Abstract
Objectives
Compression of morbidity postulates that as the populations age, the age of onset of disease is postponed. The objective of this study is to test for evidence of compression of morbidity in Spain.
Methods
We calculated the age and sex-specific incidence of myocardial infarction, heart failure, cerebrovascular disease, as well as bladder, prostate, breast, lung, and colon cancer among hospital discharges covering 99.5 % of the Spanish population, approximately 40 million inhabitants for two non-overlapping periods, 1997–2000 and 2007–2010, and estimated the length of life spent with disease using the Sullivan method.
Results
We found that expansion of morbidity due to an earlier age-specific onset of incident disease and increase in life expectancy was the norm in Spain. Notable exceptions were cardiovascular disease in women (−0.2 % time spent with disease) and lung cancer for men (−0.9 % time spent with disease) from 1997–2000 to 2007–2010.
Conclusions
Compression of morbidity is often cited by policy makers when discussing adjustments to the health-care system. If morbidity is measured by age at onset of disease, the burden of morbidity has increased in Spain.
Keywords: Compression of morbidity, Disease-free life expectancy, Cancer, Spain, Cardiovascular disease, Age of onset
Introduction
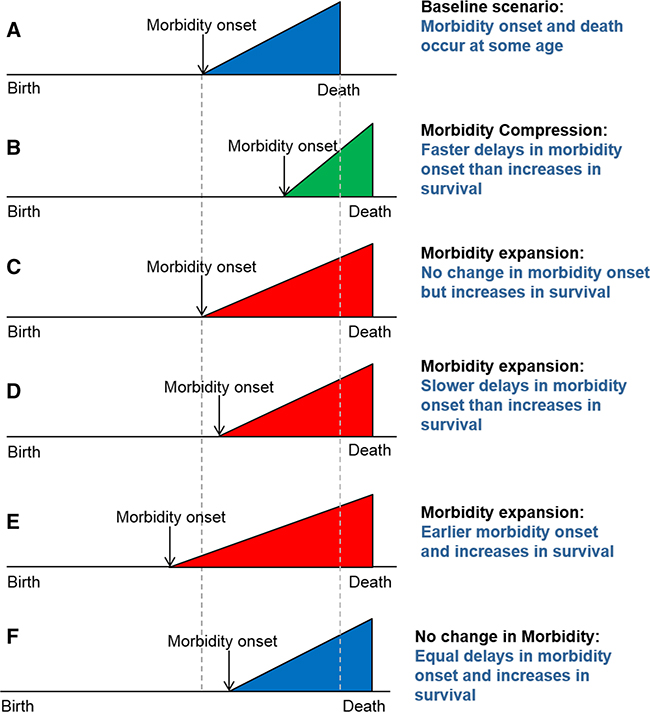
Reducing morbidity and maintaining physical functioning in old age is key to achieving healthy population aging and is currently a major research focus of the Horizon 2020 program in the European Union and Healthy People 2020 and the Patient Protection and Affordable Care Act in the United States (USA). Healthy population aging is highly related to compression of morbidity, a concept hypothesizing that increased survival in old age is invariably linked to a delay in the age at onset of chronic disease, i.e., increased longevity is accompanied by better health as the individual ages, leading to a reduction of one’s remaining life spent with morbidity (Fries 1980). Originally proposed by James Fries, the concept of compression of morbidity has been challenged empirically for more than 30 years. Global health surveys, cohort studies, and national registries have provided controversial evidence, supporting not only compression of morbidity but also its expansion (Fig. 1) (Beltran-Sanchez et al. 2014; Fries et al. 2011). Additional evidence supports the view of dynamic equilibrium (Manton 1982) in which changes in the severity and progression of disability and/or functional limitation (FL/D) have appeared to keep pace with mortality changes, so that the progression of FL/D seemed to be halted at early stages, resulting in potentially more FL/D in the population but with decreased consequences (Chatterji et al. 2015).
Fig. 1.
