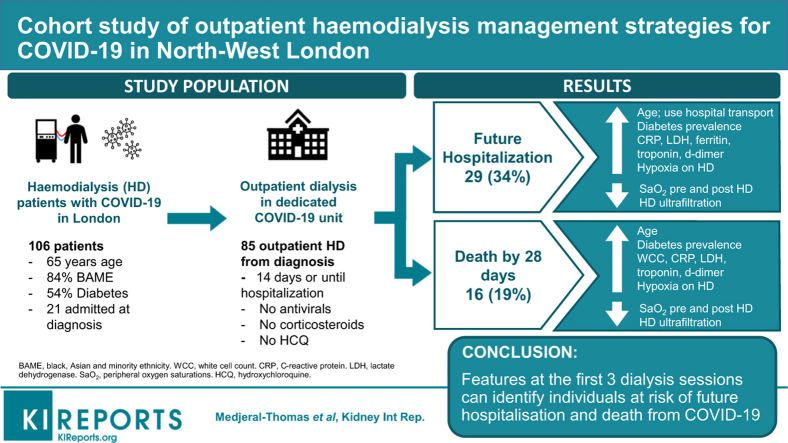
Abstract
Background
Dialysis patients are at risk of severe coronavirus disease 2019 (COVID-19). We managed COVID-19 hemodialysis outpatients in dedicated satellite dialysis units. This provided rare opportunity to study early disease progress in community-based patients. We aimed to (i) understand COVID-19 progression, (ii) identify markers of future clinical severity, and (iii) assess associations between dialysis management strategies and COVID-19 clinical outcomes.
Methods
We conducted a cohort study of all outpatients managed at a COVID-19 hemodialysis unit. We analyzed data recorded as part of providing COVID-19 clinical care. We analyzed associations between features at diagnosis and the first 3 consecutive hemodialysis sessions in patients who required future hospital admission, and those who had died at 28 days.
Results
Isolated outpatient hemodialysis was provided to 106 patients over 8 weeks. No patients received antiviral medication or hydroxychloroquine. Twenty-one patients (20%) were admitted at COVID-19 diagnosis; 29 of 85 patients (34%) were admitted after initial outpatient management; 16 patients (15%) died. By multivariate analysis, nonactive transplant list status, use of institutional transport, and increased white cell count associated with future hospitalization and increased age associated with death. Oxygen saturations progressively decreased over the first 3 dialysis sessions in the cohorts that progressed to future hospital admission or death. Mean ultrafiltration volume of the first 3 hemodialysis sessions was reduced in the same cohorts.
Conclusions
Outpatient hemodialysis in patients with COVID-19 is safe for patients and staff. Features at the first 3 dialysis sessions can identify individuals at risk of future hospitalization and death from COVID-19.
Keywords: coronavirus, COVID-19, hemodialysis, SARS-CoV-2
Graphical abstract
Patients reliant on hemodialysis renal replacement therapy for end-stage kidney disease (ESKD) are at risk of contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and developing severe coronavirus disease 2019 (COVID-19). Most hemodialysis patients attend dialysis units as outpatients, often via institutional transport, and receive therapy thrice weekly in shared clinical areas. In Brescia, Italy, 15% of the chronic hemodialysis population developed COVID-19.1 Mortality rates in dialysis patients with COVID-19 are high.2 As of May 6, 197 of the 977 (20.2%) in-center hemodialysis patients in London, UK, who contracted COVID-19 had died.3 The high prevalence of comorbidities4 associated with COVID-19 severity, such as diabetes; age; and Black, Asian, or minority ethnicity (BAME),5, 6, 7, 8, 9, 10 may contribute to both poor prognosis and limited access to inpatient intensive care for hemodialysis patients during the COVID-19 pandemic. However, the contributors to COVID-19 severity, including the influence of comorbidities on clinical outcomes, have not been established in hemodialysis patients. The effects of the dialysis prescription, including ultrafiltration volume that impacts fluid balance status, on COVID-19 clinical outcomes are also not known. Outpatient dialysis attendances provide opportunity to monitor COVID-19 progression early in community-based populations that would otherwise not encounter clinical staff. Therefore, analysis of outpatient hemodialysis patients with COVID-19 may inform dialysis prescriptions and identify risk factors for future COVID-19 severity.
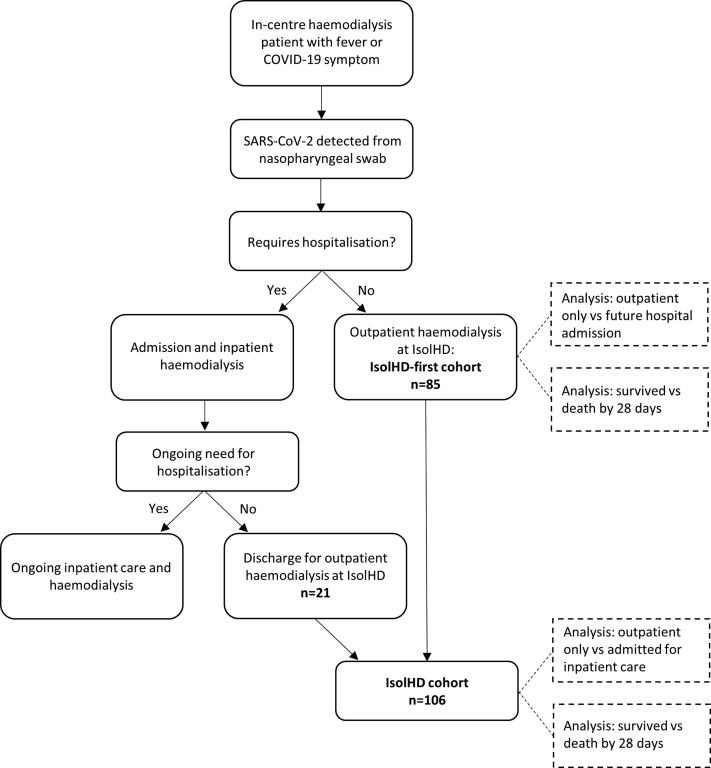
Imperial College Healthcare NHS Trust provides chronic renal replacement therapy for 1530 outpatients from a catchment population of approximately 2.5 million in North-West London. Most patients receive in-center outpatient hemodialysis at either the central Hammersmith hospital unit or 1 of 8 satellite hemodialysis units. To limit SARS-CoV-2 transmission, from March 9, 2020, we screened all in-center hemodialysis patients for fever and COVID-19 symptoms before each dialysis session.11 Patients with possible COVID-19 were segregated within their unit and tested for SARS-CoV-2 by nasopharyngeal swab. Patients with detectable SARS-CoV-2 received subsequent hemodialysis in isolated units (Figure 1) until symptom improvement and for at least 14 days.
