Abstract
Advances in stem cell technology have provided three approaches to address the demanding issue of the treatment of intractable neurological disease. One of the approaches is the screening of compounds attenuating pathological phenotypes in stem-cell based models. A second approach consists of exogenous-targeted cell supplementation to the lesion with stem cell-derived differentiated cells. A third approach involves in vivo direct programming to transdifferentiate endogenous somatic cells and to boost CNS tissue remodeling. In this review, we outline research advances in stem cell technology of direct reprogramming in vitro and in vivo and discuss the future challenge of tissue remodeling by neural transdifferentiation.
Keywords: in vivo direct reprogramming, transdifferentiation, CNS injury, neurodegenerative disease, iPSC
Introduction
Stem cell technology opened a new road for advancement of the clinical approach of regenerative medicine for incurable central nervous system (CNS) diseases.1,2 The availability of induced pluripotent stem cells (iPSCs)-derived human neurons is now leading the way toward the accessibility of novel drugs for incurable neurodegenerative diseases.3 Another regenerative medical approach with iPSCs technology is that of cell supplementation into affected CNS regions, contributing to new autologous transplantation therapy for incurable diseases.4,5
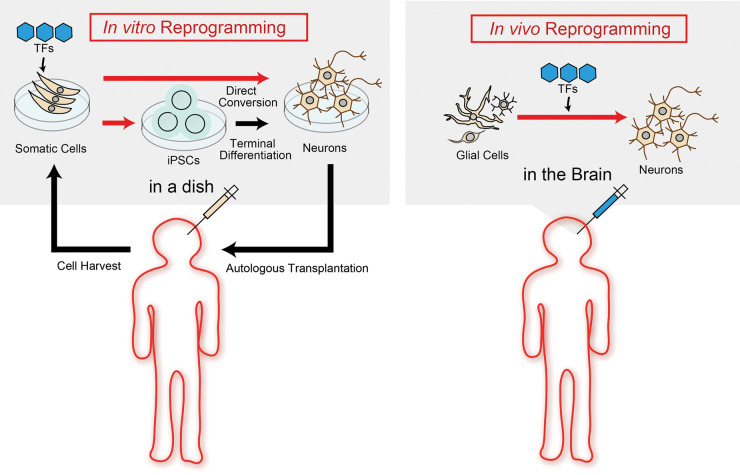
Stem cell technology has resulted in the novel research field of transcriptional factors (TFs)-mediated cell fate conversion in vitro.6 Furthermore, previous studies have demonstrated that this reprogramming technique using defined TFs can be applicable to not only in vitro but also in vivo direct reprogramming to transdifferentiate somatic cells across different cell lineages in both acute CNS injury and neurodegenerative disease model rodents.7 This should offer the next promising milestone for future regenerative medicines for CNS diseases, since it is a less invasive approach than cell transplantation and does not need to depend upon immunosuppressive therapy (Figure 1).
Figure 1.
In vitro and in vivo reprogramming for neural transdifferentiation. Left panel (In vitro reprogramming): Cells derived from patients are reprogrammed into iPSCs or targeted cells using defined transcriptional factors (TFs) transfer in a dish; Right panel (In vivo reprogramming): Glial cells are directly reprogrammed into targeted cells using defined TFs transfer in the brain.
In the acute phase of CNS diseases such as ischemia and traumatic injury, gliosis accompanying neuronal cell death is a common pathological change, where glial cells including astrocytes, oligodendrocyte precursor cells (OPC) and microglia proliferate or/and migrate to be activated for the clearance of debris or filling-out sparse space and focal tissue remodeling of injured lesion.8 In general, neurological symptoms depend on the severity of the injury to mature neurons, implying that glial cells surrounding injured core lesions could be a main cell source for in vivo direct conversion into functional neurons. In healthy CNS, an operational cellular assembly between endothelial cells, glial cells and neurons is called a neuro-vascular unit (NVU), whose crosstalk is critical for the integration of each functional activity.9 Therefore, acute neuronal loss could disrupt this NVU homeostasis, the cell-cell interaction, and also cause further delays in NVU and neural network remodeling. This suggests that early therapeutic intervention aimed at facilitating the NVU remodeling can improve the prognosis of patients with acute CNS injury.
Gliosis is also a common pathological change associated with chronic cell death in neurodegenerative diseases and might precipitate hazardous effects on disease progression and even determine the disease onset in some diseases.10,11 In contrast, gliosis may also have a protective role in pathogenesis by the clearance of pathogenic protein accumulated in the brain in other neurodegenerative diseases.12,13 Therefore, appropriate selection of the original cell source and target cells would be important for the future clinical application of in vivo reprogramming in CNS diseases.
Here, we outline the advances of stem cell technology for reprogramming and discuss the future prospects of in vivo direct reprogramming to transdifferentiate neural cells for tissue remodeling in acute CNS injury and for disease modifying for chronic neurodegenerative diseases.
In vitro reprogramming to determine cell fate
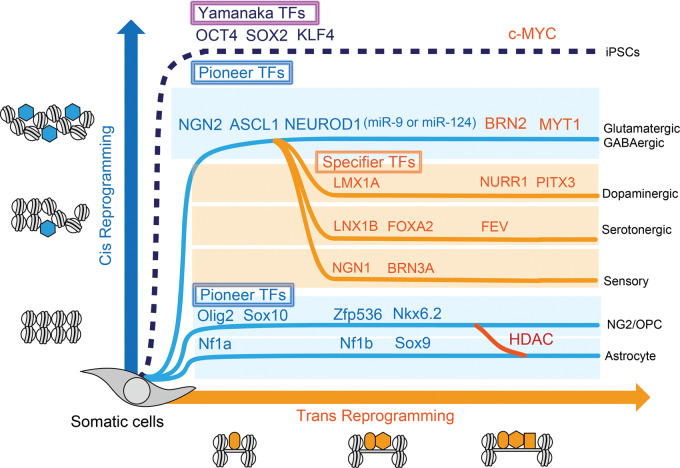
Forced ectopic expression of genes that are developmentally silenced in closed chromatin is critical for the reprogramming process. The discovery of ‘Yamanaka-TFs14 enabled us to recognize the existence of pioneer TFs that are critical chromatin modifiers engaging in targeted sites on nucleosomal DNA and opening chromatin, and they differ from conventional TFs that simply regulate gene expression by binding the promoter region of DNA.15 Pioneer TFs have cis-acting elements to access tightly packed chromatin and induce nucleosome remodeling, whereas specifier TFs have DNA-binding motifs that bind exposed trans-acting elements of promoter regions to regulate gene expression. Epigenetic regulators including histone modifiers (e.g. histone deacetylases: HDACs, histone acetyltransferases: HATs) also play cis-acting regulatory roles in gene expression that allows passive access of several TFs to the promoter region of a set of genes.16,17 Therefore, the in vitro reprogramming process to determine the cell fate of somatic cells can be divided into two operations – ‘Cis-reprogramming’ by pioneer TFs or epigenetic regulators and ‘Trans-reprogramming’ by specifier TFs. For example, among ‘Yamanaka-TFs’, Oct4, Sox2 and Klf4 are known to act by cis-reprogramming as pioneer TFs, whereas c-Myc only binds to opened chromatin to act by trans-reprogramming as secondary enhancer, which is not necessary for generating iPSCs.18 Pioneer TFs drive somatic cells to be multi-potent cells that can have the potential to differentiate into subtypes of neurons that could be determined by defined specifier TFs as additional maturation steps. Cell fate diversity can be dependent on transition via a progenitor cell (Figure 2).
