Abstract
Introduction
Interstitial pneumonia (IP) is one of the most common and poor prognostic comorbidities in patients with NSCLC and a known risk factor for pneumonitis. Atezolizumab monotherapy is an established treatment for recurrent NSCLC and reported to have a lower risk of pneumonitis than programmed cell death protein 1 inhibitors. This study aimed to assess the safety and efficacy of atezolizumab monotherapy in patients with pretreated advanced or recurrent NSCLC with idiopathic IP.
Methods
Patients with advanced or recurrent NSCLC with comorbid idiopathic, chronic fibrotic IP with % forced vital capacity of greater than 70% and no history of immune checkpoint inhibitors were enrolled. The patients received atezolizumab (1200 mg) every 3 weeks until the discontinuation criteria were met. The primary end point of this study was the 1-year survival rate. A sample size of 38 patients was set.
Results
This study was terminated early owing to high incidence of pneumonitis. A total of 17 patients were enrolled, with a median age of 70 years. The median % forced vital capacity and % diffusing capacity for carbon monoxide at baseline were 85.4% and 54.4%, respectively. The incidence of pneumonitis was 29.4% (5 of 17) for all grades, 23.5% (4 of 17) for grade greater than or equal to 3, and 5.9% (1 of 17) for grade 5. A total of 57.1% patients (4 of 7) with honeycomb lung developed pneumonitis with a grade greater than or equal to 3, whereas only one patient (10%) without honeycomb lung (n = 10) with grade 1 pneumonitis was found.
Conclusions
Patients with NSCLC with comorbid IP as defined by the selection criteria for this study might have an increased risk of immune checkpoint inhibitor–induced pneumonitis.
Keywords: Atezolizumab, Non–small cell lung cancer, Interstitial pneumonia, Pneumonitis
Introduction
Interstitial pneumonia (IP) is one of the most common comorbidities with poor prognosis in patients with NSCLC, with a prevalence of approximately 10%.1 It is also a known risk factor for pneumonitis.2 , 3 Recently, two small single-arm trials of nivolumab, an anti-programmed cell death protein 1 antibody, revealed safe and promising efficacy in patients with pretreated NSCLC with mild idiopathic IP, with an incidence of pneumonitis of 0% (n = 6)4 and 11.1% (n = 18).5 On the basis of these results, nivolumab was occasionally administered to patients with NSCLC with IP in Japanese general practice.
Atezolizumab, an anti-programmed cell death-ligand 1 antibody, is an established treatment for recurrent NSCLC.6 In addition, the incidence of pneumonitis was reported to be lower than that in previous reports of anti-programmed cell death protein 1 antibody and other cytotoxic agents.7 Therefore, some have proposed that atezolizumab might be a safer option for second-line therapy among various immune checkpoint inhibitors (ICIs).
In this regard, we launched a multicenter, single-arm phase 2 trial to assess the safety and efficacy of atezolizumab for patients with pretreated advanced or recurrent NSCLC with comorbid idiopathic IP.8 However, this study was terminated early owing to the high incidence of pneumonitis with a Common Terminology Criteria for Adverse Events (CTCAE) grade greater than or equal to 3. We have evaluated that the information regarding the risks from this trial should be promptly made available to physicians using similar treatments in general practice and to physicians planning similar studies.
Materials and Methods
Study Population
Patients who met the eligibility criteria (Supplementary Table 1) from 22 institutes in Japan were enrolled. The eligibility criteria of IP comprises the following four items: (1) high-resolution computed tomography (HRCT) result revealing reticular shadow with basal and peripheral predominance suggestive of usual IP pattern or peribronchovascular shadow suggestive of nonspecific IP pattern; (2) without known cause (e.g., infection, pneumoconiosis, drug, sarcoidosis, and collagen vascular disease); (3) % forced vital capacity (%FVC) greater than or equal to 70%; and (4) % diffusing capacity for carbon monoxide (%DLco) greater than or equal to 35%. Ordinarily, histopathologic examination and multidisciplinary discussion are recommended for the definitive diagnosis of IP.9 However, considering the fact that the patients in this study were in a situation in which treatment of advanced or recurrent NSCLC with poor prognosis was preferred over IP and that few hospitals were able to perform histopathologic examination and multidisciplinary discussion of IP satisfactorily, the diagnosis and pattern classification of IP in this study were based on the HRCT findings. Moreover, according to the postmarketing surveillance of pirfenidone and the subgroup analysis of a randomized phase 3 trial of nintedanib in patients with idiopathic pulmonary fibrosis, there is a particularly high risk of acute exacerbation in patients with a baseline %FVC of less than 70%.10 , 11 On the basis of these results, %FVC was set at greater than or equal to 70% to ensure a certain level of safety in this study.
