Abstract
The rise of emerging infectious diseases (EIDs) as well as the increase in spread of existing infections is threatening global economies and human lives, with several countries still fighting repeated onslaught of a few of these epidemics. The catastrophic impact a pandemic has on humans and economy should serve as a reminder to be better prepared to the advent of known and unknown pathogens in the future. The goal of having a set of initiatives and procedures to tackle them is the need of the hour.
Rapid detection and point-of-care (POC) analysis of pathogens causing these diseases is not only a problem entailing the scientific community but also raises challenges in tailoring appropriate treatment strategies to the healthcare sector. Among the various methods used to detect pathogens, Electrochemical Biosensor Technology is at the forefront in the development of POC devices. Electrochemical Biosensors stand in good stead due to their rapid response, high sensitivity and selectivity and ease of miniaturization to name a few advantages.
This review explores the innovations in electrochemical biosensing based on the various electroanalytical techniques including voltammetry, impedance, amperometry and potentiometry and discusses their potential in diagnosis of emerging and re-emerging infectious diseases (Re-EIDs), which are potential pandemic threats.
Keywords: Voltammetry, Impedance, Amperometry, Potentiometry, Corona Virus, COVID-19
Graphical abstract
Schematic illustration of different types of electrochemical biosensors for emerging and re-emerging infectious diseases.
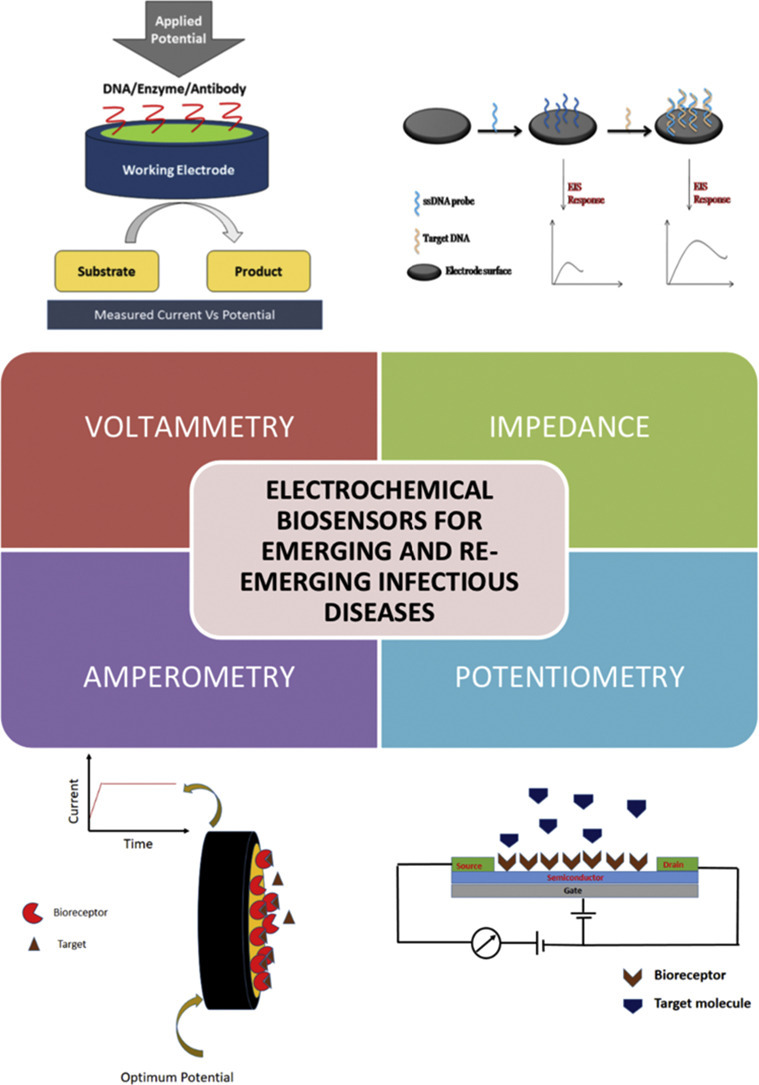
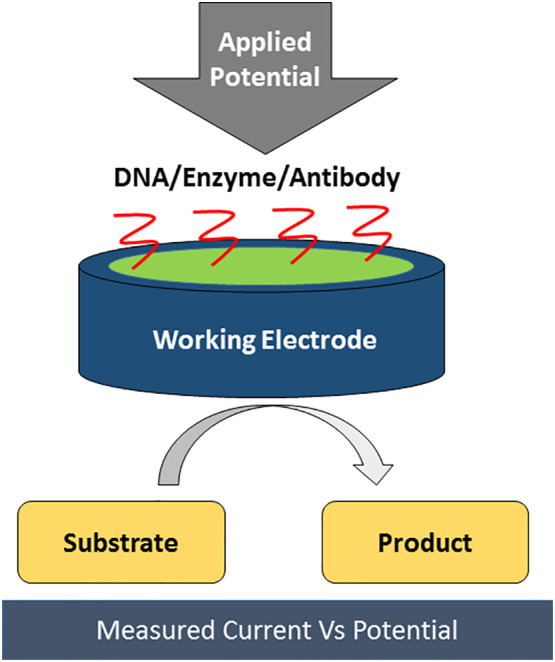
1. Introduction
The inherent risk of EID's is that the introduction of a pathogen into a community drastically decreases the percentage of healthy individuals in a society which would leave an ireversible social and economic stain. The number of potential pathogens worldwide is on the rise, while the research and development (R&D) resources are minimal [1]. Currently, medical R&D models do not account for the application of enhanced disease detection/ prevention kits to epidemics that are intermittent or unpredictable, especially when they occur in countries with minimal investment in healthcare infrastructure [1]. When faced with the challenge of a novel pathogen, the situation becomes even graver. The international community has recognized the need for innovation to improve our capacity to respond to emerging threats and the need to plan for potential epidemic outbreaks with an emerging R&D paradigm. The need for R&D in this area to be prioritized cannot be stressed enough, through which R&D could also be instrumental in planning the response to an outbreak.
In its 2007 study, the World Health Organization cautioned that infectious diseases are emerging at an alarming rate [2]. There have been about 40 infectious diseases reported since the 1970s, including Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), ebola, chikungunya, swine flu, avian flu, Zika and most recently Novel Coronavirus Disease (COVID-19) [2]. The potential for these diseases to spread rapidly and have catastrophic global impact has high probability due to the increasing international travel, population explosion in developing/ under-developed countries and ever increasing proximity with wild animals.
Re-EIDs are those caused by pathogens that are raising health concerns for a significant proportion of the population after being dormant for a while. Schistosomiasis is re-emerging in Egypt; Ebola hemorrhagic fever is making a come back in West Africa and Legionellosis had been reported in Philadelphia [3]. Tuberculosis has made a come back too as the pathogen has developed tolerance to the antibiotics used for treatment (either by genetic exchange or mutation). The prevalence of the pathogen has been the reason for the long-term usage of antibiotics (both within an organism and throughout the population) [3]. Malaria has also shown to become drug-resistant, while the mosquito host has also developed tolerance to pesticides [3]. Whooping cough (pertussis) and Diphtheria have also shown to be prevalent in communities [3].
Identification, monitoring and treatment of diseases are the primary objectives of all public health programs [4]. Effective methods of identification are paramount in preventing or mitigating the spread of a virus before the consequences make an impact to the society. Hence, the global market for diagnoses of infectious diseases is projected to increase substantially in the coming years as per a report obtained from various sources which includes expert interviews, secondary literature, market and market analysis (Fig. 1 ) [5].
Fig. 1.
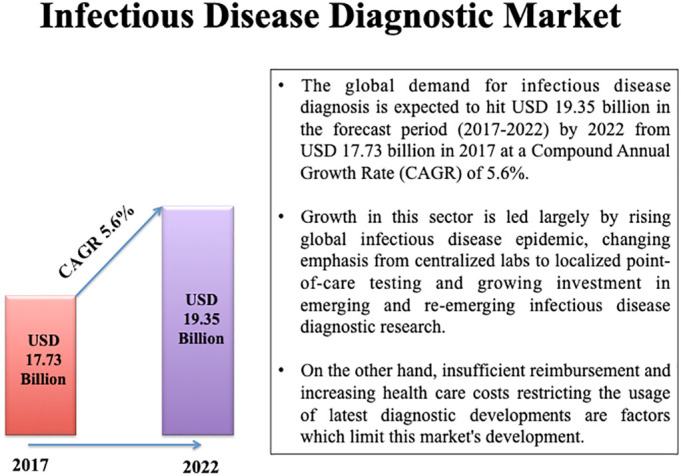
Represents the infectious disease diagnostic market (2017–2022).
Healthcare facilities usually utilize cell culture systems that require a complex of cell separation processes from their normal (in vivo) setting and subsequent growth in an artificially (in vitro) created environment with different nutrients and antibiotics [6]. Thereby pathogen recognition is visually determined based on the observed distinct growth patterns [6]. However, cell culture is increasingly losing its role and its relative importance in the diagnosis of human diseases as this technique requires expertises and trained personnel, sophisticated instruments and is also time consuming [7]. The need of the hour is the clinical diagnosis for early and successful detection and treatment. Consequently, molecular-related approaches, including nucleic acid sequencing and polymerase chain reaction (PCR) have taken center stage as methods of diagnosis to substitute strategies dependent on cell culture. Although they are more sensitive, precise and reliable in detection of microorganisms with reduced diagnosis time (1–4 h), they do have certain shortcomings in comparison with cell culture [7]. Problems that restrict the application of molecular-related approaches to routine diagnosis includes false positive and false negative results and lack of uniformity in molecular testing [8]. Moreover there are possibilities for wrongly interpreting and differentiating between a disease and an infection as the existence of nucleic acid does not necessarily indicate the presence of viable species [8].
Most of the technologies dicusssed are out of reach to majority of the world's population since they are complex, centralized and need skilled technicians to operate. The need for portability, cost reduction and ease of use is thus largely appreciable, particularly in the case of neglected diseases.
Biosensing technique is a promising diagnostic technology which has gained popularity in recent decades due to its numerous benefits [9]. Biosensors have aided in revolutionizing the treatment of various health problems since its inception, five decades ago [9]. Their effectiveness in clinical management, and characteristics like specificity, rapidity and responsiveness are considered key in initiating early diagnosis and therapy [9]. The advancements made in emerging techniques like nanotechnology and microfluidics, coupled with the identification of biomarkers would boost the efficiency of healthcare sector. Transducer integration in biomaterials has allowed for the development of interfaces capable of producing signals - aptly discussed as biosensors [10]. Biosensors, which are bio-electromechanical systems, can be classified based on transducer form, label and general configuration [10]. Biosensors are designed to suit specific functionalities and affinities. Recognition of analytes can often contribute to a transition in three-dimensional structure that is further transduced as a signal [10].
Numerous electrochemical biosensors have been proposed for identification of various diseases, citing features such as low cost, sensitivity, selectivity and rapid response, in recent years [[11], [12], [13], [14], [15], [16]]. Electrochemical biosensors, a subsidary of biological sensors, comprise of a biological sensing system and an electrochemical transducer. These devices are focused primarily on detecting actual or future changes related to interactions happening at the interface of the sensor sample matrix. The recognition factor (antibodies, enzymes, tissues, DNA/RNA or other biomolecules) interacts selectively with the target analyte, resulting in the production of an electrical signal, which is transmitted to the signal processor through the transducer [12]. The signal is further amplified and noise is separated out before relevant information is retrieved.
Electrochemical biosensing techniques will be compared to the most widely used conventional methods (Cell culture systems, immunofluorescence (IF) method and molecular approach) for the diagnosis of infectious diseases. Table 1 summarizes the advantages and disadvantages of electrochemical biosensors over these popular conventional methods.
Table 1.
Advantages and disadvantages of electrochemical techniques over widely used conventional methods.
