Abstract
OBJECTIVE:
The study is to investigate the diuretic and antiurolithiatic activities of ethanolic leaf extract of Annona squamosa Linn. in experimental animals.
MATERIALS AND METHODS:
For both studies, Wistar albino rats and two doses of extract (250 and 500 mg/kg) were used. Diuretic activity was evaluated by Lipschitz model. Urine volume and urine pH were noted, the concentration of sodium and potassium was estimated by flame photometry, and diuretic index, natriuretic index, and Lipschitz values were calculated from the results. Furosemide was used as a positive control. Ethylene glycol-induced urolithiasis model was used for antiurolithiatic study. Urine volume, urine pH, body weight, and biochemical parameters such as calcium, urea, uric acid, and creatine both from serum and urine were estimated. Antioxidant parameters and histopathological analysis of the kidney were evaluated. Cystone was used as a positive control in this study. Results were expressed as mean ± standard error of mean. Statistical analysis was carried out using one-way analysis of variance, followed by Dunnett's multiple comparison tests.
RESULTS:
In both diuretic and antiurolithiatic studies, both doses of the extract showed efficacy, and the dose of 500 mg/kg has shown a significant effect compared to positive control and negative control.
CONCLUSION:
The dose of 500 mg/kg showed a promising diuretic and antiurolithiatic activity.
Keywords: Antiurolithiatic, diuretic, ethylene glycol, triterpenes
Introduction
Drugs induce a state of increased urine flow called diuretics. Diuretics are used to increase the urinary output of electrolytes and water from the kidney by interfering with one or more reabsorptive processes occurring at different segments of the nephron.[1] A number of diuretics are available, and some have a side effect such as significant potassium loss or causing other mineral imbalances.
Urolithiasis is the formation of urinary stones (urinary calculi), formed or located anywhere in the urinary system.[2] It comprises nephrolithiasis (formation of kidney stones), ureterolithiasis (formation of stones in the ureters), and cystolithiasis (formation of bladder stones). The main causes of urolithiasis are mainly metabolic abnormality (40%), urinary tract abnormality (25%), urinary tract infection (10%), with the remaining being idiopathic.[3] The hot climate in some parts of Africa has also been reported.[4,5]
Currently, there is no synthetic drug against urolithiasis effectively. The treatment choices are surgical procedures, diuretics, analgesics, alkalinization of urine, and in some cases, allopurinol treatment also. Several herbal products have shown beneficiary effect against urolithiasis, and these products found less side effects and toxicity comparatively.
Here, we have selected that Annona squamosa Linn. belongs to the family Annonaceae for the evaluation of diuretic and antiurolithiatic studies. It has shown pharmacological effects such as analgesic,[6] anti-inflammatory,[6] smooth muscle relaxant,[7] nephroprotective,[8] antimicrobial,[9] and antioxidant[10] activities. Annona reticulata, family of Annonaceae, has shown its activities against urolithiasis.[11] Saponins and triterpenes found effective for diuresis and antiurolithiasis.[12] Based on these pharmacological properties and chemical constituents present in the leaf of A. squamosa, we have evaluated diuretic effects and antiurolithiatic efficacy against ethylene glycol (EG)-induced urolithiasis.
Materials and Methods
Animals
Twenty-four and thirty healthy Wistar albino rats of either sex weighing between 180 and 220 g were used for the diuretic and antiurolithiatic studies, respectively. The animals were housed in polypropylene cages and maintained under standard laboratory conditions. They were fed with standard pellet diet and water ad libitum. Animal-based experiments were approved by the Institutional Animal Ethics Committee (Proposal No: NCP/IAEC/No: 02/2014-2015).