Figure 1.
Flow chart demonstrating cohorts of patients managed on the isolated outpatient hemodialysis unit for individuals with COVID-19 (IsolHD). Following detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and diagnosis of coronavirus disease 2019 (COVID-19), patients received ongoing hemodialysis in isolated clinical areas. Of 106 patients managed on IsolHD, 85 did not require hospitalization at COVID-19 diagnosis and received the first hemodialysis session post COVID-19 diagnosis no IsolHD (IsolHD-first cohort). The remaining 21 patients were admitted at the time of COVID-19 diagnosis and received outpatient IsolHD dialysis after clinical improvement and receiving inpatient hemodialysis. We analyzed both the IsolHD-first and total IsolHD cohorts.
We have completed detailed analyses of serially recorded clinical features from all patients managed at our first isolated hemodialysis unit (IsolHD) for patients with COVID-19. We have demonstrated outpatient hemodialysis is appropriate for most patients with COVID-19, revealed important insight into the progression of COVID-19 in hemodialysis patients and identified clinical features and management strategies that associate with future hospital admission and death.
Materials and Methods
This was a cohort study of all hemodialysis patients with COVID-19 managed on IsolHD. Data were recorded as part of routine clinical care in electronic health records and clinical results systems. Analyzed characteristics are listed in Tables 1 and 2. Data were available from every IsolHD and inpatient dialysis session for all patients. This service analysis was approved by the renal quality and safety (governance) committee of Imperial College Healthcare NHS Trust, in view of the project being an evaluation of service change and development during the COVID-19 pandemic.
Table 1.
Characteristics at first hemodialysis session following COVID-19 diagnosis
| Characteristic | All patients | Outpatient | Admitted | P value | Survived | Death | P value |
|---|---|---|---|---|---|---|---|
| Number of patients, n | 106 | 56 | 50 | 90 | 16 | ||
| Age | 65 (54–74) | 62 (52–72) | 68 (58–77) | 0.06 | 65 (53–72) | 76 (61–80) | 0.008a |
| Female | 40 (38) | 20 (36) | 20 (40) | 0.7 | 31 (34) | 9 (56) | 0.2 |
| BAME | 89 (84) | 45 (80) | 44 (88) | 0.3 | 73 (81) | 16 (100) | 0.07 |
| Active on transplant list | 24 (23) | 19 (34) | 7 (14) | 0.02b | 24 (27) | 2 (13) | 0.3 |
| Uses institutional transport | 40 (38) | 14 (25) | 26 (52) | 0.005c | 35 (39) | 5 (31) | 0.8 |
| Diabetes | 57 (54) | 30 (54) | 27 (54) | >0.99 | 48 (53) | 9 (56) | >0.99 |
| Prescribed ACEi or ARB | 37 (35) | 22 (39) | 15 (30) | 0.4 | 35 (39) | 2 (13) | 0.05d |
| Prescribed immunosuppression | 14 (13) | 7 (13) | 7 (14) | >0.99 | 13 (14) | 1 (6) | 0.7 |
| Cause ESKD | |||||||
| Genetic | 9 (8) | 8 (14) | 1 (2) | 0.2 | 9 (10) | 0 (0) | 0.3 |
| Autoimmune | 16 (15) | 13 (23) | 3 (6) | 0.02e | 16 (18) | 0 (0) | 0.1 |
| Diabetes | 45 (42) | 18 (32) | 27 (54) | 0.03f | 34 (38) | 11 (69) | 0.01g |
| Other vascular disease | 20 (19) | 10 (18) | 11 (22) | 0.6 | 17 (19) | 4 (25) | 0.5 |
| Other | 15 (14) | 7 (13) | 8 (16) | 0.8 | 14 (16) | 1 (6) | 0.5 |
| Symptoms | |||||||
| Fever (> 37.8 °C) | 87 (82) | 45 (80) | 42 (84) | 0.8 | 72 (80) | 15 (94) | 0.3 |
| Cough | 49 (46) | 27 (48) | 22 (44) | 0.7 | 42 (47) | 7 (44) | >0.99 |
| Breathlessness | 31 (29) | 10 (18) | 21 (42) | 0.01h | 27 (30) | 4 (25) | 0.8 |
| Myalgia | 28 (26) | 17 (30) | 11 (22) | 0.4 | 24 (27) | 4 (25) | >0.99 |
| Diarrhea | 20 (19) | 14 (25) | 6 (12) | 0.1 | 20 (22) | 0 (0) | 0.04i |
| Coryza | 17 (16) | 8 (14) | 9 (18) | 0.8 | 16 (18) | 1 (6) | 0.5 |
| Nausea | 9 (11) | 7 (18) | 2 (5) | 0.09 | 8 (12) | 1 (8) | >0.99 |
| Clinical observations pre-HD | |||||||
| SaO2 | 98 (96–100) | 99 (97–100) | 98 (95–100) | 0.03j | 99 (97–100) | 98 (93–100) | 0.05k |
| SBP (mm Hg) | 150 (132–165) | 147 (130–164) | 153 (134–174) | 0.4 | 150 (131–164) | 153 (138–175) | 0.6 |
| DBP (mm Hg) | 76 (64–88) | 77 (65–88) | 73 (62–86) | 0.4 | 77 (65–88) | 69 (61–85) | 0.1 |
| Blood tests | |||||||
| HB, g/l (NR 114–150) | 109 (98–120) | 112 (96–122) | 107 (98–113) | 0.1 | 109 (97–120) | 108 (100–116) | 0.9 |
| WCC, x 109/l (NR 4.2–11.2) | 4.8 (3.9–6.7) | 4.3 (3.8–6.0) | 5.3 (4.0–8.0) | 0.02l | 4.6 (3.9–6.4) | 5.7 (4.9–9.7) | 0.02m |
| Lymphocytes, x 109/l (NR 1.1–3.6) | 1.0 (0.7–1.4) | 1.0 (0.7–1.5) | 0.9 (0.7–1.4) | 0.5 | 1.0 (0.7–1.4) | 0.9 (0.6–1.4) | 0.5 |
| PLT, x 109/l (NR 135–400) | 161 (130–228) | 166 (135–230) | 153 (130–227) | 0.6 | 160 (131–229) | 171 (110–227) | 0.8 |
| CRP, mg/l (NR <5) | 44 (13–117) | 29 (10–71) | 94 (26–164) | <0.001n | 39 (13–100) | 142 (37–205) | 0.001o |
| ALT, unit/l (NR <34) | 14 (10–20) | 14 (9–19) | 14 (10–25) | 0.5 | 14 (10–21) | 14 (8–16) | 0.4 |
| LDH, unit/l (NR 125–243) | 285 (226–375) | 251 (221–341) | 324 (247–425) | 0.008p | 269 (226–367) | 341 (290–467) | 0.02q |
| Ferritin, μg/l (NR 20–300) | 799 (502–1276) | 723 (434–1041) | 893 (589–2012) | 0.02r | 786 (494–1276) | 864 (588–1893) | 0.4 |
| CK, unit/l (NR 25–200) | 88 (61–184) | 88 (63–167) | 85 (57–226) | 0.6 | 83 (62–168) | 132 (52–334) | 0.4 |
| Troponin, ng/l (NR <15) | 35 (22–92) | 29 (19–70) | 55 (29–137) | 0.02s | 33 (20–81) | 63 (39–207) | 0.006t |
| D-dimer, μg/l (NR <500) | 1499 (942–2751) | 1171 (674–2044) | 2096 (1280–3520) | <0.001u | 1468 (799–2615) | 2166 (1209–3533) | 0.1 |
ACEi, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin 2 receptor blocker; BAME, Black, Asian and Minority Ethnic groups; CK, creatinine kinase; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DBP, diastolic blood pressure; ESKD, end-stage kidney disease; HB, hemoglobin; HD, hemodialysis; LDH, lactate dehydrogenase; Lymphocytes, lymphocyte count; NR, normal range; PLT, platelet count; SaO2, oxygen saturations measured by peripheral pulse oximetry; SBP, systolic blood pressure; WCC, white cell count.
