g-tissue/min) | 1.255 (<0.001) |
| Adjusted for age, diabetes mellitus, WMH volume (mL) and WMH-CBF (mL/100 g-tissue/min) | 1.274 (<0.001) |
| Adjusted for age, diabetes mellitus, WMH volume (mL) and NAWM-CBF (mL/100 g-tissue/min) | 1.260 (<0.001) |
| Adjusted for age, diabetes mellitus, WMH volume (mL) and WMH-FA (×10−3) | 1.244 (<0.001) |
| Adjusted for age, diabetes mellitus, WMH volume (mL) and NAWM-FA (×10−3) | 1.233 (<0.001) |
| Adjusted for age, diabetes mellitus, WMH volume (mL) and WMH-MD (×10−3 mm2/s) | 1.242 (<0.001) |
| Adjusted for age, diabetes mellitus, WMH volume (mL) and NAWM-MD (×10−3 mm2/s) | 1.241 (<0.001) |
DMVs: deep medullary veins; CBF: cerebral blood fluid; WMH: white matter hyperintensity; NAWM: normal appearing white matter; FA: fractional anisotropy; MD: mean diffusivity; OR: odds ratio.
Figure 1.
Representative images showing the relationship between deep medullary veins (DMVs) and the presence of lacunes. The first column shows the representative slice of lacunes on T2-FLAIR. The third column shows the DMVs on Susceptibility Weighted Imaging (SWI)-phase image. The second column and fourth column show the local magnified images of third column. (a) A patient had a lacuna with a DMVs score of 4. (b) A patient had four lacunes (current slice shows three lacunes) with a DMVs score of 8. (c) A patient had seven lacunes (current slice shows four lacunes) with a DMVs score of 16.
Besides, low CBF was associated with the presence of baseline lacunes independent of WMHs volume and DMVs scores. In addition, white matter microstructural parameters (WMH-FA, WMH-MD, NAWM-FA, and NAWM-MD) were not independently associated with the presence of baseline lacunes (all p > 0.05). In addition, the DVMs score was associated with WMH volume both in patients with lacunes (r = 0.567, p < 0.001) and patients without lacunes (r = 0.318, p = 0.001).
Univariate and multivariate regression analysis of occurrence of new lacunes
Supplementary Table 2 shows that patients with new lacunes had a lower proportion of female (p = 0.043), higher DMVs scores (13.1 ± 3.0 vs. 8.8 ± 4.0, p < 0.001), higher WMH-MD (1.26 ± 0.17 vs. 1.18 ± 0.12, p = 0.041), higher NAWM-MD (0.99 ± 0.17 vs. 0.88 ± 0.10, p = 0.003) and lower NAWM-FA (0.34 ± 0.04 vs. 0.37 ± 0.04, p = 0.009) than those without new lacunes. There were no significant differences in the WMHs volume and follow-up time between two groups. There were no differences in WMHs volume increase, WMH-FA and NAWM-FA decrease, and WMH-MD and NAWM-MD increase from baseline to follow-up between two groups. In addition, all of the patients with new lacunes did not have new stroke or neurological symptoms between baseline and follow-up. Supplementary Table 3 shows the comparison of characteristics between patients with and without follow-up MRI.
