Abstract
Objectives
The implantation of flow diverters, or stents in general, necessitates the use of dual anti-platelet treatment with typical regimes including aspirin and a P2Y12 inhibitor. This carries an inherent risk of haemorrhage. We sought to compare the thrombogenicity of the anti-thrombogenic p48 hydrophilic polymer coating compared to the standard uncoated p48 flow diverter using an in vitro thrombogenicity assay.
Methods
To evaluate the thrombin generation influenced by the different stent types the stents were placed in wells of a 24-well plate with the addition of plasma from healthy volunteers the thrombin calibrator respectively the PPP-reagent was added. Subsequently, the thrombin substrate was added and the thrombin generation was analysed every 60 s using a thrombinoscope. The assay is calibrated using samples containing a known amount of active thrombin in PPP. Thrombin activity is proportional to the change in fluorescence.
Results
The p48 hydrophilic polymer coating shows a significantly lower peak thrombin concentration (1.13 ± 0.21 vs. 1.41 ± 0.22) and longer time to peak thrombin concentration (0.96 ± 0.04 vs. 0.74 ± 0.07) compared to the uncoated p48 device (p < 0.01). The responses of the p48 hydrophilic polymer coating were similar to that of the negative control.
Conclusion
The hydrophilic polymer coating surface modification significantly reduces the thrombogenicity of the p48 flow diverter. These results corroborate the findings from previous in vitro studies.
Keywords: p48 MW, flow diverter, hydrophilic polymer coating
Introduction
Flow diversion has gained widespread popularity as a treatment option for intracranial aneurysms. The progressive occlusion of aneurysms over time1–3 results in very high aneurysm occlusion rates. Although a wide variety of flow diverters have entered the clinical market place the design is essentially similar and consists of braided wires with low porosity and higher metal surface coverage compared to typical braided stents. This construct promotes aneurysm healing via a two-step process. Initially, there is redirection of blood away from the aneurysm and the change in intra-aneurysmal flow promotes thrombus formation whilst a simultaneous, albeit slower process, of neo-endothelialisation along the struts of the flow diverter reconstructs the parent vessel and excludes the aneurysm permanently.4 The implantation of flow diverters, or stents in general, necessitates the use of dual anti-platelet treatment (DAPT) with typical regimes including aspirin and a P2Y12 inhibitor such as clopidogrel or prasugrel.5 Although there is no prospective evidence that platelet function testing alters outcome there is retrospective data to suggest that inadequate or hyper-responsiveness to these agents can increase thromboembolic and haemorrhagic complications respectively.6–8 In addition to the cerebral complications there are further potential haemorrhagic complications elsewhere within the body.9 Similarly, there is a reluctance to use stents and flow diversion in the setting of subarachnoid haemorrhage (SAH) because of the need for DAPT and in the potential increased risk of haemorrhagic complications10 even though flow diversion has been used successfully in cases of acute SAH.11–16 These challenges have led to a heightened interest in the development of devices with surface modification and coatings that inhibit platelet aggregation and minimise the need for anti-platelet medication. The ‘phenox Hydrophilic Polymer Coating’ (pHPC) is a proprietary multi-layer glycan-based polymer coating that has shown anti-thrombogenic properties when applied to nitinol substrates in vitro17,18 with no effect on biocompatibility in vivo.19 The coating simulates the glycocalyx and it’s anti-thrombogenic properties are related to this effect rather than a biochemical interaction.
Upon exposure of blood to an artificial surface the ‘contact system’ of coagulation is activated. It is believed that contact activation by an artificial substance may be associated with an increased risk of thrombosis near the artificial surface. Thrombin is an important enzyme involved in normal haemostasis. Increased thrombin generation predicts and increased risk of thrombosis and therefore, the measurement of thrombin is paramount in understanding the processes of thrombosis that may occur in relation to devices. Hemker and colleagues developed fluorogenic techniques that measure the course of thrombin generation, graphically represented as a ‘thrombogram’, in clotting platelet rich or poor plasma (PRP and PPP respectively) using a slow binding fluorogenic substrate with added tissue factor (TF) and phospholipids as clotting initiators.20–23 After the initial work by Hemker and colleagues an improvement in the assay was the use of chromogenic substrates that measured active thrombin levels without interfering with thrombin generation itself.22 A further improvement in the assays came with the introduction of slow binding fluorogenic substrates that have a greater dynamic range than their chromogenic counterparts as well as being able to be used in clotting PRP. These methods are now recognised as the standard method for thrombin generation measurements that is also known as the endogenous thrombin potential.23 These thrombin generation assays measure, amongst other things, the rate of thrombin formation, peak thrombin generation as the total amount of thrombin in blood.21,23,24 In order to assess coagulation activation by an artificial surface, human platelet-rich plasma (PRP) is exposed to the artificial surface followed by measurement of the rate and amount of thrombin generation over time.25 An advantage of these thrombin generation assays is that they allow an evaluation of the entire coagulation system at once including both the pro-coagulant and anti-coagulant pathways which has previously been shown to correlate with clinical outcome.26–28 These assays have been used to compare the thrombogenicity of different devices.25,29
In this study we aimed to investigate the thrombogenicity of the p48 and p48 HPC surface-modified flow diverter using an in vitro thrombogenicity assay.