Different models of compression and/or expansion of morbidity discussed in Beltran-Sanchez et al. (2014). Source: adapted from Beltran- Sanchez et al. (2014)
Most of the literature on compression of morbidity uses disability as the indicator of health problems or morbidity and this has led to morbidity being conflated with disability and/or functional limitation, which are usually assessed using questionnaires and are greatly affected by the environment in which the individual lives (Freedman et al. 2013; Verbrugge and Jette 1994). Recently, findings from the Global Burden of Disease (GBD) Study highlighted that life expectancy increased faster than disability-free life expectancy, resulting in an increased burden of disability to the society as populations age (Murray et al. 2015; Vos et al. 2015). Nonetheless, trends in many indicators of FL/ D can result from changes in the environment, because they reduce the barriers to functioning (e.g., provision of electric wheelchairs may be the cause of a reduction in FL/ D) (Freedman et al. 2006). But the concept “compression of morbidity” refers to a reduction in the intrinsic morbidity occurring within people as overall population survival increases, rather than to an improvement in environmental conditions external to the individuals living in it, as it is often captured in FL/D indicators. While the measurement of morbidity is probably more reliable in the clinical diagnosis of major diseases, it is subject to changes in clinical practice over time and to large variations in health status after a major clinical health event has been suffered and survived. Arguably, the quality of life after a heart attack today is much better than that two decades ago, but any major hospitalization still marks the onset of morbidity in a person’s life course, whether or not it results in disability or major limitations in daily living activities (Beltran-Sanchez et al. 2014). Except for small, not nationally representative, epidemiologic studies (Caran- dang et al. 2006; Ergin et al. 2004; Parikh et al. 2009), age- specific incidence rates at the population level for the major causes of death in the USA and EU are not generally known and evaluating the compression of morbidity hypothesis only within the framework of associated disability burden might be misleading when informing policy makers about the status and future of the health of a population and associated costs. At a population level, however, the comparison of age-specific incidence of major health events is very informative about changes in the age at onset in the population, which is the main tenet of compression of morbidity, and for the assessment of the morbidity dynamics.
Because the paradigm of compression of morbidity continues to influence economic models and political decisions on social security and pensions, it is important to know whether or not compression of morbidity actually occurs (Bloom et al. 2007; Romeu Gordo 2011). It is the goal of this retrospective study to (a) describe the age- and sex-specific incidence of major diseases including cardiovascular disease (CVD) and main cancers in the adult Spanish population aged 50–85; (b) compare time trends in the incidence across a 10-year time window; and (c) evaluate how the change in age-specific incidence over time links with the hypothesis of compression of morbidity by computing average years of life spent with and without disease.
Methods
Figure 1 presents different scenarios that could result from the interplay of age of onset of chronic disease and the increase in survival at older ages. Examples are given relative to Fig. 1a, a baseline case in which morbidity onset occurs at age X and death occurs at age Y. Under compression of morbidity (panel B), morbidity onset is delayed to a greater extent than increases in survival. However, three other scenarios, all suggesting expansions of morbidity are equally plausible: survival increases (i.e., increasing average age at death) that are not accompanied by delays in age at onset of morbidity (C), lesser delay in age at onset of morbidity X relative to age at death Y (D), and onset of morbidity occurring at earlier ages while survival continues to increase (E). There is also the possibility that delays in the age at onset of chronic disease and increases in survival offset each other, so there is neither expansion nor compression of morbidity (F). To test for compression of morbidity, we calculate (1) the age- and sex-specific incidence of major chronic disease and compare (2) estimates of the age of onset and (3) length of time spent with the disease between 1997–2000 and 2007–2010. These results can then be compared to Fig. 1 to assess each depicted scenario.
If the age-specific incidence level in the later period is constantly lower than in the earlier period, then for a country like Spain with increasing life expectancy compression of morbidity occurs at the level of the population. Alternatively, if the age of onset is delayed, a similar argument can be made. Lastly, compression of morbidity is evident if the fraction of the remaining life spent with chronic disease is reduced in recent times.
Data
This retrospective study used the Spanish national information system for hospital data (Conjunto Minimo Basico de Datos; CMBD) maintained by the Ministry of Health, which includes an estimated 98 % of admissions to public hospitals (accessible via http://www.ine.es/inebmenu/mnu_salud.htm). An estimated 99.5 % of the Spanish population is covered by compulsory health insurance (Peiro and Librero 1999; Rivero-Cuadrado 2001). We study two periods between 1997 and 2010 using two non-overlapping 4-year periods—1997–2000 and 2007–2010—and assess the incidence of major chronic disease for all first hospitalization events. Hospitalization events (“cases”) are extracted for the eight following codes from the 9th International Classification of Diseases (ICD-9-CM): myocardial infarction (ICD-9 410), heart failure (ICD-9 428), cerebrovascular disease (ICD-9 430–438), bladder cancer (ICD-9 188), prostate cancer (ICD-9 185), breast cancer (ICD-9 174–175), lung cancer (ICD-9 162), and colon cancer (ICD-9 153).