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Cell culture systems | Isolate wide variety of viruses (including mixed cultures & unanticipated agents); Highly sensitive over rapid antigen tests; Antiviral susceptibility testing, epidemiologic studies and serotyping possible | Technical expertise required to read cytopathic effect; Long incubation period for most viruses; Needs an in-house procurement and maintenance of a variety of cell culture forms | [6,17] |
| IF assays | Usually exhibits good sensitivity and excellent specificity | Not as sensitive as cell cultures; Not useful for all viruses; Requires trained experienced hands in reading results; poor adenovirus sensitivity | [17,18] |
| Molecular approach (Nucleic acid detection) |
Excellent specificity and sensitivity; Quick turnaround using PCR in real time; Suitable for viruses which cannot be cultivated in conventional cell cultures | FDA-cleared kits and approved protocols not commonly available for most viruses; In-house technical skills needed to establish and standardize the methods; expensive instrumentation; Highly specific probes and primers (may skip mutated virus); Detects only the sought viruses and may miss mixed infections and unexpected agents in most cases; Most assays available only at research labs | [[19], [20], [21]] |
| Electrochemical biosensors | Rapid response, cost-effective, robust, easy to miniaturize, excellent detection limits, requires less sample volume, has the ability to be used in turbid biological fluids with optically absorbing and fluorescent molecules. Conversion to a sensor device significantly reduces cost of analysis, saves time & enable regions with limited resources to perform healthcare diagnostics without the need of trained professionals |
Sensitive to sample matrix effects; Not as sensitive as conventional methods; Lower shelf life |
[[11], [12], [13], [14], [15], [16]] |
Electrochemical biosensors are being developed and manufactured in large scale nowadays owing to their increasing demand in environmental, agricultural, clinical and industrial research sectors [22]. One such success story is of an electrochemical biosensor, a Glucometer, used to monitor glucose levels in diabetic patients. Electrochemical biosensors are categorized based on parameter measured such as: Current (Amperometric/ Voltammetric biosensors), Impedance (Impedance biosensors) or Potential (Potentiometric biosensors) [12].
A few articles relevant to this subject have been previously released which includes an outline of the basic concepts of sensing, case studies and difficulties in designing point-of-care sensors for detection in a clinical environment. A comprehensive review on electrochemical biosensor-based pathogen detection has been done by Ellen and Blake [23]. Although electrochemical biosensors are broadly reviewed in the article, latest articles on the detection of pathogens causing EID's and Re-EID's have not been investigated in detail. A summary of recent advances in electrochemical sensors for virus detection and usage of these sensors to track the climate, health, and food have been presented in a recent review by Tugba et al. [24]. This review focusses on viral infections only and the biosensors presented are divided based on the biorecognition element as antibody-based, nucleic acid-based, aptamer-based and antigen-based electrochemical biosensors.
A mini-review published by Ojla and Keith in 2019 presents a detailed overview of the latest developments in the design and production of electrochemical sensing techniques for pathogenic bacteria detection over the last three years, with a special emphasis on three main clinically important pathogens, P. aeruginosa, S. Aurores and E. coli [7]. The advancement in point-of-care bacterial pathogen detection, comprehensive characteristics of bio-recognition components, immobilisation strategies and the fundamental concepts of optical-based biosensors used in bacterial pathogen detection was evaluated in a study published in 2018 by Jean and colleagues [25]. In fact, they illustrate the respective advantages and disadvantages of each methodology. In 2018, reviews on the Applications and Perspectives of Biosensors for Diagnostics in Infectious Diseases [26] and Recent advances in graphene-based biosensor technology with applications in life sciences [27] were published. The advantages of Gene Specific DNA Sensors for Diagnosis of Pathogenic Infections were presented in a review by Manali et al. [10]. The mini review discusses numerous transducer dependent sensors and their role in acute and chronic disease diagnosis. Point-of-Care Testing for Infectious Diseases- Past, Present, and Future was discussed in a mini review by Thomas and co-workers in 2017 [28]. Through this minireview, they analyzed the POC research environment, its roots, details of the variety of existing implementations, and potential technology needs. Ting-Yen-Wei presented a review on Synthetic Biology-Based Point-of-Care Diagnostics for Infectious Disease in 2016 which outlined the barriers to treatment of infectious diseases and addressed two new alternatives: biosensors and engineered virus diagnostic systems [29]. They concluded that such methods focused on synthetic biology would resolve and overcome diagnostic obstacles in infectious diseases. In the same year Bobby et al. discussed the role of biosensors in the detection of EIDs [4].This gives a description of the various forms of biosensor systems used to identify EIDs, and explains some of the techniques behind them in terms of transduction and the concepts of bioreceptors.
This presented overview offers a detailed description of recent developments in designing and improving electrochemical sensing techniques for the determination of pathogens over the last five years, with a special emphasis on EIDs and Re-EIDs. This review covers some of the important articles that have been published on the detection of EIDs and Re-EIDs and has been structured into four main sections: voltammetric biosensors, impedance biosensors, amperometric biosensors, and potentiometric biosensors. Furthermore, the respective advantages and limitations of each technique are demonstrated.
2. Review of recent electrochemical biosensors for EIDs and Re-EIDs based on various electrochemical sensing techniques
2.1. Voltammetric biosensors
Scientific community has been indebted to voltammetric biosensors as it is able to provide the information of a biological system by converting it into an electronic signal. They belong to a class of electroanalytical sensing methodologies, where the current generated is monitored between the working electrode and a counter electrode upon sweeping potential between working electrode and a reference electrode [30]. In voltammetry, the heterogeneous electron transfer takes place at the electrode-electrolyte interface, where electroactive species reaches by mass transport from the bulk of the solution. The measured current is the resultant of oxidation/reduction processes of the electroactive species, which takes place at the surface of the working electrode. Depending upon the applied potential waveform, voltammetric techniques are classified as linear weep voltammetry (LSV), cyclic voltammetry (CV), square wave voltammetry (SWV) and differential pulse voltammetry (DPV) [31]. In voltammetric biosensors, the process of bio-recognition between the recognition layer and the analyte brought to the current response either by redox processes of the analyte or via labelling. The current responses are usually observed as a peak, which corresponds to the concentration of the electroactive species [32] (Fig. 2 ). In order to overcome certain limitations of the bare electrodes, researchers have been focusing on developing modified layers on the surface of the electrodes. These modifications include an extensive class of materials such as nanomaterials, polymer films, metal complexes etc. and such transducers are generally named as chemically modified electrodes. Even though advantages like excellent sensitivity and less analysis time makes voltammetric sensors superior, the challenges suffered from the lack of selectivity (in comparison to conventional methods) and demand for the targets to be redox active in the given potential range [33] make the systems complicated than other electroanalytical techniques.
Fig. 2.
Schematic representation of functioning of a voltammetric biosensor.
The detection of toxins and pathogens of infectious diseases have received much attention, as its diagnosis is essential for human health care. Various toxins and pathogens have been detected via voltammetric biosensing approach, where simple to complex sensing platforms including specific nanostructures, nanomaterials [34], electropolymers etc. have been used. In addition to the modification layer, biomolecules like aptamers [[35], [36], [37], [38], [39]], DNAs [40,41], proteins or antibodies (depending on the mode of biorecognition) are also immobilized for the selective recognition.
The mechanism behind the immobilization of recognition elements clearly depends on the physical and chemical properties of the transducer and the environment in which the system operates. Although, physical interactions such as hydrophobic or electrostatic are simple, it causes negative impact on the analytical performance, stability and reproducibility of the sensor [33]. Another effective mode of immobilization is the covalent tethering of receptor surface to transducer exposed carboxyl or amine units. Among this, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) coupling is a popular method for receptor immobilization, wherein a covalent bridging occurs between amine groups and carboxyl groups via the amine nucleophilic attack on a generated active ester intermediate [33]. Affinity binding approaches like receptor biotinylation and its induced high affinity for pre-immobilised streptavidin, is another more functional and stable potential mode for receptor immobilization [33].
Since all biological processes are dependent on pH, it is important to put more concern on the pH of the buffer, where the studies are executed. The pH of the blood must be maintained at 7.4 and hence researchers have done most of the studies in this physiological pH.
DNA biosensing approaches have been commonly proposed in the field of biosensors for EIDs. For instance, a DNA based sensor was reported for the selective detection of dengue virus serotype 3 (DENV-3), which causes the most predominant vector-borne disease in the world, dengue fever. Oliveira et al. designed a DNA based biosensor for the detection of dengue virus with pencil graphite electrode modified with a probe specific to DENV-3 [42]. The electrochemical analysis was carried out using DPV and the sensor was able to achieve the detection limit as low as 3.09 nM in 20 mM Tris-HCl buffer (pH 7.0). Later, a genosensor for the detection of oligonucleotide sequences of avian influenza virus (AIV) type H5N1 was reported by Kurzątkowska et al., where they proposed a novel mechanism for the generation of electrochemical signal based on an ion-barrier “switch on” system and achieved picomolar detection [43]. Two types of genosensors were reported incorporating (dipyrromethene)2Cu(II) and (dipyrromethene)2Co(II) complexes on gold electrode which is covalently attached to a 20mer probe via redox-active monolayers. The (dipyrromethene)2Cu(II) incorporated genosensor was able to perform in the dynamic linear range of 1 pM −10 pM with a detection limit of 1.3 pM, whereas using (dipyrromethene)2Co(II) complex the detection limit was extended to 1.2 pM at buffer conditions of 0.9 M NaCl and 0.09 M sodium citrate (pH 7.0). An aptamer based biosensor was reported for the detection of Mycobacterium tuberculosis specific antigen MPT64 by Thakur et al. in the year 2017 [31]. They fabricated the probe by depositing graphene modified hybrid nanocomposite films of chitosan-iron oxide on fluorine tin oxide (FTO) with DNA aptamer sequence specific to MPT64 immobilized and found that the detection limit is extended in the range of 0.9 fg/mL in 0.05 M PBS (pH 7.0). The stability and the recoveries obtained by spike-in studies have shown the ability of the aptaelectrode for the diagnosis of tuberculosis at early stage. Recently, the electrochemical detection of chikungunya virus was first reported by Singhal et al., where they developed a voltammetric biosensor based on molybdenum disulphide nanosheets modified screen-printed gold electrode [45]. The detection approach adopted is based on the ability of methylene blue (MB) molecules to interact with the guanine bases in single and double stranded DNA via Vander Waals interaction. Since MB molecules are heterocyclic aromatic compound and alkaline conditions are more suited, 0.1 M PBS of pH 7.8 was chosen for all studies. The genosensor exhibited a wide linear range from 0.1 nM to 100 μM with 3.4 nM as the limit of detection. In addition to that, Ilkhani and Farhad demonstrated a DNA biosensor for the detection of ebola virus, having determination possible in the range 10 nM −75 nM with a limit of detection 4.7 nM [46]. They used gold screen-printed electrode for the detection and prior to detection, the probe DNA was immobilized on its surface. During the detection step, biotinylated target DNA strand gets hybridized with the probe DNA and gets interacted with streptavidin-alkaline phosphatase enzyme. The analytical signal is acquired by the enzymatic conversion of 4-Aminophenyl phosphate to 4-Aminophenol, which was then detected by DPV. The nanomolar detection of influenza genes was reported by Subak et al. by using a simple carbon transducer without any further modifications [47]. The label-free DNA biosensor allowed detection limit as low as 21 nM for Influenza B sequences. The electrochemical oxidation of guanine before and after probe and target DNA hybridization was evaluated using DPV in 0.5 M acetate buffer solution (ABS-pH 4.8). The rapid, direct and simple detection of the genosensor made it to be superior among other DNA biosensors and can be easily adapted for the development of POC analysis. The detection of Zika virus (ZIKV) sometimes becomes a major problem because of the cross-responses between zika and dengue viruses. A DNA based biosensor for the detection of ZIKV was revealed by Alves et al. using poly(3-amino-4-hydroxybenzoic acid) modified pencil carbon graphite electrode, which was superior over immunosensors in terms of low cost and its simple modification procedure [48]. They have validated the bioassay in human serum samples. The possibility for picomolar detection (25.4 pM) and its ability to differentiate ZIKV from dengue virus made the bioassay a promising sensor for POC applications.
