Abstract
Interferon beta (IFNβ) was the first disease-modifying therapy available to treat multiple sclerosis (MS), providing patients with a treatment that resulted in reduced relapse rates and delays in the onset of disability. Four IFNβ drugs are currently approved to treat relapsing forms of MS: subcutaneous (SC) IFNβ-1b, SC IFNβ-1a, intramuscular IFNβ-1a, and, most recently, SC peginterferon beta-1a. Peginterferon beta-1a has an extended half-life and requires less frequent administration than other available treatments (once every 2 weeks vs every other day, 3 times per week, or weekly). Large randomized controlled clinical trials have confirmed the efficacy of interferons for the treatment of relapsing MS. The most frequent adverse events in patients receiving IFNs include injection site reactions and flu-like symptoms. Patient education and mitigation strategies are key to managing these adverse events and supporting therapy adherence. With fewer injections needed, peginterferon beta-1a is associated with less frequent discomfort, which may translate to improved adherence, a major factor in treatment efficacy. Because the available interferon therapies differ in administration route and frequency of injection, switching among these therapies may be a viable option for patients who experience issues with tolerability. Although a variety of disease-modifying therapies are now available to treat relapsing MS, the efficacy and long-term safety profile of interferons make them an important first-line option for treatment.
Keywords: Efficacy, Interferon beta, Multiple sclerosis (MS), Relapsing MS, Safety
Before the 1990s, treatment for multiple sclerosis (MS) largely comprised the management of relapses, primarily with systemic corticosteroids, and short-term management of symptoms.1 The first two injectable interferon beta (IFNβ) therapies were approved in 1993 and 1996 and allowed clinicians to offer patients with MS a longer-term therapy designed to modify the course of the disease. Since then, the therapeutic landscape has expanded to include new drug targets and other disease-modifying therapies (DMTs), affording more treatment options. However, many patients continue to benefit from interferon therapy, experiencing reduced relapse rates and delayed disability.2 A large body of data supports the long-term efficacy and safety of interferons for reducing the relapse rate, slowing disability worsening, and decreasing the number of central nervous system (CNS) lesions.3–5 The role of interferons as the foundation of MS treatment is further evidenced by their use as active comparators in clinical trials evaluating newer MS therapies.6,7
Five IFNβ drugs are currently approved for the treatment of relapsing forms of MS: subcutaneous (SC) IFNβ-1b (Betaseron; Bayer HealthCare Pharmaceuticals Inc, and Extavia; Novartis Pharmaceuticals Corp), SC IFNβ-1a (Rebif; EMD Serono Inc), intramuscular (IM) IFNβ-1a (Avonex; Biogen Inc), and SC peginterferon beta-1a (Plegridy; Biogen Inc) (Table 1).8–12 The US Food and Drug Administration approved the first four therapies before 2002 and approved peginterferon beta-1a in 2014.8–12 The addition of this new formulation and dosing schedule necessitates reopening a dialogue regarding the IFNβs. Herein we provide a brief overview of the efficacy, safety, and tolerability of interferons for the treatment of MS, with an emphasis on newer clinical data, and discuss the continued role of IFNβ in the ever-expanding MS treatment algorithms.
Table 1.
| Drug | Trade name | Year of FDA approval | Route of administration | Dosing | Frequency | Pharmacokinetics |
|---|---|---|---|---|---|---|
| Interferon beta-1b | Betaseron | 1993 | Subcutaneous | 0.25 mg | Every other day | t½: 5 h Tmax: 1–8 h |
| Interferon beta-1b | Extavia | 1993 | Subcutaneous | 0.25 mg | Every other day | t½: 5 h Tmax : 1–8 h |
| Interferon beta-1a | Avonex | 1996 | Intramuscular | 30 μg | Once weekly | t½: 10 h Tmax: 5–15 h |
| Interferon beta-1a | Rebif | 2002 | Subcutaneous | 22 or 44 μg | 3 times weekly | t½: 50–60 h Tmax: 8 h |
| Peginterferon beta-1a | Plegridy | 2014 | Subcutaneous | 125 μg | Once every 2 wk | t½: mean ± SD 78 ± 15 h T max: 1–1.5 d |
Abbreviations: FDA, US Food and Drug Administration; t½, half-life; Tmax, time to maximum concentration.