Materials and methods
Devices
The p48 (phenox, Bochum, Germany) flow diverter is a low-profile next-generation device constructed from 48 braided nitinol wires and compatible with 0.021inch inner diameter (ID) microcatheters. The ‘phenox Hydrophilic Polymer Coating’ (pHPC) was applied to the surface of the p48 stents. This coating is a newly developed glycan-based multilayer polymer. The p48HPC has the same physical properties as the uncoated p48 device. All devices tested were final sterilised products. In total, 12 p48 and 12 p48HPC devices were implanted with 12 negative controls. The devices were placed into wells of a 24-well plate.
Reagents
Human plasma from citrate-anticoagulated blood, Fluo-substrate for thrombinoscope assay (Stago, Germany), thrombin calibrator (Stago, Germany) and PPP reagent (Stago, Germany) were acquired.
Test procedure
To evaluate the thrombin generation influenced by the different stent types the stents were placed in wells of a 24-well plate and after addition of plasma from different healthy volunteers and thrombin calibrator, respectively, the PPP reagent was added. Subsequently, the thrombin substrate was added and the thrombin generation was analysed every 60s using a thrombinoscope.
Thrombin generation (thrombogram)
The assay is calibrated using samples containing a known amount of active thrombin in PPP. Thrombin activity is proportional to the change in fluorescence. A plate reader fluorometer and the appropriate software (Thrombogram-Thrombinoscope™ assay, Maastricht, The Netherlands) were used. Plasmas spiked with thrombin calibrator were run in parallel with each cycle of test samples.
The following parameters were analysed using the appropriate software: (a) the time to reach the maximum concentration (tPeak) of thrombin and (b) the maximum concentration (Peak) of thrombin.
Results
Thrombogram of the p48 and p48HPC
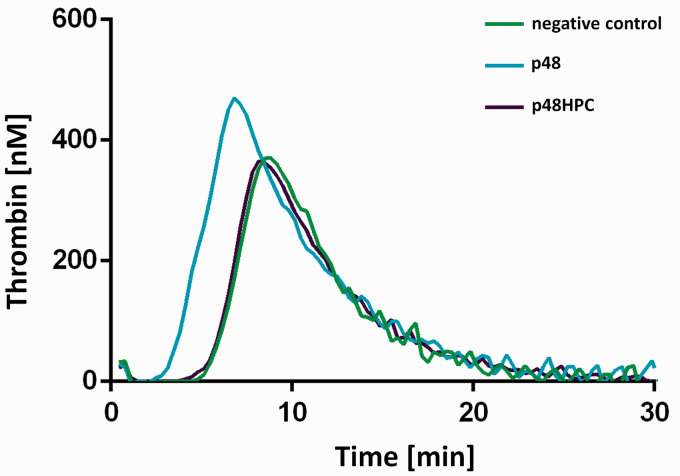
Representative thrombograms for the p48 and p48HPC together with the negative control are shown in Figure 1. Samples with lower thrombogenicity have a right shift in their thrombogram along with a reduced height to the curve. The p48 HPC shows a significantly lower peak thrombin concentration in comparison to the uncoated p48 device. The response of the p48 HPC was similar to that of the negative control. This increase in the lag time (right shift), increase in time taken to reach peak thrombin concentration (right shift), and reduced peak thrombin concentration all demonstrate the HPC surface modification results in a significant decrease in the thrombogenicity.
Figure 1.
Thrombin generation measured in platelet poor plasma (PPP) with p48 and p48HPC and the negative control (n = 12).
