Highlights
-
•
Current literature on excess mortality during the first wave of COVID-19 is limited.
-
•
We report the absolute excess risk (AER) of mortality and excess mortality rate (EMR) for weeks 2 to 20 in 2020 from surveillance network data.
-
•
AER of mortality was 197.8 per 10,000 person years.
-
•
Being male, older, of black ethnicity, more deprived, and living in a larger household increased EMR.
-
•
Presence of comorbidities also increased EMR.
Keywords: Medical record systems, computerized; General Practice; Sentinel Surveillance; Mortality
Abstract
Objectives
Few studies report contributors to the excess mortality in England during the first wave of coronavirus disease 2019 (COVID-19) infection. We report the absolute excess risk (AER) of mortality and excess mortality rate (EMR) from a nationally representative COVID-19 sentinel surveillance network including known COVID-19 risk factors in people aged 45 years and above.
Methods
Pseudonymised, coded clinical data were uploaded from contributing primary care providers (N = 1,970,314, ≥45years). We calculated the AER in mortality by comparing mortality for weeks 2 to 20 this year with mortality data from the Office for National Statistics (ONS) from 2018 for the same weeks. We conducted univariate and multivariate analysis including preselected variables. We report AER and EMR, with 95% confidence intervals (95% CI).
Results
The AER of mortality was 197.8/10,000 person years (95%CI:194.30–201.40). The EMR for male gender, compared with female, was 1.4 (95%CI:1.35–1.44, p<0.00); for our oldest age band (≥75 years) 10.09 (95%CI:9.46–10.75, p<0.00) compared to 45–64 year olds; Black ethnicity's EMR was 1.17 (95%CI: 1.03–1.33, p<0.02), reference white; and for dwellings with ≥9 occupants 8.01 (95%CI: 9.46–10.75, p<0.00). Presence of all included comorbidities significantly increased EMR. Ranked from lowest to highest these were: hypertension, chronic kidney disease, chronic respiratory and heart disease, and cancer or immunocompromised.
Conclusions
The absolute excess mortality was approximately 2 deaths per 100 person years in the first wave of COVID-19. More personalised shielding advice for any second wave should include ethnicity, comorbidity and household size as predictors of risk.
Introduction
The UK, particularly England, has experienced significant increases in all-cause mortality during the COVID-19 pandemic. England and Spain appear to have fared worst among European countries in terms of COVID-19 related mortality.1 However, cross-country mortality comparisons are difficult since some countries, like the UK, collect more complete contemporaneous mortality data, not all countries report total deaths that include community based events, and data need adjustment for population demographics. The Office for National Statistics (ONS) reported 262,237 registered deaths in England and Wales between the 10th Jan and 15th May 2020 (weeks 2 - 20). There were 49,059 additional deaths compared with the five–year average. COVID-19 was included on the death certificate in 41,105 of these deaths, leaving 7954 unaccounted deaths.2, 3, 4 These unaccounted deaths may also be related to COVID-19 infection which has not been tested for or detected.
Significant differences in risk of COVID-19 related mortality have been observed with male gender, increasing age, ethnicity, socioeconomic status, and chronic conditions such as cancer.5 , 6 Early discharge from hospital and spread of disease by asymptomatic staff members may have been contributing factors to the increased mortality rates observed in shared dwellings such as residential care homes.7, 8, 9 It is unclear whether these or other risk factors also predict excess mortality rates in 2020.
The English Oxford-Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) is a nationally representative infectious diseases sentinel surveillance network of general practices with over 4 million registered patients, established over 50 years ago providing weekly influenza and respiratory illness surveillance reports to Public Health England.10 The RCGP RSC network has adapted to include COVID-19 surveillance, including a self-swabbing programme and collecting samples for sero-surveillance.11
We report for the first time the excess mortality during the first wave of COVID-19 infection in England in the adult population 45 years old and over across the RCGP RSC. We compared mortality rates with ONS data to test representativeness, report absolute excess mortality, and excess mortality hazard ratios (EMR) for a range of demographic and clinical factors reported to be associated with increased COVID-19 related mortality, also including household size.
Research in context
Evidence before this study
We searched PubMed and Google Scholar for publications between Jan 1, 2020, and June 23rd, 2020, using combinations of the following terms: ("COVID-19″) AND ("relative risk” OR “excess mortality” OR “mortality risk”) and did not identify any estimates of the relative risk (RR) of mortality from COVID-19.
There were two publications which aimed to model COVID-19 mortality prior to the peak of the pandemic, particularly to guide policy measures. These papers aimed to model the excess mortality risk in multiple scenarios with an estimate for the RR between 1.5 and 3.0.12 The case fatality rate has also been presented across many countries and healthcare systems, however, this is subject to wide variation with rates reported between 0.3–15%,13 possibly reflecting different testing strategies and background demography.
We also searched the same sources for COVID-19 and ("absolute excess risk” OR “AER” OR “attributable risk” OR “mortality risk”) and did not identify any estimates of the absolute excess risk (AER) of mortality from COVID-19. We identified one pre-print manuscript of a study which estimated excess mortality for England and Wales in 2020, by week and region. This study utilised aggregated ONS data to report an estimated 47,243 excess deaths between March 7 and May 8, of which 9948 were not associated with COVID-19.14 Whilst an important finding on the overall impact of the COVID-19 pandemic on mortality, the underlying data relies on the reporting clinician's certification of COVID-19 and does include further investigation.