The Outpatient cohort did not require admission to hospital during the course of COVID-19 disease and recovery. The Admitted cohort received inpatient care and hemodialysis at any point during COVID-19 disease. Data are reported as n (%) for categorical variables and median (interquartile range) for continuous variables. Differences are calculated with Fisher’s exact test for categorical and Mann-Whitney test for continuous variables.
Difference between medians 11.5 years (95% confidence interval [CI] 3–17 years).
Odds ratio (OR) for admission for active transplant waiting list status = 0.3 (95% CI 0.13–0.85).
OR for admission for using hospital-provided transport = 3.3 (95% CI 1.41–7.57).
OR for death for patients prescribed ACEi or ARB = 0.2 (95% CI 0.05–0.96).
OR for admission for patients with autoimmune causes of ESKD = 0.2 (95% CI 0.06–0.73).
OR for admission for patients with diabetes as cause of ESKD = 2.5 (95% CI 1.13–5.29).
OR for death for patients with diabetes as cause of ESKD = 4.3 (95% CI 1.46–11.6).
OR for admission for breathlessness at first dialysis post severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnosis = 3.3 (95% CI 1.41–8.33).
OR for death for diarrhea at first dialysis post SARS-CoV2 diagnosis = 0.1 (95% CI: 0.001–0.75).
Difference between medians 1% SaO2 (95% CI: 0.01–2).
Difference between medians 1% SaO2 (95% CI: 0.01–3).
Difference between medians 1.0 × 109/l (95% CI: 0.2–1.7).
Difference between medians 1.1 × 109/l (95% CI: 0.3–2.9).
Difference between medians 65 mg/l (95% CI: 15–79).
Difference between medians 103 mg/l (95% CI: 20–121).
Difference between medians 73 unit/l (95% CI: 13–93).
Difference between medians 72 unit/l (95% CI: 12–122).
Difference between medians 170 μg/l (95% CI: 36–474).
Difference between medians 26 ng/l (95% CI: 3–33).
Difference between medians 30 ng/l (95% CI: 9–93).
Difference between medians 925 μg/l (95% CI: 345–1351).
Table 2.
Characteristics at consecutive isolated hemodialysis sessions following COVID-19 diagnosis
| Cohort | Characteristic | HD1 | HD2 | HD3 | P value HD1-HD2-HD3 |
|---|---|---|---|---|---|
| Total | Number of patients, n | 85 | 77 | 70 | |
| Pre-HD SaO2 (%) | 98 (97–100) | 98 (95–100) | 98 (95–100) | 0.2 | |
| Post HD SaO2 (%) | 98 (96–100) | 98 (96–100) | 98 (95–100) | 0.9 | |
| Any documented hypoxia (SaO2 <93%) | 14 (16) | 13 (17) | 13 (19) | 0.9 | |
| Pre-HD SBP (mm Hg) | 149 (133–164) | 147 (130–165) | 133 (129–164) | 0.8 | |
| Post HD SBP (mm Hg) | 144 (129–162) | 142 (130–165) | 147 (125–164) | 0.99 | |
| Lowest recorded SBP (mm Hg) | 131 (110–148) | 128 (113–147) | 133 (112–145) | 0.9 | |
| Pre-HD weight (kg) | 74 (66–1150) | 75 (67–113) | 74 (66–111) | 0.97 | |
| Net UF (l) | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 1.4 (1.0–2.0) | 0.6 | |
| Net UF / pre-HD weight (%) | 2.2 (1.2–2.7) | 2.1 (1.2–2.8) | 1.9 (1.2–4.7) | 0.6 | |
| Outpatient only | Number of patients, n | 56 (66) | 55 (71) | 55 (79) | |
| Pre-HD SaO2 (%) | 99 (97–100) | 98 (96–100) | 98 (97–100) | 0.5 | |
| Post HD SaO2 (%) | 98 (97–100) | 99 (97–100) | 99 (97–100) | 0.8 | |
| Any documented hypoxia (SaO2 <93%) | 4 (7) | 3 (5) | 6 (11) | 0.6 | |
| Pre-HD SBP (mm Hg) | 147 (130–164) | 145 (123–170) | 152 (133–164) | 0.9 | |
| Post HD SBP (mm Hg) | 144 (128–161) | 142 (127–164) | 149 (132–165) | 0.6 | |
| Lowest recorded SBP (mm Hg) | 132 (115–149) | 127 (112–146) | 136 (116–153) | 0.5 | |
| Pre-HD weight (kg) | 75 (66–85) | 75 (66–86) | 74 (66–85) | 0.98 | |
| Net UF (l) | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 0.9 | |
| Net UF / pre-HD weight (%) | 2.3 (1.3–2.7) | 2.2 (1.2–2.8) | 2.1 (1.4–2.7) | 0.8 | |
| Future hospital admission | Number of patients, n | 29 (34) | 22 (29) | 15 (21) | |
| Pre-HD SaO2 (%) | 98 (94–99) | 96 (93–98)a | 95 (89–96)b | 0.004 | |
| Post HD SaO2 (%) | 96 (94–99)c | 94 (89–96)d | 94 (91–96)e | 0.06 | |
| Any documented hypoxia (SaO2 <93%) | 10 (34)f | 10 (45)g | 7 (47)h | 0.6 | |
| Pre-HD SBP (mm Hg) | 152 (136–169) | 149 (140–164) | 126 (108–150) | 0.09 | |
| Post HD SBP (mm Hg) | 150 (134–175) | 142 (132–171) | 131 (117–163) | 0.3 | |
| Lowest recorded SBP (mm Hg) | 126 (106–146) | 130 (115–148) | 116 (103–134) | 0.4 | |
| Pre-HD weight (kg) | 73 (67–87) | 72 (68–86) | 73 (66–85) | 0.98 | |
| Net UF (l) | 1.5 (1.0–1.9) | 1.4 (1.0–1.9) | 1.0 (0.5–1.5)i | 0.3 | |
| Net UF / pre-HD weight (%) | 1.7 (1.2–2.5) | 1.9 (1.3–2.5) | 1.3 (0.8–2.1) | 0.4 | |
| Survived 28 days | Number of patients, n | 69 (81) | 65 (84) | 63 (90) | |
| Pre-HD SaO2 (%) | 99 (97–100) | 98 (96–100) | 98 (96–100) | 0.3 | |
| Post HD SaO2 (%) | 98 (97–100) | 99 (97–100) | 99 (97–100) | 0.9 | |