Mechanism of Action of Interferons
The interferon family of cytokines are secreted by many immune and nonimmune cell types, including macrophages, lymphocytes, fibroblasts, and endothelial cells.2 Interferons possess immunomodulatory effects, as well as antiviral and antitumor properties. The type I family of interferons includes the IFNβs that are used to treat MS.2
The mechanism of action of IFNβ in people with MS is complex and not completely understood. Once IFNβ binds to specific cell surface receptors, several events occur, including increased expression of anti-inflammatory cytokines (eg, interleukin [IL] 4, IL-5, IL-10, IL-13, IL-27, and transforming growth factor beta) and downregulation of expression of proinflammatory cytokines (eg, IL-17, IFNγ, and tumor necrosis factor alpha), which helps stabilize dysregulated CNS inflammation.13,14 The interferon-mediated shift from Th1/Th17 toward an anti-inflammatory profile may indirectly reduce neuronal demyelination, preventing further neuronal damage.15
Also, IFNβ acts on T cells by reducing T-cell activation as well as adhesion and penetration into the CNS through the blood-brain barrier.16 In B cells and other antigen-presenting cells, IFNβ disrupts antigen presentation.14 The overall effect of IFNβ on the brain is a shift in the balance from a proinflammatory Th1/Th17 response to a Th2 anti-inflammatory response, as well as a reduction in the number of inflammatory cells capable of crossing the blood-brain barrier.13,14
Peginterferon beta-1a is distinguished from other formulations by the addition of a polyethylene glycol (PEG) chain to the IFNβ-1a molecule.1,17–19 PEG has been appended to a variety of molecules, and clinical research supports the safety and clinical value of pegylation; specifically, the improved stability and solubility of the pegylated molecule confers pharmacologic advantages such as reduced glomerular filtration rate and prolonged half-life.20 In the case of peginterferon beta-1a, pegylation protects the IFNβ molecule from degradation and proteolysis, resulting in an extended half-life (Table 1), which, in turn, affects the pharmacokinetics and dosing interval.1
Pharmacokinetics, Dosing, and Adherence
The route of administration, dosing, and dosing frequency for the various interferons approved to treat relapsing-remitting MS (RRMS) are shown in Table 1.8–12 The dosing frequencies of the interferon formulations differ from every other day (SC IFNβ-1b) to every 2 weeks (SC peginterferon beta-1a).
The greater stability of the pegylated formulation is reflected in the pharmacokinetics of peginterferon beta-1a, specifically its longer half-life (78 hours vs 5–60 hours) and time to maximum concentration (1–1.5 days vs 1–15 hours) relative to the nonpegylated interferon formulations (Table 1).8–12 Single-dose phase 1 studies showed that peginterferon beta-1a has a longer terminal half-life, greater cumulative area under the curve, and higher maximum concentration than IM IFNβ-1a.18 In the COMPARE study, an open-label, crossover, pharmacokinetic study in healthy individuals,19 overall drug exposure over a 2-week dosing period was 60% higher after a single dose of peginterferon beta-1a than after six doses of SC IFNβ-1a. In addition, drug levels remained detectable throughout the 2-week dosing period with peginterferon beta-1a.19
Why is drug stability and dosing frequency an important issue with interferon treatment of MS? Studies of nonadherence (the proportion of patients who do not follow treatment according to the prescription) among patients receiving injectable MS therapies have shown nonadherence rates of 41% to 88%.21,22 Nonadherent patients do not achieve the full efficacy of the treatment, with a negative effect on clinical outcomes, whereas patients who are more adherent to therapy show a reduced risk of relapse, lower rates of MS-related hospitalization, and decreased medical costs.21,23,24
Although anxiety over injections, difficulty with self-injections, and adverse events (AEs) also play a role in IFNβ nonadherence in patients with MS, injection frequency is, as in other chronic conditions, a major contributor to nonadherence.22,25 Consistent with the role of injection frequency in adherence, among the nonpegylated IFNβs, a trend of declining adherence rates with increasing injection frequency has been reported, with average adherence rates of 69.4% for IM IFNβ-1a (once weekly), 63.8% for SC IFNβ-1a (three times weekly), and 58.4% for SC IFNβ-1b (every other day).22 In the COMPARE study, injection site reactions (ISRs) and flu-like symptoms were more frequent with SC IFNβ-1a than with peginterferon beta-1a19 (Figure 1) because of the higher injection frequency of SC IFNβ-1a. Based on the role of injection frequency and discomfort in leading to nonadherence,22,25 the less frequent dosing allowed by the prolonged half-life of peginterferon beta-1a may lead to improved treatment adherence compared with other injectable therapies. However, real-world studies are needed to confirm that adherence improves with peginterferon beta-1a compared with nonpegylated interferons.
Figure 1.
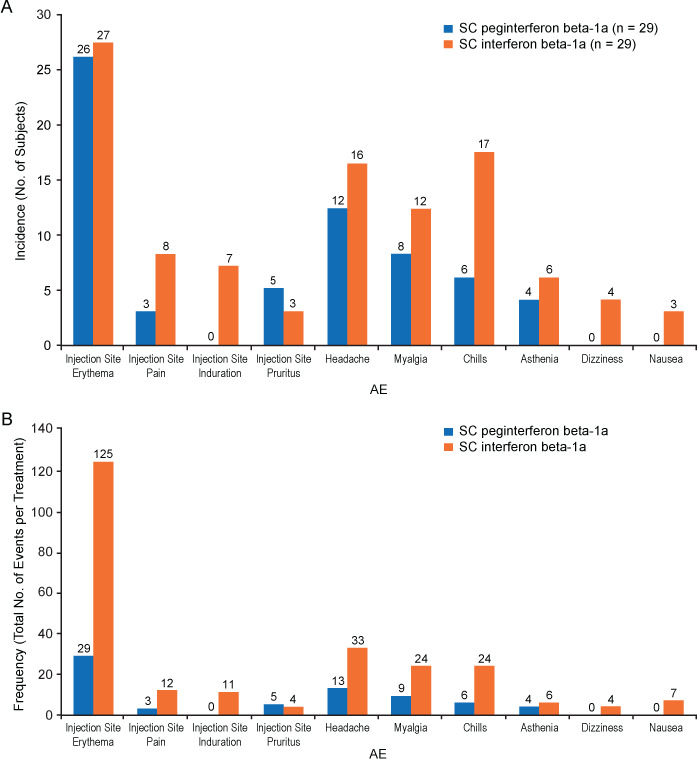
(A) Incidence and (B) frequency of adverse events (AEs) related to study drug occurring in more than 10% of participants after treatment with either peginterferon beta-1a (n = 29) or subcutaneous (SC) interferon beta-1a (n = 29) in the COMPARE study19
Reprinted with permission from Hu X, Shang S, Nestorov I, et al. COMPARE: pharmacokinetic profiles of subcutaneous peginterferon beta-1a and subcutaneous interferon beta-1a over 2 weeks in healthy subjects. Br J Clin Pharmacol. 2016;82(2):380–388. Copyright © 2016, John Wiley & Sons.