Added value of this study
This is the first study to describe risk factors associated with excess mortality during the COVID-19 pandemic in a nationally representative English population. These include male gender, increasing age, Black ethnicity, larger household size and the presence of comorbidities known to be associated with increased risk, in the population over 45 years old. Importantly, our study which presents the excess risk of COVID-19 mortality at a population level is the first to present detailed findings on mortality within patient groups, irrespective of COVID-19 status. This methodology is less liable to selection bias as it does not depend on local testing strategies, presenting a more overarching impact of the pandemic period on mortality by patient group.
Implications of all the available evidence
This study reports an absolute excess risk (AER) of mortality during the COVID-19 pandemic in the English population, at approximately 2 excess deaths per 100 person years. This is higher than the previously reported rates of confirmed COVID-19-related mortality based on hospital admissions. This study confirms previously reported disparities in confirmed COVID-19 related mortality which include male gender, older age-groups, Black ethnicity (compared to white); larger household size (which would include care homes), those in the most deprived socioeconomic quintile, and people with chronic disease being at increased relative risk of mortality. By quantifying excess risk of mortality in the first wave of infection, we can better prepare for the next.
Methods
Setting
The study population includes 4413,734 patients registered at the general practices contributing to the RCGP RSC. The Oxford RCGP RSC extracts pseudonymised data from primary health care computerised medical records (CMR) of member practices twice a week and is recruited to be nationally representative. UK general practice is a registration-based system, on patient registers with a single practice. Data includes demographics, clinical conditions, medications, and laboratory results.15
Study population
We included individuals aged 45 years old and over contributing CMRs to the RCGP RSC with at least one year's complete records prior to 6th January 2020. We selected this age, because we wanted to understand mortality in the older age-group, and our online mortality observatory, which compares the current year with the sentinel network rolling average appeared to show a difference in mortality above this age.16 We excluded 47 records due to the records being incomplete (absent dates during our observation period). The study period was between weeks 2 and 20 of 2020, the period of the first wave of COVID-19 in England.
Study variables
Variable selection was guided by our previous study of groups likely to test positive to COVID-19 and our literature review.17, 18, 19 The main outcome was all-cause mortality.
Demographic and personal characteristics included gender, age, and ethnicity divided into white, Asian, Black, mixed and other, using an established ontology,20 household size (1, 2–4,5–8 and 9+),15 , 21 , 22 and socioeconomic status as determined by the Index of Multiple Deprivation (IMD).23 We used the following body mass index (BMI) categories: (1) under weight and normal weight were grouped into “normal” (BMI<25 kg/m2); (2) overweight or pre-obese (BMI 25–29 kg/m2); (3) obese class I (BMI 30–34 kg/m2); and (4) obese class II and III (BMI ≥35 kg/m2).24
The following disease groups were included as they have been reported to be associated with poorer outcomes: hypertension, chronic kidney disease (CKD) defined as stage 3 to 5,25 heart disease (including myocardial infarction, other forms of coronary artery disease and heart failure), chronic respiratory disease (asthma, chronic obstructive pulmonary disease, bronchiectasis, and other chronic lung conditions), people undergoing treatment for cancer or who may be immunocompromised due to taking medications for inflammatory conditions.
Statistical methods
We report counts and percentage (%) for each variable included in the study cohort.
We compared mortality in the Oxford RCGP-RSC population with ONS mortality data by plotting the death rate per 100,000 population for each week during the study period - and by visually comparing survival rates using a Kaplan-Meier plot.
To calculate the AER of mortality per 10,000 population we compared the expected number of deaths between weeks 2 and 20 (reported in ONS mortality life tables for 2018)26 with the number observed deaths from the same period of 2020 (using RCGP RSC data). To calculate EMRs we fitted constant exponential Poisson survival models.27
We used the ONS mortality life tables to calculate background mortality risk for 2018. We measured excess mortality using an additive hazard model. The observed hazard of our cohort was expressed as the sum of the expected or background hazard and the excess hazard due to COVID-19, assuming that the observed and expected deaths follow Poisson distributions.28
We imputed missing data on covariates, using multiple imputation with chained equations,29 imputing five datasets (using all model covariates including outcome status) and employed Rubin's rule to pool model estimates.30
R version 3.5.331 was used for all statistical analyses together with the survival package version 2.43–3; we used the mice package version 3.9.0 for multiple imputation.
Ethical considerations
The Oxford RCGP RSC surveillance system and its work with respect to COVID-19 are approved by Public Health England's Caldicott Guardian Committee under Regulation 3 of the Health Service Control Patient Information Regulations 2002. The study was also approved by RCGP.
Results
Table 1 presents the characteristics of the study population and the amount of missing data prior to imputation. A total of 1970,314 individuals met the inclusion criteria, 48.75% were male, median age 62.95 years (IQR 53–72 years), 70.30% white, and the majority (60.64%) living in dwellings of 2–4 residents. The most common comorbidities were hypertension (33.88%) followed by CHD (13.56%) and malignancy or immunosuppression (12.06%).
Table 1.