| Any documented hypoxia (SaO2 <93%) | 9 (13) | 7 (11) | 9 (15) | 0.8 | |
| Pre-HD SBP (mm Hg) | 149 (131–163) | 145 (126–167) | 147 (129–164) | >0.99 | |
| Post HD SBP (mm Hg) | 144 (127–161) | 142 (128–163) | 149 (128–163) | 0.7 | |
| Lowest recorded SBP (mm Hg) | 132 (111–149) | 128 (112–147) | 135 (113–148) | 0.7 | |
| Pre-HD weight (kg) | 74 (65–84) | 75 (67–85) | 74 (66–85) | 0.9 | |
| Net UF (l) | 2.0 (1.5–3.3) | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 0.5 | |
| Net UF / pre-HD weight (%) | 2.3 (1.4–2.7) | 2.1 (1.3–2.8) | 1.9 (1.3–2.6) | 0.5 | |
| Death by 28 days | Number of patients, n | 16 (19) | 12 (16) | 7 (10) | |
| Pre-HD SaO2 (%) | 98 (93–99) | 96 (87–98) | 94 (86–95)j | 0.05 | |
| Post HD SaO2 (%) | 96 (93–100) | 94 (86–96)k | 93 (91–95)l | 0.02 | |
| Any documented hypoxia (SaO2 <93%) | 5 (31) | 6 (50)m | 4 (57) | 0.4 | |
| Pre-HD SBP (mm Hg) | 153 (138–175) | 151 (144–166) | 140 (108–185) | 0.5 | |
| Post HD SBP (mm Hg) | 159 (135–181) | 143 (132–184) | 131 (117–174) | 0.3 | |
| Lowest recorded SBP (mm Hg) | 127 (100–145) | 128 (119–148) | 116 (103–134) | 0.6 | |
| Pre-HD weight (kg) | 69 (66–88) | 69 (67–88) | 70 (60–89) | 0.9 | |
| Net UF (l) | 1.2 (0.8–1.5) | 1.1 (0.9–1.8) | 1.0 (0.5–1.5) | 0.6 | |
| Net UF / pre-HD weight (%) | 1.6 (1.1–2.0) | 1.5 (1.2–2.3) | 1.2 (0.8–2.8) | 0.6 |
COVID-19, coronavirus disease 2019; HD, hemodialysis; SaO2, oxygen saturations measured by peripheral pulse oximetry; SBP, systolic blood pressure; UF, ultrafiltration.
The Outpatient-only cohort did not require admission to hospital during the course of COVID-19 disease and recovery. The Admitted cohort received inpatient care and hemodialysis at any point during COVID-19 disease. Data are reported as n (%) for categorical variables and median (interquartile range) for continuous variables. Due to progressive clinical deterioration and requirement for hospitalization, the cohort sizes decreased at consecutive dialysis sessions, particularly in the future hospital admission and death by 28 days cohorts.
P value HD1-HD2-HD3 represents differences among the 3 HD sessions calculated with χ2 for categorical and repeated measures analysis of variance test for continuous variables. Differences between clinical outcome cohorts were calculated with Fisher’s exact test for categorical and Mann-Whitney test for continuous variables. P values were adjusted for multiple analysis with Bonferroni-Dunn method.
P < 0.001 compared with Outpatient-only cohort. Difference between medians 2% (95% confidence interval [CI] 1%–4%).
P < 0.001 compared with Outpatient-only cohort. Difference between medians 3% (95% CI: 3%–5%).
P = 0.007 compared with Outpatient-only cohort. Difference between medians 2% (95% CI: 1%–3%).
P < 0.001 compared with Outpatient-only cohort. Difference between medians 5% (95% CI: 3%–6%).
P < 0.001 compared with Outpatient-only cohort. Difference between medians 5% (95% CI: 3%–6%).
P = 0.01 compared with Outpatient-only cohort. Odds ratio (OR) for future hospital admission for hypoxia at HD1 = 6.8 (95% CI: 1.8–21.1).
P < 0.001 compared with Outpatient-only cohort. OR for future hospital admission for hypoxia at HD2 = 14.4 (95% CI: 3.7–52.2).
P = 0.01 compared with Outpatient-only cohort. OR for future hospital admission for hypoxia at HD3 = 7.1 (95% CI: 1.8–23.7).
P = 0.04 compared with Outpatient-only cohort. Difference between medians 0.5 l (95% CI: 0.001–0.8 l).
P < 0.001 compared with Survived cohort. Difference between medians 4% (95% CI: 3%–11%).
P < 0.001 compared with Survived cohort. Difference between medians 5% (95% CI: 3%–7%).
P < .001 compared with Survived cohort. Difference between medians 6% (95% CI: 3%–7%).
P = 0.01 compared with Survived cohort. OR for death within 28 days for hypoxia at HD2 = 8.3 (95% CI: 2.3–30.3).
All patients received hemodialysis for 3 to 4 hours thrice weekly. Ultrafiltration volume was prescribed at each dialysis session based on clinical assessment including predialysis weight. Due to patients feeling unwell, postdialysis weight, and consequentially interdialytic weight gain, was often unavailable. Peripheral oxygen saturation (SaO2) was measured by pulse oximeter. Hypoxia was defined as SaO2 less than 93% on room air. Blood tests were taken at the first dialysis session post COVID-19 diagnosis. Patients were asked about symptoms and patient experience at every dialysis session. Patient follow-up was by monitoring electronic health records and direct correspondence with responsible outpatient hemodialysis clinicians. Outcomes were recorded at 28 days post COVID-19 diagnosis.