Efficacy of Interferon for MS Treatment
The DMTs represent a breakthrough in MS treatment insofar as they can modify the course of RRMS, lowering the rate of relapses and the number of magnetic resonance imaging (MRI) brain lesions and stabilizing or delaying MS disability.2 Early studies of IFNβ therapies demonstrated reductions in relapse rates and severity of relapses and extended times to relapse and disability progression (Table S1, which is published in the online version of this article at ijmsc.org).3,26–32 Based on these early studies, the efficacy of IFNβ is generally accepted to be relatively consistent across the interferon class, yielding a decrease of approximately 30% in the annualized relapse rate (ARR).35 In addition to shortening and preventing relapses, interferon therapies have also been shown to reduce new MRI gadolinium-enhancing (Gd+) brain lesions, decrease the risk of evolution of lesions into chronic black holes (markers of irreversible tissue injury), and reduce cognitive loss.3,26,29,34,36–38 Furthermore, long-term interferon therapy has been shown to induce neuroprotectant proteins39 and increase survival rates.33
In 2014, the 2-year, double-blind, parallel-group, phase 3 ADVANCE study (with a placebo-controlled design for the first 48 weeks) was conducted at 183 sites in 26 countries to assess the safety and efficacy of peginterferon beta-1a.26 In this study, 1512 patients with RRMS were randomly assigned to receive placebo (n = 500), peginterferon beta-1a every 2 weeks (n = 512), or peginterferon beta-1a every 4 weeks (n = 500) in year 1. At year 2, all the placebo-receiving patients were randomized again to receive peginterferon beta-1a either every 2 weeks or every 4 weeks. During year 1, patients receiving peginterferon beta-1a had significantly fewer relapses than those taking placebo; the adjusted ARR was 0.256 in the every-2-weeks group, 0.288 in the every-4-weeks group, and 0.397 in the placebo group (rate ratio vs placebo: 0.644 [P = .0007] for every 2 weeks and 0.725 [P = .0114] for every 4 weeks).26 These results support the efficacy of peginterferon beta-1a for the treatment of RRMS, with less frequent administration required than with other available treatments. In year 2, the ARR was further reduced with every-2-weeks dosing (to 0.178) and was maintained with every-4-weeks dosing (at 0.291), indicating that peginterferon beta-1a efficacy is maintained beyond 1 year.16 In the phase 3 ATTAIN extension study, year-by-year ARRs remained low over 6 years in patients treated with peginterferon beta-1a every 2 weeks.40
Interferon therapies are commonly used as comparators in clinical trials of new therapies6,7,41; however, no head-to-head trials have definitively shown a difference in efficacy among the interferon therapies. Of those studies that have been conducted, most have shown no difference, and for those that have shown a difference (eg, INCOMIN, EVIDENCE),42,43 limitations in study design—namely, a lack of double-blinding—may restrict interpretation of the findings. The lack of blinding in these trials may have influenced patients and/ or investigators when measuring subjective outcomes such as relapse and disability progression.44 Results from INCOMIN and EVIDENCE were also compromised by baseline imbalances between study groups. In EVIDENCE, the groups differed in the number of active lesions, and statistical significance was mostly found in outcomes related to lesion activity. In each case, the group with better performance was the group with fewer mean lesions at baseline.44 INCOMIN study groups differed in the number of T2 lesions, disease duration, the proportion of patients with Gd+ lesions, age at disease onset, and sex. The study group in INCOMIN that had worse relapse-and MRI-related outcomes also had more T2 lesions, had longer disease duration, and were older at disease onset, and a higher percentage were male. The latter two factors have been associated with adverse disability outcomes.44
A recent meta-analysis indicated that the efficacy and safety profiles of peginterferon beta-1a are comparable with those of IFNβ-1a, IFNβ-1b, and glatiramer acetate in patients with RRMS.45 Indirect comparisons have reported better clinical outcomes with peginterferon beta-1a than with SC or IM IFNβ-1a.46,47
No evidence of disease activity (NEDA), increasingly considered the primary aim of MS treatment, is a composite of three measures of disease activity: no relapses, no disability progression, and no MRI activity (ie, new or enlarging T2 and Gd+ lesions).48–50 In post hoc analyses of the EVIDENCE study, a head-to-head trial of SC and IM IFNβ-1a, more patients achieved clinical NEDA (defined as no relapses and no increase of ≥1.0 point in Expanded Disability Status Scale score from baseline sustained for 12 weeks) and MRI NEDA (defined as no new or newly enlarging T2 lesions) over 72 weeks with SC IFNβ-1a than with IM IFNβ-1a (clinical NEDA: 46.7% vs 33.3%; MRI NEDA: 48.6% vs 25.6%).51 In the ADVANCE study, higher rates of overall NEDA, a composite of clinical NEDA (defined as no relapses and no increase in Expanded Disability Status Scale score of ≥1.0 point in patients with a baseline score of ≥1.0, or an increase of ≥1.5 points in patients with a baseline score of 0.0, confirmed after 12 weeks) and MRI NEDA (defined as no Gd+ lesions and no new or newly enlarging T2 lesions), were achieved by patients who received continuous treatment with peginterferon beta-1a every 2 weeks for 2 years (36.7%) than by patients who received peginterferon beta-1a every 4 weeks for 2 years (23.0%) or delayed treatment (placebo in year 1 and peginterferon beta-1a in year 2; 15.8%).34 Differences in study design and NEDA definitions prevent comparison of NEDA rates across studies; however, these data generally support the possibility of achieving NEDA with interferon treatment.