Figure 2.
Two operations of in vitro direct reprogramming for somatic cells. Pioneer transcriptional factors (TFs) (blue hexagons) open chromatin and involve in cis-reprogramming events and specifier TFs (orange squares) involve in trans-reprogramming events as additional cooperative factors to activate gene regulatory network to terminal differentiation. Histone deacetylase (HDAC) could modulate glial cell fate between NG2/OPC and astrocytes.
After primary de-condensing of chromatin and exposing multiple gene promoters by the use of ectopic pioneer TFs or histone modifiers, in vitro reprogramming can be achieved by either of the following processes: (1) direct binding by ectopic specifier TFs: direct reprogramming, (2) passive/non-specific binding by endogenous specifier TFs: passive/indirect reprogramming.
In vitro direct reprogramming to determine cell fate
Stem cell technology has promoted the next step of TFs-mediated conversion of somatic cells into targeted terminated cells19 (Table 1). Vierbuchen et al.6 demonstrated the TF-initiated direct conversion into neurons by defined factors from fibroblasts in vitro and opened a new avenue into the research field related to direct conversion. They used a combination of only three TFs – achaete-scute homologue 1 (Ascl1), Brn2, and myelin transcription factor 1-like protein (Myt1l) (referred to as BAM) to generate synapse-forming functional neurons from mouse fibroblasts and human fibroblasts (iNs),20–22 where Ascl1 acts as a pioneer TF, Brn2 binds to the site of Ascl1, and Myt1l later binds to the specific site for neuron maturation23 (Figure 2). The addition of pioneer TF, neurodifferentiation D1 (NEUROD1) to BAM, is necessary for efficient conversion from human fibroblasts into functional iN.20 Neurogenin-2 (Ngn2) is also a pioneer TF that can convert astrocytes into neurons in vivo and in vitro.24,25 The combination of pioneer TFs, ASCL1 and NGN2 strongly convert human fibroblasts, even if derived from aging humans, into functional iNs.26,27 BAM plus NGN2 (termed BAMN) can convert T-cell lymphocytes in peripheral blood into neurons.28 MicroRNAs (miRNAs) can convert fibroblasts into iN through NeuroD2 activation.29
Table 1.
In vitro/in vivo direct reprogramming into target cell with differential transcriptional factors.
| Type | Cell source | Transcriptional factor | Target cells | References |
|---|---|---|---|---|
| Vitro | Mouse fibroblast | Ascl1 + Brn2 + Myt1 (BAM) | Glut | Vierbuchen et al.6 |
| Vitro | Human fibroblast | ASCL1 + BRN2 + MYT1 + NEUROD1 | Glut/GABA | Pang et al.20 |
| Vitro | Mouse astrocyte | Pax6 | Immature neuron | Heins et al.24 |
| Vitro | Mouse astrocyte | Ngn2 | Glut/GABA | Heinrich et al.25 |
| Vitro | Human fibroblast | NGN2 + ASCL1* (with small molecules) | Glut/GABA | Ladewig et al.26/ Zhao et al.27 |
| Vitro | Human T-cell lymphocyte | ASCL1 + BRN2 + MYT1L + NGN2 (BAMN) | Neuron | Tanabe et al.28 |
| Vitro | Human fibroblast | Ascl1 + Brn2 + Myt1 (BAM) + FoxA2 + Lmx1a | Dopaminergic | Pfisterer et al.22 |
| Vitro | Human/Mouse fibroblast | Ascl1 + Lmx1a + Nurr1 (ALN) | Dopaminergic | Caiazzo et al.31 |
| Vitro | Mouse fibroblast/astrocyte | ASCL1 + LMX1B + NURR1 (ALN) | Dopaminergic | Addis et al.62 |
| Vitro | Human fibroblast | ASCL1 + NGN2 + NURR1 + PITX3 | Dopaminergic | Liu et al.32 |
| Vitro | Human fibroblast | ASCL1 + LMX1B + FEV + FOXA2 | Serotonergic | Xu et al.33 |
| Vitro | Human/Mouse fibroblast | Ascl1 + Brn2 + Myt1 + Hb9 + Ngn2 + Isl1 + Lhx3 | Spinal Motor neurons | Son et al.21 |
| Vitro | Human iPSC | NGN2 + ISL1 + LHX3 (NIL) | Spinal Motor neurons | Garone et al.34 |
| Vitro | Human/mouse fibroblast | Ngn1and/or Ngn2 + Brn3a | Peripheral neurons | Blanchard et al.35 |
| Vitro | Mouse fibroblast | Sox10 + Olig2 + Nkx6.2 | NG2/OPC | Najm et al.39 |
| Vitro | Mouse/rat fibroblast | Sox10 + Olig2 + ZFP536 | NG2/OPC | Yang et al.40 |
| Vitro | Mouse fibroblast | Nf1a + Nf1b + Sox9 | Astrocyte | Caiazzo et al.41 |
| Vitro | Human neural stem cell | NF1A | Astrocyte | Tchieu et al.42 |
| Vitro | Human pericyte | ASCL1 + SOX2 | Neural stem cell | Karow et al.68 |
| Vivo | Mouse astrocyte | Ngn2 | Glut | Heinrich et al.25 |
| Vivo | Mouse astrocyte | Ngn2* (with EGF + FGF2) | ND | Grande et al.58 |
| Vivo | Mouse astrocyte | Ngn2 + Bcl2 | Glut | Gascon et al.59 |
| Vivo | Mouse astrocyte | Ascl1 + Brn2 + Myt1 (BAM) | Immature/nd | Torper et al.60 |
| Vivo | Mouse astrocyte | Ascl1 | GABA | Liu et al.61 |
| Vivo | Mouse astrocyte | NeuroD1 | Glut/GABA | Guo et al.7 |
| Vivo | Mouse/human astrocyte | NEUROD1 + ASCL1 + LMX1A + miR218 (NeAL218) | Dopaminergic | Rivetti et al.57 |
| Vivo | Mouse astrocyte | SOX2* (with noggin + BDNF) | Neuroblast | Niu et al.64 |
| Vivo | Mouse NG2/OPC | NeuroD1 | Glut/GABA | Guo et al.7 |
| Vivo | Mouse NG2/OPC | Ascl1 + Lmx1a + Nurr1(ALN) | Glut/GABA | Torper et al.56 |
| Vivo | Mouse NG2/OPC | Sox2 | GABA | Heinrich et al.67 |
These iNs are prone to be characterized as glutamatergic neurons and/or a minor group of GABAergic neurons,20,26,29 suggesting that a set of endogenous specifier TFs can easily direct to these types of cells under open-chromatin condition as default iNs. To target a specific subtype of iNs such as dopaminergic neurons (iDAN),22,30–32 serotonergic neurons,33 spinal motor neurons (MNs)21,34 or peripheral neurons,35 defined sets of exogenous specifier TFs might be needed as shown in Table 1.