Characteristics of people 45 years old and above in the RCGP RSC cohort, N = 1970,314.
| Variable | Category | Number (n) | Percentage (%) |
|---|---|---|---|
| Sex | male | 960,609 | (48.75) |
| female | 1009,705 | (51.25) | |
| Age band | 45–64 | 1149,621 | (58.35) |
| 65–74 | 436,617 | (22.16) | |
| 75+ | 384,076 | (19.49) | |
| Ethnicity | White | 1385,108 | (70.30) |
| Asian | 78,243 | (3.97) | |
| Black | 44,327 | (2.25) | |
| Mixed, Other | 25,659 | (1.30) | |
| Missing | 436,977 | (22.18) | |
| Household Size | 1 | 568,530 | (28.85) |
| 2–4 | 1194,782 | (60.64) | |
| 5–8 | 129,538 | (6.57) | |
| 9+ | 30,558 | (1.55) | |
| Missing | 46,906 | (2.38) | |
| Index of multiple deprivation | 1 (Most Deprived) | 350,500 | (17.79) |
| (IMD) quintile | 2 | 448,426 | (22.76) |
| 3 | 375,423 | (19.05) | |
| 4 | 424,037 | (21.52) | |
| 5 (least Deprived) | 371,928 | (18.88) | |
| Body Mass Index (BMI) band | Normal weight | 637,287 | (32.34) |
| Overweight | 695,239 | (35.29) | |
| Obese class I | 455,990 | (23.14) | |
| Obese class II or III | 61,321 | (3.11) | |
| Missing | 120,477 | (6.11) | |
| Hypertension | Yes | 667,469 | (33.88) |
| No | 1302,845 | (66.12) | |
| Chronic Kidney Disease (CKD) | Yes | 110,877 | (5.63) |
| No | 1859,437 | (94.37) | |
| Chronic Heart Disease | Yes | 267,107 | (13.56) |
| No | 1703,207 | (86.44) | |
| Chronic Respiratory Disease | Yes | 108,799 | (5.52) |
| No | 1861,515 | (94.48) | |
| Malignancy or | Yes | 237,660 | (12.06) |
| immunocompromised | No | 1732,654 | (87.94) |
Comparison of surveillance system mortality with national statistics
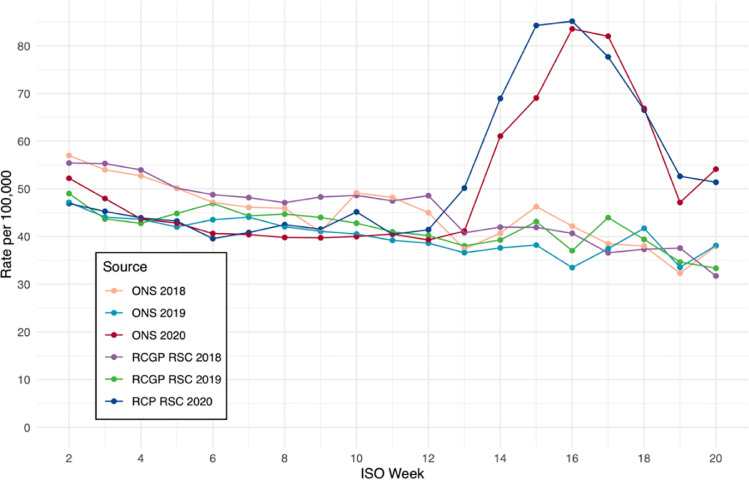
The mortality with the first wave of COVID-19 infection peaked in weeks 15 and 16 (Fig. 1 ) and followed the peak in incidence seen in the sentinel network (Supplementary file) and nationally.32 The mortality rates reported across the sentinel network were very similar to that reported nationally by ONS for the last three years (Fig. 1). The mortality rates for the three age-bands used in the study: 45 to 64 years old, 65 to 74 years, and 75 years old and over showed similar agreement with ONS (Supplementary file) mortality rates.
Fig. 1.
Mortality in people aged ≥ 45 years old per 100,000 between ISO Weeks 2 – 20 of 2018, 2019 and 2020 from sentinel network (RCGP RSC) and Office of National Statistics (ONS).
Absolute excess risk (AER) of mortality
We found the AER of mortality was just under 2 per 100 person years, in our cohort of people 45 years and older. There were 16,636 deaths in the sentinel population accrued over 703,958 person years, the average incidence was 2623 per 100,000. Based on the background mortality for the same period in 2019, we would have expected 2710.4. The absolute excess risk in this cohort was therefore 197.8 (95% CI:194.3–201.4) per 10,000 person years. We report the AER for the whole population in our supplementary file.
Univariate analysis showed disparities in mortality
Our univariate analysis showed male gender, older age-band, large household size (9 or more residents), the most deprived quintile and a range of long-term conditions were all associated with increase mortality (Table 2 ). The highest rates were age 75 years or above, EMR 22.95 (95%CI: 21.61–24.37, p<0.0001) compared to people age 45 years to 64, and household occupancy of ≥9, where the EMR compared to singly occupancy was 13.11 (95% CI:12.58–13.67, p<0.0001). Non-white ethnicities, compared with white, and obesity were not associated with increased risk in this analysis.
Table 2.
Results of the univariate analysis of associations with mortality rate (%) and excess mortality rate (EMR) reporting 95% confidence intervals (95%CI) and probability (p) in the RCGP RSC cohort of people aged 45 years and older.