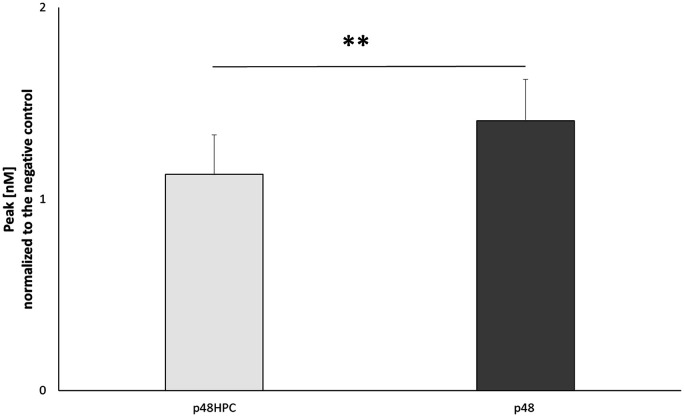
Significantly lower peak thrombin of the p48HPC
Peak thrombin was extracted from each thrombogram and averaged for all tests for each device. The results are shown as M ± SD for each device (Figure 2). The p48HPC device showed a lower peak thrombin concentration (1.13 ± 0.21 vs. 1.41 ± 0.22, p < 0.01) compared to the uncoated p48 device.
Figure 2.
Peak (in nM thrombin, standardised against the negative control) measured in the presence of p48 and p48HPC stents (n = 12).
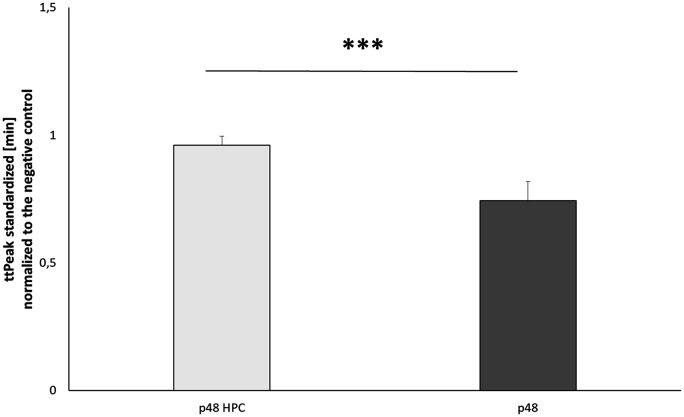
Significantly longer time to peak of p48HPC
Time to peak was extracted from each thrombogram and averaged for all tests for each device. The results are shown as M ± SD for each device (Figure 3). The p48HPC device showed a longer time to peak thrombin concentration (0.96 ± 0.04 vs. 0.74 ± 0.07, p < 0.01) compared to the uncoated p48 device.
Figure 3.
Time to peak (in minutes, standardised against the negative control) measured in the presence of p48 and p48HPC stents (n = 12).
Discussion
There are several distinct phases to a thrombogram starting with the initiation or lag phase during which only trace amounts of thrombin are generated. There is a subsequent rapid escalation phase during which there is a rapid spike in the generation of thrombin. This is rapid increase is due to a positive feedback pathway that causes an exponential increase in thrombin generation after once a threshold level has been reached. Clotting occurs at or close to the start of the escalation phase. The final termination phase is caused by the activation of anti-thrombin, which reacts with other active coagulation factors such as factor Xa, IXa etc. which slows and eventually stops the entire process. The rate of inactivation of thrombin is proportional to the total amount of thrombin produced and the anti-thrombin concentration; therefore, at peak thrombin there is a balance between the pro and anti-coagulant factors.
Our results show that the p48HPC has a significantly lower thrombogenicity compared to the uncoated p48 device. The results of these tests show that the peak thrombin concentration, time to peak and the total amount of thrombin activity were all significantly reduced compared the uncoated p48 diverter. These results are consistent with previous in vitro studies.17,18 In the initial in vitro studies, HPC-coated and uncoated nitinol plates were incubated with heparinised whole human blood for 10 min. Adherent thrombocytes were visualised using fluorescent CD61+ antibodies and a fluorescence microscope. A significantly lower number of platelets were seen on the coated surfaces compared to the uncoated surfaces (1.12 ± 0.4% vs. 48.61 ± 7.3%, p ≤ 0.001). Subsequently, studies comparing the p48 and p48HPC using the Chandler loop model demonstrated similar potent anti-thrombogenic properties associated with the surface coating. In this study, platelets in contact with the uncoated p48 device for 120 min showed a significantly reduced PAR1 activity, a measure of contact between blood and foreign surfaces, compared to the p48 HPC (65 ± 6% vs. 73 ± 9%, p < 0.05). Similarly, there was a reduction in the number of platelet microparticles, which are released on platelet activation, from blood incubated with the p48HPC compared to the p48 (1.8 ± 0.5 vs. 1.4 ± 0.4, p < 0.05). In a final series of experiments, the platelet count after 120 min circulation in the Chandler loop was significantly lower for the uncoated p48 compared to the p48HPC indicating significantly greater adherence of the platelets to the p48 (71 ± 8% vs. 87 ± 5%, p < 0.05).
