Abstract
Obesity and its related metabolic disorders, as well as infectious diseases like covid-19, are important health risks nowadays. It was recently documented that long-term fasting improves metabolic health and enhanced the total antioxidant capacity. The present study investigated the influence of a 10-day fasting on markers of the redox status in 109 subjects. Reducing power, 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt radical cation(ABTS) radical scavenging capacity, and hydroxyl radical scavenging capacity increased significantly, and indicated an increase of circulating antioxidant levels. No differences were detected in superoxide scavenging capacity, protein carbonyls, and superoxide dismutase when measured at baseline and after 10 days of fasting. These findings were concomitant to a decrease in blood glucose, insulin, glycated hemoglobin (HbA1c), total cholesterol, low-density lipoprotein (LDL) and triglycerides as well as an increase in total cholesterol/high-density lipoprotein (HDL) ratio. In addition, the well-being index as well as the subjective energy levels increased, documenting a good tolerability. There was an interplay between redox and metabolic parameters since lipid peroxidation baseline levels (thiobarbituric acid reactive substances [TBARS]) affected the ability of long-term fasting to normalize lipid levels. A machine learning model showed that a combination of antioxidant parameters measured at baseline predicted the efficiency of the fasting regimen to decrease LDL levels. In conclusion, it was demonstrated that long-term fasting enhanced the endogenous production of antioxidant molecules, that act protectively against free radicals, and in parallel improved the metabolic health status. Our results suggest that the outcome of long-term fasting strategies could be depending on the baseline values of the antioxidative and metabolic status of subjects.
Keywords: Weight loss, Antioxidant capacity, Oxidative stress, Dysmetabolism
Highlights
-
•
Long-term fasting increases the antioxidant capacity and decreases oxidative damage.
-
•
It improves the metabolic health status.
-
•
High TBARS levels at baseline limit the LDL reduction during long-term fasting.
-
•
The antioxidant status is related with the lipid lowering effect of long-term fasting.
1. Introduction
The combination of a sedentary lifestyle with easy access to processed foods rich in low-quality fats and sugars is largely responsible for the increase in obesity in modern Western culture, among others in Europe (Blundell et al., 2017). Metabolic disorders like type 2 diabetes and cardiovascular diseases as well as cancer and immune system dysfunctions have been linked to obesity (Margină et al., 2020a, 2020b). Recently during the pandemic of covid-19 obese individuals, suffering dysmetabolism, seemed to have a more severe evolution and a lower survival rate (Nasi et al., 2020; Petrakis et al., 2020; Stefan et al., 2020). Metabolic stress can be exacerbated by the exposure to environmental pollutants and disturbing among others lipid metabolism such as pesticides (Biserni et al., 2019), common food additives (Ciardi et al., 2012), or even near-roadway air pollution (Kim et al., 2018).
The redox status is intimately linked to metabolic status, especially lipid metabolism. This is supported by experiments showing that abnormal lipid metabolism is associated with the activation of oxidative and inflammatory pathways (Zhao et al., 2015). This can be due to the modulation of transcription factors such as the nuclear factor-erythroid 2 p45-related factor 2 (NRF2) which is known to coordinate the antioxidant response (Nguyen et al., 2009), but also the metabolism of lipids including their peroxidation (Dodson et al., 2019). In addition, there is a positive correlation between levels of oxidative stress and the atherogenicity of serum cholesterol fractions (Yang et al., 2008). This suggested that the level of oxidative stress is an early event influencing the evolution of dyslipidemia documenting the interplay between oxidative damages, redox and metabolic biomarkers.
There is a need to find natural ways for humans to reduce the prevalence of obesity as well as dysmetabolism in order to improve their metabolic health. In addition, it would be important to improve our oxidative status and strengthen defense mechanisms against various diseases including those caused by viruses like covid-19. A possible approach to achieve these objectives could be long-term fasting, that is defined as voluntary food abstinence from 2 days to several weeks (Wilhelmi de Toledo et al., 2020b). The study of various forms of fasting on metabolic health has been gaining ground in recent years with more and more studies showing beneficial effects such as inactivating of the mechanistic target of rapamycin (mTOR) signaling pathways as well as several others resulting in metabolic normalization, enhanced autophagy and apoptosis followed by cell regeneration, increased brain-derived neurotrophic factor (BDNF) levels in brain leading to enhanced cognition, mood as well as increased neuronal plasticity and regeneration. Fasting also enhanced the transcription of cytoprotective enzymes, mitochondrial biogenesis all of these effects leading to restoration and conservation of functional integrity of cells and tissues (de Cabo and Mattson, 2019; Wilhelmi de Toledo et al., 2020b). Moreover, it has been shown that long-term fasting improved dysmetabolism as well as the antioxidant capacity (Drinda et al., 2019; Wilhelmi de Toledo et al., 2019, 2020a).
Modern medicine is getting personalized and machine learning algorithms are increasingly used to predict the success of therapeutic interventions based on patient individual characteristics. This was the case for the prediction of postprandial lipemic and inflammatory responses (Berry et al., 2020), the development gestational diabetes (Artzi et al., 2020), or even the occurrence of heart attacks and stroke (Knott et al., 2020). Earlier studies also suggest that this can be applicable to understand redox systems as a recent study successfully developed a neural network to predict oxidative damage using measurements of antioxidants in plasma and urine (de la Villehuchet et al., 2009).
Since dysmetabolism has been repeatedly correlated to an increased oxidative stress (A Sevanian and Hochstein, 1985; Holvoet, 2008; Rani et al., 2016), it can be hypothesized that beneficial effects of long-term fasting could be affected by the baseline antioxidant status of patients. The BDNF, which has its production stimulated by ketosis during fasting (Mattson et al., 2018), is known to control the nuclear translocation of the transcription factor Nrf2 to activate antioxidant defenses (Bouvier et al., 2017). Furthermore, the mild oxidative stress caused by the reduction in adenosine triphosphate (ATP)/adenosine monophosphate (AMP) ratio has a similar effect to activate transcription of cytoprotective enzymes sulfiredoxin 1, thioredoxin reductase 1, heme oxygenase-1, as well as several others conjugation and elimination enzymes (Burton et al., 2016).
This article is a continuation of our previous study on the effect of a 10-day fasting on indicators of redox status of humans which showed that while the total antioxidant capacity (TAC) was enhanced, TBARS, an important indicator of lipid peroxidation, were reduced (Wilhelmi de Toledo et al., 2020a). The aim of the present article was to analyze additional redox parameters and to correlate the redox status with markers of glucose and lipid status, the improvement of which leads to a better metabolic health and a reduced risk of metabolic diseases. It was also evaluated if the baseline levels in markers of the redox status can predict the success of the fasting regimen using a machine learning approach.