| Category | Deaths | Denominator | Mortality rate (%) | EMR | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| Sex | Female | 8356 | 1009,705 | 0.83 | 1 | ||
| Male | 8280 | 960,609 | 0.86 | 1.04 | (1.01–1.07) | 0.02 | |
| Age band | 45–64 | 1887 | 1149,621 | 0.16 | 1 | ||
| 65–74 | 2611 | 436,617 | 0.60 | 4.12 | (3.84–4.42) | ||
| 75+ | 12,138 | 384,076 | 3.16 | 22.95 | (21.61–24.37) | <0.00 | |
| Ethnicity | White | 15,823 | 1822,085 | 0.87 | 1 | ||
| Asian | 408 | 78,243 | 0.52 | 0.58 | (0.53–0.65) | ||
| Black | 267 | 44,327 | 0.60 | 0.68 | (0.60–0.78) | ||
| Mixed, Other | 138 | 25,659 | 0.54 | 0.61 | (0.51–0.73) | <0.00 | |
| Household Size | 1 | 5899 | 573,292 | 1.03 | |||
| 2–4 | 6164 | 1236,926 | 0.50 | 0.47 | (0.45–0.48) | ||
| 5–8 | 795 | 129,538 | 0.61 | 0.58 | (0.54–0.63) | ||
| 9+ | 3778 | 30,558 | 12.36 | 13.11 | (12.58–13.67) | <0.00 | |
| IMD Quintile | 1 | 3138 | 350,500 | 0.90 | 1 | ||
| 2 | 3940 | 448,426 | 0.88 | 0.98 | (0.93–1.03) | ||
| 3 | 3049 | 375,423 | 0.81 | 0.90 | (0.86–0.95) | ||
| 4 | 3514 | 424,037 | 0.83 | 0.92 | (0.88–0.97) | ||
| 5 | 2995 | 371,928 | 0.81 | 0.89 | (0.85–0.94) | <0.00 | |
| Body Mass Index | Normal | 8070 | 681,190 | 1.18 | 1 | ||
| (BMI) | Overweight | 5231 | 771,813 | 0.68 | 0.56 | (0.54–0.58) | |
| Obese class I | 2831 | 455,990 | 0.62 | 0.51 | (0.49–0.53) | ||
| Class II/III | 504 | 61,321 | 0.82 | 0.68 | (0.62–0.75) | <0.00 | |
| Hypertension | No | 6683 | 1302,845 | 0.51 | 1 | ||
| Yes | 9953 | 667,469 | 1.49 | 3.0 | (2.93–3.10) | <0.00 | |
| Chronic Kidney | No | 12,492 | 1859,437 | 0.67 | 1 | ||
| Disease (CKD) | Yes | 4144 | 110,877 | 3.74 | 5.84 | (5.64–6.10) | <0.00 |
| Chronic Heart Disease | No | 9357 | 1703,207 | 0.55 | 1 | ||
| Yes | 7279 | 267,107 | 2.73 | 5.22 | (5.10–5.39) | <0.00 | |
| Chronic Respiratory | No | 13,650 | 1861,515 | 0.73 | 1 | ||
| Disease | Yes | 2986 | 108,799 | 2.74 | 3.89 | (3.89–4.05) | <0.00 |
| Malignancy or | No | 10,841 | 1732,654 | 0.63 | 1 | ||
| Immunocompromised | Yes | 5795 | 237,660 | 2.44 | 4.07 | (3.94–4.21) | <0.00 |
Excess mortality risk estimates from multivariate analyses
The results from the multivariable analysis showed male gender, increasing age, Black ethnicity (compared with white), poorer socioeconomic group (IMD Quintile 1), household size above 4 (compared with single occupancy), and presence of all studied comorbidities was associated with excess mortality (i.e. worse relative survival, Table 3 ). Among the chronic diseases, hypertension has a lower EMR, than CKD. CKD in turn has a lower EMR than chronic respiratory and heart disease. People with cancer and who were immunocompromised had the highest EMR.
Table 3.
Multivariable adjusted excess mortality rates for all-cause mortality across the Oxford RCGP RSC cohort of people 45 years and older including covariates contributing to excess mortality.
| Variable | Category | EMR | CI.95 | p-value |
|---|---|---|---|---|
| Sex | Female | Ref | ||
| Male | 1.40 | (1.35–1.44) | <0.00 | |
| Age band | 45–64 | Ref | ||
| 65–74 | 3.24 | (3.02–3.48) | <0.00 | |
| 75+ | 10.09 | (9.46–10.75) | <0.00 | |
| Ethnicity | White | Ref | ||
| Asian | 0.74 | (0.66–0.82) | <0.00 | |
| Black | 1.17 | (1.03–1.33) | 0.02 | |
| Mixed, Other | 1.13 | (0.94–1.35) | 0.18 | |
| Index of Multiple Deprivation | 1 (most deprived) | Ref | ||
| (IMD) Quintile | 2 | 0.88 | (0.84–0.92) | <0.00 |
| 3 | 0.80 | (0.76–0.84) | <0.00 | |
| 4 | 0.85 | (0.81–0.90) | <0.00 | |
| 5 (least deprived) | 0.81 | (0.77–0.86) | <0.00 | |
| Household size | 1 | Ref | ||
| 2–4 | 0.70 | (0.67–0.73) | <0.00 | |
| 5–8 | 1.63 | (1.51–1.77) | <0.00 | |
| 9+ | 8.01 | (7.67–8.35) | <0.00 | |
| Body Mass Index (BMI) | Normal weight | Ref | ||
| Overweight | 0.65 | (0.63–0.67) | <0.00 | |
| Obese class I | 0.62 | (0.59–0.65) | <0.00 | |
| Obese class II or III | 1.08 | (0.98–1.19) | 0.11 | |
| Hypertension | No | Ref | ||
| Yes | 1.17 | (1.13–1.22) | <0.00 | |
| Chronic Kidney Disease (CKD) | No | Ref | ||
| Yes | 1.46 | (1.41–1.52) | <0.00 | |
| Chronic Heart Disease | No | Ref | ||
| Yes | 1.73 | (1.68–1.79) | <0.00 | |
| Chronic Respiratory Disease | No | Ref | ||
| Yes | 1.62 | (1.56–1.69) | <0.00 | |
| Malignancy/ | No | Ref | ||
| immuno-compromised | Yes | 2.06 | (1.99–2.13) | <0.00 |
Discussion
Principal findings
This community-based study reports the absolute excess mortality in the population of 45 years and older was approximately 2 per 100 person years in the first wave of COVID-19 infection. In multivariate analyses, male gender, increasing age, deprivation, Black ethnicity and chronic disease were associated with an increased risk of excess mortality, confirming findings of previous studies which have focussed on confirmed COVID-19 related mortality. This study also shows that those living in a single occupancy household and those in larger households (5 or more people) have a higher risk of excess mortality compared to dwellings of 2 to 4 people. Such associations may represent older people living on their own, multigenerational occupancy or care homes which are known to be at increased risk. All of the chronic conditions examined in this cohort were associated with increased risk and further work is needed to identify how combinations of these conditions might affect an individuals’ risk, to enable better targeting of shielding strategies and vaccination programmes to prevent excess mortality if future waves of the pandemic occur.
