Abstract
Background:
Although lithium is known to cause thyroid dysfunction and increased thyroid gland volume, clinical examination and biochemical assessment are fundamental to thyroid workup of patients on lithium treatment. We aimed to determine thyroid gland volume and the Thyroid hormone levels of patients who have been receiving lithium treatment for affective disorders in comparison to voluntary healthy controls.
Methods:
This was a cross-sectional, hospital-based observational study which was performed in 43 patients on long-term lithium treatment for bipolar disorder, major depressive and schizoaffective disorders. Patients with documented continuous and adequate serum lithium levels for more than or equal to 6 months recruited consecutively underwent the ultrasonographic examination of the thyroid gland. Ultrasonographic examinations were also done in all gender- and age-matched healthy controls. All cases and controls underwent biochemical thyroid function tests.
Results:
There were no statistically significant differences in gender (P = 0.198; Chi-square = 1.654) of cases and controls. Most of the cases were married, maximum number of them unemployed and belonged to the lower socioeconomic status. Total thyroid volume was significantly greater in the lithium-treated group than the controls (9.40 ± 1.41 vs. 4.79 ± 0.45). Clinical inspection and palpation only detected goiter in six (n = 6, 13.95%) of patients on lithium and none among controls. The mean triiodothyronine, mean thyroxine, and mean scores for thyroid-stimulating hormone were significantly increased in patients receiving lithium therapy as compared to controls.
Conclusion:
It would seem wise from a clinical point of view to include ultrasonographic examination of the thyroid gland as part of the standard thyroid workup before initiating lithium treatment.
Keywords: Lithium, thyroid function test, thyroid gland ultrasonography
INTRODUCTION
Lithium an effective agent for treatment in acute mania, prophylaxis of bipolar disorder, prophylactic treatment of unipolar recurrent depression, anti-suicidal and treat resistant major depressive episodes was discovered in 1817. Lithium after its discovery was used for the treatment of gout. Patients on lithium usually require to take the drug on a long-term basis; therefore, it has long-term side effects, which include thyroid function abnormalities, renal insufficiency, persistent tremor, and dermatological effects.[1]
Treatment with lithium frequently causes hypothyroidism; however, it may also lead to hyperthyroidism on rare occasions.[2] Lithium causes hypothyroidism by affecting various aspects of thyroid gland function. The lithium-ion is concentrated in the thyroid gland three to four times greater than in plasma where it interferes with various steps in the production of thyroid hormones.[3]
It inhibits iodine uptake and is thought to alter thyroglobulin (Tg) structure, interfering with the coupling of iodotyrosine residues to form iodothyronines. It also inhibits thyroid hormone secretion, an effect which may be explained by interference with polymerization of tubulin.[4] Lithium, a well-known goitrogen, is known to induce goiter in 5.6%–60% of lithium-treated patients.[2] This difference in prevalence can be explained by different iodine content in the different geographical zones and the diagnostic tools used in these studies.[5]
Thyroid morphology, including volume in patients on lithium is usually monitored by thyroid ultrasonography as it has a definite role in the clinical settings.[6] Ultrasonographic abnormalities could be attributed to inhibition of release of thyroid hormones in the course of treatment with lithium. Female gender, weight gain, preexisting autoantibodies, higher lithium levels, rapid cycling, starting lithium at a later age are the main risk factors.[7,8,9]
The lithium-induced goiter is usually smooth and nontender. With regard to ultrasonic scan abnormalities apart from increased volume, a study by Bocchetta et al. in their cohort of 6-year of lithium treatment, found that up to 97% of women and 69% of men without evidence of thyroid circulating antibodies showed reduced echogenicity, nonhomogeneous echo pattern, and/or presence of nodules.[10]
If lithium therapy has to be continued during pregnancy, thyroid ultrasonography being safe could be carried out in the neonate besides standard thyroid function tests to check for neonatal goiter and hypothyroidism.[11] Lithium induced influence on the hypothalamic-pituitary-thyroid (HPT) axis has also been observed.[2,3]
It has been proposed that lithium acts by inhibition of cAMP activity, which results in decreased levels of serum triiodothyronine (T3) and thyroxine (T4). As a result of feedback inhibition on the pituitary, thyroid-stimulating hormone (TSH) hypersecretion occurs.[12] This increase in TSH concentration results in thyroid enlargement and goiter.[3]
Lithium-induced hypothyroidism and goiter may take weeks to years after it is started.[2,3] Studies have shown that the prevalence rates of lithium-induced hypothyroidism range from 3.4% to 52%, with a female-to-male ratio of approximately 5:1.[8,13,14,15] The clinical presentation of hypothyroidism is similar to that of the patient's not on lithium and the biochemical changes are identical to those in primary hypothyroidism.[3] It has recently been reported that there is a significant inverse relationship between mean serum free T4 level and morbidity in mentally ill patients, a low level being associated with more frequent affective episodes and greater severity of episode of depression.[16]
Our aim of conducting this study was to investigate the detailed sociodemographic profile of patients on lithium therapy, thyroid hormone levels, and thyroid gland volumes in comparison with healthy voluntary controls.