Previously Ghirdhar et al.29 compared the Pipeline Shield to several other flow diverters and aneurysm bridging devices. In this study, the authors showed that there was a significant difference in the time to peak between the Pipeline embolisation Device (PED) Shield the other Flow Diverting Stents (FDSs) tested and the negative control but not compared to the aneurysm bridging devices. In our study, the results were normalised to the negative control (no sample) and although there were differences between the HPC devices and the control these were no significant suggesting the p48 HPC has a very low thrombogenicity. This group also showed an approximately 50% reduction in mean peak thrombin (nM) between the PED Shield and the other FDSs (Shield 23.6 nM vs. Silk 59.26 nM, Flow-Redirection Endoluminal Device (FRED) 52.53 nM, PED Flex 52.37 nM), whereas the device generated higher peak thrombin levels compared to the Solitaire (20.12 nM) and the negative control (14.25 nM). In our study, we have shown an approximately 20–30% reduction in the peak thrombin concentration. The newly designed p48 flow diverter has a specifically optimised surface that is designed to reduce the thrombogenicity of the uncoated device and therefore the reduced difference seen could be due to the already reduced thrombogenicity of the p48.
The wettability of the HPC has been tailored to create a surface that is optimised for contact with blood. A contact angle (CA) of around 35° can be considered optimal for devices in contact with blood. Protein adsorption to surfaces is the starting point for cell–surface interactions and extremely hydrophilic (CA < 25°) or hydrophobic (>85°) can impair protein adsorption and hence reduce cell–surface interactions in general.30 It is known that platelet activation and adherence are decreased with increased wettability.31–33 Furthermore, endothelialisation, which is important for the long-term biocompatibility of endovascular devices and aneurysm exclusion, is decreased on extremely hydrophilic and hydrophobic surfaces due to hampered adhesion of extracellular matrix proteins (EMPs). Therefore, a moderately hydrophilic surface, like HPC, should allow the adherence of EMP and hence endothelialisation, with a maximum endothelial cell adhesions seen between 35° and 50°,30,34 whilst still reducing the thrombogenicity of the implant. The HPC is a covalently bound glycan-based multi-layer polymer surface coating that is between 10 and 20 nm thick as measured by X-ray photoelectron spectroscopy (XPS) analysis. Similarly, Shield is a 3-nm thick covalently bound phosphorylcholine whereas surface modifications include electro-polishing that aims to make the surface as smooth as possible.
Early clinical results have shown that the p48HPC and other HPC devices can be used with single anti-platelet medication;35–37 however, larger series with longer term follow-up are required and are currently underway. Although HPC devices can be used with SAPT it is yet to be deduced which is the best anti-platelet to use in different scenarios and anti-platelet responsiveness testing should be considered mandatory in all patients. Standard loading doses and maintenance doses remain unchanged when using these devices.
Our study has several limitations including a limited sample size and the inherent lack of underlying organic tissue or pathological states such as aneurysms. Similarly, we did not use anti-platelet agents in these experiments. Despite these limitations, the p48 HPC demonstrated a thrombogenic profile similar to that of the negative control; therefore, we believe these results are unlikely to be significantly affected by variability amongst blood donors.
Conclusion
The HPC surface modification significantly reduces the thrombogenicity of the p48 flow diverter which corroborates the findings from previous in vitro studies.
Author’s contributions
Pervinder Bhogal FRCR: manuscript preparation; Tim Lenz-Habijan PhD: editing and review of manuscript, review, data collection, editing; Catrin Bannewitz MSc: editing and review of manuscript, review, data collection, editing; Ralf Hannes Dipl.-Ing: editing and review of manuscript; Hermann Monstadt Dr: editing and review of manuscript; Martin Brodde PhD: design and conduct of experimental work; Beate Kehrel MD: design of experiment, review; Hans Henkes MD, PhD: study design, review, editing, guarantor.