Statistical analysis
First, we used conventional epidemiological methods for estimating the age- and sex-specific incidence of hospital admissions in the general population, dividing the number of incident hospitalizations in each 4-year period by the corresponding population at risk. The denominators for these rates were obtained from the Spanish National Institute of Statistics using the population registry (http://www.ine.es/inebmenu/mnu_padron.htm). Second, incident hospitalizations, life tables from the Human Mortality Database (HMD) (University of California, Berkeley (USA),and Max Planck Institute for Demographic Research (Germany) 2013), and Bayes Theorem were used to estimate the age of onset of disease following the methodology developed by Brinks, Landwehr, and Wal- deyer (Brinks et al. 2013). Third, the length of life spent with a disease was estimated using the Sullivan method, a prevalence-based technique that uses disease prevalence per age group to divide life expectancy into years lived with and without disease (Sullivan 1971).
To estimate the prevalence rates, the Sullivan method uses three inputs all disaggregated by age group and sex: (1) disease-specific incidence rates, (2) total population size, and (3) all-cause survival probabilities. For simplicity, all the terms defined below omit an index to identify the sex; in practice, this approach is similarly applied for men and women. We estimated annual disease-specific incidence rates of hospital admissions both in the general population and by age group and sex; let I(x, d, t) be this incidence rate for age group x, disease d, time t where x ε (50–54, 55–59, …, 85+), d ε (myocardial infarction, heart failure, stroke, bladder cancer, prostate cancer, breast cancer, lung cancer, and colon cancer), and t ε (1999, 2009). We obtained the total population size by age group and sex in each time period from the Spanish National Institute of Statistics using the population registry. Let P(x, t) be the population size at age group x and time t. Finally, survival probabilities by age and sex come from life tables from the HMD; let pr(x, x + n, t) correspond to the survival probability between ages x and x + n at time t. Let π(x, d, t) represent the prevalence rate for age group x, disease d, and time t.
Assuming that there are no prevalent cases of each disease before and at ages 50–54, the number of prevalent cases at this age can be estimated as π (50–54, d, t) = 1(50–54, d, t) × P(50–54, t) × n, where n corresponds to the length of the age group. Among these prevalent cases, a fraction of them will survive to ages 55–59 years, which is estimated with the survival probability from ages 50–54 to 55–59 years; that is, π(50–54, d, t) × pr(50–54, 55–59, t). Thus, prevalent cases at ages 5559 years correspond to the sum n(55–59, d, t) = π(50–54, d, t) × pr(50–54, 55–59, t) + 1(55–59, d, t) × P(55–59, t) × n. Put simply,
Now, let nLx(t) be the person-years lived between ages x and x + n at time t in a life table. The years lived with the disease at age x and time t are estimated as nLx(t) × π(x, d, t) and those without the disease as nLx(t) × [1 — π(x, d, t)]. Then, the years of life spent with the disease above age x are computed by summing the years lived with the disease from ages 50–54 to 85 + years; a similar computation is carried out for years of life spent without the disease.
A detailed description of all the age-, sex-, and period- specific incidence rates reported in this study and used for the life table calculations is available as Online Resource.
This study was considered exempt of ethics approval by the review board of Rey Juan Carlos University, because it only uses unidentifiable data from hospitalization and population registries.
Results
Comparison of the overall hospitalization rates for the major conditions for 1997–2000 and 2007–2010 showed a generalized increase in the overall levels of hospitalizations across all conditions for both genders (Online Resource 1). Notable exceptions were the hospitalization rate for cerebrovascular disease (1997–2000: 524.55 (95 % CI 521.74, 527.38); 515.76 (95 % CI: 513.24, 518.29) and lung cancer (1997:2000: 215.66.82 (95 % CI: 213.81, 217.53); 2007–2010: 206.93 (95 % CI: 205.28, 208.59) per 100 000 men and cerebrovascular disease (1997–2000: 321.35 (95 % CI: 319.45, 323.25); 2007–2010: 308.49 (95 % CI: 306.78, 310.20) per 100 000 women. Due to population aging, however, these overall results for the two time periods give limited information about trends in disease incidence such as age of onset and duration of disease, as they only represent the burden of disease to the society and the health-care system at a given point in time.