MATERIAL AND METHODS
Subjects
Forty-three consecutive lithium-treated patients of affective disorders of either gender, aged between 18 and 65 years, diagnosed according to the Diagnostic Criteria of International Classification of Diseases-10-Diagnostic Criteria for Research (WHO, 1992)[17] from both in and outpatient unit of Institute of mental health and neurosciences Kashmir (North-India) were taken. It was a cross-sectional, hospital-based study, in which patients were selected through nonrandomized and nonstratified sampling techniques. Forty-three age- and sex-matched healthy voluntary controls were selected from nonblood relatives of patients. Informed written consent was obtained before inclusion in the study and the subjects were explained in detail about the nature and purpose of the study. Clearance from the institute ethics committee was taken (IEC/PSY/IMHANS-K No. 20/2019). The controls were of either gender, aged between 18 and 65 years, had no current psychiatric diagnosis, scored <2 on General Health Questionnaire, were receiving no psychotropic medication and had no alcohol or substance dependence disorder, other than nicotine. The exclusion criteria for cases were:
Extremes of the age of <18 years and >65 years
Current history of substance abuse, psychosis or eating disorder, malignancies, body mass index of higher than 30 and clinical thyrotoxicosis
Persons having a known history of surgical thyroidectomy.
Assessment
All the patients who were taken for the study had used lithium carbonate for at least 6 months.[18] The treatment adherence of the patients was assured by the recommended serum lithium levels between ≥0.6 and 1.2 mmol/L during follow-up visits.
About 3–5 ml of venous blood was collected and centrifuged to separate serum from the cells as soon as the clot was formed. Serum aliquots were stored at 4°C to be run in batches. The samples were allowed to thaw before assay, mixed thoroughly. Hemolyzed and lipemic samples were rejected. Bi-level, i.e., high and low control was run with each batch. Serum was analyzed for TSH, total T4, and total T3 by electro-chemiluminescence immunoassay method using a fully automatic analyzer ECLIA 2010 (Roche Diagnostic Germany). Normal reference range was set up as T3 = 72–184 μg/dl, T4 = 5–13 μg/dl, and TSH = 0.5–4 μ-IU/l.
Clinical inspection and palpation of the thyroid gland were done in every participant by consultant physician. Further, all the participants underwent a detailed ultrasonographic examination of the thyroid gland. Blood samples for thyroid profile and ultrasonographic examination of the thyroid gland were done on the same day to save the financial burden on the participants. The findings of the thyroid volumes of both cases and controls were done and cross-checked by two consultant radiologists who were unaware of the lithium exposure and the serum lithium levels of understudied participants.