Other key inclusion criteria include the following: (1) histologically or cytologically proven NSCLC; (2) unresectable stage III/IV or recurrent NSCLC; (3) had received chemotherapy previously, including platinum doublet; (4) age greater than or equal to 20 years; and (5) Eastern Cooperative Oncology Group performance status of 0 to 1. The key exclusion criteria include the following: (1) history of acute exacerbation of preexisting IP; (2) treatment history with an ICI; (3) systemic treatment with steroids at a daily dose of greater than 10 mg of prednisolone or an equivalent immunosuppressant; (4) active autoimmune disease or history of autoimmune disease requiring treatment; (5) symptomatic brain metastasis or spinal cord metastases; and (6) treatment history with thoracic radiotherapy.
Intervention
The patients received atezolizumab (1200 mg for a day) every 3 weeks until the discontinuation criteria were met. The discontinuation criteria included the following: (1) disease progression; (2) occurrence of acute exacerbation of IP; and (3) occurrence of unacceptable immune-related adverse events, including pneumonitis, hepatotoxicity, hepatitis, nervous system disorder, renal disorder, eye disorder, myocarditis with a CTCAE grade greater than or equal to 3, colitis, diarrhea, pancreatitis, panhypopituitarism, skin disorder with a CTCAE grade greater than or equal to 2, encephalitis, meningitis, Guillain-Barre syndrome, and myasthenia gravis with a CTCAE grade greater than or equal to 1.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and the Clinical Trials Act of Japan. The Niigata University Certified Review Board of Clinical Research approved this protocol on July 23, 2019 (approval number, SP19005). This clinical trial was registered in the Japan Registry of Clinical Trials on August 26, 2019 (registry number, jRCTs031190084). All patients provided their written informed consent. In addition, consent for publication was obtained from all patients.
Evaluation of Pneumonitis
Adverse events were recorded under the CTCAE, version 5.0. In this study pneumonitis is defined as follows: (1) appearance of new shadows in the lung field after initiation of atezolizumab and (2) identification that it is not an infection, heart failure/hypertransfusion, or an exacerbation of carcinomatous lymphangitis by the investigator. A central review committee adjudicated all investigator-reported pneumonitis events. Moreover, baseline HRCTs were collected and imaging findings of IP reviewed in detail by the central review committee for post hoc risk factor analysis of pneumonitis.
Statistical Analysis
The primary end point was the 1-year survival rate. We set an expected value of 40% and a threshold value of 15%. Taking statistical points (two-sided α = 0.05; 1-β = 0.9) and ineligible patients into consideration, the sample size was set at 38 calculated by the exact binomial test.
Results
Patient Population and Disposition
The registration began on September 2, 2019. At the time of enrollment, three of the 15 patients enrolled (20%) developed grade 3 pneumonitis, so the new patient enrollment was interrupted on January 31, 2020. Two patients, for whom consent had already been obtained, were reintroduced, and a total of 17 patients were eventually registered. Subsequently, one patient who had pneumonitis worsened from grade 3 to grade 5, and one new patient developed grade 3 pneumonitis. Therefore, this study was terminated per the recommendation of the efficacy and safety evaluation committee.
For the purposes of these reports focusing on adverse events, the data cutoff date was set to April 8, 2020. The median follow-up time was 2.95 months (95% confidence interval [CI]: 1.42–4.49). The 17 patients enrolled in the trial who had received greater than or equal to one cycle of treatment were categorized as the safety analysis population (Fig. 1 ). In addition, we defined the full analysis set (efficacy analysis population) as 16 patients, excluding one patient in whom the eligibility criteria were violated.
Figure 1.