Safety and Tolerability
Treatment of MS with IFNβ has a long record of safety, with millions of patients treated across more than 2 decades.52,53 The most common AEs with a consistently higher incidence with interferon than with placebo are injection site erythema, influenza-like illness, pyrexia, headache, myalgia, chills, and injection site pain.8–12 Most reported AEs are mild or moderate in severity.16,54
The AEs that most significantly limit the use of interferon treatment are flu-like symptoms and ISRs. The exact mechanism of ISRs with interferon use is not known; however, results of a study using skin biopsy samples obtained from patients with MS suggest that IFNβ SC injection may trigger inflammatory skin reactions through local chemokine induction, followed by rapid movement of immune cells to the injection site.55 Such ISRs as injection site erythema, pain, induration, and pruritus have been observed to occur less frequently in patients receiving IM than SC MS therapies.56,57 (See the next section for further discussion on managing flu-like symptoms and ISRs.)
Peginterferon beta-1a has demonstrated a favorable tolerability profile compared with SC IFNβ-1a with respect to ISRs.18 In the COMPARE study (in healthy individuals), the most common ISR, injection site erythema, occurred with similar incidence but lower frequency with peginterferon beta-1a compared with SC IFNβ-1a. Peginterferon beta-1a was better tolerated than SC IFNβ-1a with respect to flu-like symptoms,19 with myalgia and chills as the most common AEs reported with both treatments (Figure 1). Numerically lower AE incidences, frequencies, and incidence rates with peginterferon beta-1a compared with SC IFNβ-1a were as expected given the differences in frequency of administration. In year 1 of the phase 3 ADVANCE study,26 rates of the most common ISRs and flu-like symptoms were similar in the peginterferon beta-1a every-2-weeks and every-4-weeks groups, including injection site erythema (62% and 56%, respectively), influenza-like illness (47% in both groups), pyrexia (45% and 44%, respectively), headache (44% and 41%, respectively), injection site pain (15% and 13%, respectively), and injection site pruritus (13% and 11%, respectively). The randomized phase 3b ALLOW study characterized flu-like symptoms in 201 patients with RRMS transitioning from nonpegylated IFNβ therapies to peginterferon beta-1a; it found that 90% of patients did not have new or worsening flu-like symptoms, most were mild to moderate, the median onset time after injection was 10 hours, and the median duration was 17 hours.58
Notwithstanding this long history of safety, not all patients with MS are responsive to IFNβ therapies; in fact, 30% to 50% are nonresponsive.59 Nonresponse may be attributed to genetic, pharmacologic, or pathogenic factors. One main factor in nonresponsiveness is the presence of neutralizing antibodies to IFNβ.60 Overall, the prevalence of neutralizing antibodies in patients treated with different nonpegylated interferon therapies ranges from less than 5% to almost 45%, with SC IFNβ-1b associated with the highest neutralizing antibody levels (30%–40%) and IM IFNβ-1a with the lowest neutralizing antibody levels (2%–6%).61 Pegylation of molecules has generally been shown to reduce drug immunogenicity, antigenicity, and toxicity.20 Consistent with this, in the peginterferon beta-1a phase 3 ADVANCE trial,26 the incidence of anti-interferon neutralizing antibodies remained low in both active treatment groups (<1%), and the proportion of patients developing neutralizing antibodies was at the low end of the range of immunogenicity observed with any of the nonpegylated interferon MS therapies.62,63
Management of Interferon AEs
Patients taking interferon may experience several AEs, including headaches, muscle aches, flu-like symptoms, and ISRs, which can all negatively affect the patient’s quality of life and desire to continue treatment. To maximize interferon treatment adherence and, thus, therapeutic efficacy, it is important to proactively manage AE symptoms (particularly flu-like symptoms and ISRs) in all patients, including those switching between interferon and noninterferon DMTs, those switching between IM and SC IFNβs, and those naive to DMT treatment. Table 2 summarizes recommended pharmacologic and nonpharmacologic mitigation strategies for common AEs associated with IFNβ therapies.64–66 The primary management strategy for flu-like symptoms and ISRs is gradual dose titration over 4 to 6 weeks. Analgesics and/or antipyretics, such as ibuprofen or acetaminophen, are often administered concurrently to prevent the onset of symptoms.67,68 It is especially critical that patients be educated regarding the timing and impact of flu-like symptoms and ISRs and how to reduce/prevent such AEs using over-the-counter medications and other self-care practices, such as hydration, diet, and rest.64 Most patients who experience flu-like symptoms or ISRs can administer doses in the evening, such that they sleep through the time during which they would otherwise experience symptoms.67–69 Understanding the timing of AEs may be of particular importance when considering the potential for patients treated with PEG-IFNβ-1a to potentially experience flu-like symptoms or ISRs of extended duration.58
Table 2.