Plant collection and extraction
Leaves of A. squamosa plants were collected in and around Erode in the month of January, and the plant was identified and authenticated by Dr. GVS Moorthy, Scientist “F” and Head of Office, Botanical Survey of India, Southern Regional Center, Tamil Nadu Agricultural University Campus, Coimbatore, with the reference number BSI/SRC/5/23/2015/Tech./1344. The crude A. squamosa leaves extract was prepared by the Soxhlet extraction. The yield of ethanolic extract of the leaf of A. squamosa Linn, i.e., EEAS (100 g), was 17.6% (w/w). Preliminary phytochemical evaluation of the extract was done for the detection of secondary metabolites. Acute toxicity studies were proved that the alcoholic leaf extracts of the same plant are safe up to 2000 mg/kg through oral route.[13] The doses were selected (250 and 500 mg/kg) based on the nephroprotective actions of the same plant reported.[8]
Drugs and chemicals
Furosemide (Cipla), Cystone (Himalaya), EG (Nice chemicals), and all diagnostic kits were purchased from Merck Millipore.
Diuretic activity
Lipschitz model
Diuretic activity was determined following the Lipschitz method.[14,15] The rats were fasted for 18 h before the experiment. The rats were grouped into four groups containing six rats in each.
Group I: Normal control received (NS, 5 ml/kg, p. o.)
Group II: Positive control received (furosemide 5 mg/kg p. o.)
Group III: Test I received (EEAS 250 mg/kg, p. o.)
Group IV: Test II received (EEAS 500 mg/kg, p. o.).
The bladder was emptied by pulling the base of tail of each rat before the experiment. Immediately, after the administration, the rats were placed in metabolic cages, one rat per cage. The metabolic cages were provided with a funnel for urine collection and a mesh to separate the feces from the urine. The urine was collected into a beaker covered with aluminum foils to avoid evaporation. The volume of urine was collected and recorded after 24 h, and urine output was calculated in relation to body weight and expressed as ml/100 g body weight. pH of urine was noted using pH meter (Elico). Urine was subjected to analysis for the determination of sodium and potassium ions by Flame photometry (Systronics) and expressed as mEq/L, and average values were taken. The diuretic index, Lipschitz value, and natriuretic activity were also calculated.
Ethylene glycol-induced urolithiasis
EG-induced hyperoxaluria model[16,17] was used to assess the antiurolithiatic activity in albino rats. Animals were weighed and divided into five groups containing six animals in each group.
Grouping of animals
Group I: Normal control received (normal drinking water)
Group II: Negative control received (0.75% v/v EG in drinking water)
Group III: Positive control received (0.75% v/v EG in drinking water and Cystone 750 mg/kg, p. o.)
Group IV: Test I received (0.75% v/v EG in drinking water and EEAS 250 mg/kg, p. o.)
Group V: Test II received (0.75% v/v EG in drinking water and EEAS 500 mg/kg, p. o.).
Group I served as normal control and received regular rat food and drinking water ad libitum. EG (0.75% v/v) in drinking water was fed to Groups II–V for the induction of renal calculi till the 28th day. Group II served as a negative control without treatment, Group III as a positive control received antiurolithiatic drug, Cystone (750 mg/kg body weight) from the 15th day till the 28th day, Group IV received EEAS (250 mg/kg body weight), and Group V received EEAS (500 mg/kg body weight) from the 15th day till the 28th day. All extracts and standard drugs were given once daily by oral route.
At the end of the study, the body weight of the animals was recorded individually, and all animals were kept in individual metabolic cages to collect 24-h urine samples. The volume of urine and pH were measured, and calcium content was estimated by diagnostic kit (Span Diagnostics Pvt. Ltd., India) in clinical semi-auto analyzer (Microlab). Various urine parameters[17] such as oxalate creatinine, urea, and uric acid were performed as per the manuals provided with various kits. Serum parameters such as creatinine, urea, uric acid, and calcium were analyzed after obtaining blood from retro-orbital veins under mild ether anesthesia. All the animals were killed by cervical dislocation, and kidneys of the animals were excised and washed with normal saline. Kidneys were homogenized (Remi) and centrifuged (Eppendorf) at 12,000. After centrifugation, clear supernatant was collected and analyzed for antioxidant parameters.