Every patient was assessed by a physician once per dialysis session. Oxygen was provided to all patients with SaO2 <93%. All patients received enoxaparin 20 mg as dialysis anticoagulation. Acetaminophen 1 g (unless weight was less than 40 kg) was administered for rigors, pyrexia, and symptoms of fever. Antibiotics were administered if there was clinical suspicion of superimposed bacterial infection. All staff wore personal protective equipment (PPE) allocated for aerosol-generating procedures. This included fit-tested FFP3 face mask, eye shield, head cover, disposable full body gown, and gloves for each clinical session and additional disposable apron and pair of gloves changed for every patient interaction. The unit was re-organized to create a separate entrance and exit for staff and patients with dedicated areas for PPE donning and doffing. Patients wore surgical face masks and were not allowed to eat or drink while in IsolHD.
We analyzed cohort characteristics using 2 clinical outcomes criteria: (i) patients who required hospital admission, and (ii) patients who survived 28 days from COVID-19 diagnosis. Cohort size limited our analyses to the first 3 IsolHD sessions. We used GraphPad (LaJolla, CA) Prism 8 for statistical analyses. Data were nonparametrically distributed. Categorical data were compared by χ2 or Fisher’s exact test. We used the Baptista-Pike method to calculate odds ratios and 95% confidence intervals. We compared median and interquartile ranges of continuous variables by Kruskal-Wallis test, repeated measures analysis of variance, and Mann-Whitney tests. P values were adjusted for multiple analysis with the Bonferroni-Dunn method. We used univariate and multiple logistical regression model multivariate analyses to assess whether clinical features predicted future hospital admission or death. All statistically significant associations at univariate analysis were entered in the multivariate models. We considered P < 0.05 to be significant.
Results
The first COVID-19 hemodialysis case in our catchment was identified on March 13, 2020. Subsequently, 300 hemodialysis patients (19.6% hemodialysis population) developed COVID-19 over 6 weeks.11 From March 17, IsolHD provided outpatient hemodialysis to 106 patients with COVID-19 over 8 weeks. IsolHD received all new COVID-19 cases not requiring inpatient admission until March 30, when a second unit was opened.11 After March 30, case allocation was based on hemodialysis availability.
Compared with our total dialysis population, the IsolHD cohort was of similar age (median 65 years [interquartile range (IQR) 54–74 years] for IsolHD vs. 66 years [IQR 55–75] for total population), sex ratio (38% female for IsolHD vs. 42% female for total population, P = 0.5) and proportion of patents with diabetes (53% for IsolHD vs. 45% for total population, P = 0.2). The proportion of BAME patients was higher in the IsolHD cohort than the total population (84% vs. 69%, P = 0.001).
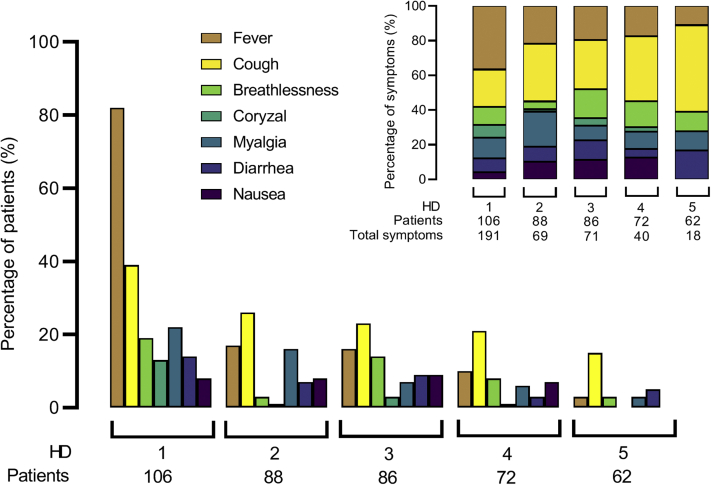
Fever and cough were the 2 most common symptoms at HD1 (Figure 2, Table 1). In general, symptom burden improved at consecutive IsolHD sessions (Figure 2); however, relative to other symptoms, cough, breathlessness, and diarrhea were described more frequently from hemodialysis sessions 3 to 5 post COVID-19 diagnosis (Figure 2). In addition, diarrhea was both a presenting symptom and a symptom that developed later on in the course of the illness.
Figure 2.
Symptom burden at consecutive isolated hemodialysis (HD) sessions post–coronavirus disease 2019 (COVID-19) diagnosis. Patients were asked about symptoms at every dialysis session. The bar chart shows the percentage of patients reporting each symptom at consecutive HD sessions 1–5 post–COVID-19 diagnosis. The stacked bar chart shows the relative proportion of each symptom as percentages of the total number of symptoms reported at each HD session.
The flow of patients through the unit is shown in Figure 1. Of the 85 patients who received outpatient hemodialysis on IsolHD immediately following COVID-19 diagnosis (IsolHD-first cohort, Figure 1), 29 patients (34%) were admitted after median 3 dialysis sessions (IQR 2–4) over 9 days (IQR 5–12 days). Twenty-one of 106 patients (20%) were admitted at the time of diagnosis for median 10 days (IQR 8–13 days) and attended IsolHD following discharge. There were 16 deaths (15% of the total cohort), all of which occurred during inpatient admission.
Characteristics at the first hemodialysis session following COVID-19 diagnosis (HD1) were associated with hospital admission and death at 28 days (Table 1). The cohort that did not require hospital admission (Outpatient only), had a greater proportion of individuals active on the transplant waiting list (P = 0.02) and with autoimmune disease as a cause of ESKD (P = 0.02). The outpatient-only cohort also had higher predialysis SaO2 (P = 0.03) and lower white cell count (P = 0.02), C-reactive protein (P < 0.001), lactate dehydrogenase (P = 0.008), ferritin (P = 0.02), troponin (P = 0.02), and D-dimer (P < 0.001) at HD1 (Table 1). Use of institutional transport (P = 0.005), diabetes as cause of ESKD (P = 0.03), and symptomatic breathlessness at HD1 (P = 0.01) were more common in the cohort requiring admission (Table 1).
Death within 28 days associated with older age (P = 0.008) and diabetes as cause of ESKD (P = 0.01). Angiotensin-converting enzyme inhibitor or angiotensin 2 receptor blocker use were more common in the cohort that survived, although the association did not reach statistical significance (P = 0.05). None of the 20 patients with diarrhea at HD1 had died at 28 days (P = 0.04). We did not detect differences between patients with and without diarrhea in demographic characteristics, antibiotic use, predialysis blood pressure or postdialysis weight loss (Supplementary Table S1). Survival also associated with lower white cell count (P = 0.02), C-reactive protein (P = 0.001), lactate dehydrogenase (P = 0.02), and troponin (P = 0.006) at HD1 (Table 1). Neither BAME ethnicity nor diabetes was associated with admission or death.