Mitigation strategies for the most common adverse events associated with interferon beta therapies64–69
| Adverse event | Pharmacologic therapies | Nonpharmacologic therapies |
|---|---|---|
| FLSs |
|
|
| ISRs |
|
|
Abbreviations: FLS, flu-like symptom; ISR, injection site reaction; NSAID, nonsteroidal anti-inflammatory drug.
Role of Interferons in Current MS Therapeutic Landscape
As in many therapeutic areas, in MS there is debate about whether to begin therapy with safer agents and then escalate treatment as necessary or to begin treatment with the most effective therapies available.70 Given the number of available therapies, recent consensus guidelines recommend individualized therapy choice made based on discussion with the patient and consideration of patient characteristics, disease severity, drug safety profiles, barriers to adherence, and accessibility of the drug.71 Guidelines from the American Academy of Neurology and the European Academy of Neurology recommend that patients with MS offered a first-line therapy such as IFNβ or glatiramer acetate be switched to a higher-efficacy DMT (eg, alemtuzumab, fingolimod, or natalizumab) if and when breakthrough disease activity occurs.71,72 However, when a treatment switch is desired for reasons unrelated to efficacy (eg, tolerability or convenience), a lateral switch from one first-line DMT to another, rather than escalating therapy, is an acceptable treatment strategy.54,73–75 In the ALLOW study, most patients (89.6%) who made a lateral switch (from a nonpegylated interferon to PEG-IFNβ-1a) did not have new or worsening flu-like symptoms.58 In that study, patients switching from a more frequent to a less frequent injection also reported increases in treatment satisfaction.65 In separate analyses, however, switching patients who were stable on an interferon therapy for at least 1 year to another interferon therapy was shown to potentially lead to worse clinical outcomes (with peginterferon beta-1a not included in the analyses) due in part to decreased adherence to the switch medication.76 Because reasons for switching were not identified and patients were followed up for only 1 year after switching, individual patient needs must continue to be weighed when considering a treatment switch.
Summary
Even with the wide array of DMTs currently available, IFNβ therapy still plays an important role in the treatment of patients with MS. Interferons have been shown to improve clinical outcomes, and their long-term safety profiles make them standard-of-care, first-line therapy for MS. Although patients with RRMS may benefit from interferon treatment, individual response, in terms of both efficacy and AEs, is unpredictable, and it is necessary in many cases to switch treatments to stabilize disease. New real-world data are continuously being collected comparing IFNβ drugs in terms of efficacy, tolerability, and the effects of switching, as well as the relative incidence of flu-like symptoms and ISRs. Both flu-like symptoms and ISRs have been shown to be manageable through patient education and monitoring and pharmacologic and nonpharmacologic intervention.
PRACTICE POINTS
Interferon beta, which is available in several formulations, plays an important role as standard-of-care, first-line therapy for relapsing forms of MS.
Interferons have demonstrated efficacy on clinical outcomes, and their long-term safety profiles are well established.
The most common adverse events associated with interferon beta therapy—flu-like symptoms and injection site reactions—can be managed through patient education and mitigation strategies.
Supplementary Material
Acknowledgments
Cindy Schultz and Alison Adams, PhD, from Ashfield Healthcare Communications (Middletown, CT), based on input from the authors, wrote the first draft and revised subsequent drafts of the manuscript, and Joshua Safran, from Ashfield Healthcare Communications, copyedited and styled the manuscript per journal requirements.
Financial Disclosures
Dr Filipi and Ms Jack have received research support from Biogen. Biogen reviewed and provided feedback on the manuscript to the authors. The authors had full editorial control of the manuscript and provided their final approval of all content.
Funding/Support
Writing and editorial support in the development of this article were funded by Biogen.