2. Materials and methods
2.1. Ethics statement
This interventional study was approved by the medical council of Baden-Württemberg, Stuttgart, on 12 February 2019 under the application number F-2018-118. The study protocol was implemented in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and registered on 20 February 2019 in the German Clinical Trials Register (DRKS-ID: DRKS00016657). The recruitment was conducted between 15 September 2019 and 18 november 2019. All participants gave their written informed consent before enrolling into the study.
2.2. Participants
The 109 study participants were recruited out of a total of 182 subjects who were admitted to the Buchinger Wilhelmi Clinic (BWC) and fulfilled the following criteria: Subjects were aged between 18 and 70 years and underwent at least 7 to maximum 13 (10 ± 3 days) days of fasting. Two blood samplings at the beginning and at the end of the experiment were conducted. One week prior as well as during the fasting period the intake of micronutrient supplements was advised to be stopped, except for magnesium supplementation. Clinical experience showed that magnesium intake during the fasting course seems to protect against muscle cramps that possibly could be provoked by the recommendation to drink sufficient liquids during fasting. Subjects were excluded when they had a predefined contraindication to fasting as described in the guidelines of fasting therapy (Wilhelmi de Toledo et al., 2013) like cachexia, anorexia nervosa, advanced kidney, liver or cerebrovascular insufficiency, dementia or other chronic psychiatric diseases, as well as pregnant or lactating women. Furthermore, subjects who could not follow the study procedure due to an inability to speak German, English or French, or subjects that were participating in another study, were also excluded. Altogether, 37 subjects did not meet the inclusion criteria, and 35 subjects declined to participate. One subject terminated the study earlier as defined in the protocol due to low hemoglobin and sodium levels.
2.3. The fasting protocol
All subjects underwent a medical supervised fasting program according to peer-reviewed guidelines (Wilhelmi de Toledo et al., 2013) that included physical exercise and individual treatments (Wilhelmi de Toledo et al., 2019). On the day prior fasting the subjects received a 600 kcal diet of either rice and vegetables or fruits. To initiate the fasting period, a laxative (20–40 g Na2SO4 in 500 ml water) was administered. During fasting, an enema was applied every other day to remove intestinal remnants and desquamated mucosal cells. A calorie intake of ~250 kcal/day was ensured by the daily intake of 250 ml freshly squeezed organic juice at midday, and 250 ml of vegetable soup in the evening, as well as 20 g honey per day. The subjects were advised to drink daily at least 2–3 L of water or non-caloric herbal teas. A stepwise reintroduction of food with an ovo-lacto-vegetarian organic diet from 800 to 1600 kcal/day followed the fasting period.
2.4. Clinical data
Clinical data were collected according to the BWC standards. Before start of the fasting program, the subjects underwent a thorough physical examination. Measurements were performed at two time-points in the morning in the fasted state. The baseline examination was conducted before starting of the fast (time point #1, pre) and the second examination was done at the 10 ± 3 fasting day (time-point #2, post). Subjects’ height was measured with seca 285 (Seca, Hamburg, Germany) and the waist circumference was assessed with a measuring tape, placed halfway between the lowest rib and the iliac crest. Body weight was measured daily (Seca 704/635, Seca, Hamburg, Germany) between 7:00 am and 9:00 am by trained nurses, while subjects were lightly dressed. Additionally, blood pressure and heart rate were measured on the non-dominant arm after a rest, while subjects were seated (boso Carat professional; BOSCH + SOHN GmbH u. Co. KG).
Subjects self-reported their energy level on a numeric rating scale from 0 (weak) to 10 (powerful) before and after fasting. Furthermore, the well-being index (WHO-5) was self-assed by answering five statements scored from 0 (at no time) to 5 (all of the time). After building the sum of the five scores and multiplying it with 4 the WHO-5 was given as a percentage between 0% and 100% (Bech, 2004). Possible adverse events were documented in a report form by the medical staff.
2.5. Blood collection and handling for the measurement of redox biomarkers
Blood samples were collected twice, at baseline in the first morning after arrival and at the 10 ± 3 fasting day by trained medical-technical assistants between 7.30 and 9.00 am Blood samples (10 ml) were drawn from a forearm vein with subjects sitting in an upright position. Blood was collected in ethylenediaminetetraacetic acid (EDTA) tubes, centrifuged immediately (1370 g, 10 min, 4 °C), and the plasma was collected and used for the measurement of the ABTS radicalscavenging activity, the reducing power, the hydroxyl radical (OH∙ -) scavenging activity, the superoxide anion (O2∙-) radical-scavenging ability of plasma and the protein carbonyls an index of protein oxidation. The remaining packed erythrocytes were lysed with dH2O (1:1 v/v), inverted vigorously, centrifuged (4020 g, 15 min, 4 °C) and the erythrocyte lysate was collected for the measurement of Superoxide Dismutase (SOD) activity. Plasma and erythrocyte lysate samples were then stored at −80 °C until the biochemical analyses were performed.
Routine laboratory blood parameters were performed in the laboratory MVZ Labor Ravensburg. The lipid parameters (total cholesterol, LDL, HDL, triglycerides), glucose, liver enzymes (serum glutamic oxaloacetic transaminase (GOT), serum glutamate pyruvate transaminase (GPT), serum gamma-glutamyl transferase (GGT), alkaline phosphatase (AP)), and kidney parameters (glomerular filtration rate (GFR), urea, creatinine, uric acid), as well as the inflammatory parameter C-reactive protein (CRP) were analyzed with ADVIA 2400 (Siemens Health care GmbH, Erlangen, Germany). Insulin was measured with Centaur XP (Siemens Healthcare GmbH) and HbA1c was assessed with TOSOHTM (Bio-Rad Laboratories GmbH, München, Germany).
2.6. Protocols for the measurement of redox biomarkers
The concentration of protein carbonyls, an index of protein oxidation, was determined based on the method described in the study by Patsoukis et al. (2004). In this assay, 50 μl of 20% TCA were added to 50 μl of plasma and this mixture was incubated in an ice bath for 15 min and centrifuged at 15,000×g for 5 min at 4 °C. The supernatant was discarded and 500 μl of 10 mM 2,4-dinitrophenylhydrazine (DNPH; Sigma-Aldrich, Munich, Germany) (in 2.5 N HCl) for the sample, or 500 μl of 2.5 N HCl for the blank, were added to the pellet. The samples were incubated in the dark at room temperature for 1 h with intermittent vortexing every 15 min and were centrifuged at 15,000×g for 5 min at 4 °C. The supernatant was discarded and 1 ml of 10% TCA was added, vortexed and centrifuged at 15,000×g for 5 min at 4 °C. The supernatant was discarded and 1 ml of ethanol-ethyl acetate (1:1 v/v) was added, vortexed and centrifuged at 15,000×g for 5 min at 4 °C. This washing step was repeated twice. The supernatant was discarded and 1 ml of 5 M urea (pH = 2.3) was added, vortexed and incubated at 37 °C for 15 min. The samples were centrifuged at 15,000×g for 3 min at 4 °C and the absorbance was read at 375 nm. The calculation of the protein carbonyls concentration was based on the molar extinction coefficient of DNPH. Total plasma protein was assayed using the Bradford protein assay.