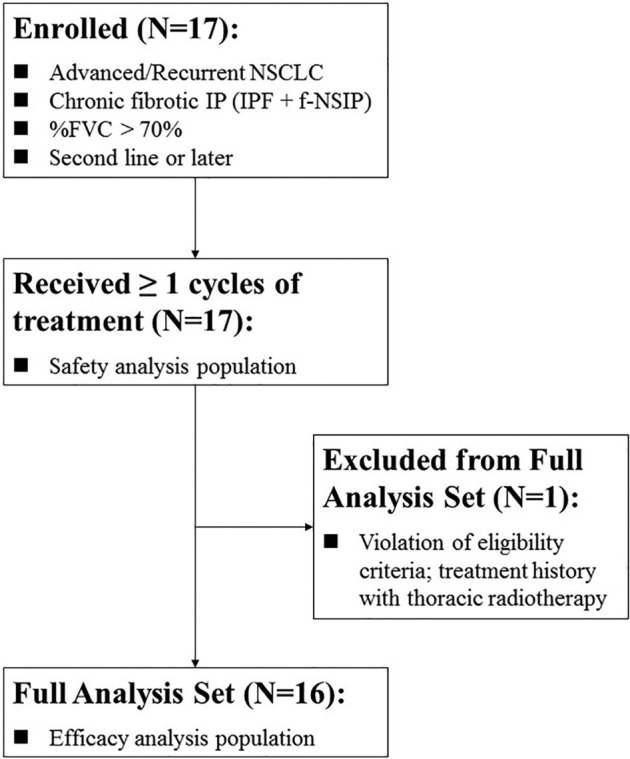
Patient disposition. f-NSIP, fibrotic nonspecific IP; %FVC, % forced vital capacity; IP, interstitial pneumonia; IPF, idiopathic pulmonary fibrosis.
Characteristics
The characteristics of the 17 enrolled patients are summarized in Table 1 and Supplementary Table 2. The median age was 70.0 years, and 16 patients (94.1%) were male. The median %FVC and %DLco at baseline were 85.4% and 54.4%, respectively.
Table 1.
Characteristics
| Characteristics | Overall Population (N = 17) | Without Pneumonitis (N = 12) | With Pneumonitis (N = 5) | p Value |
|---|---|---|---|---|
| Backgrounds | ||||
| Median age | 70.0 (66.0–73.0) | 69.0 (65.5–73.5) | 70.0 (68.0–73.0) | 0.634 |
| Sex | 1.000 | |||
| Male | 16 (94.1) | 11 (91.7) | 5 (100) | |
| Female | 1 (5.9) | 1 (8.3) | 0 | |
| Smoking history | — | |||
| Current | 3 (17.6) | 2 (16.7) | 1 (20.0) | |
| Past | 14 (82.4) | 10 (83.3) | 4 (80.0) | |
| Never | 0 | 0 | 0 | |
| Height (cm) | 167 (164–170) | 167 (165–170) | 164 (163–168) | 0.506 |
| Body weight (kg) | 66.1 (58.4–74.0) | 69.8 (65.9– 76.3) | 58.4 (56.2– 62.1) | 0.049 |
| Performance status (0/1) | 1.000 | |||
| 0 | 4 (23.5) | 3 (25.0) | 1 (20.0) | |
| 1 | 13 (76.5) | 9 (75.0) | 4 (80.0) | |
| SpO2 (%) | 96.0 (95.0–98.0) | 97.0 (95.8–98.0) | 96.0 (95.0– 96.0) | 0.422 |
| Lung cancer | ||||
| Histologic type | — | |||
| Adenocarcinoma | 9 (52.9) | 6 (50.0) | 3 (60.0) | |
| Squamous cell carcinoma | 7 (41.2) | 5 () | 2 (40.0) | |
| Not otherwise specified | 1 (5.9) | 1 (8.3) | 0 | |
| Staging | — | |||
| IIIA | 2 (11.8) | 2 (16.7) | 0 | |
| IIIB | 4 (23.5) | 3 (25.0) | 1 (20.0) | |
| IIIC | 2 (11.8) | 1 (8.3) | 1 (20.0) | |
| IVA | 3 (17.6) | 2 (16.7) | 1 (20.0) | |
| IVB | 3 (17.6) | 1 (8.3) | 2 (40.0) | |
| Recurrence | 3 (17.6) | 3 (25.0) | 0 | |
| PD-L1 expressiona [TPS (%)] | — | |||
| <1 | 4 (23.5) | 3 (25.0) | 1 (20.0) | |
| 1–49 | 3 (17.6) | 1 (8.3) | 2 (40.0) | |
| ≥50 | 7 (41.2) | 6 (50.0) | 1 (20.0) | |
| Unknown | 3 (17.6) | 2 (16.7) | 1 (20.0) | |
| No. of previous lines of therapies | — | |||
| 1 | 11 (64.7) | 6 (50.0) | 5 (100) | |
| 2 | 3 (17.6) | 3 (25.0) | 0 | |
| 3 | 2 (11.8) | 2 (16.7) | 0 | |
| 4 | 1 (5.9) | 1 (8.3) | 0 | |
| IP | ||||
| Radiologic pattern | — | |||
| UIP | 6 (35.3) | 3 (25.0) | 3 (60.0) | |
| Probable UIP | 3 (17.6) | 3 (25.0) | 0 | |
| Indeterminate for UIP | 8 (47.1) | 6 (50.0) | 2 (40.0) | |