References
- 1.Madsen C. The innovative development in interferon beta treatments of relapsing-remitting multiple sclerosis. Brain Behav. 2017;7:e00696. doi: 10.1002/brb3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dargahi N, Katsara M, Tselios T et al. Multiple sclerosis: immunopathology and treatment update. Brain Sci. 2017;7:E78. doi: 10.3390/brainsci7070078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/ remitting multiple sclerosis. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- 4.Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2015;29:207–220. doi: 10.1007/s40263-015-0238-y. [DOI] [PubMed] [Google Scholar]
- 5.Trojano M, Pellegrini F, Paolicelli D et al. Real-life impact of early interferon beta therapy in relapsing multiple sclerosis. Ann Neurol. 2009;66:513–520. doi: 10.1002/ana.21757. [DOI] [PubMed] [Google Scholar]
- 6.Hauser SL, Bar-Or A, Comi G et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376:221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 7.Kappos L, Wiendl H, Selmaj K et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2015;373:1418–1428. doi: 10.1056/NEJMoa1501481. [DOI] [PubMed] [Google Scholar]
- 8.Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2016. Betaseron (interferon beta-1b) [prescribing information] [Google Scholar]
- 9.Cambridge, MA: Biogen Inc: 2015. Avonex (interferon beta-1a) [prescribing information] [Google Scholar]
- 10.Rockland, MA: EMD Serono Inc; 2015. Rebif (interferon beta-1a) [prescribing information] [Google Scholar]
- 11.Cambridge, MA: Biogen Inc; 2015. Plegridy (peginterferon beta-1a) [prescribing information] [Google Scholar]
- 12.East Hanover, NJ: Novartis Pharmaceuticals Corp; 2016. Extavia (interferon beta-1b) [prescribing information] [Google Scholar]
- 13.Kieseier BC. The mechanism of action of interferon-beta in relapsing multiple sclerosis. CNS Drugs. 2011;25:491–502. doi: 10.2165/11591110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Kasper LH, Reder AT. Immunomodulatory activity of interferon-beta. Ann Clin Transl Neurol. 2014;1:622–631. doi: 10.1002/acn3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yong VW, Giuliani F, Xue M, Bar-Or A, Metz LM. Experimental models of neuroprotection relevant to multiple sclerosis. Neurology. 2007;68:S32–S37. doi: 10.1212/01.wnl.0000275230.20635.72. discussion S43–S54. [DOI] [PubMed] [Google Scholar]
- 16.Kieseier BC, Arnold DL, Balcer LJ et al. Peginterferon beta-1a in multiple sclerosis: 2-year results from ADVANCE. Mult Scler. 2015;21:1025–1035. doi: 10.1177/1352458514557986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker DP, Lin EY, Lin K et al. N-terminally PEGylated human interferon-beta-1a with improved pharmacokinetic properties and in vivo efficacy in a melanoma angiogenesis model. Bioconjug Chem. 2006;17:179–188. doi: 10.1021/bc050237q. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Miller L, Richman S et al. A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. J Clin Pharmacol. 2012;52:798–808. doi: 10.1177/0091270011407068. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Shang S, Nestorov I et al. COMPARE: pharmacokinetic profiles of subcutaneous peginterferon beta-1a and subcutaneous interferon beta-1a over 2 weeks in healthy subjects. Br J Clin Pharmacol. 2016;82:380–388. doi: 10.1111/bcp.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang JS, Deluca PP, Lee KC. Emerging PEGylated drugs. Expert Opin Emerg Drugs. 2009;14:363–380. doi: 10.1517/14728210902907847. [DOI] [PubMed] [Google Scholar]
- 21.Gerber B, Cowling T, Chen G, Yeung M, Duquette P, Haddad P. The impact of treatment adherence on clinical and economic outcomes in multiple sclerosis: real world evidence from Alberta, Canada. Mult Scler Relat Disord. 2017;18:218–224. doi: 10.1016/j.msard.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Menzin J, Caon C, Nichols C, White LA, Friedman M, Pill MW. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm. 2013;19:S24–S40. doi: 10.18553/jmcp.2013.19.s1.S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanova JI, Bergman RE, Birnbaum HG, Phillips AL, Stewart M, Meletiche DM. Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis in the US. J Med Econ. 2012;15:601–609. doi: 10.3111/13696998.2012.667027. [DOI] [PubMed] [Google Scholar]
- 24.Hupperts R, Ghazi-Visser L, Martins Silva A et al. The STAR study: a real-world, international, observational study of the safety and tolerability of, and adherence to, serum-free subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis. Clin Ther. 2014;36:1946–1957. doi: 10.1016/j.clinthera.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Patti F. Optimizing the benefit of multiple sclerosis therapy: the importance of treatment adherence. Patient Prefer Adherence. 2010;4:1–9. doi: 10.2147/ppa.s8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calabresi PA, Kieseier BC, Arnold DL et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol. 2014;13:657–665. doi: 10.1016/S1474-4422(14)70068-7. [DOI] [PubMed] [Google Scholar]
- 27.The IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis, I: clinical results of a multi-center, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 28.The IFNB Multiple Sclerosis Study Group. The University of British Columbia MS/MRI Group Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45:1277–1285. [PubMed] [Google Scholar]
- 29.Jacobs LD, Cookfair DL, Rudick RA et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 30.Khan OA, Tselis AC, Kamholz JA, Garbern JY, Lewis RA, Lisak RP. A prospective, open-label treatment trial to compare the effect of IFNbeta-1a (Avonex), IFNbeta-1b (Betaseron), and glatiramer acetate (Copaxone) on the relapse rate in relapsing-remitting multiple sclerosis: results after 18 months of therapy. Mult Scler. 2001;7:349–353. doi: 10.1177/135245850100700601. [DOI] [PubMed] [Google Scholar]