Implications of the findings
The excess mortality over the study period of 18 weeks, is just under a quarter (23%) of the mortality for the whole of our reference year 2018, and very similar for 2019. The mortality from the whole of 2018 in people age 45 years and above was 8.81 per 100 person years and from 2019 was 8.56 per 100 person years.33
Our findings show that risk factors for excess mortality (regardless of COVID status) are similar to those reported in studies focussing on COVID-19 confirmed mortality. This suggests unaccounted deaths captured in the present study may also be related to undetected COVID-19 infection or indirect effects of COVID-19 lockdown measures. These data also suggest that policy about staying at home may need to be more nuanced. People in single occupancy housing may have greater risk. This could be because they are less likely to have outside space or they have to break their isolation more frequently. Larger households of 5 to 8 people are also at greater risk as well as dwellings with 9 people or more. Whilst risks about care homes have been well articulated,7 , 8 increase risk within moderately large dwellings has not.
Household size, in addition to information about an individual's socio-demographic status and pre-existing conditions should be incorporated into a clinical prediction model which would enable a more personalised approach to shielding strategies and vaccination programmes if future waves of the pandemic occur.
Comparison with the literature
Several previous studies have attempted to predict excess mortality from COVID-19 in the UK. These have suggested likely age-based case fatality rates,34 relative risk (RR) of mortality,35 concluding that the increased mortality due to COVID-19 may be equivalent to “packing a year's risk of mortality into a week or two.”36 Our data suggests that over an eight week period, mortality rates were 25% higher than would usually be expected for the time of year. The mortality reported here was higher than previously reported in studies focussing on confirmed COVID-19 related mortality following hospital admission.37
In the UK, it has been suggested that those with dementia are among those who have experienced an increase in mortality despite not having a confirmed COVID-19 infection.2 Explanations for this rise include mortality from COVID-19 being present but not recorded on the death certificate, indirect causes (collateral damage) or statistical artefacts. Premature death may occur as a result of reduced hospital capacity leading to delays in people receiving life-saving care, or from people choosing not to or being prevented from seeking care.38
There seem to be consistent reports about increased risk in people of Black ethnicity, but less certainty if there is increase risk in Asian ethnicity compared to white.39 , 40 There is however, increased test positivity and hospitalisation.41 , 15 , 42
There are suggestions in the literature that household transmission is one of the ways COVID-19 is spread,43 that household transmissions is greatest from younger to older people,44 and that people who know they are quarantined are less likely to pass it on.45 Our data confirm this but also show an increase risk for those living in single occupancy households, perhaps because they are less likely to have outside space or they have to break their isolation more frequently.
There are a number of reports of confirmed COVID-19 related mortality during the first wave of COVID-19 infection. Risk factors include hypertension, cardiovascular disease, diabetes,47 respiratory disease,46 , 48 and cancer.49 These studies are primarily based on data from secondary care and speciality based rather than able to compare relative rates across a population. Our data show similar risk factors for all excess mortality, regardless of whether a COVID-19 test is given or infection is confirmed.
Strengths and limitations
The strengths of our study is that it is based on individual level data, collected from a representative national primary care surveillance network, with an emphasis on good data quality.14 , 50 We did consider a number of chronic conditions within our models, but this list was not exhaustive. For example, we did not include dementia and Alzheimer's disease in our analysis, and these conditions have since been reported as being associated with mortality, non-attributable to COVID-19 infection.2 Similarly, we did not include diabetes in our analysis, again a condition known associated with increased risk.51 There may be a small lag between death date (sentinel network) and the date death certificates were issued (ONS data).
For our estimation of excess mortality, we compared rates within the RCGP RSC to ONS Life Tables for 2018,24 the last year for which they were available. Whilst it may have been possible to construct life tables using our own data, this is unlikely to have been significantly different since mortality rates observed in the RCGP RSC were very similar to those within ONS (Fig. 1).
Fig. 2 .
Fig. 2.