The superoxide anion radical-scavenging ability of plasma was measured using a slightly modified protocol of Ak and Gülçin (2008). In this method, superoxide anion (O2 ∙−) is generated in a phenazine methosulfate and reduced nicotinamide adenine dinucleotide (PMS-NADH) system by NADH oxidation and it reduces the yellow dye of nitroblue tetrazolium (NBT2 +) to the blue colored formazan. More specifically, 125 μl of 300 μM NBT2 +, 125 μl of 468 μM NADH, and 10 μl of deproteinaized plasma were added into 625 μl of 16 mM Tris-HCl (pH = 8.0). The reaction is initiated by the addition of 125 μl of 60 μM PMS to the mixture. The samples were incubated for 5 min and the absorbance was monitored at 560 nm. Plasma antioxidants are acting as inhibitors to the blue colored formazan formation.
In the reducing power assay, a plasma sample was dissolved in phosphate buffer (0.2 M, pH = 6.6) at different concentrations. An aliquot (10 μl) of the sample solution was added to 490 μl of 1% potassium ferricyanide and incubated at 50 °C for 20 min. The samples were cooled on ice for 5 min. Then, 250 μl of 10% TCA was added and the samples were centrifuged (1700 g, 10 min, and 25 °C). Subsequently, 250 μl of dH2O and 50 μl of 0.1% ferric chloride were added to the supernatant and the samples were incubated at RT for 10 min. The absorbance was monitored at 700 nm (Yen and Duh, 1994).
Regarding the assay for hydroxyl radical (OH∙ -) scavenging activity, 10 μl of plasma dissolved in dH2O at different concentrations was added to 450 μl of 0.2 M sodium phosphate buffer (pH = 7.4), 150 μl of 10 mM 2-deoxyribose, 150 μl of 10 mM FeSO4-EDTA, 525 μl of dH2O, and 150 μl of 10 mM H2O2. Then, the samples were incubated at 37 °C for 4 h. Afterwards, 750 μl of 2.8% TCA and 750 μl of 1% TBA were added, and the samples were incubated at 95 °C for 10 min. Then, the samples were cooled on ice for 5 min, centrifuged (1700 g, 10 min, and 25 °C), and the absorbance was monitored at 520 nm. In each experiment, the sample without H2O2 was considered as blank and the sample without protein as control (Chung et al., 1997).
The free radical-scavenging activity of the samples was also determined by ABTS radical cation (ABTS•+) decolorization assay as previously described by Cano et al. (2000), with some modifications. In brief, ABTS•+ radical was produced by mixing 2 mM ABTS with 30 μM H2O2 and 6 μM horseradish peroxidase (HRP) enzyme in 50 mM PBS (pH = 7.5). Immediately, following the addition of the HRP enzyme, the contents were vigorously mixed, incubated at room temperature in the dark and the reaction was monitored at 730 nm until stable absorbance was obtained. Subsequently, 10 μl of plasma were added in the reaction mixture and the decrease in absorbance at 730 nm was measured. In each experiment, the tested sample alone containing 1 mM ABTS and 30 μM H2O2 in 50 mM PBS (pH = 7.5) was used as a blank, while the formed ABTS•+ radical solution alone with 10 μl H2O was used as a control.
The determination of SOD activity in RBCL was based on the method of nitroblue tetrazolium salt (NBT) as described in the study by Oberley and Spitz (1984). More specifically, this assay included a negative control which was prepared by mixing 800 μl of SOD buffer [1 mM diethylenetriaminepentaacetic acid (DETAPAC) in 0.05 M potassium phosphate buffer (pH = 7.8), 1 unit CAT, 5.6 × 10−5 M NBT and 10−4 M xanthine] with 200 μl of 0.05 M potassium phosphate buffer. Subsequently, ~60 mU of xanthine oxidase were added and the rate of increase in absorbance was measured at 560 nm for 1.5 min. In the test samples, 200 μl of the total 1:100 RBCL were added to 800 μl of SOD buffer followed by the addition of ~60 mU of xanthine oxidase and the rate of increase in absorbance was measured for 1.5 min at 560 nm. The calculation of SOD activity in the test samples is based on the percentage inhibition in the rate of increase in absorbance. SOD activity in the RBCL was normalized to the total cellular Hb level in each sample. The results are expressed as units (one unit of SOD inhibits the rate of increase in absorbance at 550 nm by 50%) per mg of Hb.
Previously published data were reanalyzed in order to obtain a comprehensive evaluation of the redox status. The measurement of glutathione (GSH) levels, catalase activity, TAC, TBARS, Glutathione Peroxidase (GPx) and Glutathione reductase (GR) activities were performed as previously described (Wilhelmi de Toledo et al., 2020a). In brief, GSH levels were measured according to Reddy et al. as previously descrided by Veskoukis et al., 2016. The intra- and inter-assay CVs for GSH were 3.1% and 4.5%, respectively. For catalase activity, the method of Aebi was used (Aebi, 1984). The intra- and inter-assay CVs for catalase were 6.2% and 10.0%, respectively. The determination of TAC was based on the method of Janaszewska and Bartosz as mmol of DPPH• reduced to 2, 2-diphenyl-1-picrylhydrazine (DPPH:H) by the antioxidants of plasma (Janaszewska and Bartosz, 2002). The intra- and inter-assay CVs for TAC were 2.9% and 5.4%, respectively. For TBARS determination, a slightly modified assay of Keles et al. was used (Keles et al., 2001). The intra- and inter-assay coefficients of variation (CV) for TBARS were 3.9% and 5.9%, respectively. GPx activity was measured according to Flohe and Gunzler, and calculated based on the molar extinction coefficient of NADPH (6200 L/mol/cm) as previously described by Veskoukis et al. (2016). Finally, GR activity was measured according to a protocol from Tietze et al., which was modified by Smith et al. and described previously by Veskoukis et al. (2016).
2.7. Statistical analysis
The statistical analysis was performed using R version 4.0.0. The correlations were studied using the Pearson correlation coefficient. Pair-wise comparisons were performed using a paired t-test. Associations between the different clinical parameters were evaluated using linear mixed models (R package lmerTest), considering the time point as a covariate and the repeated measure (patient grouping) as a random effect. Likelihood Ratio Tests were performed to evaluate the significance of the effects. Data was visualized using the R package ggplot2.