The maximal superior-inferior, horizontal, and ventrodorsal dimensions of each lobe were measured; by multiplying these values (cm) with each other and then by 0.479, we obtained the volume (cm3) of each thyroid lobe. The sum of the volumes of the two lobes provided the volume of the entire thyroid gland.[19] Ultrasonography, unlike other imaging techniques, does not have radiation exposure and is low in cost, fast, and relatively accurate.[20,21]
During the sonographic examinations of the thyroid gland, high-resolution color Doppler ultrasonography (USG) units (GE-P8 Refurbed 4D Ultrasound Machines CA, USA) equipped with VFX9-4 and VFX13-5 multiple (1.5-dimensional) linear-array transducers were used. The frequency bandwidths of these transducers ranged 4–9 and 5–13 MHz, respectively. In addition to these high-resolution transducers, the USG scanners were equipped with new-generation spatial compound imaging and tissue harmonic imaging technologies that are known to increase lesion conspicuity.
Statistical analysis
Descriptive statistics were used for sociodemographic and clinical variables. For detecting group differences between variables, independent samples t-test and Pearson's Chi-square test were used for continuous and categorical variables, respectively. The SPSS software version 21.0 (SPSS version 21.0 software the Statistical Package for the Social Sciences; IBM Software, NY, USA) was used for the analysis of data. A confidence level of 95%, i.e., P < 0.05 was considered statistically significant.
RESULTS
The study sample consisted of 43 patients on lithium in comparison to 43 age- and gender-matched healthy voluntary controls. Majority of the patients were males 28 (65.11%) in the lithium group as well as in the control group 29 (67.44%). There were no statistically significant differences in gender (P = 0.198; Chi-square = 1.654). The mean age of patients was 30.39 ± 2.68 years in the lithium group and 30.44 ± 2.62 years in the control group. Most of the patients were married, maximum number of them unemployed and belonged to the lower socioeconomic status [Table 1]. The clinical variables of lithium-treated patients are shown in [Table 2]. Twenty-three (53.48%) patients were receiving lithium monotherapy; 7 (16.27%) patients were using lithium and lamotrigine, 6 (13.95%) patients were using lithium and clozapine, 5 (11.63%) patients were using lithium and olanzapine, and 2 (4.65%) patients were using lithium, olanzapine, and lamotrigine. The mean duration of illness was 6.44 ± 2.32 years and mean duration of treatment was 6.33 ± 2.37 years, as shown in Table 2. The average dose of lithium was 780 ± 214.6 mg/day (600–1200 mg).
Table 1.
Comparison of Sociodemographic profiles between patients on lithium and healthy controls
| Variable | Groups | χ2 | df | P | |
|---|---|---|---|---|---|
| Lithium (n=43), n (%) | Controls (n=43), n (%) | ||||
| Gender | |||||
| Male | 28 (65.11) | 29 (67.44) | 1.654 | 1 | 0.198 |
| Female | 15 (34.88) | 14 (32.56) | |||
| Marital status | |||||
| Single | 4 (9.30) | 9 (20.93) | 2.252 | 1 | 0.133 |
| Married | 39 (90.70) | 34 (79.07) | |||
| Occupation | |||||
| Unemployed | 39 (90.70) | 30 (69.77) | 1.911 | 1 | 0.167 |
| Employed | 4 (9.30) | 13 (30.23) | |||
| Socioeconomic status | |||||
| Lower | 32 (74.42) | 11 (25.58) | 2.536 | 4 | 0.638 |
| Middle | 8 (18.60) | 29 (67.44) | |||
| Higher | 3 (6.98) | 3 (6.98) | |||
| Residence | |||||
| Urban | 36 (83.72) | 30 (69.77) | 0.632 | 1 | 0.427 |
| Rural | 7 (16.28) | 13 (30.23) | |||
| Thyroid palpation | |||||
| Palpable | 6 (13.95) | 0 | ………. | ………… | …………. |
Table 2.
Clinical variables of the lithium-treated patients and healthy controls
| Clinical variable | n | Mean (years)±SD |
|---|---|---|
| Age (cases) | 43 | 30.39±2.68 |
| Age (controls) | 43 | 30.44±2.62 |
| Duration of illness | 43 | 6.44±2.32 |
| Age of onset | 43 | 23.88±2.38 |
| Duration of treatment | 43 | 6.33±2.37 |
SD: Standard deviation
Thyroid was palpable only in 6 (13.95%) patients in the lithium group and not palpable in any of the participants in the control group [Table 2].