Other types of neuronal cells besides neurons can also be generated by a similar direct reprogramming technique. NG2 cells express NG2 chondroitin sulfate proteoglycan (CSPG4),36 and a multipotent stem cell that can proliferate itself and can differentiate mainly into oligodendrocytes which were originally purified as bipotential oligodendrocyte-type2 astrocyte progenitor cells (O-2A cells).37 Therefore, NG2 cells are also referred to as OPC or polydendrocytes.38 Induced NG2/OPC (iOPC) can be directly generated from fibroblasts by Sox10 and oligodendrocyte transcriptional factor 2 (Olig2) with either zinc-finger protein 536 (ZFP536) or Nkx6.2.39,40 Induced astrocytes (iAs) can also be generated from mouse fibroblasts through exogenous TFs of Nuclear factor 1A (Nf1a), Nf1b and Sox9.41 A previous study has demonstrated that NF1A is enough for generating iAs from human neural stem cells.42
Finally, emerging data suggest that neural crest-derived sources may also be a potential source for an endogenous transdifferentiation response. The neural crest is a unique and transient embryonic cell population that originates in the ectoderm within margins of the neural tube. After a phase of epithelial-mesenchymal transition and extensive migration, neural crest-derived stem cells (NCSCs) settle down in different parts of the body to contribute to the formation of a plethora of different organs and tissues via differentiation into neurons, glia (or Schwann cells), and mesenchymal derivatives.43 Intriguingly, a subset of NCSCs may be present in bone marrow, meninges, dental tissue, gut, heart, and skin in adults.44,45 Neural crest cells may be directly induced from human fibroblasts by reprogramming with SOX10 along with a WNT activator46 or with gene transfer of FOXD3 together with a chitosan substrate.47 Applications of patient-specific NCSCs with the reprogramming technique may provide a better understanding of the regeneration of mesodermal and ectodermal cells and tissues in various disease conditions.
In vitro passive/indirect reprogramming to determine cell fate
Combinations of methylated DNA with acetylated/deacetylated histones are involved in “open” or “closed” chromatin configuration for gene expression, implying that nucleosomal modification could work as a cis-acting element and allow passive access of endogenous specifier TFs to the specific promoter region, that is, the so-called passive/indirect conversion.
Past studies demonstrated that NG2/OPC are not only restricted to generating oligodendrocytes but they also produce astrocytes and neurons under certain conditions.48 The cell fate of NG2/OPC is regulated by DNA chromatin modifications as epigenetic regulation by epigenetic modifiers such as histone modifier, HDACs, HATs and DNA methyltransferases (DNMTs), suggesting that indirect reprogramming through epigenetic regulators could have the potential to reprogram NG2/OPC into neurons. HDAC1/2 double-knockdown suppressed terminal differentiation of NG2/OPC into oligodendrocytes.49,50 HDAC3 ablation led to an increase in astrocytes together with a loss of oligodendrocytes.51 The authors showed that HDAC3 interacted with p300 HAT to open the chromatin configuration to activate oligodendrocyte lineage-specific genes.51 These studies indicated that passive/indirect reprogramming through HDACs regulation could have the potential to transdifferentiate oligodendrocytes into NG2/OPC.
DNMTs consist of DNMT1, DNMT3A, and DNMT3B. DNMT1 is necessary for the maintenance of DNA methylation, and DNMT3A/3B are necessary for de novo DNA methylation.52 DNMT1 is upregulated in NG2/OPC differentiation during the prenatal stage in vivo and in vitro, and its activity decreases with age.53 DNMT1 supports NG2/OPC survival in vitro. Global DNA methylation by DNMT3A is required for efficient BAM-mediated in vitro reprogramming into iN,54 suggesting that the activity of DNMTs would support cellular plasticity and upregulate the neural transdifferentiation potential.
In vivo reprogramming to determine cell fate
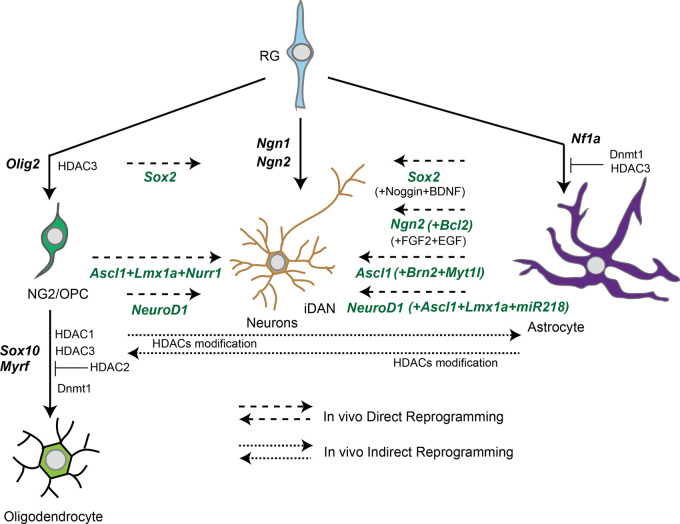
Advances of in vivo reprogramming studies have demonstrated that glial cells could be a cell source of neurogenesis after acute/chronic neuronal loss in disease progression.55 TFs involved in brain development are expected to be important pioneer TFs that determine cell fate not only in vitro but also in terminally differentiated cells in vivo. Among them, major pioneer TFs common in in vivo direct reprogramming from glial cells are Ngn2, Ascl1 and NeuroD1 (Figure 3), and all these TFs are potent converters by cis-reprogramming action in vitro as mentioned above. It should be noted that specifier TFs in vivo direct reprogramming does not function the same as in vitro. For example, Ascl1, Lmx1a, and Nurr1 (referred to as ALN) are originally used for obtaining iDAN from fibroblasts in vitro,31 but in vivo conversion using the ALN combination cannot direct iDAN, but other types of neurons, glutamatergic or GABAergic neurons.56 An additional pioneer TF, NEUROD1, is necessary to achieve in vivo conversion from glial cells into iDAN.57 These proposals suggest that a combination of potent pioneer TFs and additional specifier miRNAs, suppressors of the original TFs specifically expressed in the terminally differentiated cells, will be effective for in vivo direct reprogramming.