Marker of the redox status were used to predict the amplitude of metabolic changes with the caret package (Classification And REgression Training) in R on a training set constituting 60% of the patients. Missing values were imputed as column medians with randomForest:: na.roughfix(). Variables in the training sets were scaled and centered before a linear model was used to evaluate which are the most important predictors of the weight loss and the LDL level decrease with the function caret::train(). The machine learning model was then tested on the remaining (40%) patients. Its performance was evaluated by correlating the predicted outcomes with the actual outcomes with a correlation test.
3. Results
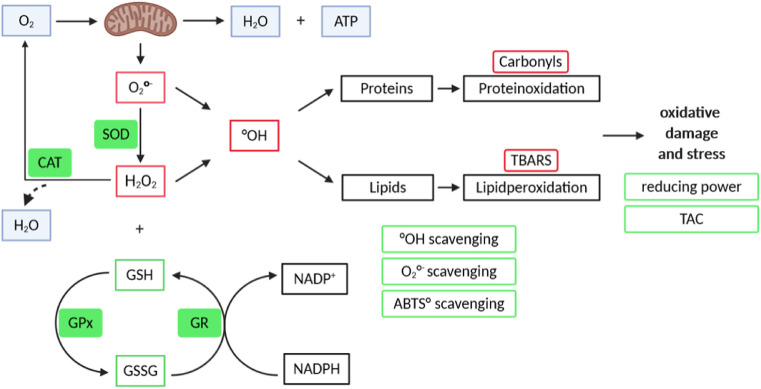
The aim of this study was to understand the interplay between markers of the redox status and the metabolic changes observed during a long-term fasting in 109 individuals. For this purpose, 12 markers of the redox status were evaluated (Fig. 1 ). This included markers of oxidative stress/damage (TBARS, carbonyls), as well as markers of the antioxidant capacity (GSH, TAC, superoxide scavenging capacity, reducing power, hydroxyl radical scavenging capacity, ABTS•+ radical scavenging capacity, as well as GPx, SOD and GR activities). The mean age of the study population was 57 years and 62% of the participants were female (Table 1 ). The cohort was predominantly overweight with an initial BMI of 28.34 ± 6.01 kg/m2, and waist circumference of 95.8 ± 14.2 cm. Baseline metabolic parameters like blood glucose, insulin, total cholesterol and triglycerides reflected a generally healthy study population (Supplementary Table 1).
Fig. 1.
Overview of the measured redox parameters.
Table 1.
Demographic characteristics of the cohort. Statistical differences between the repeated-measures comparison was determined using a paired t-test.
| Parameters | Baseline |
|---|---|
| Age, years | 57.0 (±10.5) |
| Female, n | 68 (62%) |
| Male, n | 41 (38%) |
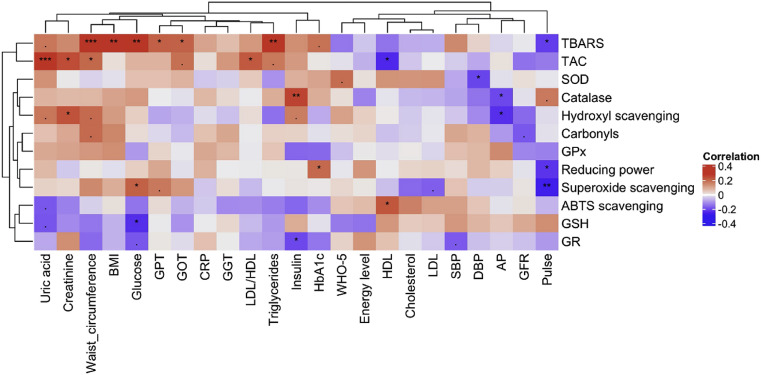
Analysis of correlations between the redox status and clinical parameters confirmed the link between markers of metabolic health and the antioxidant status established in our first study (Wilhelmi de Toledo et al., 2020a, Wilhelmi de Toledo et al., 2020b). The strongest correlation observed was between uric acid levels and the TAC (Fig. 2 ). Although most of the parameters reflecting the antioxidant capacity were poorly correlated to markers of metabolic health, there was a clear correlation between the markers of oxidative damages to lipids which had their levels the highest in individuals with a poor metabolic heath. This was reflected by the positive correlation of TBARS levels with waist circumference, BMI, glucose, GPT, GOT, GGT and triglyceride (Fig. 2).
Fig. 2.
Redox parameters correlate with metabolic parameters before fasting. The heatmap displays the correlations between the redox parameters (rows) and the metabolic parameters (columns). Dendrograms shows the relationships between the different parameters evaluated using the hierarchical clustering of Euclidean distance. The colour scale shows the coefficient of correlations. Statistical significance was tested (*p < 0.05; **p < 0.01; ***p < 0.001).
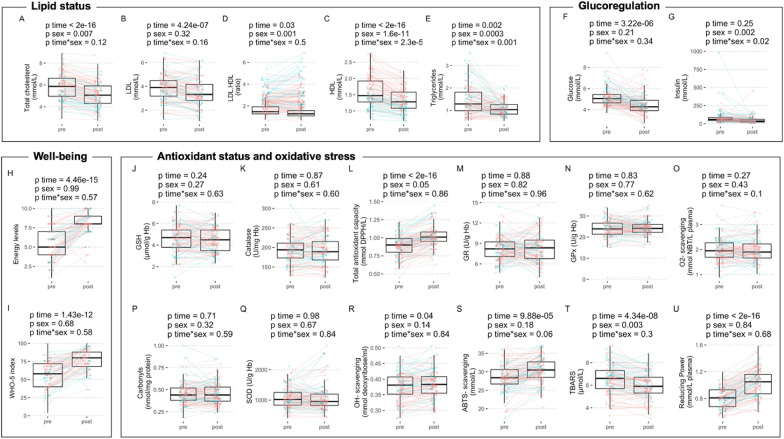
The present results confirm the improvement in lipid (Fig. 3 A to E) and glucose (Fig. 3F and G) metabolism which were evidenced in a larger cohort (Wilhelmi de Toledo et al., 2019), and show that these metabolic improvements are reproducible. In addition, well-being improvements were confirmed with new methods (i.e. energy levels, Fig. 3H; WHO-5 index, Fig. 3I). Next, the changes in 12 biomarkers of antioxidant systems caused by long-term fasting were studied (Fig. 3J to U). Gender differences were limited to TBARS levels which were higher at baselines in males. Gender did not have a substantial effect on the effects of fasting on redox parameters. Glutathione levels (Fig. 3J) and its regulation through GR (Fig. 3M) and GPx activities (Fig. 3N) were unchanged. However, the antioxidant capacity reflected by the TAC (Fig. 3L) and the reducing power (Fig. 3U) were increased. This ultimately increased ROS scavenging potential as showed by an increase hydroxyl radical scavenging activity (Fig. 3R) and an increased ABTS scavenging (Fig. 3S). This improvement of the antioxidant capacity was concomitant to a decrease in markers of oxidative stress/damage TBARS (Fig. 3T).