- 31.Etemadifar M, Janghorbani M, Shaygannejad V. Comparison of Betaferon, Avonex, and Rebif in treatment of relapsing-remitting multiple sclerosis. Acta Neurol Scand. 2006;113:283–287. doi: 10.1111/j.1600-0404.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 32.Patti F, Pappalardo A, Florio C et al. Effects of interferon beta-1a and -1b over time: 6-year results of an observational head-to-head study. Acta Neurol Scand. 2006;113:241–247. doi: 10.1111/j.1600-0404.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 33.Goodin DS, Reder AT, Ebers GC et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNbeta-1b trial. Neurology. 2012;78:1315–1322. doi: 10.1212/WNL.0b013e3182535cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold DL, Calabresi PA, Kieseier BC et al. Peginterferon beta-1a improves MRI measures and increases the proportion of patients with no evidence of disease activity in relapsing-remitting multiple sclerosis: 2-year results from the ADVANCE randomized controlled trial. BMC Neurol. 2017;17:29. doi: 10.1186/s12883-017-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 36.Paty DW, Li DKB, UBC MS/MRI Study Group. IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis, II: MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:662–667. doi: 10.1212/wnl.43.4.662. [DOI] [PubMed] [Google Scholar]
- 37.Lacy M, Hauser M, Pliskin N, Assuras S, Valentine MO, Reder A. The effects of long-term interferon-beta-1b treatment on cognitive functioning in multiple sclerosis: a 16-year longitudinal study. Mult Scler. 2013;19:1765–1772. doi: 10.1177/1352458513485981. [DOI] [PubMed] [Google Scholar]
- 38.Benesova Y, Tvaroh A. Cognition and fatigue in patients with relapsing multiple sclerosis treated by subcutaneous interferon beta-1a: an observational study SKORE. Ther Adv Neurol Disord. 2017;10:18–32. doi: 10.1177/1756285616671882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Croze E, Yamaguchi KD, Knappertz V, Reder AT, Salamon H. Interferon-beta-1b-induced short- and long-term signatures of treatment activity in multiple sclerosis. Pharmacogenomics J. 2013;13:443–451. doi: 10.1038/tpj.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiore D, Hung S, Tang W, Cui Y, Shang S, Scott T. ADVANCE phase 3 extension study (ATTAIN): peginterferon beta-1a 125 mcg every 2 weeks demonstrated sustained efficacy in RMS patients treated up to 5 years. Neurology. 2016;87:e22. [Google Scholar]
- 41.Coles AJ, Twyman CL, Arnold DL et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–1839. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- 42.Durelli L, Verdun E, Barbero P et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) Lancet. 2002;359:1453–1460. doi: 10.1016/s0140-6736(02)08430-1. [DOI] [PubMed] [Google Scholar]
- 43.Schwid SR, Panitch HS. Full results of the Evidence of Interferon Dose-Response-European North American Comparative Efficacy (EVIDENCE) study: a multicenter, randomized, assessor-blinded comparison of low-dose weekly versus high-dose, high-frequency interferon beta-1a for relapsing multiple sclerosis. Clin Ther. 2007;29:2031–2048. doi: 10.1016/j.clinthera.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 44.Vartanian T. An examination of the results of the EVIDENCE, INCOMIN, and phase III studies of interferon beta products in the treatment of multiple sclerosis. Clin Ther. 2003;25:105–118. doi: 10.1016/s0149-2918(03)90013-0. [DOI] [PubMed] [Google Scholar]
- 45.Tolley K, Hutchinson M, You X et al. A network meta-analysis of efficacy and evaluation of safety of subcutaneous pegylated interferon beta-1a versus other injectable therapies for the treatment of relapsing-remitting multiple sclerosis. PLoS One. 2015;10:e0127960. doi: 10.1371/journal.pone.0127960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hernandez L, Guo S, Toro-Diaz H, Carroll S, Syed Farooq SF. Peginterferon beta-1a versus other self-injectable disease-modifying therapies in the treatment of relapsing-remitting multiple sclerosis in Scotland: a cost-effectiveness analysis. J Med Econ. 2017;20:228–238. doi: 10.1080/13696998.2016.1247712. [DOI] [PubMed] [Google Scholar]
- 47.Coyle PK, Shang S, Xiao Z, Dong Q, Castrillo-Viguera C. Matching-adjusted comparisons demonstrate better clinical outcomes with SC peginterferon beta-1a every two weeks than with SC interferon beta-1a three times per week. Mult Scler Relat Disord. 2018;22:134–138. doi: 10.1016/j.msard.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015;72:152–158. doi: 10.1001/jamaneurol.2014.3537. [DOI] [PubMed] [Google Scholar]
- 49.Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015;4:329–333. doi: 10.1016/j.msard.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Havrdova E, Galetta S, Hutchinson M et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009;8:254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- 51.Coyle PK, Reder AT, Freedman MS, Fang J, Dangond F. Early MRI results and odds of attaining ‘no evidence of disease activity’ status in MS patients treated with interferon beta-1a in the EVIDENCE study. J Neurol Sci. 2017;379:151–156. doi: 10.1016/j.jns.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 52.Kappos L, Traboulsee A, Constantinescu C et al. Long-term subcutaneous interferon beta–1a therapy in patients with relapsing-remitting MS. Neurology. 2006;67:944–953. doi: 10.1212/01.wnl.0000237994.95410.ce. [DOI] [PubMed] [Google Scholar]