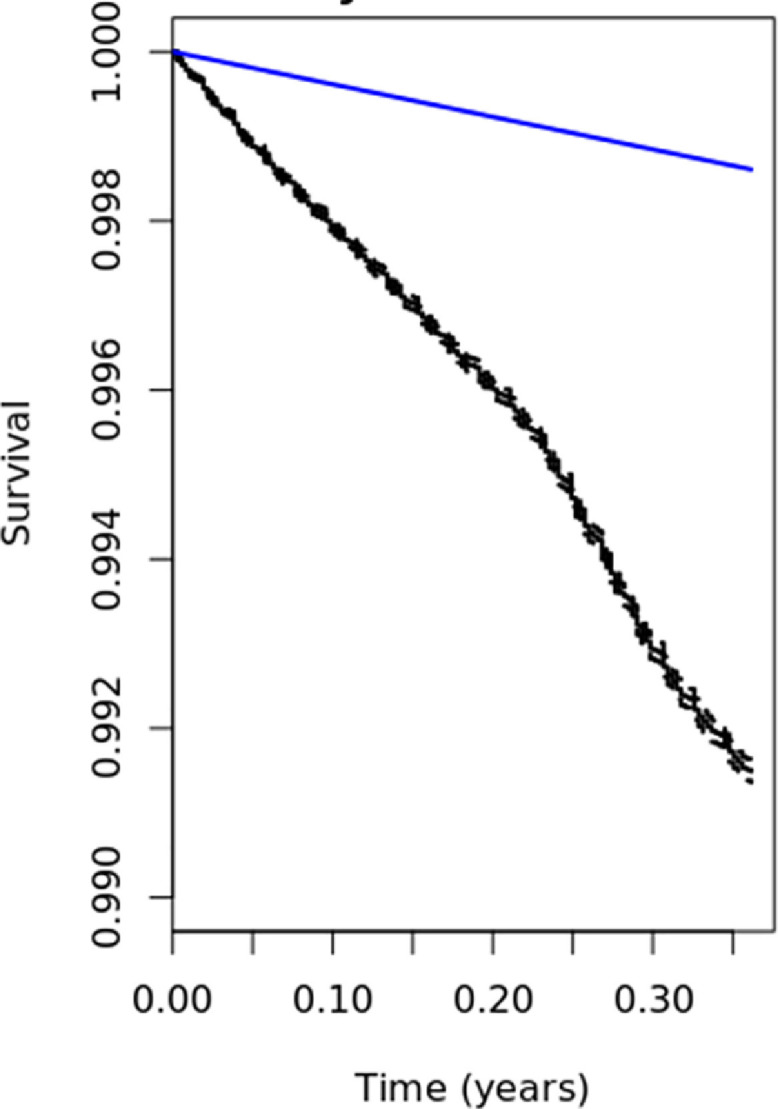
Kaplan Meier Estimates of overall survival in the RCGP RSC cohort age 45 years and above and relative survival.
Conclusions
These data show an excess in mortality rates across the first wave of COVID-19 infection, equivalent to two extra deaths per hundred person years. They also show that single occupancy and larger households are important predictors of mortality, an observation not previously seen in analyses of routine electronic health records. Household size, in addition to information about an individual's socio-demographic status and pre-existing conditions should be incorporated into a clinical prediction model to enable better targeting of strategies to prevent excess mortality in future waves of the pandemic.
Data sharing
The RCGP RSC data set can be accessed by researchers, approval is on a project-by-project basis (www.rcgp.org.uk/rsc). Ethical approval by an NHS Research Ethics Committee is needed before any data release/other appropriate approval. Researchers wishing to directly analyse the patient-level pseudonymised data will be required to complete information governance training and work on the data from the secure servers at the University of Surrey. Patient-level data cannot be taken out of the secure network. We encourage interested researchers to attend the short courses on how to analyse primary-care data/RCGP RSC data offered twice a year.
Declaration of Competing Interest
SdeL is the director of RCGP RSC. He has unrelated projects funded by GSK, Seqirus and has been a member of Global Advisory Boards for Seqirus and Sanofi.
Acknowledgements
The authors would like to thank the participating practices and patients for providing the data for this cohort. We acknowledge collaboration with the general practitioner computer system suppliers – EMIS health, The Phoenix Partnership and InPractice Systems-Apollo Medical Systems, Public Health England, Wellcome Trust and our other funders and collaborators. Funders have no role in the writing of the manuscript or the decision to submit it for publication. FDRH acknowledges part-funding from the National Institute for Health Research (NIHR) School for Primary Care Research, the NIHR Collaboration for Leadership in Health Research and Care (CLARHC) Oxford, the NIHR Oxford Biomedical Research Centre (BRC, UHT), and the NIHR Oxford Medtech and In-Vitro Diagnostics Co-operative (MIC).
The Oxford RCGP RSC is principally funded by Public Health England. CO receives funding from Wellcome Trust, which allowed her time to be repurposed for SARS-CoV-2 research. JPS receives funding from the Wellcome Trust/Royal Society via a Sir Henry Dale Fellowship (ref: 211182/Z/18/Z) and an NIHR Oxford Biomedical Research Centre (BRC) Senior Fellowship. BDN is funded by the NIHR. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. There was no specific funding for this research.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.08.037.
Appendix. Supplementary materials
References
- 1.EuroMOMO (European Mortality Monitoring). Z-scores by countries. URL:https://www.euromomo.eu/graphs-and-maps/#z-scores-by-country.
- 2.Office of National Statistics (ONS). Analysis of death registrations not involving coronavirus (COVID-19), England and Wales: 28 December2019to 1 May 2020. ONS 5th June 2020. URL:https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/analysisofdeathregistrationsnotinvolvingcoronaviruscovid19englandandwales28december2019to1may2020/technicalannex.
- 3.Kontopantelis E., Mamas M., Deanfield J., Asaria M., Doran T. Excess mortality in England and Wales during the first wave of the COVID-19 pandemic. medRxiv 2020.05.26.20113357; doi: 10.1101/2020.05.26.20113357. [DOI] [PMC free article] [PubMed]
- 4.Institute and Faculty of Actuaries. Mortality monitor – COVID-19 update – week 21 of 2020. England & Wales mortality monitor – COVID-19 update – week 21 of 2020. URL:https://www.actuaries.org.uk/system/files/field/document/Mortality%20monitor%20Week%2021%202020%20v01%202020-06-02_0.pdf.