| Honeycomb lung on HRCT | 7 (41.2) | 3 (25.0) | 4 (80.0) | 0.101 |
| %FVC (%) | 85.4 (80.6– 92.2) | 84.9 (80.2– 92.2) | 86.4 (84.0– 92.2) | 0.721 |
| %DLco (%) | 54.4 (48.2–65.2) | 57.0 (51.3–66.3) | 52.1 (47.8–63.3) | 0.646 |
Note: Categorical data are presented as numbers (percentages) and compared using the Fisher’s exact test. Continuous data are presented as medians (interquartile ranges) and compared using Mann-Whitney U test. The p value was calculated by comparing subjects with and without pneumonitis; p less than 0.05 was statistically significant.
%DLco, % diffusing capacity for carbon monoxide; %FVC, % forced vital capacity; IP, interstitial pneumonia; PD-L1, programmed cell death-ligand 1; SpO2, saturation of peripheral oxygen; TPS, tumor proportion score; UIP, usual IP; HRCT, high-resolution computed tomography.
PD-L1 expression was assessed by immunohistochemistry using 22C3 pharmDx assay (Agilent Technologies, Santa Clara, CA).
Treatment Delivery
The median delivered cycles of treatment to each patient was 3 (Table 2 ). Five of the six patients who were on treatment at the time the trial were terminated, and continued to receive atezolizumab as the usual clinical treatment outside of this trial because they understood the risks and still wanted to (as of April 8, 2020).
Table 2.
Treatment Delivery
| Characteristics | N = 17 |
|---|---|
| Total no. of treatment cycles | 3 [2, 5] |
| Cycles received, n (%) | |
| 1 | 4 (23.5) |
| 2 | 4 (23.5) |
| 3 | 2 (11.8) |
| ≥4 | 7 (41.2) |
| Reason for discontinuation of study treatment, n (%) | |
| Disease progression | 5 (29.4) |
| Pneumonitis | 5 (29.4) |
| Termination of this clinical trial | 6 (35.2)a |
| Violation of eligibility criteria | 1 (5.9) |
Five of the six patients, who were on treatment at the time the trial was terminated, continued to receive atezolizumab as a real clinical treatment outside of this trial because they understood the risks and still wanted to.
Adverse Events
The major adverse events are presented in Table 3 . The incidence of pneumonitis was 29.4% (5 of 17) for all grades, 23.5% (4 of 17) for grade greater than or equal to 3, and 5.9% (1 of 17) for grade 5. All five patients with pneumonitis had newly developed bilateral ground-glass opacities as revealed by HRCT. The median time from the initiation of treatment to the onset of pneumonitis was 36 days.
Table 3.