Multivariate analysis demonstrated nonactive transplant waiting list status (P = 0.04), use of institutional transport (P = 0.03), and high white cell count (P = 0.03) were associated with increased risk of hospital admission (Table 3). By multivariate analysis, increased age (P = 0.01) was the only feature associated with risk of death at 28 days from COVID-19 diagnosis (Table 3).
Table 3.
Univariate and multivariate analysis of associations between clinical characteristics at first hemodialysis session following COVID-19 infection and risk of hospital admission or death by 28 days
| Outcome | Characteristic | Univariate analyses |
Multivariate analyses |
||
|---|---|---|---|---|---|
| Odds ratio (95% confidence interval) | P value | Odds ratio (95% confidence interval) | P value | ||
| Hospital admission | Active transplant waiting list status | 0.3 (0.13–0.85) | 0.02 | 0.18 (0.03–0.83) | 0.04 |
| Uses institutional transport | 3.3 (1.41–7.57) | 0.005 | 4.35 (1.17–18.58) | 0.03 | |
| Autoimmune cause ESKD | 0.2 (0.06–0.73) | 0.02 | NS | ||
| Diabetes as cause ESKD | 2.5 (1.13–5.29) | 0.03 | NS | ||
| Breathlessness at HD1 | 3.3 (1.41–8.33) | 0.01 | NS | ||
| Difference between medians (95% confidence interval) | P value | ||||
| Predialysis SaO2 | 1% (0.01–2) | 0.03 | NS | ||
| Increased white cell count | 1.0 x 109/l (0.2–1.7) | 0.02 | 1.45 (1.06–2.13) | 0.03 | |
| Increased CRP | 65 mg/l (15–79) | 0.0001 | NS | ||
| Increased LDH | 73 unit/l (13–93) | 0.008 | NS | ||
| Increased ferritin | 170 μg/l (36–474) | 0.02 | NS | ||
| Increased troponin | 26 ng/l (3–33) | 0.02 | NS | ||
| Increased D-dimer | 925 μg/l (345–1351) | 0.0005 | NS | ||
| Death by 28 days | Age | 11.5 years (3–17) | 0.008 | 1.10 (1.03–1.19) | 0.01 |
| Odds ratio (95% confidence interval) | P value | ||||
| Diabetes as cause ESKD | 4.3 (1.46–11.6) | 0.01 | NS | ||
| Diarrhea at HD1 | 0.1 (0.001–0.75) | 0.04 | NS | ||
| Difference between medians (95% confidence interval) | P value | ||||
| Increased white cell count | 1.1 x 109/l (0.3–2.9) | 0.02 | NS | ||
| Increased CRP | 103 mg/l (20–121) | 0.001 | NS | ||
| Increased LDH | 72 unit/l (12–122) | 0.02 | NS | ||
| Increased troponin | 30 ng/l (9–93) | 0.006 | NS | ||
COVID-19, coronavirus disease 2019; CRP, C-reactive protein; ESKD, end-stage kidney disease; HD1, first hemodialysis session following COVID-19 diagnosis; LDH, serum lactate dehydrogenase; NS, nonsignificant; SaO2, peripheral oxygen saturations.
The multivariate analyses included all features demonstrating significant associations at univariate analysis.
We next questioned whether clinical parameters at consecutive outpatient hemodialysis following COVID-19 diagnosis were associated with clinical outcomes. We analyzed the IsolHD-first cohort only (n = 85, Figure1). The IsolHD-first cohort was similar to the total cohort (Supplementary Table S2) with the exceptions that older age and higher D-dimer at HD1 were associated with both future admission (P = 0.04 for age; P = 0.008 for D-dimer) and death (P = 0.02 for age; P = 0.01 for D-dimer), and lower predialysis SaO2 and less diarrhea at HD1 were associated with admission (P = 0.01 for SaO2; P = 0.002 for diarrhea) but not death (Supplementary Table S2).
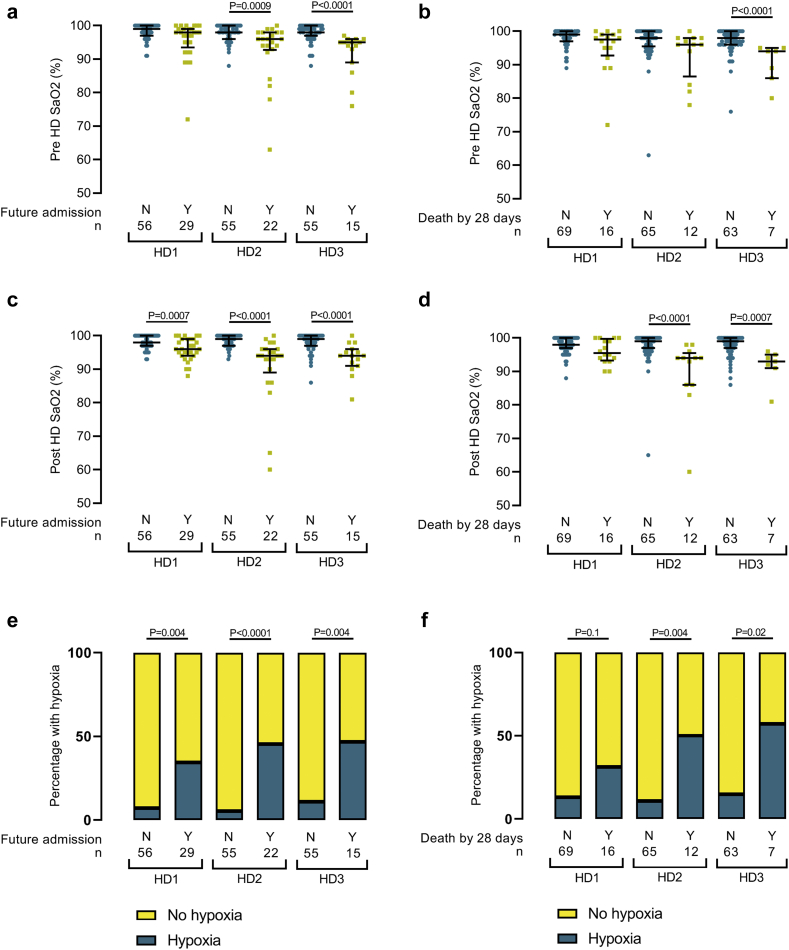
Unlike other clinical observations, pre- and postdialysis SaO2 decreased over the first 3 dialysis sessions in the future hospital admission and death by 28-day cohorts (Table 3). Consequently, the differences in SaO2 between clinical outcome cohorts was greatest at the third hemodialysis session (HD3) post COVID-19 diagnosis; SaO2 was 5% and 6% lower in the “future admission” and “death by 28 days” cohorts, respectively (Figure 3). Also, the proportion of patients with hypoxia was greater in the cohorts that would progress to hospitalization and death (Figure 3e and f). These data are potentially confounded by progressive cohort size reduction at consecutive hemodialysis sessions. Due to clinical deterioration and requirement for inpatient care, patient removal was more common in the future hospital admission and death by 28 days cohorts. The inclusion of these severe COVID-19 cases would have exaggerated differences in hypoxia and SaO2 between outcome cohorts, and therefore their loss is unlikely to explain the differences we detected. We did not detect significant differences in blood pressure or predialysis weights between outcome cohorts (Table 2). There were no significant correlations between blood pressure and SaO2 (data not shown).