Fig. 3.
The effects of the 10-day fast in a group of 109 subjects. Individuals variations are presented along with summary statistics for the markers of lipid metabolism (A–E), glucoregulation (F–G), well-being (H–I), as well as for the 12 biomarkers of the redox status (J–U) measured before (pre) and after 10 ± 3 days (post) of a long-term fasting (red, females; blue, males).
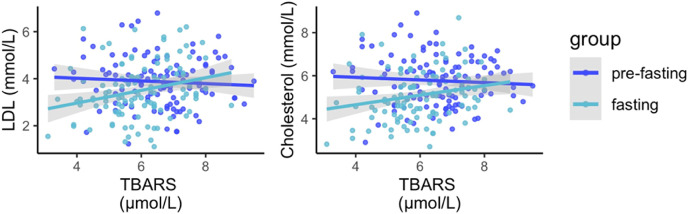
Since substantial changes in energy metabolism occurred during fasting, it was evaluated if the changes in antioxidant status interacted with the changes in markers of metabolic health caused by fasting. This was done by associating the levels of redox parameters with the metabolic responses. The strongest interactions were between markers of lipid peroxidation and lipid levels. Lipid peroxidation levels affected the effects of fasting on the normalization of lipid levels (Fig. 4 ). When lipid peroxidation is high, patients are most resistant to the normalization effect of fasting (interaction p-values 8.2e-06 and 4.1e-05 for LDL and cholesterol, respectively). This remained significant if the significance cut-off threshold was corrected by the number of comparisons (α = 0.0002). Estimate coefficients from this model suggested that LDL levels would decrease from 4.2 to 2.3 mmol/L for an hypothetical patient with no lipid peroxidation, and that every increase in lipid peroxidation by 1 μmol/L of TBARS would decrease the reduction in LDL levels by 0.25 mmol/L. The inclusion of BMI as a covariate did not change the result of this analysis, and a likelihood ratio test between a full model containing BMI as a covariate and its null model (no BMI added) was not statistically significant. It is reasonable to assume that the decreased in lipid levels caused by their use as energy substrates during fasting could be linked to the decreased lipid peroxidation. If lipid concentrations decrease, they are less available and lipid peroxidation levels decrease in return.
Fig. 4.
Lipid peroxidation levels affects the effects of long-term fasting on the normalization of lipid levels. A linear mixed model showed that LDL and TBARS levels interact with the effects of fasting on the change in lipid levels.
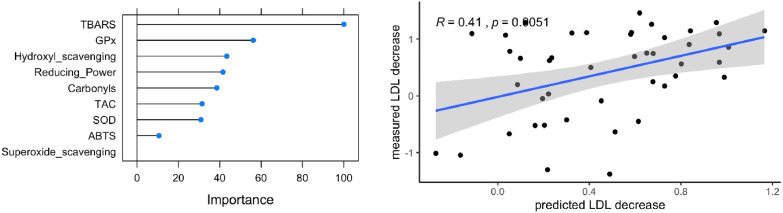
Ultimately, a machine learning algorithm was trained to predict the beneficial effects of long-term fasting from the redox status before the patients start the fast. Lipid peroxidation was the most important predictor of the changes in LDL levels on the training set made of 60% of the individuals (Fig. 5 ), confirming the results of the linear mixed models (Fig. 4). The model was validated on an independent test set consisting of 44 patients (40% of the data), with a statistically significant correlation between the predicted LDL decrease and the actual LDL decrease (p = 0.016). It was also evaluated if metabolic parameters at baseline (described in Fig. 2) could predict the changes in lipid peroxidation during fasting but failed to establish a model with good predictive abilities.
Fig. 5.
A machine learning algorithm using a combination of antioxidant parameters measured at baseline can predict changes in markers of metabolic health caused by long-term fasting. The left panel shows which redox parameters contributes to the predictive ability of the model. On the right hand side, it is shown that the values predicted by the model on an independent test set correlate well with the measured decrease in LDL during fasting.
Although the predictive ability of the redox status to predict metabolic changes caused by long-term fasting will have to be confirmed on a new cohort, and with more patients, the remarkable performance of this model considering the relatively small number of patients suggest that the antioxidant status is a crucial determinant of the normalization of lipid levels during long-term fasting. Collectively, the results show that the effects of long-term fasting on lipid metabolism are influenced by the redox status, and that these effects can be predictable by the levels of redox parameters before fasting using machine learning approaches.
4. Discussion
There is growing need of therapeutic strategies to increase the antioxidant defense mechanisms against infectious diseases like covid-19 and to improve simultaneously the metabolic health status. In the present study we expanded the insight about the effects of a 10-day fasting period on blood redox markers: Beyond the previously identified amelioration of the TAC and TBARS, it was demonstrated that indicators of the plasmatic antioxidant capacity like reducing power, ABTS radical scavenging capacity, and hydroxyl radical scavenging capacity increased significantly, which indicates an increase of the circulating antioxidant levels.
Redox changes were concomitant to a reduction in body weight and visceral fat, as well as an improvement in markers of glucose and lipid metabolism. Comparable effects were described after intermittent fasting in laboratory animals (Freire et al., 2020; Wilson et al., 2018, 2020) and human populations (Stekovic et al., 2019; Taylor et al., 2015). Energy levels and well-being index increased documenting the tolerability of this fasting program. This might be attributable to hormonal changes caused by fasting (Palmblad et al., 1977), or to the mood enhancing effects of ketosis (Mattson et al., 2018), but also to the healthy lifestyle associated with the stay at the clinic (medical care and improved well-being). In addition, the decreased body weight (Palmeira et al., 2010), and increased physical exercise (Ross and Hayes, 1988), can also increase well-being. Biochemical factors including blood glucose, HbA1c, insulin, triglycerides, total cholesterol, LDL, and HDL were lowered and total cholesterol/HDL was enhanced which in combination with the aforementioned markers, create a more favorable metabolic environment (Wilhelmi de Toledo et al., 2020a). This also showed that the effects described in a larger cohort in 2016 are reproducible (Wilhelmi de Toledo et al., 2019).