Availability of data
There is no further data available to share at this time.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Pervinder Bhogal FRCR: consulting and proctoring agreement with Phenox; Tim Lenz-Habijan PhD: employee of phenox; Catrin Bannewitz MSc: employee of phenox; Ralf Hannes Dipl.-Ing: CEO of phenox; Hermann Monstadt Dr.-Ing: CEO and shareholder of phenox GmbH; Martin Brodde: no conflict of interest declared; Beate Kehrel: no conflict of interest declared; Hans Henkes MD, PhD: co-founder and shareholder of phenox GmbH.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by phenox GmbH.
Informed consent
Informed consent was obtained from all individual participants included in the study.
ORCID iDs
Pervinder Bhogal https://orcid.org/0000-0002-5514-5237
Tim Lenz-Habijan https://orcid.org/0000-0001-5166-5692
Hans Henkes https://orcid.org/0000-0002-6534-036X
References
- 1.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 2.Becske T, Brinjikji W, Potts MB, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery 2017; 80: 40–48. [DOI] [PubMed] [Google Scholar]
- 3.Sirakov S, Sirakov A, Bhogal P, et al. The p64 flow diverter-mid-term and long-term results from a single center. Clin Neuroradiol 2019. [DOI] [PubMed] [Google Scholar]
- 4.Kadirvel R, Ding Y-H, Dai D, et al. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology 2014; 270: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta R, Moore JM, Griessenauer CJ, et al. Assessment of dual-antiplatelet regimen for pipeline embolization device placement: a survey of major academic neurovascular centers in the United States. World Neurosurg 2016; 96: 285–292. [DOI] [PubMed] [Google Scholar]
- 6.Cheung NK, Carr MW, Ray U, et al. Platelet function testing in neurovascular procedures: tool or gimmick? Interv Neurol 2019; 8: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajadi E, Kabir S, Cook A, et al. Predictive value of platelet reactivity unit (PRU) value for thrombotic and hemorrhagic events during flow diversion procedures: a meta-analysis. J NeuroInterventional Surg 2019; neurintsurg-2019-014765. [DOI] [PubMed] [Google Scholar]
- 8.Raychev R, Tateshima S, Vinuela F, et al. Predictors of thrombotic complications and mass effect exacerbation after pipeline embolization: the significance of adenosine diphosphate inhibition, fluoroscopy time, and aneurysm size. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci 2016; 22: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger PB, Bhatt DL, Fuster V, et al. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: results from the Clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance (CHARISMA) trial. Circulation 2010; 121: 2575–2583. [DOI] [PubMed] [Google Scholar]
- 10.Bodily KD, Cloft HJ, Lanzino G, et al. Stent-assisted coiling in acutely ruptured intracranial aneurysms: a qualitative, systematic review of the literature. AJNR Am J Neuroradiol 2011; 32: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhogal P, Henkes E, Schob S, et al. The use of flow diverters to treat small (≤5 mm) ruptured, saccular aneurysms. Surg Neurol Int 2018; 9: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhogal P, Brouwer PA, Söderqvist ÅK, et al. Patients with subarachnoid haemorrhage from vertebrobasilar dissection: treatment with stent-in-stent technique. Neuroradiology 2015; 57: 605–614. [DOI] [PubMed] [Google Scholar]
- 13.Mokin M, Chinea A, Primiani CT, et al. Treatment of blood blister aneurysms of the internal carotid artery with flow diversion. J NeuroInterventional Surg 2018; 10(11): 1074–1078. [DOI] [PubMed] [Google Scholar]
- 14.Hellstern V, Aguilar-Pérez M, AlMatter M, et al. Microsurgical clipping and endovascular flow diversion of ruptured anterior circulation blood blister-like aneurysms. