Dear Editor,
We read with interest the recent study of Wang et al.,1 regarding the household transmission of coronavirus disease 2019 (COVID-19). In December 2019, an outbreak of COVID-19, caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-COV-2), became a global pandemic. Many epidemiologic studies have been carried out to characterize the epidemiology of COVID-19 and inform decisions about possible interventions. Of particular early interest was the frequency of transmission from confirmed cases to their close contacts. It had been reported that about 75–80% of clustered COVID-19 infections in China were within families,2 suggesting high rates of intra-family transmission. Thus timely household studies can be highly informative for COVID-19 prevention. Here, we report a systematic review of household transmission studies of COVID-19 and try to assess the secondary attack rate of household COVID-19 transmission.
Studies containing data on household transmission of COVID-19 were retrieved from the electronic databases: PubMed, Embase, and a Chinese database, China National Knowledge Infrastructure (CNKI) on 1 July, 2020. All titles identified by the search strategy were independently screened by 2 authors (H.L. and X.X.). Eligible articles reported a household transmission for COVID-19 or sufficient data to determine a secondary attack rate, and must have reported data based on >=10 households. Case reports with only one family involved were also excluded for analysis, because the household transmissions event collection was biased towards infection caused more serious illness. The bias could be reduced with more families involved. When multiple reports of the same dataset were identified, only the most comprehensive report of the study was included. Household secondary attack rate (SAR) was calculated as the number of identified cases divided by the number of household contacts. Further, the SAR was calculated separately for adults and children, using the age criterion of the study, further, the SAR by other contacts was are summarized, when data were available. SARs were calculated as the number of cases divided by the number of contacts, using the fisher's exact test for the 95% confidence interval (CI). We assessed statistical heterogeneity among studies using the I 2 index. All analyses were performed by the software SPSS. The meta-analysis was conducted in Review Manage 2020.
A total of 463, 402 and 212 titles were identified from PubMed, Embase and CNKI, respectively; and 24 articles were included in the mata-analysis. The characteristics of 24 included articles are summarized in Supplementary Table S1. 10 studies are retrospective cohort studies, one is a prospective study, and the other 13 are case ascertainment studies. Most studies (19/24) were conducted in China, with two in South Korea, two in the USA and one in Germany. All the studies were conducted between Jan 1 and March 31, 2020.
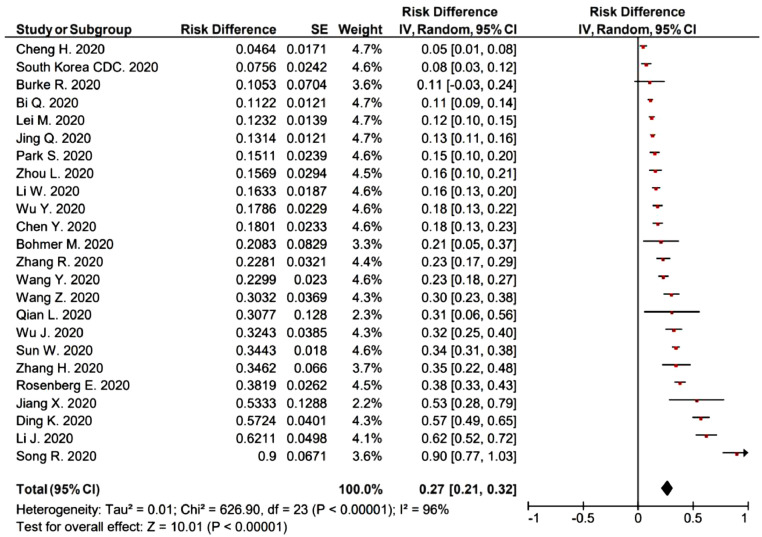
Reported SARs were substantially heterogeneous, ranging from 4.6% to 90.0% (I 2 = 96%), with pooled rate of 27% (95% CI: 21−32%) (Fig. 1 ). In the 10 retrospective cohort studies, the SARs ranged from 11.2% to 68.2%, with mean value 29.7%. In the 13 case ascertainment studies, the SARs ranged from 4.6% to 90.0%, with mean value 28.3%. There is no significantly difference between the SARs in the retrospective cohort and case ascertainment studies (p = 0.93).
Fig. 1.
Secondary attack rates (SIRs) among household contacts.
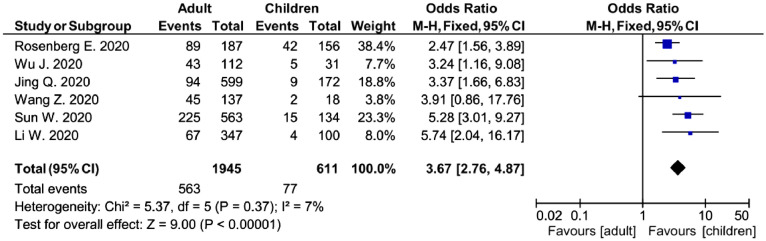
In 6 of the 24 studies, SAR was stratified by age, yielding a range of SARs from 15.7% to 47.6% in adults and from 5.2% to 26.9% in children. The meta-analysis indicates the risk of household transmission in adults is about 3-times higher than that in children (odds ratio (OR)=3.67, 95% CI: 2.76–4.87, p < 0.001) (Fig. 2 ). In 10 studies, the SAR among other contacts (not household contacts) was also reported, and ranged from 0.1% to 28.8%. The meta-analysis indicates the risk of household transmission is about 10 times higher than that from other contacts (OR = 10.72, 95% CI: 5.70–20.17, p < 0.001) (Supplementary Fig. S4).