Figure 3.
In vivo reprogramming between neurons and glial cells via pioneer transcriptional factors (TFs).Long dash lines indicate in vivo direct conversion pathway with pioneer TFs and specifier TFs. Short dash lines indicate in vivo indirect conversion pathway with epigenetic regulators. Solid lines indicate terminal differentiation with essential TFs for brain development. RG: radial glia; HDAC: histone deacetylase.
In vivo direct reprogramming from astrocytes to neurons (in vivo A-to-N)
Pax6 was the first identified TF to have the potential to reprogram glial cells into neuronal cells such as GABAergic or glutamatergic cells in vitro as the transcriptional factor expressed in radial glia and which positively regulated the expression of Ngn2.24
Ngn2 has been identified as a pioneer transcript factor in in vivo A-to-N, which converted terminally differentiated astroglia into synapse-forming glutamatergic neurons.25 A combination of the neurotrophic factors FGF2 and EGF could reprogram astrocytes into functional neurons in the neocortex and striatum even after traumatic injury.58 The co-expression of Bcl2 with Ngn2 boosts the efficiency of conversion from astrocytes to glutamatergic neurons after stab wound injury.59
Ascl1 is likely another potent pioneer TF in in vivo A-to-N. A combination of transcriptional factors of Ascl1, Brn2 and Myt1l converted mouse astrocytes into immature neuron-like cells in the mouse brain with low efficiency (∼6%).60 Another group revealed that only Ascl1 could induce astrocytes in the midbrain into functional GABAergic neurons in postnatal mouse.61 Efficient reprogramming of astrocytes to dopaminergic neurons can be achieved by a single polycistronic lentiviral vector carrying three transcription factors – ASCL1, LMX1B, and NURR1. The process was efficient, with about 18% of cells expressing markers of dopaminergic neurons after two weeks of in vitro study.62
A third pioneer transcriptional factor is NeuroD1, which plays a role in neurogenesis63 and can be used for reprogramming from reactive astrocytes into functional glutamatergic neurons with high effectivity after traumatic injury.7 A previous study demonstrated that NEUROD1 with ASCL1, LMX1A, miRNA, miR218 (termed NeAL218) directly converted striatal astrocytes into iDAN.57 They also succeeded in correcting the motor functional phenotype in a Parkinson’s disease (PD) model using iDAN.
Sox2 has also been identified as having the potential, as transcriptional factor, to reprogram glial cells into neuroblast cells. The subsequent addition of noggin and BDNF results in functionally mature neurons.64
In addition to exogenous inducers of reprogramming, the endogenous responses of trans-differentiation may also be worth further investigation. Transcription factor-mediated in vivo reprogramming in the brain is mostly performed via stereotaxic injection of viruses for over-expression in the CNS.55 Chemical approaches have also been described. Human astrocytes were sequentially treated to a cocktail of small molecules that inhibit glial but activate neuronal signaling pathways.65,66 This method successfully reprograms astrocytes into neurons in 8–10 days through epigenetic regulation and transcriptional activation.65,66 It has been reported that human astrocytes-derived neurons can survive for more than five to seven months in vitro and form functional synaptic networks with synchronous burst activities.65,66 Chemically reprogrammed human neurons can also survive for more than one month in mouse brain in vivo and integrate into local circuits.66 When administered in vivo through intracranial or intraperitoneal injection, combinations of various small molecules significantly increase hippocampal neurogenesis in mice.65 Chemical reprogramming of human astrocytes into functional neurons may therefore provide another translationally relevant approach to regenerate functional neurons from patients’ endogenous glial cells for brain repair.
In vivo direct reprogramming from NG2/OPC to neurons (in vivo O-to-N)
Guo et al.7 found that NeuroD1, which is a potent TF in A-to-N, can convert NG2/OPC into GABAergic neurons and glutamatergic neurons. Torper et al.56 demonstrated that improved delivery of the three TFs Ascl1, Lmx1a and Nurr1 (generally referred as ALN) using AAV could reprogram NG2/OPC into functional GABAergic neurons. In addition, Sox2 has also been reported to convert NG2/OPC into GABAergic neurons after stab-injury.67 Interestingly, SOX2 can convert human pericytes into functional neurons with ASCL1 (in vitro study),68,69 suggesting that SOX2 could convert cells into ‘fractional’ neural stem-like cells as weak pioneer TFs.70
In vivo reprogramming strategy for CNS diseases
In this section, we will introduce a potential therapeutic approach using an in vivo reprogramming technique to convert residential glial cells into functional CNS cells directly or to boost endogenous cellular plasticity to transdifferentiate into other types of neural cells after acute CNS injury and chronic neurodegenerative diseases. Blood–brain barrier (BBB) breakdown after disruption of NVU is a pathological feature common to both acute CNS injury and some chronic neurodegenerative diseases, creating an attractive delivery site of ectopic TFs for in vivo direct reprogramming.