It was demonstrated that long-term fasting can increase the endogenous production of a number of antioxidant molecules that act protectively against free radicals. These findings are in contrast to the preconceived idea that the antioxidant reserves would decrease during fasting due to a lack of absorbed micronutrients with known antioxidative roles (e.g. vitamin E and B, zinc, selenium) after the cessation of food intake. When exogenous antioxidants are missing, endogenous antioxidants are sufficient to maintain homeostasis. This includes uric acid and bilirubin, two important endogenous antioxidants (Ames et al., 1981; Sedlak et al., 2009). Although bilirubin was not measured in this study, we measured the increase in uric acid and its association with TAC, that was discussed in detail in the previous article (Wilhelmi de Toledo et al., 2020a). It is worth mentioning again that high uric acid levels are well-known in long-term fasting and only exceptionally lead to side-effects as long as fasting individuals drink enough water (Wilhelmi de Toledo et al., 2019). Eight-weeks intermittent fasting confirmed the increase in uric acid levels in asthmatic patients and found a reduction in oxidative stress markers like 8-prostane, nitrotyrosine, protein carbonyls and 4-hydroxynonenal adducts (Johnson et al., 2007). Bilirubin levels have been extensively studied in previous studies and, in particular, it has been observed that 48-h fasting can increase those levels by 240% while 62-h fasting by 326% (Barrett, 1971; Dohi et al., 2005; Meyer et al., 1995). It is hypothesized that organisms with protective redox systems have been selected in the course of evolution to provide protection against ROS caused aging and cancer (Ames et al., 1981), It is plausible that resilient organisms which were able to maintain antioxidative functions during periods of famine through endogenous antioxidant production were selected in the same way.
In the aforementioned methods for determining the antioxidant capacity of plasma, the various antioxidant molecules participate in different ways due to their physicochemical characteristics (Benzie and Strain, 1996; Janaszewska and Bartosz, 2002). As uric acid is involved differently in these methods, it can affect differently the levels of the determined antioxidant capacity. Something similar happens with bilirubin and other antioxidant molecules that are affected by fasting.
Besides showing that long-term fasting improves the redox status and metabolic health indicators, an interplay between these parameters was described. The strongest correlation observed was between uric acid levels and the TAC, which was expected, since much of the TAC is due to the circulating uric acid (Janaszewska and Bartosz, 2002). However, the most important biological correlation was observed between TBARS and lipid metabolism. In general, TBARS decreased significantly after long-term fasting, as did total cholesterol, LDL and triglyceride levels, with a parallel decrease in waist circumference and BMI. Higher lipid peroxidation levels at baseline, reduced the ability of long-term fasting to normalize dyslipidemia.
Our machine learning algorithm confirmed that the levels of TBARS is a reliable predictor of how well a person will respond to long-term fasting. This model was trained and evaluated on a modest sample size. Future studies would have to be done to test if the predictive ability of TBARS levels can be replicated in a different group of individuals, ideally larger in order to better understand the influence of demographic covariates (age, sex, diseases). Our results suggest that analyzing the antioxidative status before fasting could help to enhance the efficiency of the fasting regimen by preparing the patients with the poorest metabolic status through boosting their antioxidant status in a personalized and adapted way. In general, dietary guidelines are provided for an average population (European Food Safety Authority, 2017) and do not account for inter-individual differences. However, an increasing number of studies are showing that response to diet is personal and that these differences can affect disease susceptibility (Zeevi et al., 2015). This can be due to differences in lifestyle, genetics, or even gut microbiome composition (Berry et al., 2020). We suggest in this study that the response to fasting can also be individual and showed that it can depend on the baseline antioxidative status. Factors driving these differences would have to be evaluated in further studies including measurement of gut microbiome composition, as it was repeatedly showed to be an important factor driving personal susceptibility to disease (Tierney et al., 2020). In addition, it was also shown that the gut microbiome dramatically changed during fasting, and were correlated to changes in energy metabolism (Mesnage et al., 2019).
Since lipids are used as energy substrates during fasting, it is reasonable to assume that the decrease in lipid levels, could partly lead to decreased lipid peroxidation. We documented in an unpublished study that the more atherogenic, small dense LDL particles diminished significantly after 14 fasting days. Since they are the most oxidizable, it seems logical that the lipid peroxidation levels decrease (Chaudhary et al., 2017). Furthermore, HDL is the greatest antagonist of lipid oxidation and it was documented that total cholesterol/HDL increased significantly during long-term fasting (Brites et al., 2017).
5. Conclusions
The results of our study conclude that fasting improves oxidative stress indicators by increasing the antioxidant capacity of the blood plasma through the increase in TAC, reducing power, ABTS radical scavenging capacity and hydroxyl radical scavenging capacity. At the same time, fasting reduces lipid peroxidation and improves various metabolic indicators, especially lipids. Furthermore, total cholesterol/HDL improved significantly. Although the prognostic ability of the redox status to predict metabolic changes caused by long-term fasting will have to be confirmed on a larger cohort, the remarkable performance of this model considering the relatively small number of patients suggest that the antioxidant status is a crucial determinant of the normalization of lipid levels during long-term fasting.
Altogether, our results show that the effects of long-term fasting on lipid metabolism are influenced by the redox status, and that these effects can be forecasted based on the levels of redox parameters before fasting through the use of machine learning approaches. We recommend that long-term fasting strategies should be personalized and adjusted to the metabolic and antioxidative baseline status of the subjects.
Funding source
This research was funded by Amplius GmbH, Überlingen, Germany, on behalf of BWC. BWC had no role in the design, analysis or writing of this article. No additional external funding received for this study.