Heterogeneous parenchymal appearance with nodules was seen in two of the patients on lithium treatment and none in the control group.
Statistically significant higher mean volumes (5.68 ± 1.03) of the right lobe, left lobe (3.72 ± 0.53), and total thyroid volume (9.40 ± 1.41) were observed in lithium group subjects in comparison to mean right, left, and total thyroid volume in normal voluntary controls (3.30 ± 0.424, 1.49 ± 0.28, and 4.79 ± 0.45), respectively [Table 3].
Table 3.
Comparison of thyroid volumes between mood disorder subjects on lithium and healthy controls
| Variable | Mean±SD | t | df | P | |
|---|---|---|---|---|---|
| Lithium group (n=43) | Control group (n=43) | ||||
| R_L_Vol (ml) | 5.68±1.03 | 3.30±0.424 | 14.678 | 42 | 0.001 |
| L_L_Vol (ml) | 3.72±0.53 | 1.49±0.28 | 21.52 | 42 | 0.001 |
| Total _Vol (ml) | 9.40±1.41 | 4.79±0.45 | 19.16 | 42 | 0.001 |
SD: Standard deviation
Significantly higher mean scores for TSH (microgram-IU/ml) were observed in the lithium group (5.378 ± 3.88) in comparison to the normal control group (2.88 ± 1.59), and the difference was statistically significant. Similarly, statistically significant differences were also observed in mean serum T4 (5.85 ± 1.71) of cases and controls (7.39 ± 0.921) [Table 4]. Biochemical thyroid abnormalities have been significantly high in females in comparison to males. Similarly, significantly high mean scores for the right lobe and total volume of thyroid gland have been observed in females as compared to males [Table 5 and Figures 1 and 2].
Table 4.
Comparison of thyroid profiles between mood disorder subjects on lithium and healthy controls
| Variable | Lithium group | Control group | t | df | P |
|---|---|---|---|---|---|
| T3 (microgram/dl) | 134.77±23.88 | 98.89±16.59 | 9.172 | 42 | 0.001 |
| T4 (microgram/dl) | 5.85±1.71 | 7.39±0.921 | 5.211 | 42 | 0.001 |
| TSH (microgram-iu/ml) | 5.378±3.88 | 2.88±1.59 | 3.65 | 42 | 0.001 |
TSH: Thyroid stimulating hormone
Table 5.
Comparison of thyroid volumes and thyroid profiles (gender-wise) in mood disorder subjects
| Variable | Gender | n | Mean±SD | t | P |
|---|---|---|---|---|---|
| R_L_Vol (ml) | Male | 28 | 5.3942±0.851 | 0.012 | |
| Female | 15 | 6.20±1.145 | 2.618 | ||
| L_L_Vol (ml) | Male | 28 | 3.66±0.56 | 0.958 | 0.344 |
| Female | 15 | 3.8279±0.468 | |||
| Total _Vol (ml) | Male | 28 | 9.05±1.290 | 2.261 | 0.029 |
| Female | 15 | 10.03±1.45 | |||
| T3 (microgram/dl) | Male | 28 | 134.0±25.45 | 0 0.776 | |
| Female | 15 | 136.21±21.40 | 0.286 | ||
| T4 (microgram/dl) | Male | 28 | 6.46±1.70 | 3.637 | |
| Female | 15 | 4.70±0.994 | 0.001 | ||
| TSH (microgram-iu/ml) | Male | 28 | 3.88±3.20 | 4.008 | 0.001 |
| Female | 15 | 8.16±3.57 |
SD: Standard deviation, TSH: Thyroid stimulating hormone
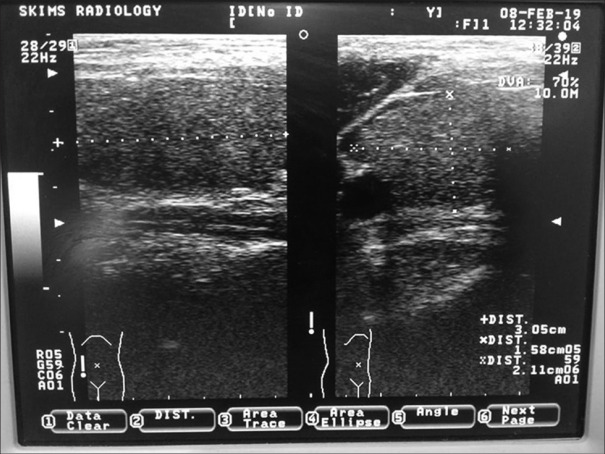
Figure 1.