Adverse Events
| Adverse Events | CTCAE Grade |
All Grade |
Grade ≥3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | % | Total | % | |
| Pneumonitis | 1 | 0 | 3 | 0 | 1 | 5 | 29.4 | 4 | 23.5 |
| Dyspnea | 2 | 0 | 4 | 0 | 0 | 6 | 35.3 | 4 | 23.5 |
| Hypoalbuminemia | 11 | 3 | 2 | 0 | 0 | 16 | 94.1 | 2 | 11.8 |
| Lung infection | 0 | 0 | 2 | 0 | 0 | 2 | 11.8 | 2 | 11.8 |
| Hyponatremia | 10 | 1 | 0 | 1 | 0 | 12 | 70.6 | 1 | 5.9 |
| General fatigue | 3 | 3 | 1 | 0 | 0 | 7 | 41.2 | 1 | 5.9 |
| Anemia | 6 | 6 | 0 | 0 | 0 | 12 | 70.6 | 0 | 0.0 |
| AST increased | 10 | 1 | 0 | 0 | 0 | 11 | 64.7 | 0 | 0.0 |
| ALT increased | 8 | 2 | 0 | 0 | 0 | 10 | 58.8 | 0 | 0.0 |
| Cough | 4 | 4 | 0 | 0 | 0 | 8 | 47.1 | 0 | 0.0 |
| Appetite loss | 5 | 2 | 0 | 0 | 0 | 7 | 41.2 | 0 | 0.0 |
| Fever | 4 | 2 | 0 | 0 | 0 | 6 | 35.3 | 0 | 0.0 |
| Hyperglycemia | 5 | 1 | 0 | 0 | 0 | 6 | 35.3 | 0 | 0.0 |
| ALP increased | 3 | 2 | 0 | 0 | 0 | 5 | 29.4 | 0 | 0.0 |
| Creatinine increased | 5 | 0 | 0 | 0 | 0 | 5 | 29.4 | 0 | 0.0 |
| Serum amylase increased | 5 | 0 | 0 | 0 | 0 | 5 | 29.4 | 0 | 0.0 |
| Hypereosinophilia | 4 | 0 | 0 | 0 | 0 | 4 | 23.5 | 0 | 0.0 |
| CPK increased | 2 | 1 | 0 | 0 | 0 | 3 | 17.6 | 0 | 0.0 |
| Proteinuria | 2 | 1 | 0 | 0 | 0 | 3 | 17.6 | 0 | 0.0 |
| Hyperkalemia | 3 | 0 | 0 | 0 | 0 | 3 | 17.6 | 0 | 0.0 |
| Hypothyroidism | 3 | 0 | 0 | 0 | 0 | 3 | 17.6 | 0 | 0.0 |
| Nausea | 3 | 0 | 0 | 0 | 0 | 3 | 17.6 | 0 | 0.0 |
| Diarrhea | 1 | 1 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Thrombocytopenia | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Blood bilirubin increased | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Hypokalemia | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Palpitations | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Vomiting | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Constipation | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Abdominal pain | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Pruritus | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Peripheral sensory neuropathy | 2 | 0 | 0 | 0 | 0 | 2 | 11.8 | 0 | 0.0 |
| Radiation recall pneumonitisa | 1 | 0 | 0 | 0 | 0 | 1 | 5.9 | 0 | 0.0 |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; CTCAE, Common Terminology Criteria for Adverse Event.
In the patient where the exclusion criteria were violated owing to a history of thoracic radiotherapy, an adverse event determined by the central judgment to be radiation recall pneumonitis with a CTCAE grade of 1 occurred. This was treated separately from pneumonitis in this report.
Risk Factors for Pneumonitis
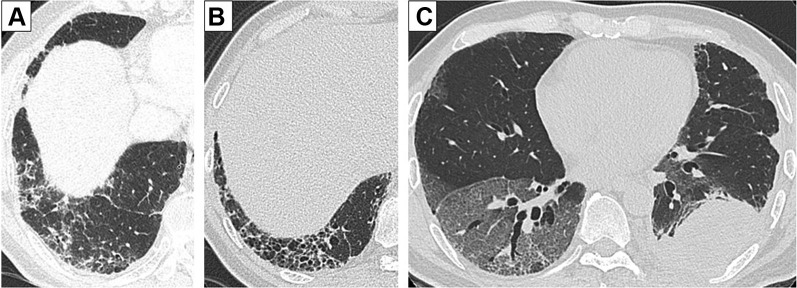
We evaluated the risk factors for pneumonitis by a post hoc analysis. The comparison of baseline characteristics between the patients who developed pneumonitis (n = 5) and those without pneumonitis (n = 12) revealed a significantly lower body weight (p = 0.049) and hemoglobin (p = 0.013) and higher serum levels of C-reactive protein (p = 0.014) in patients with pneumonitis (Table 1 and Supplemental Table 2). Subsequently, univariate logistic regression analysis did not identify statistically significant risk factor for pneumonitis but suggested that a honeycomb lung revealed on HRCT may be a candidate risk factor (OR: 12.0, 95% CI: 0.936–154.0, p = 0.056) (Table 4 ). In fact, in seven patients with a honeycomb lung on HRCT, four (57.1%) developed pneumonitis with a grade greater than or equal to 3 (Fig. 2 B and C). In contrast, in patients without a honeycomb lung (n = 10, Fig 2 A), only one patient (10%) with grade 1 pneumonitis was found.