- 53.Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011;17:423–430. doi: 10.1177/1352458510394610. [DOI] [PubMed] [Google Scholar]
- 54.Ingwersen J, Aktas O, Hartung HP. Advances in and algorithms for the treatment of relapsing-remitting multiple sclerosis. Neurotherapeutics. 2016;13:47–57. doi: 10.1007/s13311-015-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buttmann M, Goebeler M, Toksoy A et al. Subcutaneous interferon-beta injections in patients with multiple sclerosis initiate inflammatory skin reactions by local chemokine induction. J Neuroimmunol. 2005;168:175–182. doi: 10.1016/j.jneuroim.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 56.Stewart TM, Tran ZV. Injectable multiple sclerosis medications: a patient survey of factors associated with injection-site reactions. Int J MS Care. 2012;14:46–53. doi: 10.7224/1537-2073-14.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reder AT, Oger JF, Kappos L, O’Connor P, Rametta M. Short-term and long-term safety and tolerability of interferon beta-1b in multiple sclerosis. Mult Scler Relat Disord. 2014;3:294–302. doi: 10.1016/j.msard.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 58.Naismith RT, Hendin B, Wray S Risk of New Flu-like Symptoms in Patients Transitioning from Non-pegylated to Pegylated Interferon Beta-1a and Mitigation with Scheduled Naproxen. Paper presented at: Consortium of Multiple Sclerosis Centers Annual Meeting; National Harbor, MD. June 1–4, 2016. [Google Scholar]
- 59.Axtell RC, Raman C, Steinman L. Type I interferons: beneficial in Th1 and detrimental in Th17 autoimmunity. Clin Rev Allergy Immunol. 2013;44:114–120. doi: 10.1007/s12016-011-8296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bertolotto A, Gilli F. Interferon-beta responders and non-responders: a biological approach. Neurol Sci. 2008;29(suppl 2):S216–S217. doi: 10.1007/s10072-008-0941-2. [DOI] [PubMed] [Google Scholar]
- 61.Namaka M, Pollitt-Smith M, Gupta A et al. The clinical importance of neutralizing antibodies in relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2006;22:223–239. doi: 10.1185/030079906X80413. [DOI] [PubMed] [Google Scholar]
- 62.White JT, Crossman M, Richter K, Berman M, Goyal J, Subramanyam M. Immunogenicity evaluation strategy for a second-generation therapeutic, PEG-IFN-beta-1a. Bioanalysis. 2015;7:2801–2811. doi: 10.4155/bio.15.197. [DOI] [PubMed] [Google Scholar]
- 63.White JT, Newsome SD, Kieseier BC et al. Incidence, characterization, and clinical impact analysis of peginterferon beta1a immunogenicity in patients with multiple sclerosis in the ADVANCE trial. Ther Adv Neurol Disord. 2016;9:239–249. doi: 10.1177/1756285616633967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halper J, Centonze D, Newsome SD et al. Management strategies for flu-like symptoms and injection-site reactions associated with peginterferon beta-1a: obtaining recommendations using the Delphi technique. Int J MS Care. 2016;18:211–218. doi: 10.7224/1537-2073.2015-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hendin B, Huang D, Wray S et al. Subcutaneous peginterferon beta-1a injection-site reaction experience and mitigation: Delphi analysis of the ALLOW study. Neurodegener Dis Manag. 2017;7:39–47. doi: 10.2217/nmt-2016-0032. [DOI] [PubMed] [Google Scholar]
- 66.Lanzillo R, Chiodi A, Carotenuto A et al. Quality of life and cognitive functions in early onset multiple sclerosis. Eur J Paediatr Neurol. 2016;20:158–163. doi: 10.1016/j.ejpn.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 67.Filipi ML, Beavin J, Brillante RT et al. Nurses’ perspective on approaches to limit flu-like symptoms during interferon therapy for multiple sclerosis. Int J MS Care. 2014;16:55–60. doi: 10.7224/1537-2073.2013-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Girouard N, Theoret G. Management strategies for improving the tolerability of interferons in the treatment of multiple sclerosis. Can J Neurosci Nurs. 2008;30:18–25. [PubMed] [Google Scholar]
- 69.Munschauer FE, III, Kinkel RP. Managing side effects of interferon-beta in patients with relapsing-remitting multiple sclerosis. Clin Ther. 1997;19:883–893. doi: 10.1016/s0149-2918(97)80042-2. [DOI] [PubMed] [Google Scholar]
- 70.Fenu G, Lorefice L, Frau F, Coghe GC, Marrosu MG, Cocco E. Induction and escalation therapies in multiple sclerosis. Antiinflamm Antiallergy Agents Med Chem. 2015;14:26–34. doi: 10.2174/1871523014666150504122220. [DOI] [PubMed] [Google Scholar]
- 71.Montalban X, Gold R, Thompson AJ et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol. 2018;25:215–237. doi: 10.1111/ene.13536. [DOI] [PubMed] [Google Scholar]
- 72.Rae-Grant A, Day GS, Marrie RA et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 73.Michel L, Larochelle C, Prat A. Update on treatments in multiple sclerosis. Presse Med. 2015;44:e137–e151. doi: 10.1016/j.lpm.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 74.D’Amico E, Leone C, Zanghi A, Fermo SL, Patti F. Lateral and escalation therapy in relapsing-remitting multiple sclerosis: a comparative study. J Neurol. 2016;263:1802–1809. doi: 10.1007/s00415-016-8207-z. [DOI] [PubMed] [Google Scholar]
- 75.Gajofatto A, Bacchetti P, Grimes B, High A, Waubant E. Switching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosis. Mult Scler. 2009;15:50–58. doi: 10.1177/1352458508096687. [DOI] [PubMed] [Google Scholar]
- 76.Cohan S, Smoot K, Kresa-Reahl K Stable Multiple Sclerosis Patients on Intramuscular Interferon beta-1a Therapy Have Better Outcomes When Staying on Therapy than Patients Who Switch to Another Interferon. Paper presented at: 32nd Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); London, UK. September 14, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