- 5.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention [published online ahead of print, 2020 Feb 24] JAMA. 2020 doi: 10.1001/jama.2020.2648. 10.1001/jama.2020.2648. doi:10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Yarza R., Bover M., Paredes D., López-López F., Jara-Casas D., Castelo-Loureiro A. SARS-CoV-2 infection in cancer patients undergoing active treatment: analysis of clinical features and predictive factors for severe respiratory failure and death. Eur J Cancer. 2020 Jun 6 doi: 10.1016/j.ejca.2020.06.001. S0959-8049(20)30313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burki T. England and Wales see 20 000 excess deaths in care homes. Lancet. 2020;395(10237):1602. doi: 10.1016/S0140-6736(20)31199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Temkin-Greener H., Gao S., Cai X. COVID-19 infections and deaths among Connecticut nursing home residents: facility correlates [published online ahead of print, 2020 Jun 18] J Am Geriatr Soc. 2020 doi: 10.1111/jgs.16689. 10.1111/jgs.16689. doi:10.1111/jgs.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham N.S.N., Junghans C., Downes R., Sendall C., Lai H., McKirdy A. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020 Jun 3 doi: 10.1016/j.jinf.2020.05.073. S0163-4453(20)30348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lusignan S., Correa A., Smith G.E. RCGP Research and Surveillance Centre: 50 years surveillance of influenza, infections, and respiratory conditions. Br J Gen Pract. Oct 2017;67(663):440–441. doi: 10.3399/bjgp17X692645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lusignan S., Lopez Bernal J., Zambon M. Emergence of a Novel Coronavirus (COVID-19): protocol for extending surveillance used by the royal college of general practitioners research and surveillance centre and public health England. JMIR Public Health Surveill. 2020 Apr 2;6(2):e18606. doi: 10.2196/18606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. Jun 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. Epub 2020 Mar 30. Erratum in: Lancet Infect Dis. 2020 Apr 15;: Erratum in: Lancet Infect Dis. 2020 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajgor D.D., Lee M.H., Archuleta S., Bagdasarian N., Quek S.C. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. Jul 2020;20(7) doi: 10.1016/S1473-3099(20)30244-9. 776–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontopantelis E., Mamas M., Deanfield J., Asaria M., Doran T. Excess mortality in England and Wales during the first wave of the COVID-19 pandemic. MedRxiv. 2020 doi: 10.1016/S1473-3099(20)30244-9. doi: 10.1016/S1473-3099(20)30244-9. 05.26.20113357. Published online 2020 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa A., Hinton W., McGovern A. Royal College of General Practitioners Research and Surveillance Centre (RCGP RSC) sentinel network: a cohort profile. BMJ Open. 2016;6(4) doi: 10.1136/bmjopen-2016-011092. Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical Informatics and Health Outcomes Research Group, Nuffield department of primary care health sciences. Mortality in the Oxford-RCGP RSC sentinel network. Available at: https://app.powerbi.com/view?r=eyJrIjoiNDQ4NjA1MDktODBjZC00MjkxLThjZTEtZDQ3YzllYTgwYWRkIiwidCI6IjZiOTAyNjkzLTEwNzQtNDBhYS05ZTIxLWQ4OTQ0NmEyZWJiNSIsImMiOjh9 Accessed 20/06/2020.
- 17.de Lusignan S., Dorward J., Correa A. Risk factors for SARS-CoV-2 among patients in the oxford royal college of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30371-6. May 15, S1473-3099(20)30371-6 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Public Health England . PHE Publications; London: June 2020. Disparities in the risk and outcomes of COVID-19. Gateway number GW-1311 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/890258/disparities_review.pdf. [Google Scholar]
- 19.Niedzwiedz C.L., O'Donnell C.A., Jani B.D. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. Version 2. BMC Med. 2020;18(1):160. doi: 10.1186/s12916-020-01640-8. May 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tippu Z., Correa A., Liyanage H. Ethnicity recording in primary care computerised medical record systems: an ontological approach. J Innov Health Inform. 2017;23(4):920. doi: 10.14236/jhi.v23i4.920. Mar 14. [DOI] [PubMed] [Google Scholar]
- 21.Hoang U., James A.C., Liyanage H. Determinants of inter-practice variation in ADHD diagnosis and stimulant prescribing: cross-sectional database study of a national surveillance network. BMJ Evid Based Med. Aug 2019;24(4):155–161. doi: 10.1136/bmjebm-2018-111133. Epub 2019 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lusignan S., Sherlock J., Ferreira F., O'Brien S., Joy M. Household presentation of acute gastroenteritis in a primary care sentinel network: retrospective database studies. BMC Public Health. 2020;20(1):445. doi: 10.1186/s12889-020-08525-8. Published 2020 Apr 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UK Government, National Statistics, English indices of deprivation2015. URL:https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015
- 24.World Health Organisation. What is overweight and obesity?. URL:https://www.who.int/dietphysicalactivity/childhood_what/en/ (accessed 18/06/2020).
- 25.Cole N.I., Liyanage H., Suckling R.J. An ontological approach to identifying cases of chronic kidney disease from routine primary care data: a cross-sectional study. BMC Nephrol. 2018 Apr 10;19(1):85. doi: 10.1186/s12882-018-0882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Max Planck Institute for Demographic Research. The Human Mortality Database [Internet]. 2020. [cited 2020 Jun 8]. Available from: https://www.mortality.org/ (accessed 15/06/2020).