Ultrasonographic picture of thyroid gland of a patient on lithium showing normal echo texture
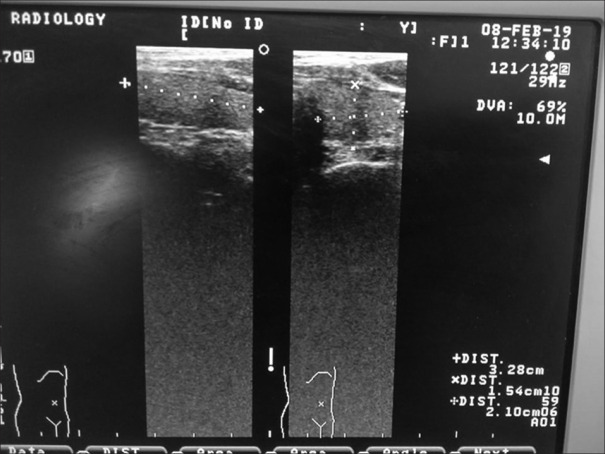
Figure 2.
Ultrasonographic picture showing dimensions of thyroid gland of a patient on lithium
DISCUSSION
To our knowledge, this is the first study from Kashmir (J and K) and second from India in which USG was used to determine thyroid volume and morphology in lithium-treated patients in comparison to age- and gender-matched healthy volunteers. Male predominance in our study can be possibly explained by the stigma associated with the mental illnesses and societal shame and rejection in females who infrequently visit government setup though the results were statistically insignificant. Majority of the cases and controls were from urban area which can be explained by the geographical location of our institute.
Although examination of the thyroid gland by clinical inspection and palpation is vital to any physical examination of patients on lithium treatment, the results of findings varies widely depending on the examiner.[22] Ultrasonographic measurement of thyroid volume has therefore been performed in patients on lithium and found to be very sensitive.[23] Unlike other imaging techniques, USG does not involve any radiation exposure and is low in cost, fast, and relatively accurate.[21]
In our study, ultra-sonographically measured thyroid volume was found significantly increased in lithium-treated patients in comparison to voluntary healthy controls. The volumes of the right, the left thyroid lobe and the overall thyroid volumes were significantly higher in the lithium-treated than among normal controls. Several mechanisms have been proposed to explain the development of increase thyroid volume like lithium is concentrated by the thyroid and inhibits thyroidal iodine uptake.[24]
The most important clinically relevant action purposed is the inhibition of thyroid hormone release. This may result in the development of goiter and hypothyroidism. Independent effects on the HPT axis and the receptor-mediated mechanism of thyroid hormone action may contribute. The immunological influence of lithium on thyroid antibody concentrations leading to a more rapid onset of thyroid autoimmunity resulting in goiter and hypothyroidism and possibly also a state of hyperthyroidism in some cases has been hypothesized.[23] It has been proposed that mechanism of the proliferation of thyrocytes in patients treated with lithium is activation of tyrosine kinase by lithium-ion and lithium effects on intracellular signaling connected with adenylate cyclase and that of Wnt/beta-catenin.[23]
Our study results are in unison with the only Indian study by Alam, et al. were it was found that lithium treatment is associated with increased individual mean thyroid lobe volume and overall mean thyroid volumes.[25]
In 1990, the assessment made by the Danish Research Group of 100 patients with BD showed that goiter occurs in 44% of patients treated for 1–5 years and in 50% of those treated for more than 10 years, compared to 16% in the control group.[26,27]
Besides anti-thyroid peroxidase (TPO) and anti-Tg antibodies, autoimmune thyroid disease could be detected by USG as well. Since there was no identifiable autoimmune thyroid disease detected by ultrasound in the lithium-treated group and the control group, therefore this parameter would not interference between autoimmune thyroid disease and thyroid volume.