Histopathological examinations
At the completion of the experiment, animals from each group were randomly selected and sacrificed and kidneys were identified. A portion of the kidneys were excised and fixed in 10% formalin and processed for histological studies stained with hematoxylin and eosin for histological evaluation using microscopy (Leica).
Statistical analysis
Results were expressed as mean ± standard error of mean. Statistical analysis was carried out using one-way analysis of variance, followed by Dunnett's multiple comparison tests. P < 0.01 considered as statistically significant.
Results
The phytochemical analysis of the plant extract has shown the presence of carbohydrates, flavonoids, triterpenoids, tannins, phenolic compounds, and saponins.
Results of diuretic activity
The effect of ethanolic leaf extract of A. squamosa Linn. showed a significant (**P < 0.01) increase in urinary volume and urinary pH when compared with normal control, and it possesses potent diuretic activity for both the doses (250 and 500 mg/kg) [Table 1]. Hence, the tested parameters and measurement of urinary electrolyte concentrations [Table 2] resulted in a significant action in case of sodium and potassium excretion. The diuretic index and Lipschitz values [Table 1] also showed a positive effect. It was calculated for 24 h, where furosemide 5 mg/kg was used as standard.
Table 1.
Effect of ethanolic leaf extract of Annona squamosa Linn. on diuretic index and Lipchitz value
| Groups | Treatments | Urine volume (ml/100 g/24 h) | Urinary pH | Diuretic index | Lipchitz value |
|---|---|---|---|---|---|
| Group I | Normal saline 5 (ml/kg) | 8.46±0.23 | 7.13±0.13 | - | - |
| Group II | Furosemide 20 (mg/kg) | 13.21±0.21** | 8.13±0.117** | 2.46 | - |
| Group III | EEAS 250 (mg/kg) | 9.85±0.19** | 8.08±0.122** | 1.80 | 0.74 |
| Group IV | EEAS 500 (mg/kg) | 11.91±0.28** | 8.53±0.098** | 2.18 | 0.90 |
Values are mean±SEM; n=6 in each group; **P<0.01when compared to normal control (one-way ANOVA followed by Dunnett’s test). ANOVA=Analysis of variance, SEM=Standard error of mean, EEAS= Ethanolic extract of annona squamosa
Table 2.
Effect of ethanolic leaf extract of Annona squamosa Linn. on natriuretic activity and index
| Groups | Treatment | Urinary electrolyte concentration (mEq/24 h) | Natriuretic activity (Na+/K+ ratio) | Natriuretic index | |
|---|---|---|---|---|---|
| Na+ | K+ | ||||
| Group I | Normal saline 5 (ml/kg) | 79.23±0.64 | 39.2±0.313 | 2.02 | - |
| Group II | Furosemide 5 (mg/kg) | 186.06±0.867** | 85.96±0.414** | 2.26 | 1.11 |
| Group III | EEAS 250 (mg/kg) | 153.35±0.922** | 74.16±0.30** | 2.06 | 1.01 |
| Group IV | EEAS 500 (mg/kg) | 178.76±0.569** | 80.35±0.076** | 2.19 | 1.08 |
Values are mean±SEM; n=6 in each group; **P<0.01when compared to normal control (one-way ANOVA followed by Dunnett’s test). ANOVA=Analysis of variance, SEM=Standard error of mean, EEAS= Ethanolic extract of annona squamosa
The ratio of Na+/K+ is calculated for natriuretic activity values [Table 2] is >2 indicates favorable natriuretic activity. It means that ethanolic extract of A. squamosa Linn showed potassium sparing effect and slightly loop diuretic effect.
Results of antiurolithiatic activity
The toxicity of EG has been related to it's metabolic breakdown product oxalic acid, causes crystallization as calcium oxalate in the body tissues. In this experiment, when EG has given through the drinking water produce calcium oxalate stone in the kidney or urinary tract of the animals.