CRediT authorship contribution statement
Franziska Grundler: Conceptualization, Data curation, Investigation, Project administration, Writing - original draft, Writing - review & editing. Robin Mesnage: Formal analysis, Software, Writing - original draft, Writing - review & editing. Nikolaos Goutzourelas: Data curation, Validation, Writing - original draft, Writing - review & editing. Fotios Tekos: Data curation, Validation, Writing - review & editing. Sotiria Makri: Data curation, Writing - review & editing. Michel Brack: Conceptualization, Writing - review & editing. Demetrios Kouretas: Conceptualization, Methodology, Writing - review & editing. Françoise Wilhelmi de Toledo: Conceptualization, Supervision, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: F.W.d.T. is managing director of Amplius GmbH. R.M. is consultant of Amplius GmbH and receive financial compensation for this role. F.W.d.T. and F.G. are employees of BWC. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Acknowledgments
The authors thank the study patients for their participation as well as Fabienne Zugmantel, Paraskevi Kouka, and Alexander Gumbinger for their support in data collection. Special thanks are expressed to the medical, nurse and laboratory team who conducted the measurements.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fct.2020.111701.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aebi H. Elsevier; 1984. [13] Catalase in Vitro, Methods in Enzymology; pp. 121–126. [DOI] [PubMed] [Google Scholar]
- Ak T., Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Ames B.N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant-and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. Unit. States Am. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artzi N.S., Shilo S., Hadar E., Rossman H., Barbash-Hazan S., Ben-Haroush A., Balicer R.D., Feldman B., Wiznitzer A., Segal E. Prediction of gestational diabetes based on nationwide electronic health records. Nat. Med. 2020;26:71–76. doi: 10.1038/s41591-019-0724-8. [DOI] [PubMed] [Google Scholar]
- Barrett P.V. Hyperbilirubinemia of fasting. J. Am. Med. Assoc. 1971;217:1349–1353. [PubMed] [Google Scholar]
- Bech P. Measuring the dimension of psychological general well-being by the WHO-5. Quality of Life Newsletter. 2004:15–16. [Google Scholar]
- Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Berry S.E., Valdes A.M., Drew D.A., Asnicar F., Mazidi M., Wolf J., Capdevila J., Hadjigeorgiou G., Davies R., Al Khatib H. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020:1–10. doi: 10.1038/s41591-020-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biserni M., Mesnage R., Ferro R., Wozniak E., Xenakis T., Mein C.A., Antoniou M.N. Quizalofop-p-ethyl induces adipogenesis in 3T3-L1 adipocytes. Toxicol. Sci. 2019;170:452–461. doi: 10.1093/toxsci/kfz097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J.E., Baker J.L., Boyland E., Blaak E., Charzewska J., De Henauw S., Frühbeck G., Gonzalez-Gross M., Hebebrand J., Holm L. Variations in the prevalence of obesity among European countries, and a consideration of possible causes. Obesity facts. 2017;10:25–37. doi: 10.1159/000455952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier E., Brouillard F., Molet J., Claverie D., Cabungcal J.H., Cresto N., Doligez N., Rivat C., Do K.Q., Bernard C., Benoliel J.J., Becker C. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatr. 2017;22:1701–1713. doi: 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- Brites F., Martin M., Guillas I., Kontush A. Antioxidative activity of high-density lipoprotein (HDL): mechanistic insights into potential clinical benefit. BBA clinical. 2017;8:66–77. doi: 10.1016/j.bbacli.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A., Burton N., Krachler B. 2016. Fasting Supervision and Lifestyle Care in the Tradition of Natural Hygiene Vulkan. [Google Scholar]
- Cano A., Acosta M., Arnao M. A method to measure antioxidant activity in organic media: application to lipophilic vitamins. Redox Rep. 2000;5:365–370. doi: 10.1179/135100000101535933. [DOI] [PubMed] [Google Scholar]
- Chaudhary R., Mathew D., Bliden K., Tantry U.S., Sharma T., Gesheff M.G., Franzese C.J., Pandya S., Toth P.P., Gurbel P.A. Low-density lipoprotein 4: a novel predictor of coronary artery disease severity. Curr. Med. Res. Opin. 2017;33:1979–1984. doi: 10.1080/03007995.2017.1365052. [DOI] [PubMed] [Google Scholar]
- Chung S.-K., Osawa T., Kawakishi S. Hydroxyl radical-scavenging effects of spices and scavengers from Brown mustard (Brassica nigra) Biosc. Biotech. Biochem. 1997;61:118–123. doi: 10.1271/bbb.61.118. [DOI] [Google Scholar]
- Ciardi C., Jenny M., Tschoner A., Ueberall F., Patsch J., Pedrini M., Ebenbichler C., Fuchs D. Food additives such as sodium sulphite, sodium benzoate and curcumin inhibit leptin release in lipopolysaccharide-treated murine adipocytes in vitro. Br. J. Nutr. 2012;107:826–833. doi: 10.1017/s0007114511003680. [DOI] [PubMed] [Google Scholar]
- de Cabo R., Mattson M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019;381:2541–2551. doi: 10.1056/NEJMra1905136. [DOI] [PubMed] [Google Scholar]
- de la Villehuchet A.M., Brack M., Dreyfus G., Oussar Y., Bonnefont-Rousselot D., Chapman M., Kontush A. A machine-learning approach to the prediction of oxidative stress in chronic inflammatory disease. Redox Rep. 2009;14:23–33. doi: 10.1179/135100009X392449. [DOI] [PubMed] [Google Scholar]
- Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi K., Satoh K., Ohtaki H., Shioda S., Miyake Y., Shindo M., Aruga T. Elevated plasma levels of bilirubin in patients with neurotrauma reflect its pathophysiological role in free radical scavenging. In Vivo. 2005;19:855–860. [PubMed] [Google Scholar]
- Drinda S., Grundler F., Neumann T., Lehmann T., Steckhan N., Michalsen A., Wilhelmi de Toledo F. Effects of periodic fasting on fatty liver index—a prospective observational study. Nutrients. 2019;11:2601. doi: 10.3390/nu11112601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority Dietary reference values for nutrients summary report. EFSA Supporting Publications. 2017;14 doi: 10.2903/sp.efsa.2017.e15121. [DOI] [Google Scholar]
- Freire T., Senior A.M., Perks R., Pulpitel T., Clark X., Brandon A.E., Wahl D., Hatchwell L., Le Couteur D.G., Cooney G.J. Sex‐specific metabolic responses to 6 hours of fasting during the active phase in young mice. J. Physiol. 2020 doi: 10.1113/JP278806. [DOI] [PubMed] [Google Scholar]
- Holvoet P. Relations between metabolic syndrome, oxidative stress and inflammation and cardiovascular disease. Verh. - K. Acad. Geneeskd. Belg. 2008;70:193–219. [PubMed] [Google Scholar]
- Janaszewska A., Bartosz G. Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Invest. 2002;62:231–236. doi: 10.1080/003655102317475498. [DOI] [PubMed] [Google Scholar]