In our study, significantly increased mean T3, decreased mean T4, and increased mean TSH values were observed in the lithium group as compared to control group and the trend was towards hypothyroidism.
It is important to note that discontinuation of lithium is not required when lithium-induced subclinical or clinical hypothyroidism supervenes. Lithium if indicated should be continued along with thyroid replacement therapy. Supplementation with T4 was done in 4 of the studied patients and none in the control group to maintain them in euthyroid state. The common side effects of lithium treatment include overt hypothyroidism in 8%–10% and subclinical hypothyroidism in 20%–25% of patients[23] assumed to results from lithium interfering in the synthesis and release of thyroid hormones from the thyroid gland.[23]
A study found substantial number of patients on long-term lithium therapy receiving additional treatment with levothyroxine.[28] Hypothyroidism is described as commonly known effect of lithium use as has been quoted by other studies.[5,29]
However our study results are in contradiction to study done by Alam, et al. where hyperthyroidism was the most common finding. Hyperthyroidism associated with long-term lithium use has also been reported by many other authors.[5,30] However, the most common reason cited by all these authors was the chronic use of lithium.
In this study, higher biochemical thyroid dysfunction was found in females in comparison to males. Similarly, higher mean volumes of the thyroid gland were found in women in comparison to men. Our study results are in concordance with some earlier studies. Özerdem et al.[31] showed a higher frequency of TSH abnormalities in patients with bipolar disorders, with lithium-induced thyroid dysregulation occurring more frequently in female patients. Kirov et al.[32] also found a higher frequency of lithium induced thyroid dysfunction (mostly hypothyroidism) in females than in male patients hence results match our study.
Strengths of the study
As it is known fact that valproic acid, carbamazepine, escitalopram, beta-blockers, and quetiapine treatment can lead to hypothyroidism patients using these medications were excluded
Measurement of thyroid gland volume was done by two consultant radiologists who were unaware of lithium exposure.
Limitation
Because impairment of the HPT axis stemming from the illness may influence the changes observed in the thyroid state and thus influence results
This was a cross-sectional study conducted at a random point in time during long-term lithium treatment
Small sample size, night-shifting, infection, and smoking status could be confounding factors
Kashmir region with heavy rainfall or snowfall is particularly likely to be iodine-deficient as the superficial layer of soil is washed away may be confounding factor
We could not eliminate the possibility that patients had undiagnosed thyroid disorders before lithium treatment onset because their prelithium baseline values were not known
Nonavailability of TPO and Tg antibodies may be considered among the limitations of the study.
CONCLUSION
The results of our study confirm a greater susceptibility of female patients for disturbances in thyroid hormone levels and thyroid morphologies during lithium therapy with increased volume and features of hypothyroidism. It would seem wise from a clinical point of view to include ultrasonographic examination of thyroid volume as part of the standard thyroid workup before initiating lithium treatment.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Sayar Ahmad Talay and Dr. Iqbal Hussain Dar consultant radiologists for valuable support. I would also like to thank Dr. Umar Khurshid consultant physician for his valuable support and inputs.