In urolithiasis, the decreased volume of urine output was observed in the urolithiatic group. It may be due to the obstruction of formed stones in the urinary system. The urinary output of the negative control is significantly reduced when compared to normal control group showed that lithiasis was induced successfully in the animal models. However, the treatment groups Cystone (750 mg/kg) and EEAS (250 and 500 mg/kg) showed a significant (P < 0.01) increase in the urine output or urine volume when compared to the negative control (urolithiatic control). It is believed that this is due to diuretic activity and stone-dissolving action of ethanolic leaf extract of A. squamosa Linn.
The type of stones formed can be predicted from the pH of the fasting urine. Crystalluria is pH dependent. In the present study [Table 3], a decrease in pH was observed in all the urolithiatic groups. Treatment with EEAS reversed acidic pH to slightly alkaline pH at a dose of 500 mg/kg, and this shows a significant (P < 0.01) increase in urinary pH, when compared to the urolithiatic group, whereas in the normal control group, pH of urine was observed neutral. This increase in urinary pH might be responsible for dissolving the complexes of ammonium and oxalate. Thus, some of the antiurolithiatic effects of EEAS are possibly due to its effect on urinary pH.
Table 3.
Effect of ethanolic leaf extract of Annona squamosa Linn. on urinary volume, urinary pH, and body weight in ethylene glycol-induced urolithiatic rats
| Groups | Urinary volume (ml/24 h) | Urinary pH | Body weight (g) | |
|---|---|---|---|---|
| Initial | Final | |||
| Normal control | 8.03±0.33 | 7.1±0.129 | 150.32±1.45 | 164.66±1.11 |
| Negative control (EG) | 4.625±0.202a | 5.75±0.076a | 157.58±0.76 | 150.16±1.13a |
| Positive control (Cystone 750 mg/kg) | 8.31±0.139b | 7.5±0.09b | 161.62±1.2 | 168.16±0.87b |
| Test I (EEAS 250 mg/kg) | 6.58±0.060b | 6.75±0.66b | 169.54±1.14 | 172.83±0.477b |
| Test II (EEAS 500 mg/kg) | 7.98±0.094b | 7.43±0.143b | 169.21±3.3 | 176.12±0.47b |
Values are mean±SEM; n=6 in each group; aP<0.01 when compared to normal control; bP<0.01 when compared to urolithiatic control (one-way ANOVA followed by Dunnett’s test). EG=Ethylene glycol, ANOVA=Analysis of variance, SEM=Standard error of mean, EEAS= Ethanolic extract of annona squamosa
The change in body weight of the normal and the treatment groups is found to be increased compared to the urolithiatic group [Table 3]. The loss of body weight observed is due to anorexia due to disturbance in carbohydrates, proteins, or fat metabolism which is affected by calcium oxalate imbalance in the body. The treatment with EEAS (250 and 500 mg/kg) restored the elevated body weight in the treatment groups significantly (<0.01).
Due to the presence of stones, there is an obstruction to the outflow of urine, and because of this, the glomerular filtration rate also decreases. This is also an indication which shows renal function impairment. These parameters urea, creatinine, and uric acid are restored in the treatment groups treated with EEAS as well as the Cystone-treated group significantly (P < 0.01) compared to the urolithiatic group in both serum and urine [Figures 1 and 2]. There was a hike in the concentration of calcium in the urolithiatic group (P < 0.01) compared to the normal group, in both serum and urine. The treatment shows that calcium was also restored in the treatment groups with both the doses significantly (P < 0.01) when compared to the urolithiatic group [Figures 1 and 2].
Figure 1.