Yet another DNA based sensor for dengue virus was proposed by Tripathy et al. in 2016, where they developed a label free sensing platform using Manganese (III) oxide nanofiber modified Glassy carbon electrode which allowed a zeptomolar electrochemical detection of dengue virus (120 × 10−21 M) using DPV in PBS pH 7.4 [49]. The sensing mechanism was based on DNA hybridization and its efficiency was investigated in blood serum samples. Their proposed sensor was superior to other labelled and label free sensors for the detection of dengue viruses in respect to sensitivity and detection without any complex labelling and sophisticated instrumentation.
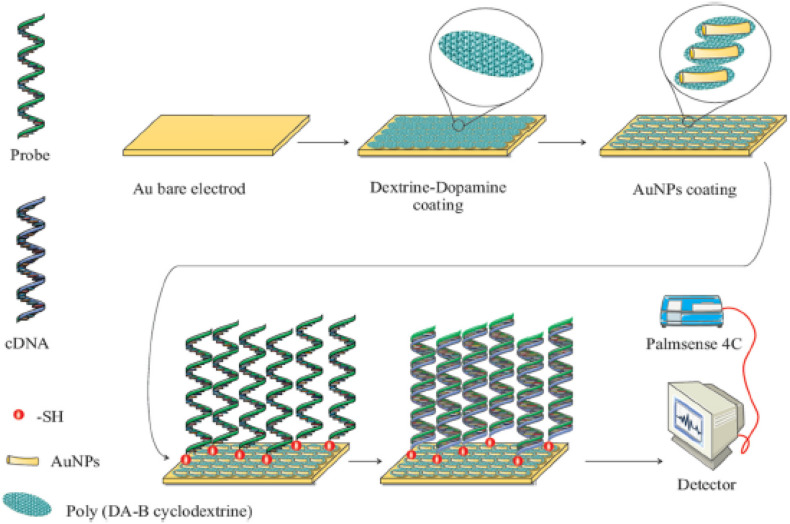
In addition to the above-mentioned biosensor, zeptomolar detection was also achieved for the detection of bacterial pathogens. Mobed et al. in 2019 designed the ultrasensitive DNA biosensor for the detection of bacterial pathogen Legionella pneumonia, which causes severe form of pneumonia in humans (Fig. 3 ) [50]. The fabrication was done by assembling a gold nano architecture (AuNPs) on the surface of poly (dopamine-β-Cyclodextrin) modified gold electrode. Further immobilization and hybridization of probe and target DNA leads to the generation of voltammetric signal using toluidine blue redox indicator. The ultrasensitive bioassay permits linear range and low limit of quantification as 1 ZM to 1 μM and 1 ZM, respectively, in Tris-HCl buffer (pH 7.4).
Fig. 3.
Schematic representation of DNA biosensor for the detection of legionella pneumonia using AuNPs/poly (dopamine-β-Cyclodextrin) modified gold electrode. Reprinted from Int. J. Biol. Macromol., 128, Mobed et al., DNA-based bioassay of legionella pneumonia pathogen using gold nanostructure: A new platform for diagnosis of legionellosis, 692–699, Copyright (2019), with permission from Elsevier, License number 4850890192113.
Researches have also been done for developing voltammetric biosensors capable for simultaneous determination, as it needs less analysis time, cost effective detection and comparatively lower sample size. Yan et al. reported a novel biosensor for the simultaneous electrochemical detection of two DNA targets related to human immune deficiency virus (HIV) and tuberculosis (TB) in 2015 [51]. The bioassay is based on quantum dots-polymer nanotracers on the surface of glassy carbon electrode and the detection was made by measuring square wave voltammetric signals from metal ions (Pb or Cd). The excellent signal amplification capacity of the polymer nanotracers caused the assay to detect target DNAs as low as 0.2 fM and exhibited dynamic concentration range from 0.5 fM to 500 pM.
An important immunosensing based bioassay for the detection of MERS-CoV, a highly pathogenic virus was first reported by Layqah et al. [52]. The novel competitive immunosensor was fabricated by electrodepositing AuNPs on carbon disposable array electrodes and immobilized with MERS-CoV antigen – 725 Spike protein S1. The single step detection was achieved by measuring the redox peak current of 5 mM ferro/ferri cyanide redox system in 0.1 M PBS (pH 7.4). The sensitive and selective detection of MERS-CoV enable linear response in the concentration range 0.001 ng/mL to 100 ng/mL and detection limit as low as 1.0 pg/mL, which is lower than the reported ELISA (enzyme linked immunosorbent assay) approach. They have also mentioned that the proposed sensing strategy can be extended in future for the multiplex detection of different CoVs. Similarly, for ZIKV detection, Faria et al. reported an electrochemical immunosensor, where bioassay is based on arranging ZnO nanostructures immobilized with ZIKV-NS1 antibody on printed circuit board [53]. The analytical responses were evaluated using CV in 10 mmolL−1 K4 [Fe(CN)6] and 0.5 molL−1 NaNO3 solution as mediator and the bioassay permits a rapid detection in the concentration range 0.1 ng/mL −100 ng/mL and limit of detection as low as1.00 pg/mL.
Though a good number of researches are going on in the field of voltammetric biosensors, the platform for miniaturization to a portable device is necessary for POC applications. In an approach to develop an immuno based voltammetric sensor device for the detection of cholera toxin, Archibald et al. in 2015 designed a vertically oriented, nanocoaxial electrodes in array format [54]. The linear range was obtained as 10 ng/mL - 1 μg/mL and the limit of detection was found to be 2 ng/mL, which is comparable to the optical ELISA approach. The fabricated sensor array proved to be an excellent platform for diagnosis of infectious cholera toxin in the POC scenario. Recently, SARS-CoV-2 or COVID-19 outbreak has emerged as a global pandemic and resulting as a serious public health issue all over the world. Mahari et al. fabricated a biosensor device for COVID-19 detection in spiked saliva samples using nCOVID-19 antibody (nCOVID-19Ab) immobilized screen printed electrode (eCovSens) and compared it with a potentiostat based sensor fabricated using FTO electrode modified with gold nanoparticles and immobilized with nCovid-19Ab [55]. Both the sensing strategies achieved a femtomolar detection and the eCovSens device offers as a stable and rapid diagnostic tool for POC applications.
The rapid and sensitive POC detection has become the need of the hour. From the conventional methods of detection of infectious diseases through RT-PCR (Reverse transcription polymerase chain reaction), which involves time consuming and cumbersome procedures, the voltammetric biosensors, be it DNA or immuno based sensors, stands out as it possess some unique advantages. Nevertheless, a study of recent literatures clearly reveals that DNA sensors are more exploited than immuno based sensors and has emerged as an effective alternate method for the conventional techniques. Some limitations like limited shelf life, temperature sensitivity and thereby denaturation of antibodies makes immunosensors a less preferred technique for on-site clinical diagnosis [44]. Labeling in DNA sensors also often lead to change in the properties of the macromolecules, which results in loss of bioactivity and affinity to the target [42,56]. Currently, the researchers are focusing on developing label free DNA sensing, where the inherent electrochemical properties of DNA like electro-oxidation of purines, are relied for signal generation. These label free DNA sensors have many advantages such as rapidness in detection, simplicity in fabrication, and least pre sample requirements. Though voltammetric immunosensors have been designed to portable device for onsite monitoring, many of the label-free DNA sensors reported are also promising approaches for the development of POC diagnostic applications.
2.2. Impedance biosensors
Electrochemists have been familiar with the Electrochemical Impedance Spectroscopic (EIS) technique for over a century [57]. The fact that EIS can be used effectively for the label free detection makes it a powerful tool in biosensing applications [4]. The occurrence of a bio-recognition process is always followed by some changes in various physical and chemical properties at the electrode electrolyte interface, and EIS technique makes use of the changes in charge transfer resistance (Rct) or interfacial capacitance to mark the biochemical changes occurring at the sensor surface [4,57].
Unlike other electrochemical methods such as cyclic voltammetry which involves large amplitude perturbations, small amplitude perturbation in EIS makes it a non-destructive technique [58]. In spite of being an ideal method for understanding dynamics of biochemical reactions, EIS technique suffers from several challenges. One among them is the sensitivity of the so developed label free biosensor [4,58]. Studies reveal that sensitivity of these sensors is lower compared to the biosensors that utilize labels. Though labelling can enhance selectivity and sensitivity, it consumes extra time, involves difficult sample handling and is also expensive [59]. Another major challenge to be addressed is whether the technique works good in real samples such as blood serum, where there is a significant amount of non-target molecules. [60]. Now a great deal of effort has been done by researchers in an attempt to improve the sensitivity by modifying electrodes with nanocomposites, conducting polymers, metal nanoparticles etc. [61,62].
In simpler terms impedance can be regarded as a resistance to the flow of current in an electrical circuit. Basically, information from impedance measurement consists of resistive and capacitive parts based on which there are faradaic and non-faradaic sensors [59]. In faradaic biosensors the electrode containing the bioreceptor is immersed in a solution of an electrochemical redox probe and electron transfer resistance of the electrode is measured whereas in the case of non-faradaic sensors it does not require a redox probe because it measures the interfacial capacitance (ie, the charge storage of the system when voltage is applied). Impedance can be represented as Nyquist (for faradaic process) and Bode plots (for capacitive or non-faradaic process) [63]. This section focuses on impedance biosensors based on faradaic process. Literatures reveal that most of the studies use (Fe(CN)6 4−/ Fe(CN)6 3−) as the redox probe for monitoring the charge transfer resistance (Rct) before and after the bio-recognition event. Other redox probes used include [Ru(NH3)6]3+/2+ and ferrocene (Fc+/Fc) [64].
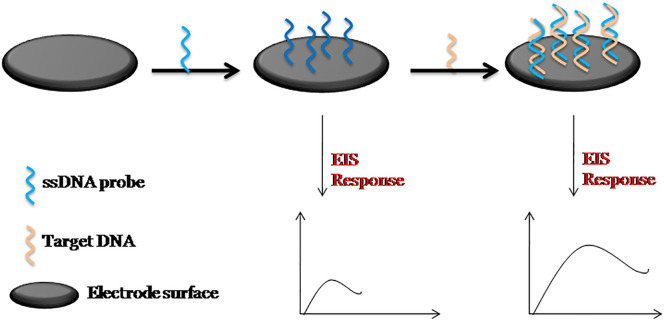
Currently active research is progressing on impedance biosensors for infectious diseases. When the bioreceptor immobilized on the electrode surface captures the target analyte, there occurs a change in the Rct value of the redox probe, which can be linked to the concentration of the target analyte. EIS technique has been found very much successful in monitoring hybridization of probe DNA with the target DNA in DNA based biosensors [[65], [66], [67]]. A general schematic illustration of the fabrication of an impedance DNA biosensor is given in Fig. 4 .
Fig. 4.
General schematic illustration of the fabrication of an impedance DNA biosensor.