- Johnson J.B., Summer W., Cutler R.G., Martin B., Hyun D.-H., Dixit V.D., Pearson M., Nassar M., Tellejohan R., Maudsley S. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keles M., Taysi S., Sen N., Aksoy H., Akcay F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol. Sci. 2001;28:141–143. doi: 10.1017/s0317167100052823. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Alderete T.L., Chen Z., Lurmann F., Rappaport E., Habre R., Berhane K., Gilliland F.D. Longitudinal associations of in utero and early life near-roadway air pollution with trajectories of childhood body mass index. Environ. Health. 2018;17:64. doi: 10.1186/s12940-018-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott K.D., Seraphim A., Augusto J.B., Xue H., Chacko L., Aung N., Petersen S.E., Cooper J.A., Manisty C., Bhuva A.N. The prognostic significance of quantitative myocardial perfusion: an artificial intelligence–based approach using perfusion mapping. Circulation. 2020;141:1282–1291. doi: 10.1161/CIRCULATIONAHA.119.044666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margină D., Ungurianu A., Purdel C., Nitulescu G.M., Tsoukalas D., Sarandi E., Thanasoula M., Burykina T.I., Tekos F., Buha A. Analysis of the intricate effects of polyunsaturated fatty acids and polyphenols on inflammatory pathways in health and disease. Food Chem. Toxicol. 2020:111558. doi: 10.1016/j.fct.2020.111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margină D., Ungurianu A., Purdel C., Tsoukalas D., Sarandi E., Thanasoula M., Tekos F., Mesnage R., Kouretas D., Tsatsakis A. Chronic inflammation in the context of everyday life: dietary changes as mitigating factors. Int. J. Environ. Res. Publ. Health. 2020;17:4135. doi: 10.3390/ijerph17114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P., Moehl K., Ghena N., Schmaedick M., Cheng A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018;19:63–80. doi: 10.1038/nrn.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnage R., Grundler F., Schwiertz A., Le Maho Y., Wilhelmi de Toledo F. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J. Nutr. Sci. 2019;8:e36. doi: 10.1017/jns.2019.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B., Scholtz H., Schall R., Muller F., Hundt H., Maree J. The effect of fasting on total serum bilirubin concentrations. Br. J. Clin. Pharmacol. 1995;39:169–171. doi: 10.1111/j.1365-2125.1995.tb04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasi A., McArdle S., Gaudernack G., Westman G., Melief C., Kouretas D., Arens R., Sjölin J., Mangsbo S. 2020. Reactive Oxygen Species as an Initiator of Toxic Innate Immune Responses in Retort to SARS-CoV-2 in an Ageing Population, Consider N-Acetylcysteine as Early Therapeutic Intervention. Toxicology Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley L.W., Spitz D.R. Elsevier; 1984. [61] Assay of Superoxide Dismutase Activity in Tumor Tissue, Methods in Enzymology; pp. 457–464. [DOI] [PubMed] [Google Scholar]
- Palmblad J., Levi L., Burger A., Melander A., Westgren U., von Schenck H., Skude G. Effects of total energy withdrawal (fasting) on the levels of growth hormone, thyrotropin, cortisol, adrenaline, noradrenaline, T4, T3, and rT3 in healthy males. Acta Med. Scand. 1977;201:15–22. doi: 10.1111/j.0954-6820.1977.tb15648.x. [DOI] [PubMed] [Google Scholar]
- Palmeira A.L., Branco T.L., Martins S.C., Minderico C.S., Silva M.N., Vieira P.N., Barata J.T., Serpa S.O., Sardinha L.B., Teixeira P.J. Change in body image and psychological well-being during behavioral obesity treatment: associations with weight loss and maintenance. Body Image. 2010;7:187–193. doi: 10.1016/j.bodyim.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Patsoukis N., Zervoudakis G., Panagopoulos N.T., Georgiou C.D., Angelatou F., Matsokis N.A. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004;357:83–86. doi: 10.1016/j.neulet.2003.10.080. [DOI] [PubMed] [Google Scholar]
- Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., Kouretas D., Spandidos D.A., Tsatsakis A. Obesity-a risk factor for increased COVID-19 prevalence, severity and lethality. Mol. Med. Rep. 2020;22:9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani V., Deep G., Singh R.K., Palle K., Yadav U.C. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Ross C.E., Hayes D. Exercise and psychologic well-being in the community 1. Am. J. Epidemiol. 1988;127:762–771. doi: 10.1093/oxfordjournals.aje.a114857. [DOI] [PubMed] [Google Scholar]
- Sedlak T.W., Saleh M., Higginson D.S., Paul B.D., Juluri K.R., Snyder S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106:5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanian A., Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu. Rev. Nutr. 1985;5:365–390. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020;16:1–2. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stekovic S., Hofer S.J., Tripolt N., Aon M.A., Royer P., Pein L., Stadler J.T., Pendl T., Prietl B., Url J. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metabol. 2019;30:462–476. doi: 10.1016/j.cmet.2019.07.016. e5. [DOI] [PubMed] [Google Scholar]
- Taylor R.W., Roy M., Jospe M.R., Osborne H.R., Meredith-Jones K.J., Williams S.M., Brown R.C. Determining how best to support overweight adults to adhere to lifestyle change: protocol for the SWIFT study. BMC Publ. Health. 2015;15:861. doi: 10.1186/s12889-015-2205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney B.T., He Y., Church G.M., Segal E.J., Kostic A.D., Patel C.J. The predictive power of the microbiome exceeds that of genome-wide association studies in the discrimination of complex human disease. bioRxiv. 2020;2019 12. 31.891978. [Google Scholar]
- Veskoukis A.S., Kyparos A., Paschalis V., Nikolaidis M.G. Spectrophotometric assays for measuring redox biomarkers in blood. Biomarkers. 2016;21:208–217. doi: 10.3109/1354750X.2015.1126648. [DOI] [PubMed] [Google Scholar]
- Wilhelmi de Toledo F., Buchinger A., Burggrabe H., Hölz G., Kuhn C., Lischka E., Lischka N., Lützner H., May W., Ritzmann-Widderich M. Fasting therapy-an expert panel update of the 2002 consensus guidelines. Forschende Komplementärmedizin/Research in Complementary Medicine. 2013;20:434–443. doi: 10.1159/000357602. [DOI] [PubMed] [Google Scholar]
- Wilhelmi de Toledo F., Grundler F., Bergouignan A., Drinda S., Michalsen A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PloS One. 2019;14 doi: 10.1371/journal.pone.0209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmi de Toledo F., Grundler F., Goutzourelas N., Tekos F., Vassi E., Mesnage R., Kouretas D. Influence of long-term fasting on blood redox status in humans. Antioxidants. 2020;9:496. doi: 10.3390/antiox9060496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmi de Toledo F., Grundler F., Sirtori C.R., Ruscica M. Unravelling the health effects of fasting: a long road from obesity treatment to healthy life span increase and improved cognition. Ann. Med. 2020 doi: 10.1080/07853890.2020.1770849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.A., Deasy W., Stathis C.G., Hayes A., Cooke M.B. Intermittent fasting with or without exercise prevents weight gain and improves lipids in diet-induced obese mice. Nutrients. 2018;10:346. doi: 10.3390/nu10030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.A., Stathis C.G., Hayes A., Cooke M.B. Intermittent fasting and high-intensity exercise elicit sexual-dimorphic and tissue-specific adaptations in diet-induced obese mice. Nutrients. 2020;12:1764. doi: 10.3390/nu12061764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.-L., Shi Y.-H., Hao G., Li W., Le G.-W. Increasing oxidative stress with progressive hyperlipidemia in human: relation between malondialdehyde and atherogenic index. J. Clin. Biochem. Nutr. 2008;43:154–158. doi: 10.3164/jcbn.2008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen G.C., Duh P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994;42:629–632. [Google Scholar]
- Zeevi D., Korem T., Zmora N., Israeli D., Rothschild D., Weinberger A., Ben-Yacov O., Lador D., Avnit-Sagi T., Lotan-Pompan M. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Zhao Y.-Y., Wang H.-L., Cheng X.-L., Wei F., Bai X., Lin R.-C., Vaziri N.D. Metabolomics analysis reveals the association between lipid abnormalities and oxidative stress, inflammation, fibrosis and Nrf2 dysfunction in aristolochic acid-induced nephropathy. Sci. Rep. 2015;5:12936. doi: 10.1038/srep12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.