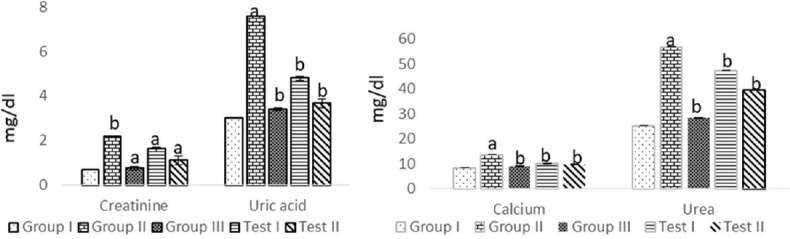
Effect of ethanolic leaf extract of Annona squamosa Linn. on serum creatinine and uric acid in ethylene glycol-induced urolithiatic rats. Values are mean ± standard error of mean; n = 6 in each group; aP < 0.01 when compared to normal control; bP < 0.01 when compared to negative control (one-way ANOVA followed by Dunnett's test)
Figure 2.
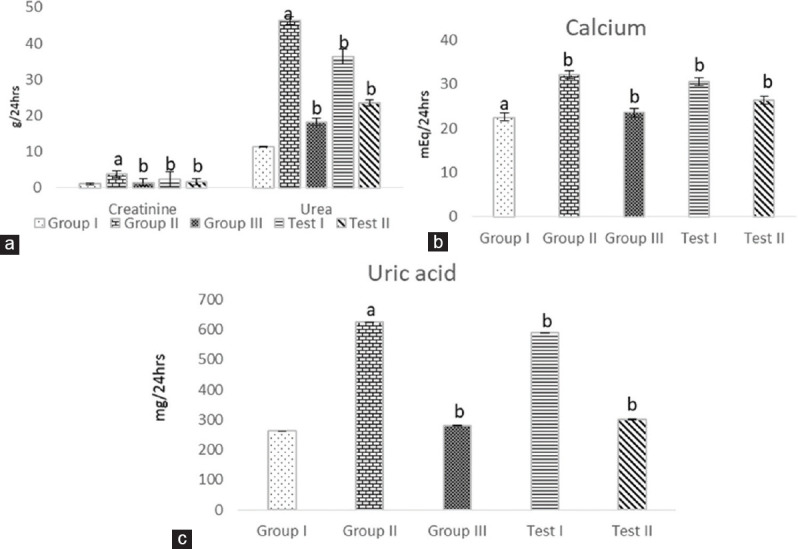
Effect of ethanolic leaf extract of Annona squamosa Linn. on urine (a) creatinine and urea, (b) calcium, and (c) uric acid in ethylene glycol-induced urolithiatic rats. Values are mean ± standard error of mean; n = 6 in each group; aP < 0.01 when compared to normal control; bP < 0.01 when compared to negative control (one-way ANOVA followed by Dunnett's test)
Antioxidant therapy with Vitamin E has prevented calcium oxalate precipitation in the rat kidney and decreased urinary oxalate excretion in patients with kidney stones. In this regard, there was a rise in lipid peroxidation and malon dialdehyde, however, super oxide dismutase (SOD), catalase (CAT) and glutathione (GSH) were decreased in the kidney homogenate in negative control in comparison to normal control [Figure 3]. However, in the groups treated with EEAS (250 and 500 mg/kg) and Cystone (750 mg/kg), it is observed that a significant attenuation in lipid peroxidation and malon dialdehyde restores SOD, CAT and GSH [Figure 3]. Thus, treatment with EEAS can inhibit complications of urolithiasis caused by oxidative stress.
Figure 3.