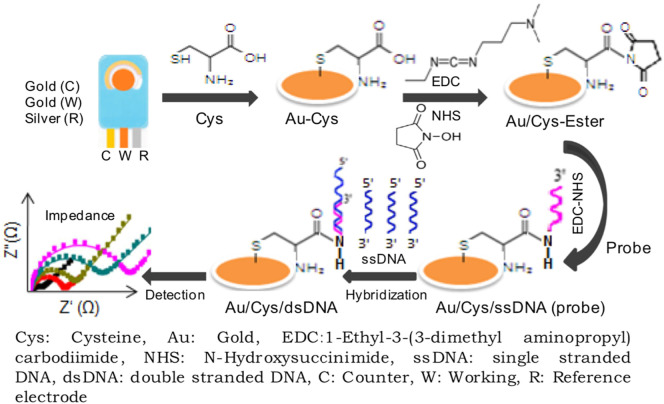
In an approach by Tamayo and co-workers, self-assembled monolayer of biotin modified 19 base-oligonucleotide probe on a gold electrode was used to detect complementary proviral sequences of HIV-1 [68]. Surface Enhanced Raman Spectroscopy (SERS) confirmed the direct linking of biotin- DNA to the gold electrode. EIS was used to monitor the hybridization of proviral sequences with the oligonucleotide probe where Rct value showed an increase upon hybridisation and is explained to be due to an increase in blocking effect at the electrode surface. Using Rct value as a measure of the blocking capacity of the monolayer to the electron transfer reactions of (Fe(CN)6 4−/ Fe(CN)6 3−) probe, the surface coverage of the biotin-DNA monolayer on the gold electrode was calculated to be 89%. Yet another impedance DNA biosensor for HIV-1 gene was proposed by Gong et al. using graphene-nafion composite film modified glassy carbon electrode as the sensing platform [69]. The sensor responded to the HIV-1 gene over a concentration range of 1.0 × 10 −13 M - 1.0 × 10 −10 M and achieved a detection limit of 2.3 × 10−14 M. The single stranded capture probe DNA was adsorbed onto the graphene-nafion modified electrode surface via п-п* stacking interactions and this led to an increased electron transfer resistance value of the (Fe(CN)6 4−/ Fe(CN)6 3−) redox couple. The authors reported that in the presence of HIV-1 target DNA, the probe DNA gets hybridized, forming the double stranded helix DNA (dsDNA) and this strong and effective binding forces the dsDNA to leave the electrode surface, which resulted in a decreased electron transfer resistance of the redox couple. In a recent work done by Ravina et al., the authors demonstrate a genosensor for early detection of swine flu (H1N1) infection in human [70]. The sensor was fabricated by immobilizing amino labelled single stranded DNA specific to the glycoprotein hemagglutinin (HA) found on the surface of H1N1 virus onto the cysteine modified screen printed gold electrode (Fig. 5 ). The hybridization of the single stranded complementary DNA (ss-cDNA) of H1N1 to the probe DNA was carried out in Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, pH 8.0) and was confirmed by EIS. They also studied the specificity of the biosensor towards H1N1 in presence of human DNA and ssDNA of other infectious pathogens. More importantly they validated the biosensor with samples from H1N1 infected patients.
Fig. 5.
Schematic representation of the fabrication of gene specific impedance biosensor for determination of swine flu (H1N1) in human. Reprinted from Int. J. Biol. Macromol., 130, Ravina et al., Hemagglutinin gene based biosensor for early detection of swine flu (H1N1) infection in human, 720–726, Copyright (2019), with permission from Elsevier, License number 4850890410830.
Most recently, a label free, impedance DNA biosensor was developed by Antonio et al. for the detection of synthetic antibody sequences of Zika virus [71]. The thiol-probe DNA was immobilized on the gold working electrode (exploiting the strong affinity of sulphur towards gold) of the planar three contact disposable electrode fabricated on a polyethylene terephthalate (PET) substrate. The sensor was able to directly detect the Zika virus sequences selectively over a concentration range of 25 nM −340 nM with a detection limit of 25 nM.
In addition to DNA biosensors, significant research has been dedicated to the field of impedance immunosensors for infectious diseases [72]. Kaushik et al. reports a more rapid, selective and sensitive micro immunosensor for Zika virus protein [73]. The immunosensor was developed using interdigitated gold array micro electrode (IDE-Au). The electrode was functionalized with self-assembled monolayer of dithiobis(succinimidyl propionate) (DTSP). The Zika virus specific envelop protein antibody were then immobilized onto this modified electrode via electrostatic interactions. The response of the immunosensor towards different concentrations of Zika virus protein was analyzed by EIS technique in 5 mM PBS (pH = 7.4) containing 5 mM Fe(II)/Fe(III) and it allowed the detection in the range of 10 pM to 1 nM with a detection limit of 10 pM. The authors compared the developed sensor and it showed better performance with those reported in literature. However the presented sensor was not tested in real samples and it questions its real life applicability. Similarly, a label free impedance immunosensor was fabricated by Darwish and co-workers for the direct detection of Non-structural protein (NS1) biomarker for dengue virus [72]. They modified the indium tin oxide (ITO) electrode surface with antifouling agents derived from aryl-diazonium cations, which was followed by the electrodeposition of gold nanoparticles and its functionalization with 1,4-phenylenediamine. The anti-NS1 IgG antibodies where then immobilized onto the modified electrode surface. The immunosensor responded to the NS1 antigen over a wide range from 5 ng/mL-4000 ng/mL. They evaluated reproducibility, selectivity and stability of the sensor and also assessed it's cross-reaction with malaria infected human sera.
Although there have been considerable research works reported in the area of biosensors for infectious diseases, the ultimate aim is miniaturization to provide POC diagnosis [74]. Sepulveda et al. designed, constructed and tried bio-microsystems based on printed circuit board platforms containing independent electro-immunosensors for early secretary antigen target-6 (ESAT-6) produced by Mycobacterium tuberculosis [75]. They fabricated three bio-microsystems with each microsystem containing 40 electro-immunosensors. The so developed electro-immunosensor consisted of polyclonal antibodies immobilized onto the gold nano layer surface by self-assembly. As a non-destructive technique, EIS was used to monitor all the stages involved and it enabled rapid detection of the disease. Indeed the work done by Soraya et al. [76] reported the development of an ultrasensitive and label free sensor for histidine-rich protein 2 produced by the malaria parasite Plasmodium falciparum (fpHRP2) and the sensor showed enough potential to be developed into a POC diagnostic device for eliminating malaria. The impedance sensor demonstrated the use of an interdigitated electrode sensor surface for immobilizing the capture probe anti- fpHRP2 monoclonal antibodies. They validated the sensor in human saliva samples for ensuring applicability in real world and it showed sensitivity as low as 25 pg/mL for the detection of fpHRP2.
Nidzworski et al. [77] developed a novel biosensor for the specific detection of M1 proteins, the universal biomarker of influenza disease, at ultralow concentrations. Boron doped diamond (BDD) electrode, due to its rapid electrochemical response, low background current and broad potential window, was used as the sensing surface. The anti-M1antibodies were immobilized onto the surface of the BDD electrode functionalized with 4-aminobenzoic acid via self assembly. In order to avoid nonspecific binding during analysis, bovine serum albumin (BSA) molecules were capped onto the open sites of the modified BDD electrode. The response of the electrode after incubating in M1 proteins was followed by EIS analysis and the sensor presented lower detection time of less than 5 min. Furthermore, this rapid, label free biosensor showed a limit of detection of 1 fg/mL and exhibited high specificity towards strains of influenza virus in presence of bacteria and yeast.
Recently, Chowdhury and Park reported another promising work [78]. They developed an ultrasensitive impedance immunosensor for hepatitis E virus (HEV) detection. Notable significance is that the sensor was able to attain sensitivity comparable to that achieved by real time quantitative reverse transcription polymerase chain reaction. The fabrication of the immunosensor consisted of modifying the glassy carbon electrode with graphene quantum dots and gold nanoparticles enclosed polyaniline nanowires (GQDs@AuNP-PAni) onto which the anti-hepatitis E antibody was easily loaded. The AuNP-PAni increases electron transfer process and also provides high surface area. They ensured the sensitive detection of HEV by applying an external pulse at the time of loading virus. The sensor exhibited good linear range of concentration from 1 fg/mL to 10 pg/mL with a detection limit of 0.8 fg/mL.
Infectious diseases have emerged as a major health problem worldwide and electrochemical biosensors have evolved as an attractive tool as it enables rapid, sensitive and selective detection. For the past few years, there has been considerable progress in research works in the area of impedance DNA biosensors and immunosensors for several EIDs and Re-EIDs. Possibility of label-free detection and thus reduction of assay complexity thereby favours miniaturization and this differentiates impedance technique from other existing methods. Compared to the fabrication of immunosensors, which use antibodies, DNA biosensors show several advantages. High purity DNAs can be synthesized and can be easily modified with functional groups such as thiols or biotin so that they can be easily captured onto the electrode surface. Moreover, they are highly stable and can be regenerated from the double stranded DNA, formed after hybridization with target DNA of the sample, by thermally melting it [79]. On the contrary, the sensitivity of DNA biosensors was found to be lesser compared to impedance immunosensors. This may be attributed to the repulsion between the redox probe Fe(CN)6 4−/ Fe(CN)6 3− and the negatively charged sugar phosphate backbone of DNA, which may significantly affect the resulting EIS signals. Moreover, the extent of interaction of the target molecule with the immobilized probe, which in turn determines the performance of the biosensor, depends on various factors such as pH and ionic strength of the reaction medium, temperature, duration of probe immobilization etc. Optimization of these parameters is of utmost importance while developing a biosensor. Instead of merely acting as a platform for sensing, the structure and configuration of the electrode plays a key role in the performance of a sensor and interdigitated microelectrodes is found to be more advantageous over conventional electrodes in miniaturization. Although several advances have been made in impedance biosensors for infectious diseases, a number of hurdles still exist and further studies need to be done before these sensors can find real-time applicability in future.
2.3. Amperometric biosensors
Literature clearly reveals that the era of electrochemical biosensors was established on amperometric and potentiometric transducer platforms [80,81]. An amperometric glucose biosensor itself was the stepping stone towards the development of cost effective, reliable, rapid and handy POC diagnostic devices, which then created a revolution in medical diagnosis [82]. The current-time response of electro-oxidation/reduction of an electroactive species, at an optimal potential is monitored in amperometry [83]. The current generated will be proportional to the concentration of the electroactive species and the excellent selectivity in detection offered by this potentiostatic technique made it the widely used one in chemical sensor development [84]. In addition, wide concentration range of detection and low-cost instrumentation of amperometric technique has made it a preferred one for sensor developers [85]. Minimization of charging current (current needed to apply potential) which affects the detection limit is also an advantage of amperometry [32]. Indeed, the blend of selectivity provided by amperometry and the specificity in binding offered by biorecognition elements have led to the successful development of numerous highly sensitive and reliable biosensors so far [86]. In amperometric biosensors, specific bioreceptor-target binding produces amperometric signal either directly or indirectly. Charge transfer from electron rich label attached to the target molecule can produce direct signals. On the other hand, indirect signalling is possible through redox processes catalysed by enzyme labels on the target molecule. Natural polymers like glycans also act as a bioreceptors that selectively react with certain proteins to give indirect amperometric signals. Conducting polymers and nanomaterials have been also incorporated in these biosensors to enhance the immobilization of the recognition element and thereby stability and sensitivity [87,88]. Simplicity in design of an amperometric detector paves possibilities for miniaturisation of these biosensors as mentioned earlier [89]. However, signal reduction due to interference from sample matrix exists as a challenge of amperometric enzyme based sensors [90]. Fig. 6 is a general schematic representation of an amperometric biosensor.
Fig. 6.
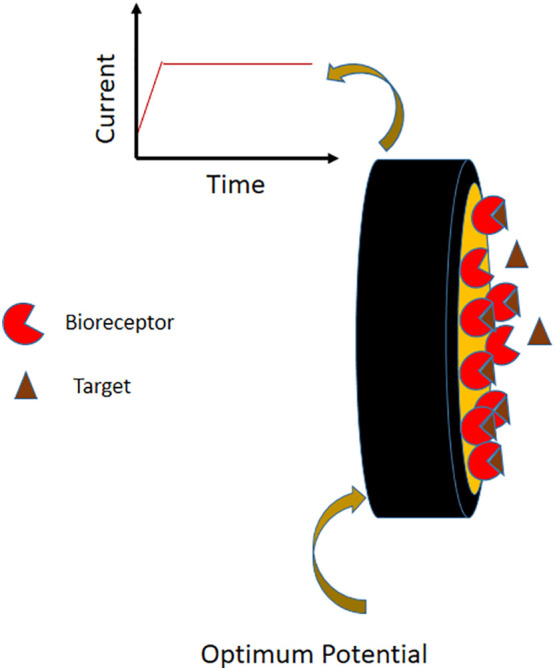
Schematic representation of an amperometric biosensor.