Table 4.
Risk Factors for Pneumonitis
| Risk Factors | OR | 95% CI |
p Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Body weight | 0.882 | 0.765 | 1.020 | 0.083 |
| %FVC | 1.040 | 0.957 | 1.130 | 0.372 |
| %DLco | 0.998 | 0.910 | 1.070 | 0.771 |
| Hemoglobin | 0.238 | 0.053 | 1.080 | 0.063 |
| C-reactive protein | 2.500 | 0.814 | 7.670 | 0.109 |
| Krebs von den Lungen-6 | 1.000 | 0.998 | 1.000 | 0.732 |
| Honeycomb lung on HRCT | 12.00 | 0.936 | 154.0 | 0.056 |
Note: A univariate logistic regression analysis was performed to verify the risk of pneumonitis.
CI, confidence interval; %DLco, % diffusing capacity for carbon monoxide; %FVC, % forced vital capacity; HRCT, high-resolution computed tomography.
Figure 2.
Representative images of HRCT. (A) Probable UIP pattern; baseline HRCT revealed a subpleural reticular shadow predominant in lower lobes without a honeycomb lung. This patient continued atezolizumab without developing pneumonitis. (B) UIP pattern; baseline HRCT revealed a subpleural basal predominant reticular shadow and a honeycomb lung. (C) This patient developed grade 3 pneumonitis on day 20 of atezolizumab initiation. HRCT, high-resolution computed tomography; UIP, usual interstitial pneumonia.
Efficacy
As of April 8, 2020, when the data were cutoff at this time, the primary end point, 1-year survival rate, could not be assessed. The objective response rate and disease control rate were 6.3% and 62.5%, respectively. The median progression-free survival time was 3.38 months (95% CI: 0.84–5.93).
Discussion
The high incidence of pneumonitis indicated in this study deviates from previously reported results of two prospective trials of nivolumab for patients with pretreated NSCLC with IP. Because all these trials had a small number of patients, it is possible that this difference was accidental. However, we suspect that the discrepancy in the results may be owing to the following two differences in the eligibility criteria of IP between the trials: (1) acceptance or exclusion of patients with a honeycomb lung and (2) a lower limit of %FVC.
Previously reported nivolumab trials excluded patients with a honeycomb lung,4 , 5 but in this study, 41.1% of the patients (7 of 17) had honeycomb lungs. In terms of pneumonitis or acute exacerbation of preexisting IP induced by cytotoxic chemotherapy, a honeycomb lung is one of the most common risk factors.2 However, the risk factors of ICI–induced pneumonitis in patients with comorbid IP remain unclear. Moreover, identifying a honeycomb lung is difficult for nonspecialists, and there is often disagreement about the identification of honeycomb lung, even among experienced chest radiologists.12 Therefore, we did not include the presence of honeycomb lungs in the exclusion criteria for this study, in view of simplicity and generalization of the study results. However, the risk factor analysis of pneumonitis in this study revealed the possibility that exclusion of patients with honeycomb lung on baseline computed tomography images may reduce the risk of developing pneumonitis. Nonetheless, careful consideration of a larger number of patients is mandatory for the validation of this result.
Low %FVC was also a common risk factor for cytotoxic chemotherapy–induced pneumonitis or acute exacerbation of preexisting IP.13 Previously reported trials of nivolumab have included patients with %FVC greater than or equal to 80%, whereas, this trial included patients with %FVC greater than or equal to 70%. However, the median %FVC of our patients was 85.4%, and neither %FVC nor %DLco had a significant difference when compared with the presence or absence of pneumonitis. Because most of the patients enrolled in this study are smokers, the effect of emphysema may lead to an overestimation of %FVC.
In the present situation in which coronavirus disease 2019 is rapidly spreading worldwide, an additional difficulty of differentiating between ICI-induced pneumonitis and coronavirus disease 2019–associated pneumonia can arise. Therefore, more prudence should be exercised when considering the administration of ICIs for patients with NSCLC with IP.
As a limitation of this study, the baseline imaging findings were assessed by a central review committee who were not completely blinded. Patients with known cause of IP (e.g., infection, pneumoconiosis, drug, sarcoidosis, and collagen vascular disease) at present were excluded, but it cannot be ruled out that some of the enrolled patients had IP secondary to subclinical collagen vascular disease, which may have contributed to the high incidence of pneumonitis in this study. The frequency and severity of adverse events may change in the future owing to the short observation period and the fact that some patients continue to receive atezolizumab outside of the study.