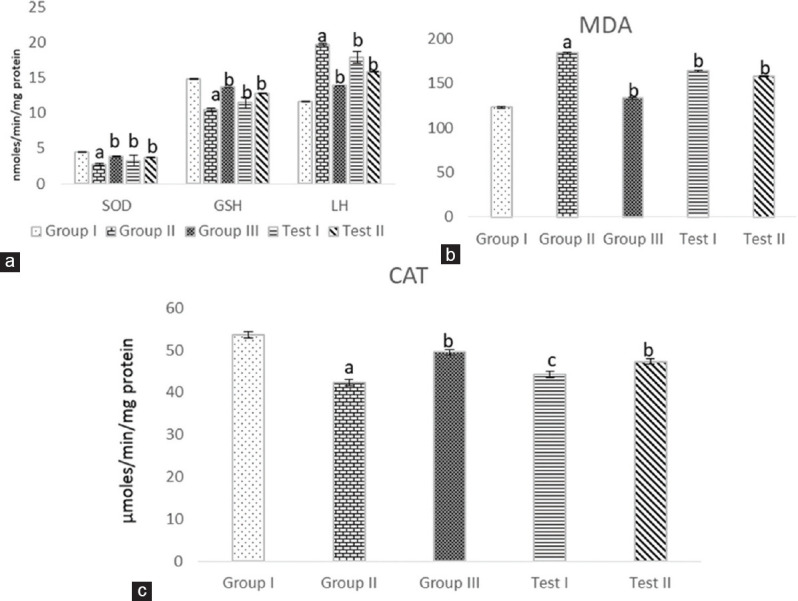
Effect of ethanolic leaf extract of Annona squamosa Linn. on kidney antioxidant parameters in ethylene glycol-induced urolithiatic rats. (a) Superoxide dismutase, glutathione and lipid hydroperoxidase. (b) Malondialdehyde. (c) Catalase
Histopathological examination [Figure 4] of normal control Group I showed normal size tubules with single epithelial lining along the margin. In the urolithiatic group, there were glomerular atrophy, infiltration of the inflammatory cells into the interstitial space, and deposition of crystals. In the positive control rats, there were marked dilatation of the tubules and total degeneration of the epithelial lining with infiltration of the inflammatory cells into the interstitial space. In Groups IV (EEAS 250 mg/kg) and V (EEAS 500 mg/kg), the specimen showed characters similar to normal control Group I rats.
Figure 4.
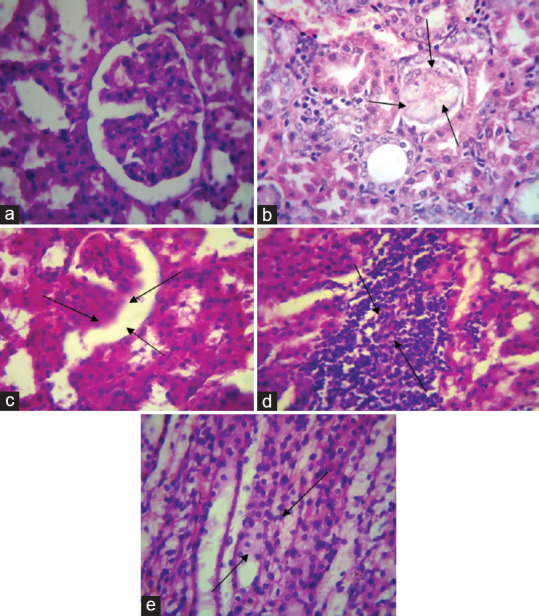
Histopathological examination of the kidney. (a) Kidney section of normal rats which shows normal morphology of kidney tissues, (b) rat kidney section treated with ethylene glycol which shows the presence of calcium oxalate crystals with mild tubular injury and lymphocytic infiltration, (c) rat kidney section of Cystone (750 mg/kg) that shows similar morphology to normal rat kidney section and mild interstitial inflammation, (d) rat kidney treated with EEAS (250 mg/kg) which shows mild tubular injury and light congestion of blood vessels, (e) rat kidney treated with EEAS (500 mg/kg) that shows normal kidney tissue morphology and mild swelling of glomeruli
Discussion
The plant A. squamosa Linn. is used traditionally and has various medicinal uses. It has shown pharmacological effects such as analgesic,[6] anti-inflammatory,[6] smooth muscle relaxant,[7] nephroprotective,[8] antimicrobial,[9] and antioxidant[10] activities. Preliminary phytochemical analysis showed that its leaf contains various phytoconstituents such as carbohydrates, flavonoids, triterpenoids, tannins, phenolic compounds, and saponins.
