Introduction
Multisystem inflammatory syndrome in children (MIS-C) has recently been described in pediatric patients associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the coronavirus disease 2019 (COVID-19) pandemic.1, 2, 3 Although MIS-C is commonly associated with cardiovascular findings, the occurrence of arrhythmias is rare. We present the case of a pediatric patient who developed severe hemodynamic instability and high-grade heart block associated with MIS-C, requiring transvenous pacing.
Key Teaching Points.
-
•
Multisystem inflammatory syndrome in children (MIS-C) has recently been described in pediatric patients associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the coronavirus disease 2019 (COVID-19) pandemic.
-
•
Although cardiovascular involvement is common with MIS-C, rhythm disturbances are less commonly associated with the syndrome.
-
•
High-grade heart block is a potential complication of MIS-C, requiring close observation and the need for advanced invasive cardiac intervention, such as transvenous pacing.
Case report
In early April 2020, an 11-year-old previously healthy male child (weight 59.3 kg, body mass index 24.2 kg/m2) presented with a week of fever to 39.5oC, dyspnea and a 4-week history of cough. One day prior to admission, he developed a rash and conjunctival injection. Vital signs on admission included a heart rate of 138 beats per minute, a blood pressure of 87/57 mm Hg, a respiratory rate of 28 breaths per minute, a saturation in room air of 98%, and a temperature of 38oC. Pertinent physical exam findings revealed perilimbal conjunctival injection, diminished breath sounds with crackles in the right upper lobe, mild distress, and a polymorphic maculopapular rash over the torso and trunk. The patient had no significant past medical history.
The differential diagnosis included bacterial and viral pneumonia. Toxic shock syndrome, early Stevens-Johnson syndrome, and Kawasaki disease were also considered. Possible infectious etiologies included an adenovirus, Mycoplasma pneumoniae, Group A Streptococcus, and Staphylococcus aureus. Given the COVID-19 pandemic, infection with SARS-CoV-2 was also considered.
An initial radiograph demonstrated a multifocal right-sided pneumonia. His respiratory panel was positive for Mycoplasma pneumoniae, and treatment with azithromycin was begun. His SARS-CoV-2 RNA RT-PCR testing was negative. Laboratory investigations demonstrated a hyperinflammatory state (CRP 330.9 mg/L, ESR 110 mm/h, ferritin 509 ng/mL, and D-dimer 7.67 mcg/mL FEU), neutrophilia, and lymphopenia. Interleukin-2 receptor and interleukin-6 were markedly elevated at 56,818 PG/mL and 534.71 pg/mL, respectively.
Within 24 hours of admission, he developed worsening hypoxia and hypotension, requiring high-flow nasal cannula support (15 liters per minute, 50% FiO2) and 80 mL/kg crystalloid fluid resuscitation, prompting transfer to the pediatric intensive care unit. Antibiotic coverage was broadened to levofloxacin and linezolid. An echocardiogram demonstrated an ectatic right coronary artery (RCA) with a z-score of 5.16 with loss of distal tapering, a normal left main coronary artery (LMCA), and an ectatic left anterior descending coronary artery (LAD) with a z-score of 2.4 with loss of distal tapering. There was normal cardiac function. He received 2 mg/kg intravenous immunoglobulin (IVIG) and medium-dose aspirin, consistent with Kawasaki disease treatment.
During hospital days 2–3, the patient developed vasogenic shock and rising lactate levels, requiring intubation and a norepinephrine infusion. He had persistent fevers (up to 39.9oC) and worsening rash. Small bilateral pleural effusions developed. Serial echocardiograms showed further coronary artery dilation and ectasia, with an RCA z-score of 13.5, a left main coronary artery z-score of 4.8 and an LAD z-score of 7.05. The apical 4-chamber left ventricular ejection fraction was normal at 74%. Active anticoagulation commenced with unfractionated heparin, and a second dose of IVIG was given. Early electrocardiograms demonstrated normal sinus rhythm without evidence of ischemia. Serial troponin levels remained <0.01 ng/mL.