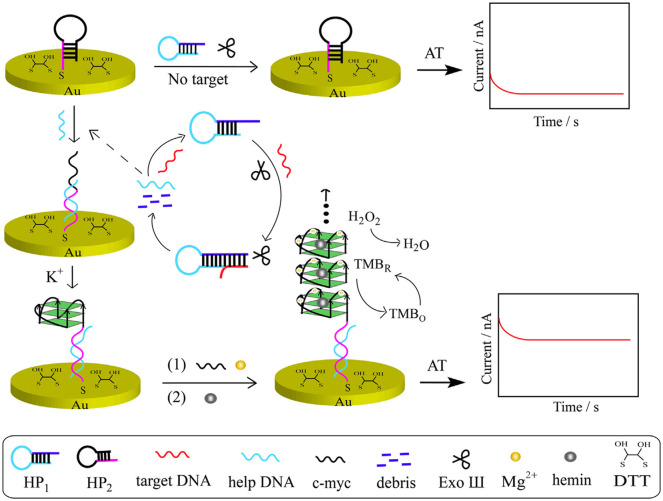
For the last few years, very staunch research has been progressing in amperometric DNA based, enzyme based and immunosensors for clinical analysis. A number of biosensors for the selective detection of biomarkers of some EIDs and Re-EIDs have become a part of the above. It is quite interesting that DNA based sensors are now in the forefront than conventional enzyme and immunosensors for EID detection, which might be due to the remarkable stability inherent to nucleic acids [91]. Recently, Chen et al. developed a dual probe amperometric biosensor which can distinguish hepatitis B virus genotypes, B and C through temperature control [92]. B and C type capture DNA probes were simultaneously immobilized on BSA based probe carrier and the current produced by an enzyme attached to the target DNA (horseradish peroxidase (HRP)) mediated reduction of H2O2 was used for detection. The BSA monolayer is capable of enhancing spatial positioning and controlling the inter probe and probe-target interactions in a better way [92]. Detection of B and C genotypes of hepatitis using this single sensing platform is possible due to the difference in the temperature of hybridization exhibited by these genotypes with their respective capture single stranded DNAs in phosphate buffer of pH 7.4. The sensor is applicable in real biological samples with a limit of detection 1.12 pM (B type) and 1.64 pM (C type). A different strategy involving an enzyme was used by Li and co-workers for the development of a DNA based biosensor for HIV gene [93]. This DNA hybridisation based label free electrochemical sensor, involves a series of processes and two hair pin probes HP1 and HP2 and only the later one is immobilized on the gold working electrode through self assembled monolayers. The enzyme exonuclease (III) induces selective digestion of duplex DNA formed by the hybridization of target ssDNA with HP1. A help DNA formed as the digestion product release a c-myc template DNA from HP2 and the guanine nanowire formed from the c-myc provides amperometric detection of HIV gene upto concentration of 3.60 pM (Fig. 7 ). All the solutions were prepared and studies were done in phosphate buffer (pH 7.4) and TECEP buffer (pH 6.5) to maintain physiological pH. Possibility of target DNA recycling and the good accuracy exhibited in complex biological samples by this biosensor clearly indicates its potential in real sample analysis [93].
Fig. 7.
Schematic representation of DNA based biosensor for HIV. Reprinted from Sensors Actuators, B Chem., 238, Huang et al., Sensitive detection of HIV gene by coupling exonuclease III-assisted target recycling and guanine nanowire amplification, 1017–1023, Copyright (2017), with permission from Elsevier, License number 4850890539754.
Recently, another DNA hybridization based amperometric sensor was reported by Bouhemadou and co-workers, for dengue virus serotype-2 [94]. In this sensor, Cu2CdSnS4 alloy nanostructure based silver coated interdigitated electrode allows more stable immobilization of the probe DNA. The carboxyl terminated probe DNA was covalently linked to the electrode using (3-Aminopropyl)triethoxysilane and its hybridization with dengue viral DNA alter the electrical property of the electrode. It is shown that there exists an inverse relationship between the amperometric signal and concentration of target DNA in the range 100 fM to 10 nM at a working voltage 1.5 V. Inspite of the rapidness and low power consumption claimed for this biosensor, lack of any mention about the medium of studies or application in real samples may affect the reliability of the sensor. In addition, Dong et al. developed a very sensitive amperometic sensor which specifically detect AIV type H7N9 using a very similar strategy [95]. Here they have utilized a tetrahedral DNA nanostructure containing the capture DNA, immobilized on a gold electrode via Au-S bond to enhance the molecular recognition (DNA hybridisation), and thereby sensitivity to H7N9 virus gene could reach 100 fM. Moreover, signal amplification was done through oxidation of 3,3′ 5,5′–tetramethylbenzidine dihydrochloride (TMB) catalysed by HRP linked to the target DNA. The practical utility of this assay is clear from its successful application in throat- swab samples containing the virus. It is notable that a good reduction in assay times (~ 1 h) can be seen in all these biosensors, when long time consumption of DNA based sensors exist as a drawback in POC analysis.
The possibilities of glycans (carbohydrate based polymers) were also employed for the detection of ID biomarkers recently. A glycan based amperometric biosensor was reported for the specific and sensitive detection of influenza viruses, by Cui et al. [96]. The glycoprotein present on the influenza viral surface can selectively release galactose from glycans. The strategy of indirect detection of virus through the detection of released galactose amperometrically via glucose strips bearing dehydrogenase was used in this assay. Successful application in human nasal swab samples and very less assay time (15 min) are the salient features of this sensor. It is clear that the rapidity and simplicity in design of this sensor enables to present it in a POC device form. In fact, the detection via glycan based sensors are limited to glycan-binding proteins and viruses [97].
Furthermore, Hiraiwa et al. developed an immunosensor for Mycobacterium tuberculosis in human sputum using a microtip microtip with performance comparable to PCR [98]. The amperometric signals were obtained by the capture of Mycobacterium tuberculosis cells spiked in sputum on anti-Mycobacterium tuberculosis functionalized microtip followed by binding of fluorescein labelled antibodies over the former. Charge transfer from electron rich fluorescein label to the electrode results in the amperometric signal, which is further, amplified through a duplicated coffee ring effect. It is shown that detection of Mycobacterium tuberculosis cells upto a concentration of 100 CFU/mL is possible with the immunoassay.
Long assay time, low target DNA levels in biological fluids (need for previous amplification of sequences) still remains as the main obstacle for miniaturisation of DNA biosensors, in spite of the ease of synthesis, high chemical stability and reusability of DNA sequences [99]. The coupling of simple glycan bioreceptor with amperometry rather than the usual impedance technique has opened facile possibilities for POC settings. It can be expected to make it more specific through the incorporation of different glycan chains. Likewise, the integration of microtip and amperometry in the immunoassay for tuberculosis widens the possibilities for on-site analysis of infectious diseases. However, in the current situation of the emergence of deadly mutated pathogens, it is expected that the research in the near future will be focussing on the excellent specificity offered by genosensing for rapid bedside testing.
2.4. Potentiometric biosensors
The working principle behind potentiometric sensors is that, the potential difference between working and reference electrodes varies directly with the concentration of the analyte under study, at zero current flow [100]. Ion selective, gas sensitive electrodes and Field Effect Transistors (FET) are the usually employed transducers in potentiometric sensors, which are selected, based on the nature of the species under study. This tuning of the working electrode can enhance the selectivity and sensitivity [101] and which in combination with other key features of potentiometric technique such as non-invasion, cost effectiveness and sensitivity have led to the development of many biosensors important in biomedical analysis [102,103]. Since fabrication of biosensors to POC devices have been the prime goal among researchers, FET based potentiometric sensors have gained very much attention recently, which might be due to FET's built-in potential for miniaturisation [104]. Ease of fabrication, rapid response and highly sensitive label-free detection by FET has also amplified the interest of researchers for this transducer platform [105]. FET is a semiconductor (transducer) based potentiometric device containing three terminals- source, drain and gate. In biologically sensitive field effect transistors, gate terminal (dielectric material) is modified with target specific bioreceptors. The surface-charge changes on the gate terminal through the binding of charged biomolecules like proteins, DNA, RNA etc. can alter the gate voltage and thereby the charge transport properties of FET channel [106]. In fact, defects like impurities of the semiconductor exist as a limitation of FET based sensors [107]. A typical schematic representation of Field effect transistor based biosensor is given in Fig. 8 .
Fig. 8.
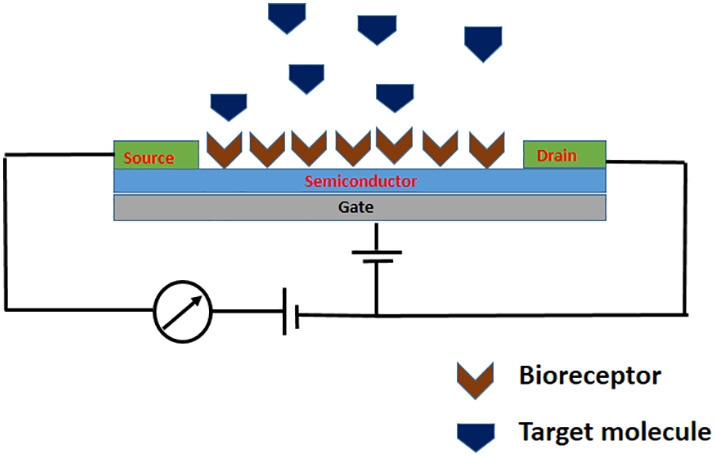
Schematic representation of Field effect transistor based biosensor.
Three efficient FET based biosensors were developed recently for infectious diseases. The most interesting and important one in the present scenario is the FET based immunosensor for the very pandemic COVID-19 causative virus developed by Seo et al. [108] Graphene based FET functionalized with highly immunogenic SARS- COV-2 spike protein specific antibodies act as the early detection platform for the Corona virus upto 1 fg/mL. The antibodies were immobilized on the graphene surface and the protein binding induced response of the FET is monitored for the SARS-COV-2 detection using PBS of pH 7.4 as the electrolyte. The successful and rapid real time application of the immunosensor in human nasopharyngeal swab specimens, SARS-COV-2 cultured cells and clinical samples make it very significant in this crucial situation. In addition, the assay responds specifically to SARS CoV-2 in presence of MERS CoV. Hideshima et al. reported a glycan based FET biosensor which can detect both human H1N1 and avian H5N1 influenza viruses in nasal mucus [109]. Rapid detection in the wide concentration range 100.5 to 108.5 TCID 50/mL was made possible by functionalizing the gate terminals with two different sialic acid containing glycans that recognize human and avian viruses respectively. Feasibility of the assay was also successfully demonstrated by connecting it to a smartphone. All the measurements were carried out at physiological pH (pH 7.4). The remarkable stability and sensitivity exhibited by glycan-immobilized FET biosensor over an antibody-immobilized (antibodies are the widely used recognition elements with FET) [110] one is also discussed. In addition, Goswami and co-workers developed an aptamer based extended gate FET biosensor for malaria biomarker, Plasmodium falciparum glutamate dehydrogenase (Pfgd) [105]. The extended gate was used for increasing the sensitivity and biocompatibility if the FET. Anti-Pfgd aptamer was immobilized on an interdigitated gold microelectrode, which was attached to gate terminal of the FET. The net charge produced on the electrode surface due to aptamer-Pfgd binding led to detection of Pfgd in human serum samples in the linear range 100 fM to 10 nM with a limit of 48.6 pM within a minimal response time (~ 5 s). The developed device has a very good potential for POC settings as well (Fig. 9 ).
Fig. 9.
Schematic representation of aptamer based FET biosensor for malaria biomarker. Reprinted from Biosens. Bioelectron. 123, Singh et al., Development of an aptamer-based field effect transistor biosensor for quantitative detection of Plasmodium falciparum glutamate dehydrogenase in serum samples, 30–35, Copyright (2019), with permission from Elsevier, License number 4850890651665.