As emphasized in the result, diuretic properties of ethanolic leaf extract of A. squamosa Linn. could be due to other active principles such as flavonoids and saponins. It is also possible that the diuretic effect could be due to other secondary active metabolites.[18] The other possibility for the observed diuretic effect of ethanolic leaf extract of A. squamosa Linn. could be due to indirect changes of some physiological parameters before blood filtration step.[18,19]
There is a possibility that diuretic effect may be produced by stimulation of regional blood flow or initial vasodilatation, or by producing inhibition of tubular reabsorption of water and anions. The result in both cases being diuresis[20] and the plant has shown vasorelaxant activity in vivo in earlier studies.[7]
Naturally occurring triterpenes of plant origin have been identified as possessing a wide range of pharmacological effects. Among this, the most effective pharmacological action is antiurolithiatic activity. Triterpenes are found efficient in minimizing crystal-induced renal peroxidative changes measured in terms of MDA and subsequent tissue damage. The antioxidant status, comprising the enzymatic and nonenzymatic components, was found to be significantly depleted in the kidney and bladder of stone-forming animals. However, triterpenes have the ability to restore antioxidant enzymes such as SOD, CAT, and glutathione peroxidase. The mechanism by which the triterpenoids render protection against oxalate-induced toxic manifestations and free radical production may involve the inhibition of calcium oxalate crystal aggregation and enhancement of the body defense systems.[12]
Because of these pharmacological actions, ethanolic extract of A. squamosa Linn. exhibits almost the same result compared to the clinical management of urolithiasis. Analgesic and anti-inflammatory activities of A. squamosa can reduce the pain and inflammation of the urinary tract. Smooth muscle relaxant activity of A. squamosa reduces urinary tract muscle contraction, nephroprotective action of the plant can restore the elevated serum and urine parameters and restoration of impaired renal function and cellular damage, antimicrobial action can inhibit further infections of urinary system, and antioxidant activity restored the elevated antioxidant parameters and MDA and lipid hydroperoxidase. And also, the diuretic activity of the plant may hasten the process of dissolving or by flushing of the preformed stones.
The mechanism involved in observed activity profile may be improving the renal antioxidant status and cell membrane integrity, inhibition of crystal nucleation, aggregation, and growth, by increasing urine volume, pH, and anticalcifying activity and regulation of oxalate metabolism.
Conclusion
The present study “Evaluation of diuretic efficacy and antiurolithiatic potential of ethanolic leaf extract of Annona squamosa Linn. in experimental animal models” was found promising in both diuretic and antiurolithiatic activities. This study strongly suggests that ethanolic leaf extract of A. squamosa Linn. can be used to treat in renal insufficiency and other related disorders as diuretic and effective against urinary stones or urinary calculi associated renal damage and altered biochemical parameters. The present research study reveals that the ethanolic leaf extract of A. squamosa Linn. has a potent role in relieving renal calculi and its therapy will be more efficacious and beneficial than surgical interference.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful for the management of Nandha College of Pharmacy for providing the facilities and also extend our regards to staffs in the Department of Pharmaceutical analysis, Nandha College of Pharmacy, for helping in flame photometry analysis.