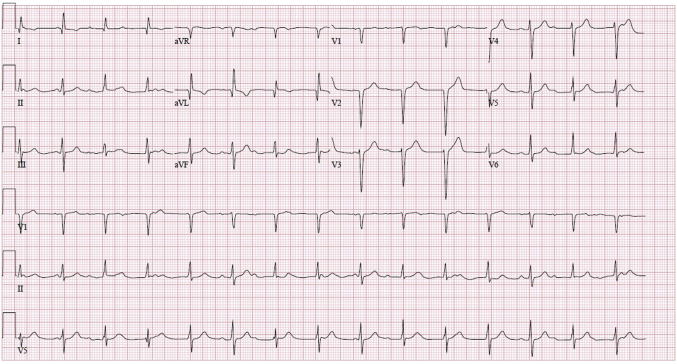
On hospital day 4, the patient’s clinical status worsened further, requiring escalation in vasomotor support and the addition of a vasopressin infusion to maintain a systolic blood pressure > 80 mm Hg. He developed conduction abnormalities, including sinus bradycardia with varying degrees of atrioventricular conduction abnormalities (first- and second-degree heart block), intraventricular conduction abnormalities with varying QRS morphologies, and periods of an idioventricular rhythm. Despite a normal left ventricular ejection fraction, the relative bradycardia contributed to decreased cardiac output, oliguric renal failure, and a rising lactate level, which peaked at 14.7 mmol/L. He had a brief run of nonsustained ventricular tachycardia (7 beats with cycle length of 220 ms). He then developed second-degree type II heart block with variable nonspecific intraventricular conduction delay (Figure 1). His heart rate ranged from 75 to 89 beats per minute. This was deemed an insufficient heart rate based on his age, severity of illness, and markers of inadequate oxygen delivery. An emergent transvenous bipolar pacing catheter (Edwards Lifesciences, Irvine, CA) was inserted at the bedside, and ventricular pacing was initiated at a VVI rate of 110 beats per minute. One gram of methylprednisolone was given. Repeat SARS-CoV-2 RNA RT-PCR testing was negative, and antibiotics were changed to ceftaroline and doxycycline. The extracorporeal membrane oxygenation team was alerted for possible cannulation, should the patient’s status continue to decline.
Figure 1.
Electrocardiogram demonstrating second-degree type II heart block.
A few hours after the initiation of transvenous pacing and methylprednisolone, the lactate level decreased, and his blood pressure stabilized. The following day, his conduction system abnormalities improved, progressing to second-degree type I block (Figure 2). Approximately 48 hours later, he was noted to have first-degree atrioventricular block, and we were able to safely discontinue transvenous pacing. This was followed by complete resolution of atrioventricular and intraventricular conduction abnormalities, a return to normal sinus rhythm (Figure 3), cessation of vasoactive support, and extubation.
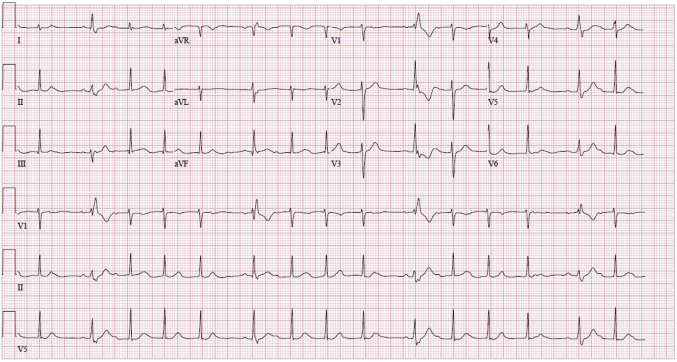
Figure 2.
Electrocardiogram demonstrating second-degree type I heart block with intermittent aberrant conduction.
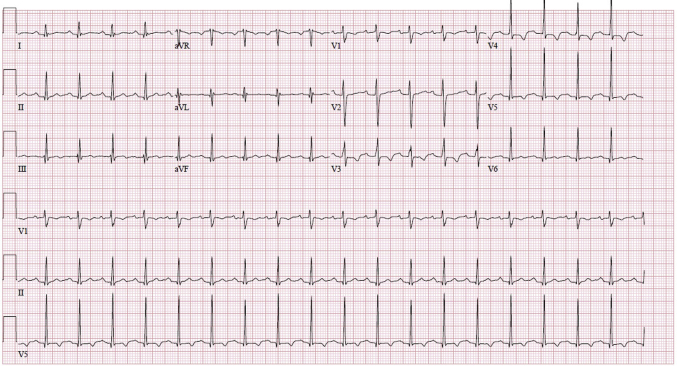
Figure 3.
Electrocardiogram demonstrating normal sinus rhythm.
Despite continued steroid administration and clinical recovery of all other organs, his coronary artery dilation progressed to severe diffuse coronary ectasia with an aneurysm of the proximal RCA and an aneurysm of the LAD. Maximum z-scores were 13.95 for the RCA and 19.9 for the proximal LAD. This prompted administration of infliximab. Magnetic resonance imaging of the chest and abdomen showed no evidence of systemic vasculitis. His conduction remained intact throughout the rest of his hospitalization, and he was discharged home in good condition.