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci 2018; 24: 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AlMatter M, Aguilar Pérez M, Hellstern V, et al. Flow diversion for treatment of acutely ruptured intracranial aneurysms : a single center experience from 45 consecutive cases. Clin Neuroradiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cagnazzo F, di Carlo DT, Cappucci M, et al. Acutely ruptured intracranial aneurysms treated with flow-diverter stents: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2018; 39: 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenz-Habijan T, Bhogal P, Peters M, et al. Hydrophilic stent coating inhibits platelet adhesion on stent surfaces: initial results in vitro. Cardiovasc Intervent Radiol 2018; 41(11): 1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz-Habijan T, Brodde M, Kehrel BE, et al. Comparison of the thrombogenicity of a bare and antithrombogenic coated flow diverter in an in vitro flow model. Cardiovasc Intervent Radiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhogal P, Lenz-Habijan T, Bannewitz C, et al. The pCONUS HPC: 30-day and 180-day in vivo biocompatibility results. Cardiovasc Intervent Radiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemker HC. The application of thrombin generation in real life clinical situations. Thromb Res 2015; 136: 3–4. [DOI] [PubMed] [Google Scholar]
- 21.Hemker HC, Béguin S. Thrombin generation in plasma: its assessment via the endogenous thrombin potential. Thromb Haemost 1995; 74: 134–138. [PubMed] [Google Scholar]
- 22.Hemker HC, Giesen P, AlDieri R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb 2002; 32: 249–253. [DOI] [PubMed] [Google Scholar]
- 23.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb 2003; 33: 4–15. [DOI] [PubMed] [Google Scholar]
- 24.Chandler WL, Roshal M. Optimization of plasma fluorogenic thrombin-generation assays. Am J Clin Pathol 2009; 132: 169–179. [DOI] [PubMed] [Google Scholar]
- 25.Girdhar G, Read M, Sohn J, et al. In-vitro thrombogenicity assessment of polymer filament modified and native platinum embolic coils. J Neurol Sci 2014; 339: 97–101. [DOI] [PubMed] [Google Scholar]
- 26.Young G, Sørensen B, Dargaud Y, et al. Thrombin generation and whole blood viscoelastic assays in the management of hemophilia: current state of art and future perspectives. Blood 2013; 121: 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hron G, Kollars M, Binder BR, et al. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA 2006; 296: 397–402. [DOI] [PubMed] [Google Scholar]
- 28.Besser M, Baglin C, Luddington R, et al. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin-generating potential in a prospective cohort study. J Thromb Haemost JTH 2008; 60: 1720–1725. [DOI] [PubMed] [Google Scholar]
- 29.Girdhar G, Li J, Kostousov L, Wainwright J, et al. In-vitro thrombogenicity assessment of flow diversion and aneurysm bridging devices. J Thromb Thrombolysis 2015; 40: 437–443. [DOI] [PubMed] [Google Scholar]
- 30.Faucheux N, Schweiss R, Lützow K, et al. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 2004; 25: 2721–2730. [DOI] [PubMed] [Google Scholar]
- 31.Wan GJ, Huang N, Yang P, et al. Platelet activation behavior on nitrogen plasma-implanted silicon. Mater Sci Eng C 2007; 27: 928–932. [Google Scholar]
- 32.Yang P, Huang N, Leng YX, et al. Wettability and biocompatibility of nitrogen-doped hydrogenated amorphous carbon films: effect of nitrogen. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater At 2006; 242: 22–25. [Google Scholar]
- 33.Tzoneva R, Groth T, Altankov G, et al. Remodeling of fibrinogen by endothelial cells in dependence on fibronectin matrix assembly. Effect of substratum wettability. J Mater Sci Mater Med 2002; 13: 1235–1244. [DOI] [PubMed] [Google Scholar]
- 34.Arima Y, Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007; 28: 3074–3082. [DOI] [PubMed] [Google Scholar]
- 35.Colgan F, Aguilar Pérez M, Hellstern V, et al. Vertebral artery aneurysm: ruptured dissecting aneurysm, implantation of telescoping p48_HPC flow diverter stents under antiaggregation with ASA only In: H Henkes, P Lylyk, O Ganslandt. (eds) The Aneurysm Casebook: A Guide to Treatment Selection and Technique [Internet]. Cham: Springer International Publishing, 2018, pp. 1–16. Available at: 10.1007/978-3-319-70267-4_80-1 (accessed 19 April 2019). [Google Scholar]
- 36.Bhogal P, Bleise C, Chudyk J, et al. The p48_HPC antithrombogenic flow diverter: initial human experience using single antiplatelet therapy. J Int Med Res 2019; 48: 300060519879580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez MA, AlMatter M, Hellstern V, et al. Use of the pCONus HPC as an adjunct to coil occlusion of acutely ruptured aneurysms: early clinical experience using single antiplatelet therapy. J NeuroInterventional Surg 2020. Available at: http://jnis.bmj.com/content/early/2020/02/26/neurintsurg-2019-015746 (accessed 31 March 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no further data available to share at this time.