References
- 1.Tripathy KD. Essentials of Medical Pharmacology. 6th ed. New Delhi: Jaypee Brother's Medical Publishers (P) Ltd; 2010. p. 561. [Google Scholar]
- 2.Pearle MS, Calhoun EA, Curhan GC. Urolithiasis. In: Litwin MS, Saigal CS, editors. Urologic Diseases in America (NIH Publication No 07–5512) 8. Bethesda, Maryland: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. pp. 283–319. [Google Scholar]
- 3.Sellaturay S, Fry C. The metabolic basis for urolithiasis. Surgery (Oxford) 2008;26:136–40. [Google Scholar]
- 4.Duvie SO, Endeley EM, Dahinya MA. Urolithiasis in Maiduguri. The Nigerian Savannah belt experience. West Afr J Med. 1988;7:148–56. [Google Scholar]
- 5.Jones TW, Henderson TR. Urinary calculi in children in Western Australia: 1972-86. Aust Paediatr J. 1989;25:93–5. [PubMed] [Google Scholar]
- 6.Manvi FV, Nanjawade BK, Shing S. Pharmacological screening of combined extract of Annona squamosa and Nigella sativa. Int J Pharm Bio Sci. 2011;2:520–9. [Google Scholar]
- 7.Morita H, Iizuka T, Choo CY, Chan KL, Takeya K, Kobayashi J. Vasorelaxant activity of cyclic peptide, cyclosquamosin B, from Annona squamosa. Bioorg Med Chem Lett. 2006;16:4609–11. doi: 10.1016/j.bmcl.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Pandit SB, Pagar HJ, Patel TR, Darwade AP, Jadhav SA. In vivo study of Nephroprotective potential of Shilajit by using Cisplatin induced nephrotoxicity model. Asian J Pharm Pharmacol. 2018;45:680–5. [Google Scholar]
- 9.Vijayalakshmi R, Nithiya T. Antimicrobial activity of fruit extract of Annona squamosa l. World J Res Pharm Pharm Sci. 2015;4:1257–67. [Google Scholar]
- 10.Mariod AA, Abdelwahab SI, Elkheir S, Ahmed YM, Fauzi PN, Chuen CS. Antioxidant activity of different parts from Annona squamosa, and Catunaregam nilotica methanolic extract. Acta Sci Pol Technol Aliment. 2012;11:249–58. [PubMed] [Google Scholar]
- 11.Pujar KK, Akki S, Kulkarni PV, Kulkarni MV. Synthesis, anti-inflammatory and analgesic activity of oxime-ethers from benzaldoxime and 4-bromomethyl coumarins. J Adv Pharm Educ Res. 2011;1:12–44. [Google Scholar]
- 12.Malini MM, Lenin M, Varalakshmi P. Protective effect of triterpenes on calcium oxalate crystal-induced peroxidative changes in experimental urolithiasis. Pharmacol Res. 2000;41:413–8. doi: 10.1006/phrs.1999.0601. [DOI] [PubMed] [Google Scholar]
- 13.Onwusonye JC, Uwakwe A, Patrick A, Iwuanyanwu KC. Acute and sub-acute toxicity studies of methanol leaf extracts of Annona squamosa Linn. In mice. Sky J Biochem Res. 2014;3:053–9. [Google Scholar]
- 14.Lipschitz WL, Haddian Z, Kerscar A. Bioassay of diuretics. J Pharmacol Exp Ther. 9:97–110. 194. [Google Scholar]
- 15.Jeffery GH, Basett J, Mendham J, Denny RC. Vogels Text of Quantitative Chemical Analysis. 5th ed. England: Addison Wesley Longmann Ltd; 1989. [Google Scholar]
- 16.Anbu J, Suman S, Swaroop KS, Kumar Slvvsn SK, Kannadhasan R. Antiurolithiatic activity of ethyl acetate root extract of Ichnocarpus frutescens using ethylene glycol induced method in rats. J Pharm Sci Res. 2011;3:1182–9. [Google Scholar]
- 17.Ratnam KV, Ravishankar K, Priyabhandavi P. Evaluation of in vitro antioxidant activity of ethanolic root extract of curculigoorchioides. Int J Res Pharm Chem. 2013;3:364–9. [Google Scholar]
- 18.Agunu A, Abdurahman EM, Andrew GO, Muhammed Z. Diuretic activity of the stem-bark extracts of Steganotaenia araliacea hochst [Apiaceae] J Ethnopharmacol. 2005;96:471–5. doi: 10.1016/j.jep.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Stanic G, Samaržija I. Diuretic activity of Satureja montana subsp. Montana extracts and oil in rats. Phytother Res. 1993;7:363–6. [Google Scholar]
- 20.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–11. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]