Figure 3.
Progression of oxygen saturations (SaO2) and hypoxia at consecutive hemodialysis sessions in patients with coronavirus disease 2019 (COVID-19) who required future hospital admission or died. Pre- (a and b) and post- (c and d) dialysis peripheral SaO2 are shown from the first (HD1), second (HD2), and third (HD3) hemodialysis sessions post COVID-19 diagnosis and divided by the presence (Y, yes, yellow squares) or absence (N, no, blue circles) of future hospital admission or death at 28 days form COVID-19 diagnosis. n, number. (e and f) Percentage of patients with hypoxia (peripheral oxygen saturations <93%) at consecutive COVID-19 isolated hemodialysis sessions who progress to (a) future hospital admission or (b) death within 28 days.
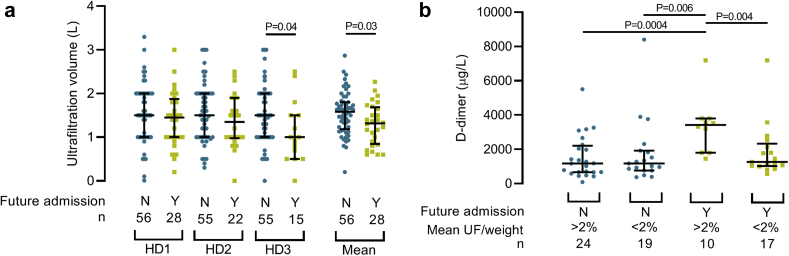
We next interrogated fluid balance management. The mean ultrafiltration volume for HD3 was significantly less than HD1 in cohorts subsequently requiring hospital admission (P = 0.03) and the reduction seemed to be progressive from HD1 to HD2 to HD3 (Table 2 and Figure 4a). We saw a similar pattern when the volume was expressed as a percentage of predialysis weight (P = 0.07) (Supplementary Figure S1). Similar associations were identified in the cohort that died (Supplementary Figure S2). We did not identify associations between ultrafiltration volume and age or blood pressure (data not shown).
Figure 4.
Ultrafiltration volume and D-dimer associated with future hospital admissions and death in hemodialysis patients with coronavirus disease 2019 (COVID-19). (a) Net dialysis ultrafiltration (UF) at the first 3 consecutive isolated hemodialysis sessions (HD1, HD2, HD3) and the mean volume of HD1–HD3 in patients who required future hospital admission with COVID-19. UF was not available from HD1 for 1 patient who required future admission. (b) D-dimer at first dialysis post COVID-19 diagnosis and future hospital admission despite mean UF volume (mean UF/weight) from HD1–HD3 of more than 2% predialysis weight. All patients with available D-dimer results were included. N, no, blue circles. Y, yes, yellow squares. n, number.
There was significant overlap in ultrafiltration volumes between the outcome cohorts (Figure 4a and Supplementary Figures S1 and S2) and some required admission despite significant net ultrafiltration. We therefore questioned whether C-reactive protein and D-dimer, as surrogates of COVID-19 severity, were raised in these individuals. D-dimer was significantly higher in patients requiring admission despite net ultrafiltration of 2% predialysis weight (P = 0.004) (Figure 4b). We did not detect similar associations for C-reactive protein (Supplementary Figure S3). Mean ultrafiltration did not correlate significantly with D-dimer in the total IsolHD population (data not shown).
None of our patients received antiviral medication, hydroxychloroquine, or corticosteroids. Antibiotics were administered to 19 of 85 (22%) IsolHD-first patients. Antibiotic use was more common in the future hospital admission cohort (11 of 29 [58%] patients) than the outpatient-only cohort (8 of 56 [14%] patients, P = 0.03; odds ratio: 3.7; 95% confidence interval: 1.3–10.2). There was no association between antibiotic administration and death at 28 days. In addition to routine anticoagulation administered on dialysis, 5 patients took oral coumarin for preexisting clinical conditions. One patient admitted at COVID-19 diagnosis had pulmonary embolus. No venous thromboemboli were detected in the IsolHD-first cohort. Acetaminophen was administered to 25% of the cohort at HD1 and 26% of the cohort at HD2. Acetaminophen use decreased over subsequent IsolHD sessions.
We asked patients about their hemodialysis experience at IsolHD. Despite 50% of the 78 patient survey responders feeling scared or sad when first moved to IsolHD unit, 91% were happy or very happy with their overall treatment. Physician allocation to the unit increased from 1 doctor for 1 day weekly to 3 doctors for 6 days weekly. None of the nursing or medical staff at IsolHD developed symptoms of COVID-19.
Discussion
The risk factors associated with hospital admission and death from COVID-19 have not been established in dialysis patients.1 Importantly, safe and effective management protocols for COVID-19 hemodialysis patients have not been identified. Our study provides essential insight into early markers of clinical severity and effective management strategies for COVID-19 in an urban, outpatient hemodialysis cohort.
Similar to non-ESKD populations, age and frailty, as represented by nonactive transplant waiting list status and dependence on institutional transport, associated with worse clinical outcomes. Given that transplant waiting list status and use of hospital-organized transport are generally easily accessible and clearly documented in patient records, we were interested whether these surrogate markers of frailty would associate with COVID-19 severity. This information may allow rapid identification of patients particularly at risk of disease severity. BAME individuals were overrepresented in the COVID-19 cohort and all the patients who died were of BAME ethnicity. This supports growing evidence that BAME ethnicity independently associates with COVID-19 disease severity and demands urgent research investment.10,12
We identified novel associations with COVID-19 severity that may be specific to hemodialysis patients and our urban, multi-ethnic patient population. Our data suggest diabetes does not significantly influence risk of death from COVID-19 and therefore should not influence access to intensive care resources. We detected a trend toward angiotensin-converting enzyme inhibitor or angiotensin 2 receptor blocker use and survival. This contradicts concerns that angiotensin-converting enzyme inhibitor use may contribute to COVID-19 severity. The association of diarrhea early in COVID-19 disease with survival was not seen in a similar dialysis population from Italy1 and may be anomalous to population size. However, if seen in other populations, the possibility it represents differences in, for example, immune responses or dietary intake should be considered.