Even though the excellent sensitivity and specificity offered by immunosensors have been utilized in bioassays, last two assays discussed above have presented viable alternatives (glycans, aptamers) for antibodies in FET based biosensing. Glycan based sensors developed so far are really rays of hope for the effective control of the pandemic influenza viruses at its source itself. But the sensing activity of glycan based assays are limited to glycan binding viruses, as discussed earlier. Since aptamers have already been proved as effective bio-recognition elements for the detection of many infectious diseases, its combination with the potential FET transducer can lead to the development of accurate, rapid and portable analysis devices for infectious diseases in future.
Table 2 provides a detailed summary of the literature discussed in the four different sub-sections.
Table 2.
A summary of selected biosensors referenced in this review for the determination of EIDs and Re-EIDs.
| Sl. No. | Transducer | Target | Biosensor format | Linear range | Detection Limit | Reference |
|---|---|---|---|---|---|---|
| 1. | Differential Pulse Voltammetry | Dengue Virus Serotype 3 | DNA sensor | – | 3.09 nM | [42] |
| 2. | Square Wave Voltammetry | Avian Influenza | DNA sensor | 1 - 10 pM | 1.39 pM | [43] |
| 3. | Differential Pulse Voltammetry | Mycobacterium Tuberculosis | DNA aptasensor | 1.00 - 1 × 105 fg/mL | 0.9 fg/mL | [44] |
| 4. | Cyclic Voltammetry | Chikungunya Virus | DNA sensor | 0.1 nM - 100 μM | 3.4 nM | [45] |
| 5. | Differential Pulse Voltammetry | Ebola Virus | DNA sensor | 10-75 nM | 4.7 nM | [46] |
| 6. | Differential Pulse Voltammetry | Influenza genes | DNA sensor | – | 21 nM | [34] |
| 7. | Square Wave Voltammetry | Zika Virus | DNA sensor | 84 pM -1.41 nM | 25.4 pM | [48] |
| 8. | Differential Pulse Voltammetry | Dengue Virus | DNA sensor | 1 ZM-1μM | 120 ZM | [49] |
| 9. | Square Wave Voltammetry | Legionella pneumophilia | DNA sensor | 1 ZM - 1 μM | 1 ZM | [50] |
| 10. | Square Wave Voltammetry | DNA related to Human Immuno Deficiency Virus and Tuberculosis | DNA sensor | 0.5 fM - 500 pM | 0.2 fM | [51] |
| 11. | Square Wave Voltammetry | Middle East Respiratory Syndrome Corona Virus | Immunosensor | 0.001 - 100 ng/mL | 1.0 pg/mL | [52] |
| 12. | Cyclic Voltammetry | Zika Virus | Immunosensor | 0.1 - 100 ng/mL | 1.00 pg/ mL | [53] |
| 13. | Square Wave Voltammetry | Cholera toxin | Immunosensor | 10mng/mL - 1 μg/mL | 1 ng/mL | [54] |
| 14 | Differential Pulse voltammetry | Severe Acute Respiratory Syndrome Corona virus | Immunosensor | 1 fm -1 μM | 90 fM | [55] |
| 15. | Impedance | Human immune deficiency virus -1 | DNA biosensor | – | – | [68] |
| 16. | Impedance | Human immune deficiency virus -1 | DNA biosensor | 1.0 × 10 -13 M - 1.0 × 10 -10 M | 2.3 × 10-14 M | [69] |
| 17. | Impedance | H1N1 | DNA biosensor | – | 6.6 ×10-4 ng/ul |
[70] |
| 18. | Impedance | Zika virus | DNA biosensor | 25-340 nM | 25 nM | [71] |
| 19. | Impedance | Zika virus | Immunosensor | 10 pM-1 nM | 10 pM | [73] |
| 20. | Impedance | Dengue virus | Immunosensor | 5- 4000 ng/mL | [72] | |
| 21. | Impedance | Mycobacterium Tuberculosis | Immunosensor | [75] | ||
| 22. | Impedance | Plasmodium Falciparum Histidine rich peotein 2 | Immunosensor | 25 pg/mL | [76] | |
| 23. | Impedance | Influenza virus | Immunosensor | 1fg/mL | [77] | |
| 24. | Impedance | Hepatitis E virus |
Immunosensor | 1 fg/mL - 10 pg/mL | 0.8 fg/mL | [78] |
| 25. | Amperometry | B/C genotyping of hepatitis B virus | DNA based sensor | 100 - 800 fM (B type) 100 – 800 fM (C type) |
1.12 fM (B type) 1.64 fM (C type) |
[92] |
| 26. | Amperometry | Human immune deficiency virus | DNA based sensor | 10 fM- 100 nM | 3.60 fM | [93] |
| 27. | Amperometry | Dengue virus | DNA based sensor | _ | 17 nM | [94] |
| 28. | Amperometry | Avian influenza virus type H7N9 | DNA based sensor | 1.0 fM – 2.5 nM | 0.75 fM | [95] |
| 29. | Amperometry | Influenza viruses | Glycan based | _ | _ | [96] |
| 30. | Amperometry | Mycobacterium Tuberculosis | Immuno sensor | 102-105 CFU/mL | 102 CFU/mL | [98] |
| 31. | Potentiometry | Plasmodium falciparum glutamate dehydrogenase (malaria biomarker) | Aptamer based FET biosensor | 100 fM – 10 nM | 48.6 nM | [105] |
| 32. | Potentiometry | Corona virus | Immuno sensor | _ | Culture medium-1.6 pfu/mL Clinical samples- 242 copies/ mL |
[108] |
| 33. | Potentiometry | Influenza virus | Glycan based FET biosensor | 100.5 - 108.5 TCID 50/mL | 100.5 TCID 50/mL | [109] |
3. Conclusions and future prospects
Shortfalls in epidemic management, disease diagnosis & healthcare facilities have had devastating impact on humans in recent memory, be it in terms of life or the economic burden on nations. Throughout this study, we have highlighted the applications of electrochemical biosensor systems in detecting EIDs and Re-EIDs in an attempt to tackle this menace.
The research discussed in this review highlight the ongoing paradigm shift in health monitoring and disease diagnosis sector, via increased application of electrochemical biosensing diagnostic systems. Despite promising research and advancements made towards electrochemical biosensors in recent years, availability of commercially viable devices in the real-world setting is far from a reality.
As discussed in the review, every modality does have pros and cons, but they can all facilitate in disease diagnosis at a POC level with immaculate planning. The glucometer is an example of the scope of electrochemical biosensors, with sound research, meticulous planning and clinical execution the future of POC detection would be along similar lines.
The electrochemical biosensors discussed have been identified as prototypes and have been evaluated under laboratory conditions only thus far. The implementation was restricted to a minimum number of actual samples that were inadequate to establish a satisfactory validation. Optimizing the stability, storage, and logistics of electrochemical biosensors, as well as maintaining their optimum functionality in diverse samples that are barely treated or diluted, are challenges to be addressed prior to developing marketable products.
Additional work into microfluidic techniques & microfabrication, cost-effective materials and electronics are required to promote the manufacture of smaller, compact and cheaper sensors. The miniaturization of electrochemical cells and deployment of more autonomous handheld readers are also manadated to improve the efficiency of POC systems, ensuring these would also tick the guidelines required for large scale production such as complete integration, automation, large-scale miniaturization, flexibility and cost reduction.
In addition, there is an imminent need to deepen collaboration between hospitals and research laboratories, in making use of funds in improving disease diagnosis and treatment, while also aiding in checking the efficacy of newly designed electrochemical biosensors with adequate numbers of real samples.
Declaration of Competing Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
Authors, Shalini Menon and Sonia Sam hereby acknowledge Council of Scientific & Industrial Research (CSIR), India, for financial aid in the form of Research Fellowship. Authors Manna Rachel Mathew and Keerthi K. would like to acknowledge the University Grants Commission (UGC), India for financial aid in the form of Research Fellowship.
References
- 1.WHO Blueprint for R & D preparedness and response to public health emergencies due to highly infectious pathogens. Work. Prioritisation Pathog. 2015:1–7. http://www.who.int/medicines/ebola-treatment/WHO-list-of-top-emerging-diseases/en/ [Google Scholar]
- 2.Baylor College of Medicine, Emerging Infectious Diseases. 2020. https://www.bcm.edu/departments/molecular-virology-and-microbiology/emerging-infections-and-biodefense/emerging-infectious-diseases
- 3.National Institutes of Health Understanding Emerging and Re-emerging Infectious Diseases. 2020. https://www.ncbi.nlm.nih.gov/books/NBK20370
- 4.Pejcic B., De Marco R., Parkinson G. The role of biosensors in the detection of emerging infectious diseases. Analyst. 2006;131:1079–1090. doi: 10.1039/b603402k. [DOI] [PubMed] [Google Scholar]
- 5.Medical Sensors Market. 2020. https://www.marketsandmarkets.com/Market-Reports/sensors-market-healthcare-applications-372.html
- 6.Hudu S.A., Alshrari A.S., Syahida A., Sekawi Z. Cell culture, technology: enhancing the culture of diagnosing human diseases. J. Clin. Diagn. Res. 2016;10:DE01–DE05. doi: 10.7860/JCDR/2016/15837.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simoska O., Stevenson K.J. Electrochemical sensors for rapid diagnosis of pathogens in real time. Analyst. 2019;144:6461–6478. doi: 10.1039/c9an01747j. [DOI] [PubMed] [Google Scholar]
- 8.Speers D.J. Clinical applications of molecular biology for infectious diseases. Clin. Biochem. Rev. 2006;27:39–51. http://www.ncbi.nlm.nih.gov/pubmed/16886046%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC1390794 [PMC free article] [PubMed] [Google Scholar]
- 9.Rodovalho V., Alves L., Castro A., Madurro J., Brito-Madurro A., Santos A. Biosensors applied to diagnosis of infectious diseases – An update. Austin J. Biosens. Bioelectron. 2015;1:1–12. http://austinpublishinggroup.com/biosensors-bioelectronics/ [Google Scholar]
- 10.Datta M., Desai D., Kumar A. Gene Specific DNA Sensors for Diagnosis of Pathogenic Infections. Indian J. Microbiol. 2017;57:139–147. doi: 10.1007/s12088-017-0650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jesny S., Menon S., Girish K. Simultaneous determination of guanine and adenine in the presence of uric acid by a poly(para toluene sulfonic acid) mediated electrochemical sensor in alkaline medium. RSC Adv. 2016;6:75741–75748. doi: 10.1039/c6ra13567f. [DOI] [Google Scholar]
- 12.Lazcka O., Del Campo F.J., Muñoz F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007;22:1205–1217. doi: 10.1016/j.bios.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Malecka K., Menon S., Palla G., Girish Kumar K., Daniels M., Dehaen W., Radecka H., Radecki J. Redox-active monolayers self-assembled on gold electrodes—effect of their structures on electrochemical parameters and DNA sensing ability. Molecules. 2020;25:1–17. doi: 10.3390/molecules25030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathew M.R., Girish Kumar K. Poly(Amino Hydroxy Naphthalene Sulphonic Acid) modified glassy carbon electrode; an effective sensing platform for the simultaneous determination of xanthine and hypoxanthine. J. Electrochem. Soc. 2020;167 doi: 10.1149/1945-7111/ab74c1. [DOI] [Google Scholar]
- 15.Menon S., Jesny S., Girish Kumar K. A voltammetric sensor for acetaminophen based on electropolymerized-molecularly imprinted poly(o-aminophenol) modified gold electrode. Talanta. 2018;179:668–675. doi: 10.1016/j.talanta.2017.11.074. [DOI] [PubMed] [Google Scholar]
- 16.Menon S., Girish Kumar K. Simultaneous voltammetric determination of acetaminophen and its fatal counterpart nimesulide by gold nano/L-cysteine modified gold electrode. J. Electrochem. Soc. 2017;164:B482–B487. doi: 10.1149/2.0181712jes. [DOI] [Google Scholar]