Conclusions
Patients with NSCLC with comorbid IP as defined by the selection criteria for this study may have an increased risk of ICI–induced pneumonitis. As the safety of ICIs for NSCLC with comorbid IP is still unclear, further safety data are warranted from a clinical trial with more carefully selected and a larger number of patients.
Acknowledgments
This study was supported by the Chugai Pharmaceutical Co., Ltd. (Tokyo, Japan). The authors thank the patients, their families, the Thoracic Oncology Research Group (TORG) data center staff, and all the investigators who participated in the TORG1936/AMBITIOUS study. All data generated or analyzed in this study are included in this published article. Dr. Ikeda contributed to the conceptualization, methodology, investigation, visualization, project administration, funding acquisition, and writing (original draft) of the manuscript. Dr. Kato contributed to the conceptualization, methodology, project administration, funding acquisition, and writing (review and editing) of the manuscript. Drs. Kenmotsu and Ogura contributed to the conceptualization, methodology, and writing (review and editing) of the manuscript. Dr. S. Iwasawa contributed to the methodology, investigation, and writing (review and editing) of the manuscript. Drs. Sato, Harada, Kubota, Tokito, I. Okamoto, Furuya, Yokoyama, and Hosokawa contributed to the investigation and writing (review and editing) of the manuscript. Drs. T. Iwasawa and Yamanaka contributed to the methodology, formal analysis, and writing (review and editing) of the manuscript. Dr. H. Okamoto contributed to the methodology, supervision, project administration, and writing (review and editing) of the manuscript.
Footnotes
Disclosure: Dr. Ikeda reports receiving grants and personal fees from Chugai Pharmaceuticals, during the conduct of the study; grants and personal fees from AstraZeneca; and personal fees from Boehringer Ingelheim, Ono Pharmaceuticals, Taiho Pharmaceuticals, Bristol-Myers Squibb, and Eli Lilly, outside of the submitted work. Dr. Kato reports receiving grants and personal fees from Chugai Pharmaceuticals, during the conduct of the study; grants and personal fees from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Biopharma, Merck Sharp & Dohme, Novartis, Ono Pharmaceuticals, Pfizer, and Taiho Pharmaceuticals; personal fees from Daiichi Sankyo, F. Hoffmann-La Roche, Nippon Kayaku, Nitto Denko, Shionogi, Sumitomo Dainippon, and Takeda Pharmaceuticals; and grants from Astellas, Kyorin, Kyowa Kirin, and Regeneron, outside of the submitted work. Dr. Kenmotsu reports receiving grants and personal fees from Chugai Pharmaceuticals, during the conduct of the study; grants and personal fees from AstraZeneca and Novartis; and personal fees from Ono Pharmaceuticals, Boehringer Ingelheim, Eli Lilly, Kyowa Hakko Kirin, Bristol-Myers Squibb, Merck Sharp & Dohme, Daiichi Sankyo, and Pfizer, outside of the submitted work. Dr. Ogura reports receiving personal fees from Shionogi, Nippon Boehringer Ingelheim, and Eisai, outside of the submitted work. Dr. S. Iwasawa reports receiving personal fees from AstraZeneca, Daiichi Sankyo, Merck Sharp & Dohme, and Chugai Pharmaceuticals; and grants and personal fees from Ono Pharmaceuticals, outside of the submitted work. Dr. Sato reports receiving personal fees from Ono Pharmaceuticals, Novartis, Taiho Pharmaceuticals, and Chugai Pharmaceuticals, outside of the submitted work. Harada reports receiving personal fees from Chugai Pharmaceuticals, outside of the submitted work. Dr. Kubota reports receiving grants and personal fees from Taiho Pharmaceuticals; grants from Boehringer Ingelheim and Ono Pharmaceuticals; and personal fees from Merck Sharp & Dohme and Chugai Pharmaceuticals, outside of the submitted work. Dr. Tokito reports receiving personal fees from AstraZeneca, Merck Sharp & Dohme, Boehringer Ingelheim, and Chugai Pharmaceuticals, outside of the submitted work. Dr. I. Okamoto reports receiving grants and personal fees from AstraZeneca, Taiho Pharmaceuticals, Boehringer Ingelheim, Ono Pharmaceuticals, Merck Sharp & Dohme, Eli Lilly, Bristol-Myers Squibb, and Chugai Pharmaceuticals; grants from Astellas, Novartis, and AbbVie; and personal fees from Pfizer, outside of the submitted work. Dr. Furuya