REFERENCES
- 1.McInnis MG. Lithium for bipolar disorder: Are emerging treatment for mood instability: Low dosage, slow titration might mitigate side effects. Curr Psychiatry. 2014;13:38–45. [Google Scholar]
- 2.Livingstone C, Rampes H. Lithium: A review of its metabolic adverse effects. J Psychopharmacol. 2006;20:347–55. doi: 10.1177/0269881105057515. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus JH. Lithium and thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23:723–33. doi: 10.1016/j.beem.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Burrow GN, Burke WR, Himmelhoch JM, Spencer RP, Hershman JM. Effect of lithium on thyroid function. J Clin Endocrinol Metab. 1971;32:647–52. doi: 10.1210/jcem-32-5-647. [DOI] [PubMed] [Google Scholar]
- 5.Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: A current perspective. Thyroid Res. 2013;6:3. doi: 10.1186/1756-6614-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiemann U, Hengst K. Thyroid echogenicity in manic-depressive patients receiving lithium therapy. J Affect Disord. 2002;70:85–90. doi: 10.1016/s0165-0327(00)00374-8. [DOI] [PubMed] [Google Scholar]
- 7.Vincent A, Baruch P, Vincent P. Early onset of lithium-associated hypothyroidism. J Psychiatry Neurosci. 1993;18:74–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Kirov G. Thyroid disorders in lithium-treated patients. J Affect Disord. 1998;50:33–40. doi: 10.1016/s0165-0327(98)00028-7. [DOI] [PubMed] [Google Scholar]
- 9.Johnston AM, Eagles JM. Lithium-associated clinical hypothyroidism. Prevalence and risk factors. Br J Psychiatry. 1999;175:336–9. doi: 10.1192/bjp.175.4.336. [DOI] [PubMed] [Google Scholar]
- 10.Bocchetta A, Cherchi A, Loviselli A, Mossa P, Velluzzi F, Derai R, et al. Six-year follow-up of thyroid function during lithium treatment. Acta Psychiatr Scand. 1996;94:45–8. doi: 10.1111/j.1600-0447.1996.tb09823.x. [DOI] [PubMed] [Google Scholar]
- 11.Frassetto F, Tourneur Martel F, Barjhoux CE, Villier C, Bot BL, Vincent F. Goiter in a newborn exposed to lithium in utero. Ann Pharmacother. 2002;36:1745–8. doi: 10.1345/aph.1C123. [DOI] [PubMed] [Google Scholar]
- 12.Kusalic M, Engelsmann F. Effect of lithium maintenance therapy on thyroid and parathyroid function. J Psychiatry Neurosci. 1999;24:227–33. [PMC free article] [PubMed] [Google Scholar]
- 13.Gracious BL, Findling RL, Seman C, Youngstrom EA, Demeter CA, Calabrese JR. Elevated thyrotropin in bipolar youths prescribed both lithium and divalproex sodium. J Am Acad Child Adolesc Psychiatry. 2004;43:215–20. doi: 10.1097/00004583-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Aliasgharpour M, Abbassi M, Shafaroodi H, Razi F. Subclinical hypothyroidism in lithium-treated psychiatric patients in Tehran, Islamic republic of Iran. East Mediterr Health J. 2005;11:329–33. [PubMed] [Google Scholar]
- 15.Fagiolini A, Kupfer DJ, Scott J, Swartz HA, Cook D, Novick DM, et al. Hypothyroidism in patients with bipolar I disorder treated primarily with lithium. Epidemiol Psichiatr Soc. 2006;15:123–7. doi: 10.1017/s1121189x00004322. [DOI] [PubMed] [Google Scholar]
- 16.Frye MA, Denicoff KD, Bryan AL, Smith-Jackson EE, Ali SO, Luckenbaugh D, et al. Association between lower serum free T4 and greater mood instability and depression in lithium-maintained bipolar patients. Am J Psychiatry. 1999;156:1909–14. doi: 10.1176/ajp.156.12.1909. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 18.Sofuogğlu S, Tutusş A, Gönül AS, Basştürk M, Köse K, Esşel E. Thyroidal hormone profile in bipolar patients who were in short and long-term lithium treatment. Eur Neuropsychopharmacol. 1997;1002:S140–1. [Google Scholar]