Oxygen saturations decreased progressively at consecutive dialysis sessions in cohorts who would progress to hospitalization and death. Whether this is a modifiable observation or marker of established severe pneumonitis is unclear. Progressive decreases in ultrafiltration volume at consecutive dialysis sessions corresponded with SaO2 patterns. Fluid balance management of COVID-19 patients has attracted debate.13,14 Although causality cannot be inferred, our data indicate maintained ultrafiltration volumes associate with better clinical outcomes in COVID-19 dialysis patients.
We administered standard hemodialysis anticoagulation only to 94% of patients. Despite detecting raised D-dimer in 90% patients, no venous thromboembolism were detected in the IsolHD-first population. Furthermore, we observed significant overlap in D-dimer levels between outcome cohorts (Figure 4b). Our findings suggest D-dimer–based anticoagulation regimens for COVID-19 should not be prescribed to dialysis patients. Raised D-dimer could indicate pulmonary endothelial dysfunction and thrombosis in a subset of patients with deteriorating COVID-19 unresponsive to fluid balance management.15
Most of our patients recovered from COVID-19 with outpatient hemodialysis management alone (Supplementary Figure S4). Excluding SaO2, clinical observations remained stable at consecutive dialysis sessions (Table 2). Despite the use of no antiviral or immunomodulatory agents and a comorbid, urban, multi-ethnic population, our admission (47%) and mortality (15%) rates are comparable to other published studies. A cohort of 94 hemodialysis patients from Brescia, Italy, documented admission and mortality rates of 54% and 29%, respectively.1 The UK Renal Registry Survey reported mortality rates of 22% for in-center hemodialysis patients in London.3 A kidney center that provides care to 670 hemodialysis patients in South London reported a smaller proportion of patients (11.3%) who tested positive for COVID-19 and similar rates of admission (40.8%) and death (9.2%).16 Similar to our cohort, COVID-19 was more common in patients who attended hemodialysis using hospital-organized patient transport.16 Determining whether features specific to our IsolHD regimen, which involved physician review at every dialysis session and liberal use of intradialytic acetaminophen and supplemental oxygen, contributed to our relatively high survival rate requires further research.
Unlike other clinical areas for dialysis patients with COVID-19 at our hospital trust,11 no IsolHD staff developed COVID-19 symptoms. National UK PPE guidelines were not followed on IsolHD; staff on IsolHD used PPE normally reserved for clinical areas with aerosol-generating procedures, such as intensive care units. Our data suggest the provision of comprehensive PPE, including FFP3 masks, eye shields, and full body gowns, is essential for protecting health care staff in clinical areas with known cases from COVID-19 transmission.
Our data are limited by the cohort nature of the study and the significant patient loss at consecutive dialysis sessions. However, our study provides a rare opportunity to analyze comprehensive, thrice-weekly assessments of patients with COVID-19 who, without the unavoidable need for outpatient hemodialysis, would not have interacted with clinical services, but of whom 34% progressed to hospitalization or death (Supplementary Table S2).
In conclusion, we have identified novel features at diagnosis and consecutive dialysis sessions that associate with future hospitalization and death from COVID-19. We have demonstrated outpatient hemodialysis is safe for patients with COVID-19 and highlighted strategies that will improve patient outcomes and staff safety. These results are important for the management of dialysis patients during the COVID-19 pandemic and will inform practice in the event of subsequent waves of the disease.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We acknowledge the contributions of a large number of senior clinicians at the Imperial College Healthcare NHS Trust Renal and Transplant Centre caring for these patients. We also acknowledge the participation of the patients involved.
NRM-T is funded by a Wellcome Trust and Imperial College London Research Fellowship (WIII/PS3459). We acknowledge the support of the NIHR Imperial Biomedical Research Centre.
Author Contributions
ML designed and was responsible for the study; TT, ML, AM, and MN collected data; NRM-T, TT, and DA analyzed the data; NRM-T made the figures and drafted the paper; NRM-T, ML, DA, and ND revised the paper; all authors approved the final version of the manuscript.
Footnotes
Table S1. Characteristics of patients with diarrhea at first hemodialysis session following COVID-19 diagnosis.
Table S2. Characteristics at first hemodialysis session following COVID-19 diagnosis of patients without initial hospital admission (IsolHD-first cohort).
Figure S1. Net dialysis ultrafiltration as percentage of predialysis weight (ultrafiltration [UF]/weight) at the first 3 consecutive isolated hemodialysis sessions (HD1, HD2, HD3) in cohorts of patients with COVID-19 who required future hospital admission.
Figure S2. Hemodialysis ultrafiltration (UF) at the first 3 consecutive hemodialysis sessions following COVID-19 diagnosis (HD1, HD2, HD3) and survival at 28 days.
Figure S3. C-reactive protein (CRP) at first dialysis post COVID-19, ultrafiltration volumes of more than 2% predialysis weight and future hospital admission.
Figure S4. Clinical status of patients managed at the isolated outpatient hemodialysis unit dedicated to patients with COVID-19 at each day from COVID-19 diagnosis
Supplementary Appendix. Senior clinicians at the Imperial College Healthcare NHS Trust Renal and Transplant Centre caring for these patients and collaborating with this investigation.
Supplementary Material
References
- 1.Alberici F., Delbarba E., Manenti C. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98:20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Renal Association BU . 2020. UK Renal Registry (2020) COVID-19 surveillance report for renal centres in the UK: All regions and centres. [Google Scholar]
- 4.Miskulin D.C., Meyer K.B., Athienites N.V. Comorbidity and other factors associated with modality selection in incident dialysis patients: the CHOICE Study. Choices for Healthy Outcomes in Caring for End-Stage Renal Disease. Am J Kidney Dis. 2002;39:324–336. doi: 10.1053/ajkd.2002.30552. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khunti K., Singh A.K., Pareek M. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 11.Corbett R.W., Blakey S., Nitsch D. Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol. 2020;31:1815–1823. doi: 10.1681/ASN.2020040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pareek M., Bangash M.N., Pareek N. Ethnicity and COVID-19: an urgent public health research priority. Lancet. 2020;395:1421–1422. doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koratala A., Ronco C., Kazory A. Need for objective assessment of volume status in critically ill patients with COVID-19: the Tri-POCUS approach. Cardiorenal Med. 2020;10:209–216. doi: 10.1159/000508544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phua J., Weng L., Ling L. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;25:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roper N., Kumar N., Lewis-Morris T. Delivering dialysis during the COVID-19 outbreak: strategies and outcomes. Kidney Int Rep. 2020;5:1090–1094. doi: 10.1016/j.ekir.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.