Fig. 2.
Transmission risk of COVID-19 to adults and children in household.
There were substantial heterogeneities in estimated SAR from the various studies, with point estimates of the SAR ranging from 4.6% to 90.0%. The intrinsic transmissibility is not thought to have various transmissibility in different regions, indeed, as a comparison, review studies reported 2009 pandemic influenza A(H1N1) in household SAR with H1N1 virus were also widely varying, from 3% to 38%.3 In these studies, with 10 or more families involved, the highest SAR were observed in Wuhan, China, which also had the greatest number of COVID-19 confirmed cases in China. Generally, the data-based SAR estimated from literatures would be higher than the real SAR since data-based estimates might have included some untraced exposures from outside.
Household SAR were greater than SAR by other contacts (OR = 10.72, 95% CI: 5.70–20.17, p < 0.001), suggesting much higher rates of intra-family transmission of COVID-19. Several studies have reported that adults were more vulnerable to SARS-CoV-2.4 In this study, we also found that within households, adults were about 4 times as susceptible to COVID-19 from a household member as children (OR = 3.67, 95% CI: 2.76–4.87, p < 0.001). In the study with the highest SAR (62.1%, 95% CI: 52.4−71.9%) among the 10 retrospective cohort studies, the mean age of the households (58.7 ± 16.0)5 was also much higher than these in other studies with detailed age of the households. The age of the households might partly explain the varying SARs. However, because the age of the households was not available in most studies, the quantitative analysis of the SARs and age of the households were not done.
For SARS-CoV, the SAR was estimated to be 6.2–10.2% in Beijing, Hong Kong, Toronto and Singapore.6, 7, 8 Information about the household transmission of MERS-CoV is relatively rare. In a study in Saudi Arabia, the household SAR of MERS-CoV was estimated to be 4% (95% CI: 2–7).9 We conclude that SARS-CoV-2 is more transmissible than SARS-CoV and MERS-CoV in households. In addition, pre-symptomatic and asymptomatic cases were estimated to contribute 53% of COVID-19 transmission.10 All these challenge the value of home isolation for COVID-19 patients, as it may put household members at high risk of infection, propagating the disease. When the hospital isolation of all cases becomes unfeasible, other sheltering facilities, such as the Fangcang Shelter Hospital used in Wuhan, China, might be a better option.2
Author's contributions
HL and XX contributed equally. HL conceived the study, HL and XX designed the study. YS and XW supervised the study. HL, XX and SX searched the references, collected and cleaned the data. HL wrote the drafts of the manuscript. YS, XW, XX and SX commented on and revised drafts of the manuscript. All authors read and approved the final report.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
Acknowledgements
We thank Prof. Racheal Mary Jones (School of Medicine, University of Utah) for helpful discussion and comments.
Funding
This project was supported by Natural Science Foundation of Zhejiang Province (Grant no. LQ20H260009).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.08.033.
Appendix. Supplementary materials
References
- 1.Wang Z., Ma W., Zheng X., Wu G., Zhang R. Household transmission of SARS-CoV-2. J Infect. 2020;81(1):179–182. doi: 10.1016/j.jinf.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S., Zhang Z., Yang J., et al. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau L.L.H., Nishiura H., Kelly H., et al. Household transmission of 2009 pandemic influenza A(H1N1): a systematic review and meta-analysis. Epidemiol. 2012;23(4):531–542. doi: 10.1097/EDE.0b013e31825588b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Litvinova M., Liang Y., et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020 doi: 10.1126/science.abb8001. DOI: 11.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J., Gong X., Wang Z., et al. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 2020;286 doi: 10.1016/j.virusres.2020.198043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.China C.D.C. Efficiency of quarantine during an epidemic of severe acute respiratory syndrome—Beijing, China, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1037. [PubMed] [Google Scholar]
- 7.Wilson-Clark S.D., Deeks S.L., Gournis E., et al. Household transmission of SARS, 2003. CMAJ. 2006;175(10):1219–1223. doi: 10.1503/cmaj.050876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goh D.L.M., Lee B.W., Chia K.S., et al. Secondary Household transmission of SARS, Singapore. Emerg Infect Dis. 2004;10(2):232–234. doi: 10.3201/eid1002.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drosten C., Meyer B., Müller M.A., et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 10.Ferretti L., Wymant C., Kendall M., et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368(619):eabb6936. doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.