- 19.Brunn J, Block U, Ruf G, Bos I, Kunze WP, Scriba PC. Volumetric analysis of thyroid lobes by real-time ultrasound (author's transl) Dtsch Med Wochenschr. 1981;106:1338–40. doi: 10.1055/s-2008-1070506. [DOI] [PubMed] [Google Scholar]
- 20.Knudsen N, Bols B, Bülow I, Jørgensen T, Perrild H, Ovesen L, et al. Validation of ultrasonography of the thyroid gland for epidemiological purposes. Thyroid. 1999;9:1069–74. doi: 10.1089/thy.1999.9.1069. [DOI] [PubMed] [Google Scholar]
- 21.Braverman LE, Cooper D. Werner and Ingbar's the thyroid: A fundamental and clinical text. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2012. [Google Scholar]
- 22.Jarløv AE, Nygaard B, Hegedüs L, Hartling SG, Hansen JM. Observer variation in the clinical and laboratory evaluation of patients with thyroid dysfunction and goiter. Thyroid. 1998;8:393–8. doi: 10.1089/thy.1998.8.393. [DOI] [PubMed] [Google Scholar]
- 23.Lazarus JH, Kirov G, Harris BB. Lithium in Neuropsychiatry. United States: CRC, Division of Taylor and Francis, Press; 2006. Effect of lithium on the thyroid and endocrine glands; pp. 279–90. [Google Scholar]
- 24.Lazarus JH. Pharmacotherapeutics of the Thyroid Gland. Berlin, Heidelberg: Springer; 1997. Effect of lithium on the thyroid gland; pp. 207–23. [Google Scholar]
- 25.Alam SA, Sinha VK, Nizamie H. Ultrasonographically measured change in thyroid status in lithium treated adult patients with mood disorder. Indian J Psychol Med. 2016;38:120–6. doi: 10.4103/0253-7176.178774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrild H, Hegedüs L, Baastrup PC, Kayser L, Kastberg S. Thyroid function and ultrasonically determined thyroid size in patients receiving long-term lithium treatment. Am J Psychiatry. 1990;147:1518–21. doi: 10.1176/ajp.147.11.1518. [DOI] [PubMed] [Google Scholar]
- 27.Ozsoy S, Mavili E, Aydin M, Turan T, Esel E. Ultrasonically determined thyroid volume and thyroid functions in lithium-naïve and lithium-treated patients with bipolar disorder: A cross-sectional and longitudinal study. Hum Psychopharmacol. 2010;25:174–8. doi: 10.1002/hup.1093. [DOI] [PubMed] [Google Scholar]
- 28.Shulman KI, Sykora K, Gill SS, Mamdani M, Anderson G, Marras C, et al. New thyroxine treatment in older adults beginning lithium therapy: Implications for clinical practice. Am J Geriatr Psychiatry. 2005;13:299–304. doi: 10.1176/appi.ajgp.13.4.299. [DOI] [PubMed] [Google Scholar]
- 29.Bauer M, Blumentritt H, Finke R, Schlattmann P, Adli M, Baethge C, et al. Using ultrasonography to determine thyroid size and prevalence of goiter in lithium-treated patients with affective disorders. J Affect Disord. 2007;104:45–51. doi: 10.1016/j.jad.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Siyam FF, Deshmukh S, Garcia-Touza M. Lithium-associated hyperthyroidism. Hosp Pract (1995) 2013;41:101–4. doi: 10.3810/hp.2013.08.1073. [DOI] [PubMed] [Google Scholar]
- 31.Özerdem A, Tunca Z, Çımrın D, Hıdıroǧlu C, Ergör G. Female vulnerability for thyroid function abnormality in bipolar disorder: Role of lithium treatment. Bipolar Disord. 2014;16:72–82. doi: 10.1111/bdi.12163. [DOI] [PubMed] [Google Scholar]
- 32.Kirov G, Tredget J, John R, Owen MJ, Lazarus JH. A cross-sectional and a prospective study of thyroid disorders in lithium-treated patients. J Affect Disord. 2005;87:313–7. doi: 10.1016/j.jad.2005.03.010. [DOI] [PubMed] [Google Scholar]