In vivo reprogramming strategy for acute CNS injury
Representative acute focal injuries in CNS are traumatic injury and ischemic stroke. Both trauma and stroke result in vascular damage and the following BBB breakdown. There are three phases in tissue remodeling after acute CNS injury, as follows.8 The first phase of acute CNS injury (within 1 day after injury) is cell death of parenchymal cells (i.e. neurons and glial cells) and platelet aggregation to serve the recruitment of inflammatory cells and residual/circulating immune cells through a leaky BBB. Neuronal cell death and apoptosis result from an insufficiency of energy such as lactate supplied by damaged glial cells and from massive Ca2+ influx into neurons. Infiltrated immune cells/microglia phagocytose cellular debris and damaged cells release danger-associated molecular patterns (DAMPs) and alarmins that promote non-infectious inflammation. Efficient removal of damaged glial cells protects neurons from myelin-associated inhibitory proteins for axonal regeneration secreted by damaged glial cells.71
The second phase of acute CNS injury (2–10 days after injury) is tissue replacement by proliferation of intrinsic CNS cells such as endothelial progenitors, fibroblast-lineage cells and various glial cells.72 Finally, the third phase of CNS injury (over 10 days after injury) is tissue remodeling under scar formation by functional astrocytes to surround fibroblast-lineage cells and pericytes and promote angiogenesis. Astrocyte scar formation serves as a border that protects healthy neurons in peri-lesions from further migration of inflammatory cells and cytotoxic cytokines and molecules.73
An in vivo direct reprogramming approach may be effective during the second and third phases of active cellular proliferation for replacement in acute CNS injury. Endothelial progenitors, fibroblast-lineage cells and various types of glial cells and pericytes would be the source of cells of direct reprogramming by delivery of TFs. Conventional delivery methods consist of direct injection of virus vectors such as retrovirus and lentivirus. Previous studies have highlighted the potential application of exosome, which is a nanosized vesicle, as miRNA delivery.74–76 Delivery of engineered exosome, the half-life of which is increased by CD47 expression,77 suppressed pancreatic cancer by silencing oncogenic KRAS in vivo.78 Delivery of such engineered exosome packing TFs through leaky BBB in vivo might be a safe and promising approach for direct reprogramming into target neurons during the second and third phases after acute CNS injury.
Besides in vivo direct reprogramming, in vivo passive/indirect reprogramming using epigenetic modifiers may also be effective for early tissue remodeling in all phases after acute CNS injury. HATs bind to transformation-related protein (TRP53) to form a transcriptional complex, which enhances the accessibility of the promoter of regeneration-associated genes (RAGs), such as RAB13, CORO1B and growth-associated protein (GAP43). Increasing histone acetylation by HDAC inhibitors induced GAP43 expression as well as the improvement of axon regeneration after injury.79–81 HDAC5 may play an important role in axonal regeneration after injury via regulating microtubule dynamics.82,83 In addition, HDAC6 also contributes to axon regeneration, as its inhibition enhanced tubulin acetylation levels to promote axon regeneration of DRG neurons in the presence of myelin-associated glycoprotein.84 Therefore, the epigenetic regulation of gene expression related to axon regeneration could offer a promising approach toward increasing the neuronal network after CNS injury.
BBB modeling using iPSC-derived cells has been intensively studied to mimic a neurovascular unit. A combination of endothelial cells derived from iPSCs with human primary pericytes and astrocytes in Transwell® or a microfluidic chip setting could mimic a BBB co-culture model with formation of a tight junction, suggesting that physiological contact between heterogenous cells derived from iPSCs could achieve self-BBB remodeling.85,86 BBB modeling using iPSC-derived cells can provide us with a novel platform where the delivery of TFs toward in vivo reprogramming can be examined.
Looking at cell-based regenerative medicine via cell-reprogramming in the clinical field of stroke, three transplantation clinical trials have largely succeeded in lab-to-clinic translational application of reprogrammed cells. The pioneering study by Layton Bioscience Inc. used a cocktail of growth factors and mitotic inhibitors that turned teratocarcinoma cells into post-mitotic-like neurons called NT2N.87–89 Subsequently, SanBio Inc. initiated clinical trials of intracerebral implantation of SB623® into patients in chronic stage of stroke. SB623® are bone marrow-derived cells that exhibit a neuronal phenotype by reprogramming with Notch-1 gene transfer.90–92 Reneuron Inc. started trials of transplanting immortalized fetal cortical cells called CTX0E03® via control of c-mycERTAM.93,94 These stem cell trials were intended to boost CNS tissue repair that supports the survival of intrinsic neurons, and combination with reprogramming technology could enhance the CNS tissue remodeling during the second and third phases after acute CNS injury.
Reprogramming strategy for neurodegenerative diseases
Tissue damage in neurodegenerative diseases accumulates gradually during chronic periods. Multifocal pathogenic processes evoke multifocal gliosis and multicellular response similar to those caused by acute injury with a breakdown of BBB and recruitment of neural cells and leukocytes, but on a smaller scale.
Pathogenic molecules such as beta-amyloid (Aβ) produced in Alzheimer’s disease (AD) can also act just as alarmins to trigger microglial activation to release IL-1β, TNF-α and IL-6.95 Cytokine signaling links to activation of kinase that phosphorylates tau, another pathogenic protein.96 However, microglia and astrocytes have a biphasic role in the AD pathological process, as they are known to serve as Aβ scavengers to clear out Aβ,12,13 a burden that precedes cognitive decline in AD patients by about 20 years. Attenuation of reactive astrocytes increased the Aβ load in AD model mice.97 Therefore, direct programming of microglia and astrocytes into functional neurons in AD might not be effective for the prevention of disease progression.
Dysfunctional glial cells may trigger the onset, or facilitate progression of amyotrophic lateral sclerosis (ALS). Oligodendrocytes, microglia and astrocytes with mutation of responsive gene SOD1 for familial ALS mutation accelerate disease progression.10 However, astrocytic NF-kB activation drives microglial proliferation that is rather protective against ALS disease progression in the pre-symptomatic phase.98 Actually, exogenous glial-rich cells derived from iPSCs extended the survival of mutant SOD1 ALS mice,99 implying that stage-dependent glial replacement would be effective for disease-modifying therapy.
Parkinson’s disease (PD) is characterized by a loss of dopaminergic neurons that innervate the striatum, where astrocytes are chronically activated. Microglia activate to convert normal astrocytes into neurotoxic A1 astrocytes, which kill neurons and other glial cells.100 Pharmacological inhibition of A1 astrocyte conversion was neuroprotective for dopaminergic neurons in a PD rodent model,101 suggesting that reprogramming astrocytes could be beneficial against the pathogenic process in PD. In vivo direct reprogramming from striatal astrocytes into iDAN in PD model mice reverted the motor dysfunction phenotype via generation of functional iDAN.57
Collectively, the role of glia in the pathogenetic process is pivotal in neurodegenerative diseases, and selection of subtypes of glial cells depending on the disease stage is essential for future in vivo direct programming therapy for each neurodegenerative disease. In vivo direct reprogramming therapy towards gaining pericytes would be attractive for the neurodegenerative disease. Since about 50% of pericytes in BBB are lost in ALS,102 the blood-spinal cord barrier in ALS would be permeable, suggesting that intravenous delivery of TFs might achieve in vivo direct conversion of toxic glial cells into target motor neurons in the spinal cord. Pericyte transplantation extended the survival of SOD1 mice and iPSC-derived neuronal cells by expression of anti-oxidant enzymes,103 implying that cell replacement could revert the protective effects on motor neurons. Pericyte degeneration precedes the disease progression not only in ALS and but also in AD.104 Pericyte loss slows the blood flow and affects the accumulation of Aβ,105 which in turn induces pericyte loss, further burdening the Aβ load,106 suggesting that in vivo direct conversion of adjunct astrocytes beside the BBB into functional pericytes could contribute to efficient remodeling of NVU to prevent AD pathological progression.