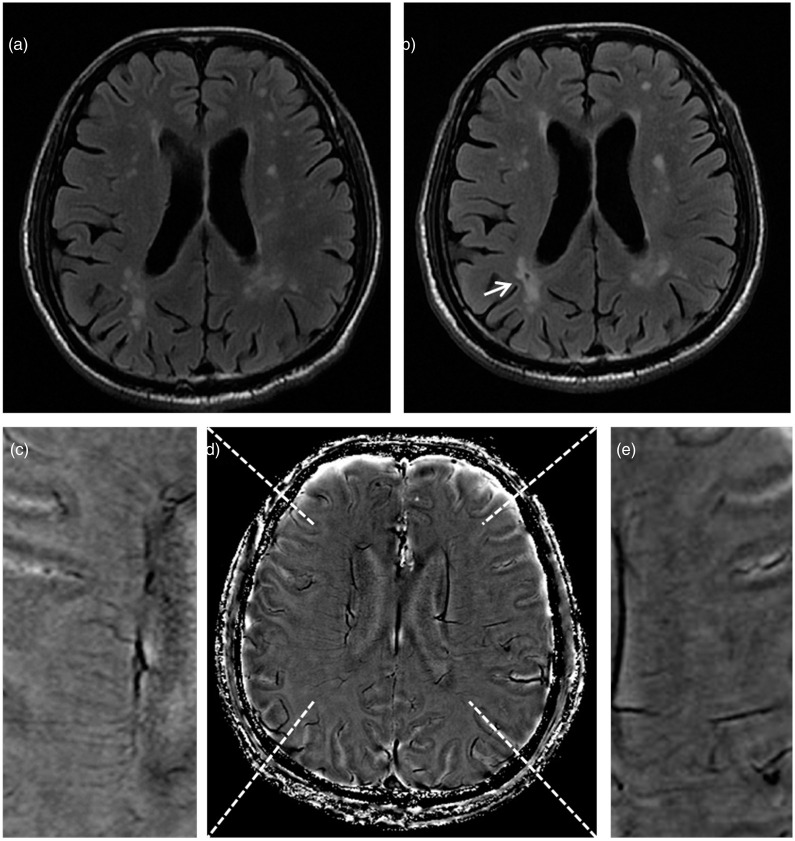
As Table 3 shows, high DMVs score was independently associated with new lacunes (odds ratio = 1.569, p < 0.001) after adjusting for gender. The association was also independent of WHMs volumes, CBF or white matter microstructural parameters (Table 4). Figure 2 shows representative images of the relationship between DMVs and the presence of new lacunes.
Table 3.
Binary logistic regression analysis for the presence of new lacunes.
| Adjusting for female, OR (p) | Adjusting for DMVs score, odds ratio (p) | |
|---|---|---|
| DMVs score, ±SD | 1.569 (<0.001) | 1.350 (0.001) |
| WMH volume, mL, ±SD | 1.025 (0.047) | 1.002 (0.905) |
| Brain-CBF, mL/100 g-tissue/min, ±SD | 1.019 (0.542) | 0.985 (0.590) |
| WMH-CBF, mL/100 g-tissue/min, ±SD | 1.019 (0.713) | 0.986 (0.744) |
| NAWM-CBF, mL/100 g-tissue/min, ±SD | 1.048 (0.187) | 1.003 (0.929) |
| WMH-FA, ×10−3 | 0.991 (0.305) | 0.993 (0.453) |
| NAWM-FA, ×10−3 | 0.979 (0.007) | 0.996 (0.670) |
| WMH-MD, ×10−3 mm2/s, ±SD | 109.743 (0.066) | 6.578 (0.461) |
| NAWM-MD, ×10−3 mm2/s, ±SD | 2778.736 (0.012) | 92.937 (0.150) |
DMVs: deep medullary veins; CBF: cerebral blood fluid; WMH: white matter hyperintensity; NAWM: normal appearing white matter; FA: fractional anisotropy; MD: mean diffusivity; OR: odds ratio.
Table 4.
Binary logistic regression analysis for the presence of new lacunes (DMVs score).
| Adjusting variable | OR (p) |
|---|---|
| WMH volume, mL, ±SD | 1.343 (0.005) |
| Brain-CBF, mL/100 g-tissue/min, ±SD | 1.350 (0.001) |
| WMH-CBF, mL/100 g-tissue/min, ±SD | 1.347 (0.001) |
| NAWM-CBF, mL/100 g-tissue/min, ±SD | 1.351 (0.001) |
| WMH-FA, ×10−3 | 1.337 (0.001) |
| NAWM-FA, ×10−3 | 1.316 (0.011) |
| WMH-MD, ×10−3 mm2/s, ±SD | 1.319 (0.004) |
| NAWM-MD, ×10−3 mm2/s, ±SD | 1.284 (0.008) |
DMVs: deep medullary veins; CBF: cerebral blood fluid; WMH: white matter hyperintensity; NAWM: normal appearing white matter; FA: fractional anisotropy; MD: mean diffusivity; OR: odds ratio.