- 17.Leland D.S., Ginocchio C.C. Role of cell culture for virus detection in the age of technology. Clin. Microbiol. 2007;20:49–78. doi: 10.1128/CMR.00002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar P. Methods for rapid virus identification and quantification. Mater. Methods. 2013;3:207. [Google Scholar]
- 19.Gomes Z. Danielle Alves. Perspectives on Polymerase Chain Reaction. IntechOpen; 2019. PCR and Infectious Diseases. [Google Scholar]
- 20.Shieh W.J. Advanced Techniques in Diagnostic Microbiology. Springer; Cham: 2018. Advanced pathology techniques for detecting emerging infectious disease pathogens; pp. 543–561. [Google Scholar]
- 21.Powell S., Zachary C. The impact of molecular approaches to infectious disease diagnostics. MLO Med. Lab. Obs. 2015;47:18–19. [PubMed] [Google Scholar]
- 22.Huang Y., Xu J., Liu J., Wang X., Chen B. Disease-related detection with electrochemical biosensors: A review. Sensors. 2017;17:1–30. doi: 10.3390/s17102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesewski E., Johnson B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020;159:112214. doi: 10.1016/j.bios.2020.112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozer T., Geiss B.J., Henry C.S. Review—chemical and biological sensors for viral detection. J. Electrochem. Soc. 2020;167 doi: 10.1149/2.0232003jes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Dieu Habimana Jean, Ji J., Sun X. Minireview: trends in optical-based biosensors for point-of-care bacterial pathogen detection for food safety and clinical diagnostics. Anal. Lett. 2018;51:2933–2966. doi: 10.1080/00032719.2018.1458104. [DOI] [Google Scholar]
- 26.Etchegaray A. Applications and perspectives of biosensors for diagnostics in infectious diseases. Cohesive J. Microbiol. Infect. Dis. 2018;1:1–4. doi: 10.31031/cjmi.2018.01.000503. [DOI] [Google Scholar]
- 27.Peña-Bahamonde J., Nguyen H.N., Fanourakis S.K., Rodrigues D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018;16:1–17. doi: 10.1186/s12951-018-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozel T.R., Burnham-marusich A.R. crossm diseases: past, present, and future. J. Clin. Microbiol. 2017;55:2313–2320. doi: 10.1128/JCM.00476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei T.Y., Cheng C.M. Synthetic biology-based point-of-care diagnostics for infectious disease. Cell Chem. Biol. 2016;23:1056–1066. doi: 10.1016/j.chembiol.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Chesney D.J. In: Laboratory techniques in electroanalytical chemistry. second ed. Kissinger P.T., Heineman W.R., editors. CRC Press; 1996. [Google Scholar]
- 31.Gao C., Huang X.J. Voltammetric determination of mercury(II) TrAC - Trends Anal. Chem. 2013;51:1–12. doi: 10.1016/j.trac.2013.05.010. [DOI] [Google Scholar]
- 32.Ronkainen N.J., Halsall H.B., Heineman W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010;39:1747–1763. doi: 10.1039/b714449k. [DOI] [PubMed] [Google Scholar]
- 33.Luo X., Davis J.J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 2013;42:5944–5962. doi: 10.1039/c3cs60077g. [DOI] [PubMed] [Google Scholar]
- 34.Thomas A., Girish Kumar K. Electro-oxidation of Dopamine at CoNP-pAHNSA modified electrode: A sensitive approach to its determination. J. Electrochem. Soc. 2018;165:B466. [Google Scholar]
- 35.Shahriar J., Benvidi A. Comparison of two fabricated aptasensors based on modified carbon paste/oleic acid and magnetic bar carbon paste/Fe3O4@ oleic acid nanoparticle electrodes for tetracycline detection. Biosens. Bioelectron. 2016;85:553–562. doi: 10.1016/j.bios.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 36.Ali B., Tezerjani M.D., Moshtaghiun S.M., Ardakani M.M. An aptasensor for tetracycline using a glassy carbon modified with nanosheets of graphene oxide. Microchim. Acta. 2016;183:1797–1804. doi: 10.1007/s00604-016-1810-y. [DOI] [Google Scholar]
- 37.Samira Y., Banaei M., Nikukar H., Tezerjani M.D., Azimzadeh M. Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly (glutamic acid)/MWNT nanocomposite. Microchim. Acta. 2018;185:405. doi: 10.1007/s00604-018-2918-z. [DOI] [PubMed] [Google Scholar]
- 38.Ali B., Yasdanparast S., Rezaeinasab M., Tezerjani M.D., Abbasi S. Designing and fabrication of a novel sensitive electrochemical aptasensor based on poly (L-glutamic acid)/MWCNTs modified glassy carbon electrode for determination of tetracycline. J. Electroanal. Chem. 2018;808:311–320. doi: 10.1016/j.jelechem.2017.12.032. [DOI] [Google Scholar]
- 39.Ali B., Banaei M., Tezerjani M.D., Molahosseini H., Jahanbani S. Impedimetric PSA aptasensor based on the use of a glassy carbon electrode modified with titanium oxide nanoparticles and silk fibroin nanofibers. Microchim. Acta. 2018;185:50. doi: 10.1007/s00604-017-2589-1. [DOI] [PubMed] [Google Scholar]
- 40.Benvidi A., Jahanbani S. Self-assembled monolayer of SH-DNA strand on a magnetic bar carbon paste electrode modified with Fe3O4@Ag nanoparticles for detection of breast cancer mutation. J. Electroanal. Chem. 2016;768:47–54. doi: 10.1016/j.jelechem.2016.02.038. [DOI] [Google Scholar]
- 41.Benvidi A., Firouzabadi A., Tezerjani M.D., Moushtaghioun S.M., Ardakani M.M., Ansarin A. A highly sensitive and selective electrochemical DNA biosensor to diagnose breast cancer. J. Electroanal. Chem. 2015;750:57–64. doi: 10.1016/j.jelechem.2015.05.002. [DOI] [Google Scholar]
- 42.Oliveira N., Souza E., Ferreira D., Zanforlin D., Bezerra W., Borba M.A., Arruda M., Lopes K., Nascimento G., Martins D., Cordeiro M., Lima-Filho J. A sensitive and selective label-free electrochemical DNA biosensor for the detection of specific dengue virus serotype 3 sequences. Sensors. 2015;15:15562–15577. doi: 10.3390/s150715562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurzatkowska K., Sirko A., Zagórski-Ostoja W., Dehaen W., Radecka H., Radecki J. Electrochemical label-free and reagentless genosensor based on an ion barrier switch-off system for DNA sequence-specific detection of the avian influenza virus. Anal. Chem. 2015;87:9702–9709. doi: 10.1021/acs.analchem.5b01988. [DOI] [PubMed] [Google Scholar]
- 44.Thakur H., Kaur N., Sabherwal P., Sareen D., Prabhakar N. Aptamer based voltammetric biosensor for the detection of Mycobacterium tuberculosis antigen MPT64. Microchim. Acta. 2017;184:1915–1922. doi: 10.1007/s00604-017-2174-7. [DOI] [Google Scholar]
- 45.Singhal C., Khanuja M., Chaudhary N., Pundir C.S., Narang J. Detection of chikungunya virus DNA using two-dimensional MoS2 nanosheets based disposable biosensor. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-25824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ilkhani H., Farhad S. A novel electrochemical DNA biosensor for Ebola virus detection. Anal. Biochem. 2018;557:151–155. doi: 10.1016/j.ab.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Subak H., Ozkan-Ariksoysal D. Label-free electrochemical biosensor for the detection of Influenza genes and the solution of guanine-based displaying problem of DNA hybridization. Sensors Actuators B Chem. 2018;263:196–207. doi: 10.1016/j.snb.2018.02.089. [DOI] [Google Scholar]
- 48.da Fonseca Alves R., Franco D.L., Cordeiro M.T., de Oliveira E.M., Fireman Dutra R.A., Sotomayor M. Del Pilar Taboada. Novel electrochemical genosensor for Zika virus based on a poly-(3-amino-4-hydroxybenzoic acid)-modified pencil carbon graphite electrode. Sensors Actuators, B Chem. 2019;296:126681. doi: 10.1016/j.snb.2019.126681. [DOI] [Google Scholar]
- 49.Tripathy S., Krishna Vanjari S.R., Singh V., Swaminathan S., Singh S.G. Electrospun manganese (III) oxide nanofiber based electrochemical DNA-nanobiosensor for zeptomolar detection of dengue consensus primer. Biosens. Bioelectron. 2017;90:378–387. doi: 10.1016/j.bios.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Mobed A., Hasanzadeh M., Babaie P., Agazadeh M., Mokhtarzadeh A., Rezaee M.A. DNA-based bioassay of legionella pneumonia pathogen using gold nanostructure: a new platform for diagnosis of legionellosis. Int. J. Biol. Macromol. 2019;128:692–699. doi: 10.1016/j.ijbiomac.2019.01.125. [DOI] [PubMed] [Google Scholar]
- 51.Yan Z., Gan N., Zhang H., Wang D., Qiao L., Cao Y., Li T., Hu F. A sandwich-hybridization assay for simultaneous determination of HIV and tuberculosis DNA targets based on signal amplification by quantum dots-PowerVisionTM polymer coding nanotracers. Biosens. Bioelectron. 2015;71:207–213. doi: 10.1016/j.bios.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 52.Layqah L.A., Eissa S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta. 2019;186 doi: 10.1007/s00604-019-3345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faria H.A.M., Zucolotto V. Label-free electrochemical DNA biosensor for zika virus identification. Biosens. Bioelectron. 2019;131:149–155. doi: 10.1016/j.bios.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Archibald M.M., Rizal B., Connolly T., Burns M.J., Naughton M.J., Chiles T.C. A nanocoaxial-based electrochemical sensor for the detection of cholera toxin. Biosens. Bioelectron. 2015;74:406–410. doi: 10.1016/j.bios.2015.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahari S., Roberts A., Shahdeo D., Gandhi S. eCovSens-ultrasensitive novel in-house built printed circuit board based electrochemical device for rapid detection of nCovid-19. BioRxiv. 2020 doi: 10.1101/2020.04.24.059204. [DOI] [Google Scholar]
- 56.Souada M., Piro B., Reisberg S., Anquetin G., Noël V., Pham M.C. Label-free electrochemical detection of prostate-specific antigen based on nucleic acid aptamer. Biosens. Bioelectron. 2015;68:49–54. doi: 10.1016/j.bios.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 57.Chang B.-Y., Park S.-M. Electrochemical impedance spectroscopy of composite adhesive joints. Annu. Rev. Anal. Chem. 2010;3:207–229. doi: 10.1146/annurev.anchem.012809.102211. [DOI] [PubMed] [Google Scholar]
- 58.Han L., Liu P., Petrenko V.A., Liu A.H. A label-free electrochemical impedance cytosensor based on specific peptide-fused phage selected from landscape phage library. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daniels J.S., Pourmand N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis. 2007;19(12):1239–1257. doi: 10.1002/elan.200603855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manickam A., Johnson C.A., Kavusi S., Hassibi A. Interface design for CMOS-integrated Electrochemical Impedance Spectroscopy (EIS) biosensors. Sensors. 2012;12:14467–14488. doi: 10.3390/s121114467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali B., Firouzabadi A.D., Moshtaghiun S.M., Mazloum-Ardakani M., Tezerjani M.D. Ultrasensitive DNA sensor based on gold nanoparticles/reduced graphene oxide/glassy carbon electrode. Anal. Biochem. 2015;484:24–30. doi: 10.1016/j.ab.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Mazloum-Ardakani M., Rajabzadeh N., Benvidi A., Mehdi Heidari M. Sex determination based on amelogenin DNA by modified electrode with gold nanoparticle. Anal. Biochem. 2013;443:132–138. doi: 10.1016/j.ab.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 63.Sohag M.M.H., et al. Improved micro-impedance spectroscopy to determine cell barrier properties. The EuroBiotech. J. 2020;4:150–155. [Google Scholar]