reports receiving personal fees from Eli Lilly, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim Japan, Taiho Pharmaceuticals, Ono Pharmaceuticals, Pfizer Japan, and Chugai Pharmaceuticals, outside of the submitted work. Dr. Yokoyama reports receiving personal fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Ono Pharmaceuticals, Taiho Pharmaceuticals, Merck Sharp & Dohme, Pfizer, Novartis, and Chugai Pharmaceuticals, outside of the submitted work. Dr. T. Iwasawa reports receiving personal fees from Ono Pharmaceuticals and Boehringer Ingelheim and grants from Canon Medical Systems, outside of the submitted work. Dr. Yamanaka reports receiving grants and personal fees from Takeda Pharmaceuticals, Chugai Pharmaceuticals, Boehringer Ingelheim, Taiho Pharmaceuticals, Daiichi Sankyo, and Bayer; grants from Ono Pharmaceuticals, Merck, Astellas, and Eli Lilly; and personal fees from Pfizer, Sysmex, Huya Biosciences, and Gilead Sciences, outside of the submitted work. Dr. H. Okamoto reports receiving grants from AMED, Takeda Pharmaceuticals, Merck, and Daiich Sankyo; grants and personal fees from Merck Sharp & Dohme, Ono Pharmaceuticals, AstraZeneca, Chugai Pharmaceuticals, Taiho Pharmaceuticals, Bristol-Myers Squibb, and Eli Lilly; and personal fees from Kyorin, Boehringer Ingelheim, and Novartis, outside of the submitted work. Dr. Hosokawa declares no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2020.08.018.
Supplementary Data
References
- 1.Raghu G., Nyberg F., Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer. 2004;91(suppl 2):S3–S10. doi: 10.1038/sj.bjc.6602061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenmotsu H., Naito T., Kimura M., et al. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol. 2011;6:1242–1246. doi: 10.1097/JTO.0b013e318216ee6b. [DOI] [PubMed] [Google Scholar]
- 3.Ogura T., Takigawa N., Tomii K., et al. Summary of the Japanese Respiratory Society statement for the treatment of lung cancer with comorbid interstitial pneumonia. Respir Investig. 2019;57:512–533. doi: 10.1016/j.resinv.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Fujimoto D., Morimoto T., Ito J., et al. A pilot trial of nivolumab treatment for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer. 2017;111:1–5. doi: 10.1016/j.lungcan.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto D., Yomota M., Sekine A., et al. Nivolumab for advanced non-small cell lung cancer patients with mild idiopathic interstitial pneumonia: a multicenter, open-label single-arm phase II trial. Lung Cancer. 2019;134:274–278. doi: 10.1016/j.lungcan.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Rittmeyer A., Barlesi F., Waterkamp D., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial [published correction appears in Lancet. 2017;389:e5] Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khunger M., Rakshit S., Pasupuleti V., et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda S., Kato T., Kenmotsu H., et al. A phase II study of atezolizumab for pretreated advanced/recurrent non-small cell lung cancer with idiopathic interstitial pneumonias: rationale and design for the TORG1936/AMBITIOUS study. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920922022. 1758835920922022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis W.D., Costabel U., Hansell D.M., et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogura T., Azuma A., Inoue Y., et al. All-case post-marketing surveillance of 1371 patients treated with pirfenidone for idiopathic pulmonary fibrosis. Respir Investig. 2015;53:232–241. doi: 10.1016/j.resinv.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Costabel U., Inoue Y., Richeldi L., et al. Efficacy of nintedanib in idiopathic pulmonary fibrosis across prespecified subgroups in INPULSIS. Am J Respir Crit Care Med. 2016;193:178–185. doi: 10.1164/rccm.201503-0562OC. [DOI] [PubMed] [Google Scholar]
- 12.Watadani T., Sakai F., Johkoh T., et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 13.Enomoto Y., Inui N., Kato T., et al. Low forced vital capacity predicts cytotoxic chemotherapy-associated acute exacerbation of interstitial lung disease in patients with lung cancer. Lung Cancer. 2016;96:63–67. doi: 10.1016/j.lungcan.2016.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.