Figure 2.
Representative images showing the relationship between deep medullary veins (DMVs) and the presence of new lacunes. The patient not presenting lacunes on baseline T2-FLAIR images (a) and developed a new lacune (white arrow) on follow-up T2-FLAIR images (b). To note, the white matter hyperintensities also expand, with a lacune appearing within them (b). The patient had DMVs disruption on baseline, with a DMVs score of 14 on baseline Susceptibility Weighted Imaging (SWI)-phase image (d). (c) and (e) are local magnified images of (d).
Discussion
In the current longitudinal study, we observed that the severity of DMVs disruption (not continuous or with inhomogeneous signal) was not only associated with the presence of baseline lacunes, but also the occurrence of new lacunes after about 2.5 years, independent of WMHs volume, CBF and white matter microstructure damages. Interestingly, reduced CBF was not related to the occurrence of new lacunes, although it was associated with the presence of baseline lacunes.
As the visibility of the venous vessels on SWI-Phase images depends on the de-oxygenation of the blood, the venous heterogeneous signal could be a result of altered venous hemodynamics or venous occlusion.11 Thickening and obstruction of the periventricular veins might result in an increased venous pressure, venular dilatation or venular blood–brain barrier (BBB) disruption.12 Since the disruption of BBB has been implicated as one of the mechanisms involved in the development of lacunes in previous studies,4 it could be speculated that the effects of DMVs on the pathogenesis of lacunes may be mediated by BBB disruption. In addition, it is also possible that venous ischemia due to the venous outflow obstruction may contribute to the development of lacunes. Previously, Keith et al. found that venous collagenosis of both small and large caliber veins was frequent in the periventricular infarcts cohort, and was especially common and severe when infarct was histologically confirmed, suggesting that the stenosis of veins may be a potential pathogenic mechanism underlying periventricular infarcts.5 As a probable common mechanism of lacunes and periventricular infarcts, it might be an evidence in support.13–15
Previously, the assessment of DMVs disruption on images was usually based on the number or thickness of DMVs. For example, Yan et al. demonstrated that increased numbers of voxels of DMVs on SWI-Phase was independently associated with WMHs volume,6 while Keith et al. found that greater stenosis due to collagenosis of large caliber veins correlated with higher WMH scores.13 Actually, postmortem MRI and histopathologic findings have revealed that pathological changes of veins were dynamic, ranging from intramural thickening, stenosis to luminal occlusion, which may result in different voxels and visibility of veins on images during different stage.16 Therefore, in the current study, to assess the role of DMVs in pathogenesis of lacunes, we used the visual score which accords with the pathological dynamic changes of DMVs in the different phases. The changes of DMVs from continuous and homogeneous signal to uncontinuous and spot-like hypointensity represented the pathological changes of DMVs from thickening, stenosis to occlusion. The accuracy of this DMVs score has been approved in our previous study.7
Our findings about the relationship of reduced CBF with the presence of baseline lacunes, but not with the occurrence of new lacunes indicate that lacunes are the reason of reduced CBF, rather than the result of reduced CBF, which is also consistent with previous studies.17–20 Lacunes might lead to down-regulation of CBF because of reduced metabolic demand from lacunes.21 In addition, the reduced CBF could be the results of total cSVD damage, as the disruption of DMVs might lead to the total cSVD damage of tissue. Further studies with longer observation period and follow-up CBF data are thus needed to illustrate them.