Discussion
Just prior to discharge, the United Kingdom National Health Service released an alert regarding “a new phenomenon affecting previously asymptomatic children with SARS-CoV-2 infection manifesting as a hyperinflammatory syndrome with multiorgan involvement similar to Kawasaki disease shock syndrome,” now termed MIS-C.1,2 Serologic testing for SARS-CoV-2 using an ELISA assay (Epitope Diagnostics, Inc, San Diego, CA) confirmed the presence of anti-COVID-19 nucleocapsid IgG. The serologic ELISA assay testing for the presence of IgM was negative. Suggested exclusion criteria for the diagnosis of MIS-C is evidence of another infectious etiology.4 Although this patient tested positive for Mycoplasma pneumoniae, his hyperinflammatory state, coronary artery dilation, and high-grade heart block were not consistent with Mycoplasma. COVID-19 coinfection with Mycoplasma pneumoniae has been previously reported.5, 6, 7, 8 MIS-C has been associated with cardiac involvement and negative SARS-CoV-2 testing.9,10
While the development of conduction system abnormalities including high-grade heart block is a known complication of coronary ischemia,11 fulminant myocarditis,12 and Kawasaki Disease with severe coronary involvement,13 this is the first case report describing high-grade heart block as a complication of MIS-C associated with SARS-CoV-2 requiring transvenous pacing. In 1 reported cohort of 186 MIS-C patients from the United States, 12% had an associated arrhythmia; however, no specifics regarding the types of arrhythmias seen were described in the publication.3 In another MIS-C publication describing 35 children from France and Switzerland, 1 patient with an unspecified ventricular arrhythmia was reported.10 Severe local inflammation and/or insufficiency of the coronary arterial supply to the atrioventricular node and specialized conduction system are the presumed mechanisms of the conduction system abnormalities in our patient. Interestingly, during his high-grade heart block, he had at least 2 different QRS morphologies, suggesting either rare intermittent atrioventricular conduction with some degree of aberrancy or competing junctional and ventricular escape mechanisms. Regardless of the exact mechanism of the variable intraventricular conduction during bradycardia, the resulting cardiac output was insufficient to meet his metabolic demands.
Although the therapeutic strategy was unproven in this clinical scenario, we speculate that the treatment aimed at mitigating a profound inflammatory response, namely the combination of methylprednisolone and IVIG, likely reversed the conduction system insult in our patient. Transvenous pacing was initiated on hospital day 4 and stopped on hospital day 6. Given the previously unknown entity of MIS-C at the time, inflammatory markers were not all closely trended. However, on hospital days 4, 5, and 7, the patient’s CRP was 266.5, 210, and 32.2 mg/L, respectively. In addition, on hospital days 3, 5, 6, and 7, the patient’s D-dimer was 8.80, 3.71, 3.14, and 2.76 mcg/mL FEU, respectively.
After a 4-week hospital stay, the patient was discharged home in good condition on enoxaparin and aspirin. At cardiology follow-up 6 weeks after the onset of illness, his coronary arteries remained significantly dilated, but sinus node rate, atrioventricular conduction, and intraventricular conduction remained normal.
Conclusions
To our knowledge, this is the first reported case of the development of high-grade heart block associated with MIS-C during the COVID-19 global pandemic to require transvenous pacing. The initiation of transvenous pacing along with the combination of aggressive anti-inflammatory therapies seemed to rapidly improve the patient’s condition and ultimately reverse the high-grade heart block. This case highlights that high-grade conduction system disease, including high-grade heart block, variable atrioventricular block, and intraventricular conduction abnormalities, is a potential complication of MIS-C, requiring close observation and the need for advanced invasive cardiac intervention.
Acknowledgments
The Children’s Hospital of Orange County Institutional Review Board waived approval for this case report, as this is a deidentified case report.
We would like to thank Anjan S. Batra, MD, FHRS, for his assistance with this case.
Footnotes
Funding: This case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PICS Statement Increased number of reported cases of novel presentation of multi-system inflammatory disease. https://picsociety.uk/wp-content/uploads/2020/04/PICS-statement-re-novel-KD-C19-presentation-v2-27042020.pdf Available at.
- 3.Feldstein L.R., Rose E.B., Horwitz S.M. (2020). Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittaker E., Bamford A., Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. https://doi.org/10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed]
- 5.Jiang S., Liu P., Xiong G. Coinfection of SARS-CoV-2 and multiple respiratory pathogens in children. Clin Chem Lab Med. 2020;58:1160–1161. doi: 10.1515/cclm-2020-0434. [DOI] [PubMed] [Google Scholar]
- 6.Fan B.E., Lim K.G.E., Chong V.C.L., Chan S.S.W., Ong K.H., Kuperan P. COVID-19 and mycoplasma pneumoniae coinfection. Am J Hematol. 2020;95:723–724. doi: 10.1002/ajh.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng F., Liao C., Fan Q. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40:1–6. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim D., Quinn J., Pinsky B., Shah N., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) https://emergency.cdc.gov/coca/calls/2020/callinfo_051920.asp Available at.
- 10.Bellhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. https://doi.org/10.1161/circulationaha.120.048360 2020 May 17. [DOI] [PubMed]
- 11.Cardoso R., Alfonso C.E., Coffey J.O. Reversibility of high-grade atrioventricular block with revascularization in coronary artery disease without infarction: a literature review. Case Rep Cardiol. https://doi.org/10.1155/2016/1971803 2016;2016:1971803. [DOI] [PMC free article] [PubMed]
- 12.Batra A.S., Epstein D., Silka M.J. The clinical course of acquired complete heart block in children with acute myocarditis. Pediatr Cardiol. 2003;24:495–497. doi: 10.1007/s00246-002-0402-2. [DOI] [PubMed] [Google Scholar]
- 13.Liu C., Li J., Cui Z. Kawasaki disease: multiple giant coronary aneurysms intervention and pacemaker implantation due to complete heart block-a case report. J Thorac Dis. 2018;10:E108–E112. doi: 10.21037/jtd.2018.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]