A three-dimensional (3D) cultured system of the CNS microenvironment could provide an attractive research platform for reprogramming technology.107,108 A scaffold-based 3D system of neuronal cells derived from iPSCs has promoted the AD pathological recapitulation of Aβ.109 In a scaffold-free system, a 3D spheroid structure derived from AD-iPSCs can produce extracellular Aβ, replicating AD phenotypes of the cellular niche in vivo.110 These evolving in vitro modellings using a 3D cultured system could contribute to the development of in vivo direct reprogramming technology applicable for neurodegenerative diseases.
Conclusion and future direction
Collectively, rapid advances and growing evidence of stem cell technology for cell regeneration and CNS tissue repair have been accumulating. TFs-mediated direct reprogramming into functional neurons and other types of neural cells could be a promising approach for early remodeling of NVU in both acute CNS injury and neurodegenerative diseases.
However, there are several limitations. First, the delivery method for TFs must be considered for targeted cell conversion into intended functional cells. Careful selection of target cells as a source of reprogramming is needed because locally delivered TFs can affect the cells surrounding the target cells and rather ruin the tissue remodeling by functional endogenous CNS cells. Therefore, cell heterogeneity in the intended tissue remodeling would be a primary hurdle for in vivo direct reprogramming. One resolution is a local injection of viral vectors carrying the promoter specifically expressed in target cells, which would be effective for minimizing the off-target effect. Adeno-associated virus vector has been reported to be relatively safe and applicable to gene therapy for CNS diseases.111 Intravenous delivery of miRNA/mRNA enwrapped by exosome via leaky BBB reprogramming would be another attractive approach aimed at remodeling the micro-circulating system including pericyte assembly in acute CNS injury and neurodegeneration.112
Second, the safety and functional role of reprogrammed cells should be clarified and their tracking and monitoring must be addressed. Reprogramming is mainly aimed at (1) compensation for loss of function of neurons secreting neurotransmitters to receiver cells by cell replacement, (2) rebuilding the neuronal circuit to convey bioelectrical signaling via synapse formation, (3) and remodeling early assembly among neurons, glial cells and endothelial cells by improving the microcirculation and metabolic homeostasis. Ideally, reprogrammed cells should maintain cellular specialization, survive for a long period, and form a synaptic connection with the correct target, but these ‘three S (Specialization, Survival, Synapse)’ are all challenging. To address the therapeutic effect of in vivo direct reprogramming, we need to define appropriate biomarkers for neurotransmitters, functional imaging for axonal connectivity, and cerebral blood/CSF flow for cerebral perfusion in the patient after in vivo direct reprogramming.
Third, direct reprogramming is insufficient for the radical treatment of diseases resulting from genetic mutation. Although replenishment by regenerative neurons could be transiently effective for the loss of function in neurodegenerative diseases, the disease-causing mutation carried in reprogrammed cells will cause gradual neuronal cell loss by its gain of toxic function. One promising solution may be the combinational delivery of both additional genes for functional restoration and TFs for direct reprogramming in vivo.113 Technological progress in the drug-delivery system, imaging and gene editing will boost in vivo direct reprogramming to become future promising regenerative medicine.
Fourth, reprogramming could induce immature iNs that have the potential to transdifferentiate into other types of cells than the aimed-for cells. For example, Ascl1 is sufficient to induce a heterogenic cell population including neuron progenitor cells.23 Further, elucidation of hierarchical mechanisms for both in vivo and in vitro reprogramming would be necessary for efficient and safe regenerative medicine.70 In vivo direct conversion into GABAergic, glutamatergic and dopaminergic neurons can be achieved, although the precise mechanism of conversion into other types of neurons (i.e. cholinergic, serotoninergic, sensory) has not yet been elucidated. Utilization of computational strategy for predicting lineage specifiers with single cell analysis might facilitate our understanding of the complex transcriptional network to manipulate cell identity for in vivo direct reprogramming.114,115
Acknowledgements
We would like to express our sincere gratitude to all our coworkers and collaborators: Kayoko Tsukita, Takako Enami, Ayako Nagahashi, Ikuyo Inoue, Ran Shibukawa, Yukako Sagara, Yasue Okanishi, and Kazuma Kamata for technical assistance; Mikie Iijima, Nozomi Kawabata, Ayumi Suzuki, Miwa Fujita, Mayu Okuda, and Makiko Yasui for valuable administrative support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded in part by a grant for Core Center for iPS Cell Research of Research Center Network for Realization of Regenerative Medicine from AMED to H.I. and from KAKENHI (18K15347, 20K07883) to N.E. and KAKENHI (18K18452, 18H02717) to H.I. and supported in part by NIH to K.A.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Takahashi K, Yamanaka S.Induced pluripotent stem cells in medicine and biology. Development 2013; 140: 2457–2461. [DOI] [PubMed] [Google Scholar]
- 2.Inoue H, Nagata N, Kurokawa H, et al. iPS cells: a game changer for future medicine. EMBO J 2014; 33: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imamura K, Izumi Y, Watanabe A, et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med 2017; 9: pii: eaaf3962. [DOI] [PubMed] [Google Scholar]
- 4.Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 2017; 376 : 1038–1046. [DOI] [PubMed] [Google Scholar]
- 5.Kikuchi T, Morizane A, Doi D, et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature 2017; 548: 592–596. [DOI] [PubMed] [Google Scholar]
- 6.Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010; 463: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Z, Zhang L, Wu Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 2014; 14: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burda JE, Sofroniew MV.Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014; 81: 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egawa N, Lok J, Arai K.Mechanisms of cellular plasticity in cerebral perivascular region. Prog Brain Res 2016; 225: 183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka K, Chun SJ, Boillee S, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci 2008; 11: 251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.di Domenico A, Carola G, Calatayud C, et al. Patient-specific iPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinson’s disease. Stem Cell Rep 2019; 12: 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prokop S, Miller KR, Heppner FL.Microglia actions in Alzheimer’s disease. Acta Neuropathol 2013; 126: 461–477. [DOI] [PubMed] [Google Scholar]
- 13.Wyss-Coray T, Loike JD, Brionne TC, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 2003; 9: 453–457. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872. [DOI] [PubMed] [Google Scholar]
- 15.Bosnali M, Munst B, Thier M, et al. Deciphering the stem cell machinery as a basis for understanding the molecular mechanism underlying reprogramming. Cell Mol Life Sci 2009; 66: 3403–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu P, Li L.Histone acetylation and recruitment of serum responsive factor and CREB-binding protein onto SM22 promoter during SM22 gene expression. Circ Res 2002; 90: 858–865. [DOI] [PubMed] [Google Scholar]
- 17.Khochbin S, Verdel A, Lemercier C, et al. Functional significance of histone deacetylase diversity. Curr Opin Genet Dev 2001; 11: 162–166. [DOI] [PubMed] [Google Scholar]