We also found that white matter microstructures damages (high FA and low MD) were related to the presence of lacunes, which could be explained by the total cSVD damage of tissue. However, the relationship was less significant after adjusting for WMHs volume. It might be explained by the intimate connection of lacunes and WMHs. Ghaznawi et al. also found that patients with lacunes had greater WMHs volumes than patients without lacunes. Meanwhile, previous studies found significant association between WMHs volume and white matter microstructure damages, that is, subjects with larger WMHs burden were more likely to show lower FA and higher MD in both NAWM and WMHs.22 Therefore, we speculated that the relationship between white matter microstructures and lacunes might be partly mediated by WMHs injury. Notably, when only adjusting for DMVs, both white matter microstructures damages and WMH volumes were not related to the occurrence of new lacunes, which highlights the major contribution of DMVs to the development of lacunes.
Strengths of our study include the longitudinal design and the available data on quantitative measurements of CBF, DTI parameters, WMHs, and lacunes, which allowed us to examine the relationships among CBF, white matter microstructure, WMHs, and lacunes over time. The findings about the relationship between DMVs and the development of lacunes in a sample of cSVD offer new insights into the mechanisms underlying lacunes, whereas most studies focused on the role of arteries.
Our study had limitations. First, the signal to noise ratio (SNR) on SWI Phase images is relatively lower than that on minimum intensity projection (mIP) venograms, but mIP images are lack of three-dimensional information of blood vessels. Besides, our assessment of DMVs was on a 3T brain MRI and the comparatively lower field strength may reduce the detection quality of DMVs. Second, the visualization of DMVs could depend critically on the tissue function itself. For example, slow flow in healthy tissue would lead to high visibility of DMVs, but in necrotic or compromised tissue would lead to low visibility. Third, due to the lack of data on BBB, the mechanism between DMVs and lacunes could not be elaborated clearly. Our hypothesis still needs further investigation. Fourth, the relatively small follow-up sample might lead to a potential risk of selection bias. The limited case numbers with follow-up and the relatively short follow-up time might also contribute to the non-significant relationship between WMH worsening and the presence of new lacunes. Future study is needed to clarify it. In addition, patients with follow-up had milder WMH lesions than patients without follow-up MRI, which could affect the analysis results of new lacunes. Fifth, our cohort only included Chinese patients at a single institution, it may not represent the full spectrum of lacunes, and the generalizability of our results need confirmation and extension in larger and multicenter cohorts.
In summary, our finding suggested that DMVs disruption might be involved in the pathogenesis of lacunes. It should be further confirmed in large cohorts and in prospective studies as other cSVD markers such as WMHs might also participate in the evolution.
Supplemental Material
Supplemental material, JCB882918 Supplemental Material for Role of deep medullary veins in pathogenesis of lacunes: Longitudinal observations from the CIRCLE study by Ying Zhou, Qingqing Li, Ruiting Zhang, Wenhua Zhang, Shenqiang Yan, Jinjin Xu, Shuyue Wang, Minming Zhang and Min Lou in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Key Research and Development Program of China (2016YFC1300504), National Natural Science Foundation of China (81622017, 81701150), Science Technology Department of Zhejiang Province (2018C04011), Chinese Cardiovascular Association-V.G found (2017-CCA-VG-004), and Basic Public Interests of Research Plan of Zhejiang Province (GF18H090006).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
YZ, QL and RZ drafted the manuscript, participated in study design and data collection, conducted the statistical analyses, analyzed, and interpreted the data. ML participated in study design and data collection, data interpretation and made a major contribution in revising the manuscript. JX, WZ and SW participated in the data collection and made contribution in revising the manuscript. SY and MZ assisted in designing the MRI sequences, data collection and imaging analysis.