- 64.Leva-Bueno J., Peyman A.S., Millner P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 2020;209:343–362. doi: 10.1007/s00430-020-00668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ali B., Tezerjani M.D., Jahanbani S., Ardakani M.M., Moshtaghioun S.M. Comparison of impedimetric detection of DNA hybridization on the various biosensors based on modified glassy carbon electrodes with PANHS and nanomaterials of RGO and MWCNTs. Talanta. 2016;147:621–627. doi: 10.1016/j.talanta.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 66.Shahriar J., Benvidi A. A novel electrochemical DNA biosensor based on a modified magnetic bar carbon paste electrode with Fe3O4NPs-reduced graphene oxide/PANHS nanocomposite. Mater. Sci. Eng. C. 2016;68:1–8. doi: 10.1016/j.msec.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 67.Ali B., Saucedo N.M., Ramnani P., Villarreal C., Mulchandani A., Tezerjani M.D., Jahanbani S. Electro-oxidized Monolayer CVD Graphene Film Transducer for Ultrasensitive Impedimetric DNA Biosensor. Electroanalysis. 2018;30:1791–1800. [Google Scholar]
- 68.Balbin A.I., López L.S., Blanco M., Armas D., Aparecido A., Ferreira P., Manzani D., Yamanaka H., Margarita A., Guas E. Biotin self-assembled monolayer for impedimetric genosensor for direct detection of HIV-1. Microchem. J. 2019;104462 doi: 10.1016/j.microc.2019.104462. [DOI] [Google Scholar]
- 69.Gong Q., Wang Y. Biosensors and Bioelectronics A Sensitive Impedimetric DNA Biosensor for the Determination of the HIV Gene Based on Graphene-Na fi on Composite Film. Vol. 89. 2017. H. Yang; pp. 565–569. [DOI] [PubMed] [Google Scholar]
- 70.Mohan H., Singh P., Kumar A. International Journal of Biological Macromolecules Hemagglutinin gene based biosensor for early detection of swine fl u (H1N1) infection in human. Int. J. Biol. Macromol. 2019;130:720–726. doi: 10.1016/j.ijbiomac.2019.02.149. [DOI] [PubMed] [Google Scholar]
- 71.Antonio H., Faria M., Zucolotto V. Biosensors and Bioelectronics Label-free electrochemical DNA biosensor for zika virus identi fi cation. Biosens. Bioelectron. 2019;131:149–155. doi: 10.1016/j.bios.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Darwish N.T., Alrawi A.H., Sekaran S.D., Alias Y., Mei S. Electrochemical Immunosensor Based on Antibody-Nanoparticle Hybrid for Specific Detection of the Dengue Virus NS1 Biomarker. J. Electrochem. Soc. 2016;163:19–25. doi: 10.1149/2.0471603jes. [DOI] [Google Scholar]
- 73.Kaushik A., Yndart A., Kumar S., Jayant R.D., Vashist A., Brown A.N., Li C., Nair M. OPEN A sensitive electrochemical immunosensor for label-free detection of Zika-virus protein. Sci. Rep. 2018:3–7. doi: 10.1038/s41598-018-28035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel S., Nanda R., Sahoo S., Mohapatra E. Biosensors in health care: The milestones achieved in their development towards lab-on-chip-analysis. Biochem. Res. Int. 2016;2016 doi: 10.1155/2016/3130469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sepulveda D., Aroca M.A., Osma J.F. Bioelectrochemical Detection of Mycobacterium tuberculosis ESAT-6 in an Antibody-Based Biomicrosystem. Sensors. 2017;17:2178. doi: 10.3390/s17102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soraya G.V., Abeyrathne C.D., Buffet C., Huy D.H., Uddin S.M., Chan J., Skafidas E., Kwan P., Rogerson S.J. Ultrasensitive and label-free biosensor for the detection of Plasmodium falciparum histidine- rich protein II in saliva. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-53852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nidzworski D., Siuzdak K., Niedziałkowski P., Bogdanowicz R., Sob M., Ryl J., Weiher P., Sawczak M., Wnuk E. A rapid-response ultrasensitive biosensor for influenza virus detection using antibody modified boron-doped diamond. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-15806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park E.Y. Electrical pulse-induced electrochemical biosensor for hepatitis E virus detection. Nat. Commun. 2019;10:4–7. doi: 10.1038/s41467-019-11644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y., Ye Z., Ying Y. New Trends in Impedimetric Biosensors for the Detection of Foodborne Pathogenic Bacteria. Sensors. 2012;12:3449–3471. doi: 10.3390/s120303449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lowe C.R. Biosensors. 1984:59–65. [Google Scholar]
- 81.Bhalla N., Jolly P., Formisano N., Estrela P. Introduction to biosensors. Essays Biochem. 2016;60:1–8. doi: 10.1042/EBC20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliver N.S., Toumazou C., Cass A.E.G., Johnston D.G. Glucose sensors: A review of current and emerging technology. Diabet. Med. 2009;26:197–210. doi: 10.1111/j.1464-5491.2008.02642.x. [DOI] [PubMed] [Google Scholar]
- 83.Moon J.M., Thapliyal N., Hussain K.K., Goyal R.N., Shim Y.B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018;102:540–552. doi: 10.1016/j.bios.2017.11.069. [DOI] [PubMed] [Google Scholar]
- 84.Malhotra B.D., Ali M.A. Nanomater. Biosensors. 2018:1–74. doi: 10.1016/b978-0-323-44923-6.00001-7. [DOI] [Google Scholar]
- 85.Amine A., Mohammadi H. Amperometry. Encycl. Anal. Sci. 2019:85–98. doi: 10.1016/B978-0-12-409547-2.14204-0. [DOI] [Google Scholar]
- 86.Morales M.A., Halpern J.M. Guide to Selecting a Biorecognition Element for Biosensors. Bioconjug. Chem. 2018;29:3231–3239. doi: 10.1021/acs.bioconjchem.8b00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fitzpatrick D. Glucose Biosensors. Implant. Electron. Med. Devices. 2015:37–51. doi: 10.1016/b978-0-12-416556-4.00004-8. [DOI] [Google Scholar]
- 88.Wu X., Hou L., Lin X., Xie Z. Elsevier Inc.; 2018. Application of Novel Nanomaterials for Chemo- and Biosensing of Algal Toxins in Shellfish and Water. [DOI] [Google Scholar]
- 89.Yashin Y.I., Yashin A.Y., Walton H.F. Ion exchange | Ion chromatography instrumentation. Encycl. Anal. Sci. 2019;5:170–177. doi: 10.1016/B978-0-12-409547-2.11498-2. [DOI] [Google Scholar]
- 90.Rocchitta G., Spanu A., Babudieri S., Latte G., Madeddu G., Galleri G., Nuvoli S., Bagella P., Demartis M.I., Fiore V., Manetti R., Serra P.A. Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sensors (Switzerland) 2016;16 doi: 10.3390/s16060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sohrabi N., Valizadeh A., Farkhani S.M., Akbarzadeh A. Basics of DNA biosensors and cancer diagnosis, Artif. Cells. Nanomed. Biotechnol. 2016;44:654–663. doi: 10.3109/21691401.2014.976707. [DOI] [PubMed] [Google Scholar]
- 92.Chen J., Liu Z., Zheng Y., Lin Z., Sun Z., Liu A., Chen W., Lin X. B/C genotyping of hepatitis B virus based on dual-probe electrochemical biosensor. J. Electroanal. Chem. 2017;785:75–79. doi: 10.1016/j.jelechem.2016.12.017. [DOI] [Google Scholar]
- 93.Huang Y.L., Gao Z.F., Luo H.Q., Li N.B. Sensitive detection of HIV gene by coupling exonuclease III-assisted target recycling and guanine nanowire amplification. Sensors Actuators B Chem. 2017;238:1017–1023. doi: 10.1016/j.snb.2016.07.144. [DOI] [Google Scholar]
- 94.Odeh A.A., Al-Douri Y., Voon C.H., Mat Ayub R., Gopinath S.C.B., Odeh R.A., Ameri M., Bouhemadou A. A needle-like Cu2CdSnS4 alloy nanostructure-based integrated electrochemical biosensor for detecting the DNA of Dengue serotype 2. Microchim. Acta. 2017;184:2211–2218. doi: 10.1007/s00604-017-2249-5. [DOI] [Google Scholar]
- 95.Dong S., Zhao R., Zhu J., Lu X., Li Y., Qiu S., Jia L., Jiao X., Song S., Fan C., Hao R.Z., Bin Song H. Electrochemical DNA biosensor based on a tetrahedral nanostructure probe for the detection of avian influenza A (H7N9) virus. ACS Appl. Mater. Interfaces. 2015;7:8834–8842. doi: 10.1021/acsami.5b01438. [DOI] [PubMed] [Google Scholar]
- 96.Cui X., Das A., Dhawane A.N., Sweeney J., Zhang X., Chivukula V., Iyer S.S. Highly specific and rapid glycan based amperometric detection of influenza viruses. Chem. Sci. 2017;8:3628–3634. doi: 10.1039/c6sc03720h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Belick S., Katrl J. Glycan and lectin biosensors. Essays Biochem. 2016;60:37–47. doi: 10.1042/EBC20150005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hiraiwa M., Kim J., Lee H., Inoue S., Becker A.L., Weigel K.M., Cangelosi G.A., Lee K., Chung J. Mycobacterium Tuberc. 2016:1–20. doi: 10.1088/0960-1317/25/5/055013.Amperometric. [DOI] [Google Scholar]
- 99.Teles F.R.R., Fonseca L.P. Talanta Trends DNA Biosensors. 2008;77:606–623. doi: 10.1016/j.talanta.2008.07.024. [DOI] [Google Scholar]
- 100.Bi H., Han X. Elsevier Inc.; 2019. Chemical sensors for environmental pollutant determination. [DOI] [Google Scholar]
- 101.Su L., Jia W., Hou C., Lei Y. Microbial biosensors: a review. Biosens. Bioelectron. 2011;26:1788–1799. doi: 10.1016/j.bios.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 102.Koncki R. Recent developments in potentiometric biosensors for biomedical analysis. Anal. Chim. Acta. 2007;599:7–15. doi: 10.1016/j.aca.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y., Chen Q., Zeng X. Potentiometric biosensor for studying hydroquinone cytotoxicity in vitro. Biosens. Bioelectron. 2010;25:1356–1362. doi: 10.1016/j.bios.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kaisti M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017;98:437–448. doi: 10.1016/j.bios.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 105.Singh N.K., Thungon P.D., Estrela P., Goswami P. Development of an aptamer-based field effect transistor biosensor for quantitative detection of Plasmodium falciparum glutamate dehydrogenase in serum samples. Biosens. Bioelectron. 2019;123:30–35. doi: 10.1016/j.bios.2018.09.085. [DOI] [PubMed] [Google Scholar]
- 106.Pachauri V., Ingebrandt S. Biologically sensitive field-effect transistors: from ISFETs to NanoFETs. Essays Biochem. 2016;60:81–90. doi: 10.1042/EBC20150009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee C.S., Kyu Kim S., Kim M. Ion-sensitive field-effect transistor for biological sensing. Sensors. 2009;9:7111–7131. doi: 10.3390/s90907111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seo G., Lee G., Kim M.J., Baek S., Choi M., Ku K.B., Lee C., Jun S., Park D., Kim H.G., Kim S., Lee J., Kim B.T., Park E.C., Il Kim S. 2020. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. [DOI] [PubMed] [Google Scholar]
- 109.Hideshima S., Hayashi H., Hinou H., Nambuya S., Kuroiwa S., Nakanishi T., Momma T., Nishimura S.I., Sakoda Y., Osaka T. Glycan-immobilized dual-channel field effect transistor biosensor for the rapid identification of pandemic influenza viral particles. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-48076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vu C.A., Chen W.Y. Predicting future prospects of aptamers in field-effect transistor biosensors. Molecules. 2020;25:680. doi: 10.3390/molecules25030680. [DOI] [PMC free article] [PubMed] [Google Scholar]