- 27.Elie C., De Rycke Y., Jais J., Landais P. Appraising relative and excess mortality in population-based studies of chronic diseases such as end-stage renal disease. Clin Epidemiol. 2011;3:157–169. doi: 10.2147/CLEP.S17349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murad H., Dankner R., Berlin A., Olmer L., Freedman L.S. Imputing missing time-dependent covariate values for the discrete time Cox model. Stat Methods Med Res. 2019 doi: 10.1177/0962280219881168. 962280219881168. [DOI] [PubMed] [Google Scholar]
- 29.Murad H., Dankner R., Berlin A., Olmer L., Freedman L.S. Imputing missing time-dependent covariate values for the discrete time Cox model [published online ahead of print, 2019 Nov 3] Stat Methods Med Res. 2019 doi: 10.1177/0962280219881168. 962280219881168. [DOI] [PubMed] [Google Scholar]
- 30.Eekhout I., van de Wiel M.A., Heymans M.W. Methods for significance testing of categorical covariates in logistic regression models after multiple imputation: power and applicability analysis. BMC Med Res Methodol. 2017;17(1):129. doi: 10.1186/s12874-017-0404-7. Published 2017 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Core Team . R Foundation for Statistical Computing. Vienna; Austria: 2013. R: a language and environment for statistical computing. URL http://www.R-project.org/ (accessed 10/06/2020) [Google Scholar]
- 32.Public Health England (PHE). Weekly Coronavirus Disease 2019 (COVID-19) Surveillance Report Summary of COVID-19 surveillance systems: year:2020Week: 20 URL:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/885877/COVID19_Epidemiological_Summary_w20.pdf(accessed 20/06/2020).
- 33.Office for National Statistics. Deaths registered weekly in England and Waleshttps://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/weeklyprovisionalfiguresondeathsregisteredineng. (accessed 12/06/2020).
- 34.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. Jun 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. Epub 2020 Mar 30. Erratum in: Lancet Infect Dis. 2020 Apr 15;: Erratum in: Lancet Infect Dis. 2020 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee A., Pasea L., Harris S. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395(10238):1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiegelhalter D. How much ‘normal’ risk does Covid represent? URL:https://medium.com/wintoncentre/how-much-normal-risk-does-covid-represent-4539118e1196(accessed 14/06/2020).
- 37.Williamson E., Walker A., Bhaskaran K. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. MedRxiv. 2020 doi: 10.1101/2020.05.06.20092999. [DOI] [Google Scholar]
- 38.Baldi E., Sechi G.M., Mare C. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J. 2020 Jun 20 doi: 10.1093/eurheartj/ehaa508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santorelli G., Sheldon T., West J., Cartwright C., Wright J. COVID-19 in-patient hospital mortality by ethnicity. Wellcome Open Res. 2020;5:86. doi: 10.12688/wellcomeopenres.15913.1. Published 2020 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan D., Sze S., Minhas J. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100404. published online June 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niedzwiedz C.L., O'Donnell C.A., Jani B.D. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. Version 2. BMC Med. 2020 May 29;18(1):160. doi: 10.1186/s12916-020-01640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel A.P., Paranjpe M.D., Kathiresan N.P., Rivas M.A., Khera A.V. Race, Socioeconomic Deprivation, and Hospitalization for COVID-19 in English participants of a National Biobank. medRxiv[Preprint]. 2020 May 2:2020.04.27.20082107. doi: 10.1101/2020.04.27.20082107. [DOI] [PMC free article] [PubMed]
- 43.Jing Q.L., Liu M.J., Zhang Z.B. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou. China: A Retrospect Cohort Study. Lancet Infect Dis. 2020 Jun 17 doi: 10.1016/S1473-3099(20)30471-0. S1473-3099(20)30471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Gu Z., Xia S. What are the underlying transmission patterns of COVID-19 outbreak? - An age-specific social contact characterization. Version 2. EClinicalMedicine. 2020 Apr 18;22 doi: 10.1016/j.eclinm.2020.100354. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W., Zhang B., Lu J. The characteristics of household transmission of COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa450. Apr 17:ciaa450Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barron E., Bakhai, C., Kar P., et al. Type 1 and Type 2 Diabetes and COVID-19 Related Mortality in England: a WholePopulation URL: 10.2139/ssrn.3605225(accessed 17/05/2020). [DOI] [PMC free article] [PubMed]
- 47.Michelozzi P., de'Donato F., Scortichini M. Mortality impacts of the coronavirus disease (COVID-19) outbreak by sex and age: rapid mortality surveillance system, Italy, 1 February to 18 April 2020. Euro Surveill. 2020;25(19) doi: 10.2807/1560-7917.ES.2020.25.19.2000620. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deiana G., Azara A., Dettori M. Deaths in SARS-Cov-2 positive patients in italy: the influence of underlying health conditions on lethality. Int J Environ Res Public Health. 2020 Jun 21;17(12):E4450. doi: 10.3390/ijerph17124450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuderer N.M., Choueiri T.K., Shah D.P. COVID-19 and cancer consortium. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020 Jun 20;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. Epub 2020 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinton W., McGovern A., Coyle R. Incidence and prevalence of cardiovascular disease in English primary care: a cross-sectional and follow-up study of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) BMJ Open. 2018 Aug 20;8(8) doi: 10.1136/bmjopen-2017-020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Z.H., Tang Y., Cheng Q. Diabetes increases the mortality of patients with COVID-19: a meta-analysis [published online ahead of print, 2020 Jun 24] Acta Diabetol. 2020:1–6. doi: 10.1007/s00592-020-01546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.