- 18.Soufi A, Garcia MF, Jaroszewicz A, et al. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 2015; 161: 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mertens J, Marchetto MC, Bardy C, et al. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat Rev Neurosci 2016; 17: 424–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang ZP, Yang N, Vierbuchen T, et al. Induction of human neuronal cells by defined transcription factors. Nature 2011; 476: 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son EY, Ichida JK, Wainger BJ, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 2011; 9: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfisterer U, Kirkeby A, Torper O, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 2011; 108): 10343–10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wapinski OL, Vierbuchen T, Qu K, et al. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 2013; 155: 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heins N, Malatesta P, Cecconi F, et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat Neurosci 2002; 5: 308–315. [DOI] [PubMed] [Google Scholar]
- 25.Heinrich C, Blum R, Gascon S, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol 2010; 8: e1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladewig J, Mertens J, Kesavan J, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods 2012; 9: 575–578. [DOI] [PubMed] [Google Scholar]
- 27.Zhao P, Zhu T, Lu X, et al. Neurogenin 2 enhances the generation of patient-specific induced neuronal cells. Brain Res 2015; 1615: 51–60. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe K, Ang CE, Chanda S, et al. Transdifferentiation of human adult peripheral blood T cells into neurons. Proc Natl Acad Sci U S A 2018; 115: 6470–6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo AS, Sun AX, Li L, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011; 476: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson E, Tryggvason U, Deng Q, et al. Identification of intrinsic determinants of midbrain dopamine neurons. Cell 2006; 124: 393–405. [DOI] [PubMed] [Google Scholar]
- 31.Caiazzo M, Dell’Anno MT, Dvoretskova E, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011; 476: 224–227. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, Li F, Stubblefield EA, et al. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res 2012; 22: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Jiang H, Zhong P, et al. Direct conversion of human fibroblasts to induced serotonergic neurons. Mol Psychiatry 2016; 21: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garone MG, de Turris V, Soloperto A, et al. Conversion of human induced pluripotent stem cells (iPSCs) into functional spinal and cranial motor neurons using PiggyBac vectors. J Vis Exp 2019; 147: e59321. [DOI] [PubMed] [Google Scholar]
- 35.Blanchard JW, Eade KT, Szucs A, et al. Selective conversion of fibroblasts into peripheral sensory neurons. Nat Neurosci 2015; 18: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishiyama A, Chang A, Trapp BD.NG2+ glial cells: a novel glial cell population in the adult brain. J Neuropathol Exp Neurol 1999; 58: 1113–1124. [DOI] [PubMed] [Google Scholar]
- 37.Raff MC, Miller RH, Noble M.A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 1983; 303: 390–396. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama A, Komitova M, Suzuki R, et al. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 2009; 10: 9–22. [DOI] [PubMed] [Google Scholar]
- 39.Najm FJ, Lager AM, Zaremba A, et al. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol 2013; 31: 426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang N, Zuchero JB, Ahlenius H, et al. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol 2013; 31: 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caiazzo M, Giannelli S, Valente P, et al. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem Cell Rep 2015; 4: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchieu J, Calder EL, Guttikonda SR, et al. NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat Biotechnol 2019; 37: 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee G, Kim H, Elkabetz Y, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol 2007; 25: 1468–1475. [DOI] [PubMed] [Google Scholar]
- 44.Achilleos A, Trainor PA.Neural crest stem cells: discovery, properties and potential for therapy. Cell Res 2012; 22: 288–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decimo I, Fumagalli G, Berton V, et al. Meninges: from protective membrane to stem cell niche. Am J Stem Cells 2012; 1: 92–105. [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YJ, Lim H, Li Z, et al. Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell 2014; 15: 497–506. [DOI] [PubMed] [Google Scholar]
- 47.Tseng TC, Hsieh FY, Dai NT, et al. Substrate-mediated reprogramming of human fibroblasts into neural crest stem-like cells and their applications in neural repair. Biomaterials 2016; 102: 148–161. [DOI] [PubMed] [Google Scholar]
- 48.Kondo T, Raff M.Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 2000; 289: 1754–1757. [DOI] [PubMed] [Google Scholar]
- 49.Ye F, Chen Y, Hoang T, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci 2009; 12: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egawa N, Shindo A, Hikawa R, et al. Differential roles of epigenetic regulators in the survival and differentiation of oligodendrocyte precursor cells. Glia 2019; 67: 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, He X, Liu L, et al. Hdac3 Interaction with p300 histone acetyltransferase regulates the oligodendrocyte and astrocyte lineage fate switch. Dev Cell 2016; 36: 316–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu SC, Zhang Y.Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 2010; 11: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moyon S, Huynh JL, Dutta D, et al. Functional characterization of DNA methylation in the oligodendrocyte lineage. Cell Rep 2016; 15: 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo C, Lee QY, Wapinski O, et al. Global DNA methylation remodeling during direct reprogramming of fibroblasts to neurons. Elife 2019; 8: e40197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Chen G.In vivo reprogramming for CNS repair: regenerating neurons from endogenous glial cells. Neuron 2016; 91: 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torper O, Ottosson DR, Pereira M, et al. In vivo reprogramming of striatal NG2 glia into functional neurons that integrate into local host circuitry. Cell Rep 2015; 12: 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivetti di Val Cervo P, Romanov RA, Spigolon G, et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat Biotechnol 2017; 35: 444–452. [DOI] [PubMed] [Google Scholar]
- 58.Grande A, Sumiyoshi K, Lopez-Juarez A, et al. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun 2013; 4: 2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gascon S, Murenu E, Masserdotti G, et al. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell 2016; 18: 396–409. [DOI] [PubMed] [Google Scholar]
- 60.Torper O, Pfisterer U, Wolf DA, et al. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A 2013; 110: 7038–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y, Miao Q, Yuan J, et al. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J Neurosci 2015; 35: 9336–9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Addis RC, Hsu FC, Wright RL, et al. Efficient conversion of astrocytes to functional midbrain dopaminergic neurons using a single polycistronic vector. PLoS One 2011; 6: e28719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuwabara T, Hsieh J, Muotri A, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci 2009; 12: 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niu W, Zang T, Zou Y, et al. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol 2013; 15: 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin JC, Zhang L, Ma NX, et al. Chemical conversion of human fetal astrocytes into neurons through modulation of multiple signaling pathways. Stem Cell Rep 2019; 12: 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Yin JC, Yeh H, et al. Small molecules efficiently reprogram human astroglial cells into functional neurons. Cell Stem Cell 2015; 17: 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinrich C, Bergami M, Gascon S, et al. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Rep 2014; 3: 1000–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karow M, Camp JG, Falk S, et al. Direct pericyte-to-neuron reprogramming via unfolding of a neural stem cell-like program. Nat Neurosci 2018; 21: 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karow M, Sanchez R, Schichor C, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell 2012; 11: 471–476. [DOI] [PubMed] [Google Scholar]