Supplemental material
Supplemental material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roman GC, Erkinjuntti T, Wallin A, et al. Subcortical ischaemic vascular dementia. Lancet Neurol 2002; 1:426–436. [DOI] [PubMed] [Google Scholar]
- 3.Arboix A, Font A, Garro C, et al. Recurrent lacunar infarction following a previous lacunar stroke: a clinical study of 122 patients. J Neurol Neurosur Ps 2007; 78:1392–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Sandercock PAG, Dennis MS, et al. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003; 34:806–812. [DOI] [PubMed] [Google Scholar]
- 5.Keith J, Gao FQ, Noor R, et al. Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropath Exp Neur 2017; 76:299. [DOI] [PubMed] [Google Scholar]
- 6.Yan S, Wan J, Zhang X, et al. Increased visibility of deep medullary veins in leukoaraiosis: a 3-T MRI study. Front Aging Neurosci 2014; 6:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Zhou Y, Yan S, et al. A brain region-based deep medullary veins visual score on susceptibility weighted imaging. Front Aging Neurosci 2017; 9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C, Pennington MA.MR evaluation of developmental venous anomalies: medullary venous anatomy of venous angiomas. Am J Neuroradiol 1996; 17:61–70. [PMC free article] [PubMed] [Google Scholar]
- 9.Gouw AA, Wm VDF, Fazekas F, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the leukoaraiosis and disability study. Stroke 2008; 39:1414. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 2012; 59:3774–3783. [DOI] [PubMed] [Google Scholar]
- 11.Alexander R, Jan S, Markus B, et al. Noninvasive assessment of vascular architecture and function during modulated blood oxygenation using susceptibility weighted magnetic resonance imaging. Magn Reson Med 2010; 54:87–95. [DOI] [PubMed] [Google Scholar]
- 12.Moody DM, Brown WR, Challa VR, et al. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 1995; 194:469–476. [DOI] [PubMed] [Google Scholar]
- 13.Keith J, Gao FQ, Noor R, et al. Collagenosis of the deep medullary veins: an underrecognized pathologic correlate of white matter hyperintensities and periventricular infarction? J Neuropath Exp Neur 2017; 76:299–312. [DOI] [PubMed] [Google Scholar]
- 14.Caplan LR.Lacunar infarction and small vessel disease: pathology and pathophysiology. J Stroke 2015; 17:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardlaw JM, Smith C, Dichgans M.Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black S, Gao F, Bilbao J.Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke 2009; 40(3 Suppl):S48–52. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki Y, Oishi M, Takasu T.Cerebral blood flow in single and multiple lacunar infarctions. Stroke 1997; 28:1458–1460. [DOI] [PubMed] [Google Scholar]
- 18.Vernooij MW, Aad VDL, Mohammad Arfan I, et al. Total cerebral blood flow and total brain perfusion in the general population: the Rotterdam Scan Study. J Cereb Blood Flow Metab 2007; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 19.van der Veen PH, Muller M, Vincken KL, et al. Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow: the second manifestations of arterial disease-magnetic resonance study. Stroke 2015; 46:1233–1238. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Thrippleton MJ, Blair GW, et al. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J Cereb Blood Flow Metab 2018: 271678X18803956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nezu T, Yokota C, Uehara T, et al. Preserved acetazolamide reactivity in lacunar patients with severe white-matter lesions: 15O-labeled gas and H2O positron emission tomography studies. J Cereb Blood Flow Metab 2012; 32:844–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moscufo N, Wakefield DB, Meier DS, et al. Longitudinal microstructural changes of cerebral white matter and their association with mobility performance in older persons. PLoS One 2018; 13:e194051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JCB882918 Supplemental Material for Role of deep medullary veins in pathogenesis of lacunes: Longitudinal observations from the CIRCLE study by Ying Zhou, Qingqing Li, Ruiting Zhang, Wenhua Zhang, Shenqiang Yan, Jinjin Xu, Shuyue Wang, Minming Zhang and Min Lou in Journal of Cerebral Blood Flow & Metabolism