- 70.Morris SA.Direct lineage reprogramming via pioneer factors; a detour through developmental gene regulatory networks. Development 2016; 143: 2696–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egawa N, Lok J, Washida K, et al. Mechanisms of axonal damage and repair after central nervous system injury. Transl Stroke Res 2017; 8: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casella GT, Marcillo A, Bunge MB, et al. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp Neurol 2002; 173: 63–76. [DOI] [PubMed] [Google Scholar]
- 73.Wanner IB, Anderson MA, Song B, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 2013; 33: 12870–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chopp M, Zhang ZG.Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. Expert Opin Emerg Drugs 2015; 20: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki N, Noguchi E, Nakashima N, et al. The Saccharomyces cerevisiae small GTPase, Gsp1p/Ran, is involved in 3’ processing of 7S-to-5.8S rRNA and in degradation of the excised 5’-A0 fragment of 35S pre-rRNA, both of which are carried out by the exosome. Genetics 2001; 158: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang ZG, Chopp M.Exosomes in stroke pathogenesis and therapy. J Clin Invest 2016; 126: 1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koh E, Lee EJ, Nam GH, et al. Exosome-SIRPalpha, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 2017; 121: 121–129. [DOI] [PubMed] [Google Scholar]
- 78.Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017; 546: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finelli MJ, Wong JK, Zou H.Epigenetic regulation of sensory axon regeneration after spinal cord injury. J Neurosci 2013; 33: 19664–19676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaub P, Tedeschi A, Puttagunta R, et al. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ 2010; 17: 1392–1408. [DOI] [PubMed] [Google Scholar]
- 81.Tedeschi A, Nguyen T, Puttagunta R, et al. A p53-CBP/p300 transcription module is required for GAP-43 expression, axon outgrowth, and regeneration. Cell Death Differ 2009; 16: 543–554. [DOI] [PubMed] [Google Scholar]
- 82.Cho Y, Sloutsky R, Naegle KM, et al. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell 2013; 155: 894–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho Y, Cavalli V.HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J 2012; 31: 3063–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rivieccio MA, Brochier C, Willis DE, et al. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc Natl Acad Sci U S A 2009; 106: 19599–19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Appelt-Menzel A, Cubukova A, Gunther K, et al. Establishment of a human blood-brain barrier co-culture model mimicking the neurovascular unit using induced pluri- and multipotent stem cells. Stem Cell Reports 2017; 8: 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vatine GD, Barrile R, Workman MJ, et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019; 24: 995–1005.e6. [DOI] [PubMed] [Google Scholar]
- 87.Borlongan CV, Tajima Y, Trojanowski JQ, et al. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol 1998; 149: 310–321. [DOI] [PubMed] [Google Scholar]
- 88.Hara K, Yasuhara T, Maki M, et al. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol 2008; 85: 318–334. [DOI] [PubMed] [Google Scholar]
- 89.Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000; 55: 565–569. [DOI] [PubMed] [Google Scholar]
- 90.Yasuhara T, Matsukawa N, Hara K, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev 2009; 18: 1501–1514. [DOI] [PubMed] [Google Scholar]
- 91.Stonesifer C, Corey S, Ghanekar S, et al. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 2017; 158: 94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke 2016; 47: 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Borlongan CV.Age of PISCES: stem-cell clinical trials in stroke. Lancet 2016; 388: 736–738. [DOI] [PubMed] [Google Scholar]
- 94.Kalladka D, Sinden J, Pollock K, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet 2016; 388: 787–796. [DOI] [PubMed] [Google Scholar]
- 95.Wang WY, Tan MS, Yu JT, et al. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med 2015; 3: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bernhardi RV, Nicholls JG.Transformation of leech microglial cell morphology and properties following co-culture with injured central nervous system tissue. J Exp Biol 1999; 202(Pt 6): 723–728. [DOI] [PubMed] [Google Scholar]
- 97.Kraft AW, Hu X, Yoon H, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J 2013; 27: 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ouali Alami N, Schurr C, Olde Heuvel F, et al. NF-kappaB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J 2018; 37: e98697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kondo T, Funayama M, Tsukita K, et al. Focal transplantation of human iPSC-derived glial-rich neural progenitors improves lifespan of ALS mice. Stem Cell Rep 2014; 3: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017; 541: 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yun SP, Kam TI, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 2018; 24: 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Winkler EA, Sengillo JD, Sullivan JS, et al. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 2013; 125: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coatti GC, Frangini M, Valadares MC, et al. Pericytes extend survival of ALS SOD1 mice and induce the expression of antioxidant enzymes in the murine model and in IPSCs derived neuronal cells from an ALS patient. Stem Cell Rev 2017; 13: 686–698. [DOI] [PubMed] [Google Scholar]
- 104.Sengillo JD, Winkler EA, Walker CT, et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol 2013; 23: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nortley R, Korte N, Izquierdo P, et al. Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science 2019; 365: pii: eaav9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun 2013; 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Penney J, Ralvenius WT, Tsai LH.Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol Psychiatry 2019; 25: 148--167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kadoshima T, Sakaguchi H, Nakano T, et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A 2013; 110: 20284–20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Choi SH, Kim YH, Hebisch M, et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 2014; 515: 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raja WK, Mungenast AE, Lin YT, et al. Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS One 2016; 11: e0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kojima K, Nakajima T, Taga N, et al. Gene therapy improves motor and mental function of aromatic l-amino acid decarboxylase deficiency. Brain 2019; 142: 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–345. [DOI] [PubMed] [Google Scholar]
- 113.Yao K, Qiu S, Wang YV, et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 2018; 560: 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stubbington MJT, Rozenblatt-Rosen O, Regev A, et al. Single-cell transcriptomics to explore the immune system in health and disease. Science 2017; 358: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Okawa S, del Sol A.A computational strategy for predicting lineage specifiers in stem cell subpopulations. Stem Cell Res 2015; 15: 427–434. [DOI] [PubMed] [Google Scholar]