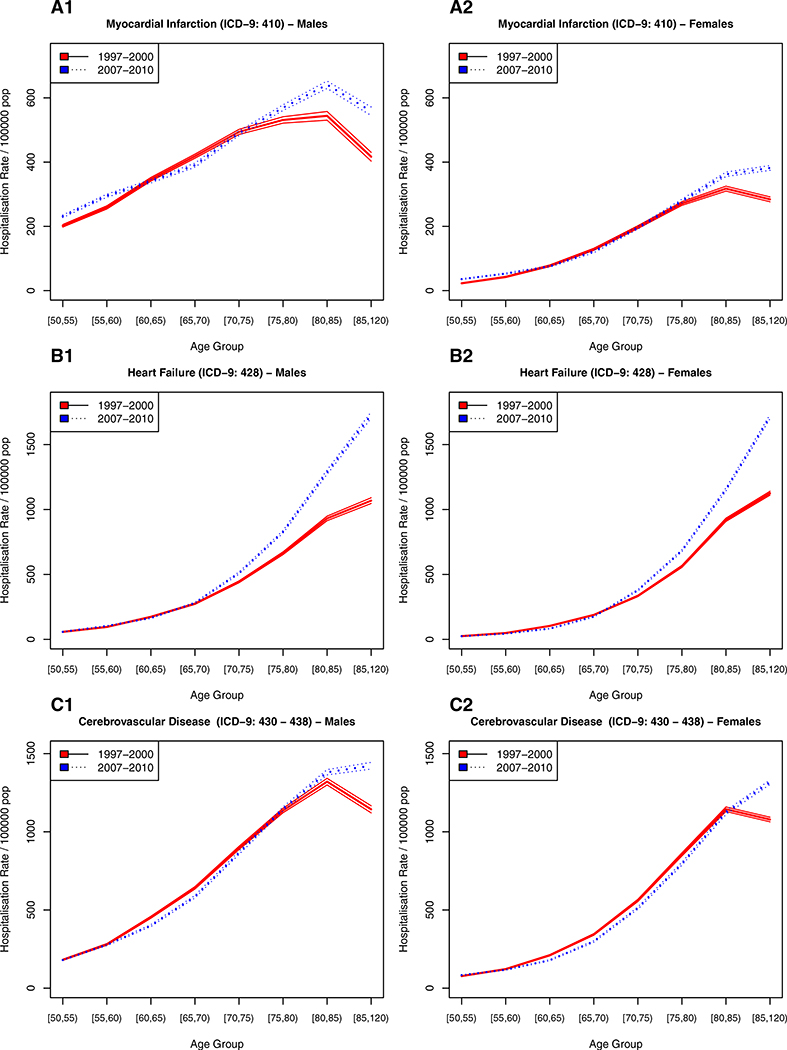
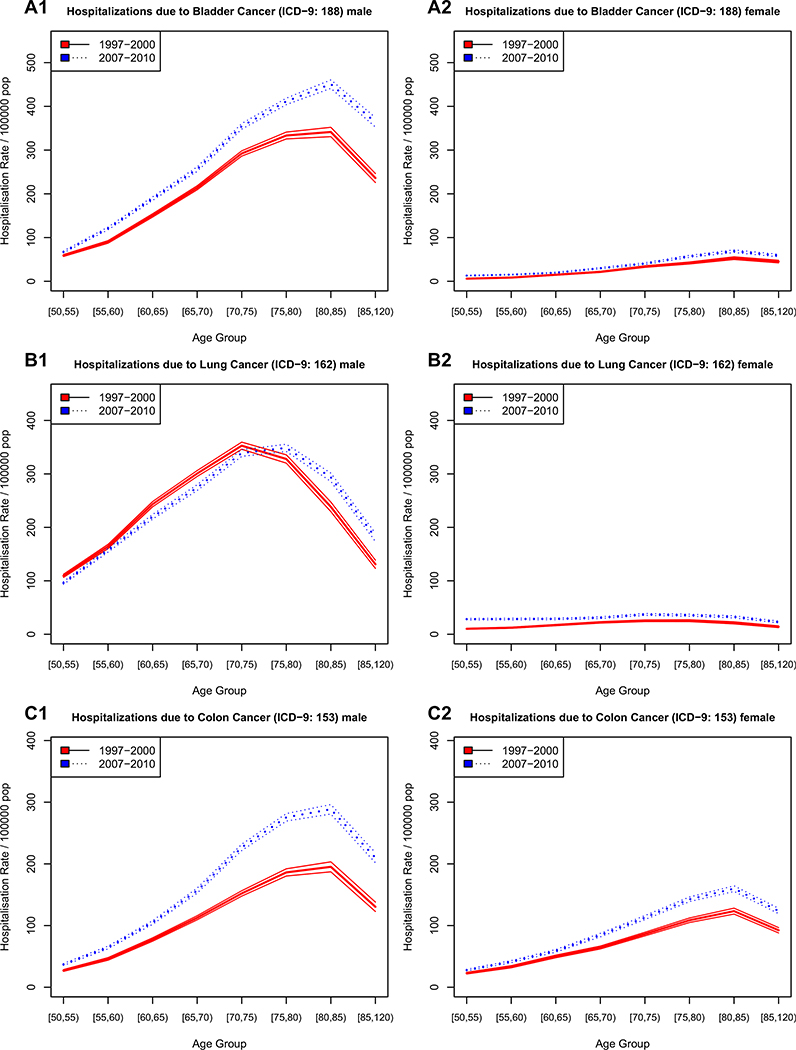
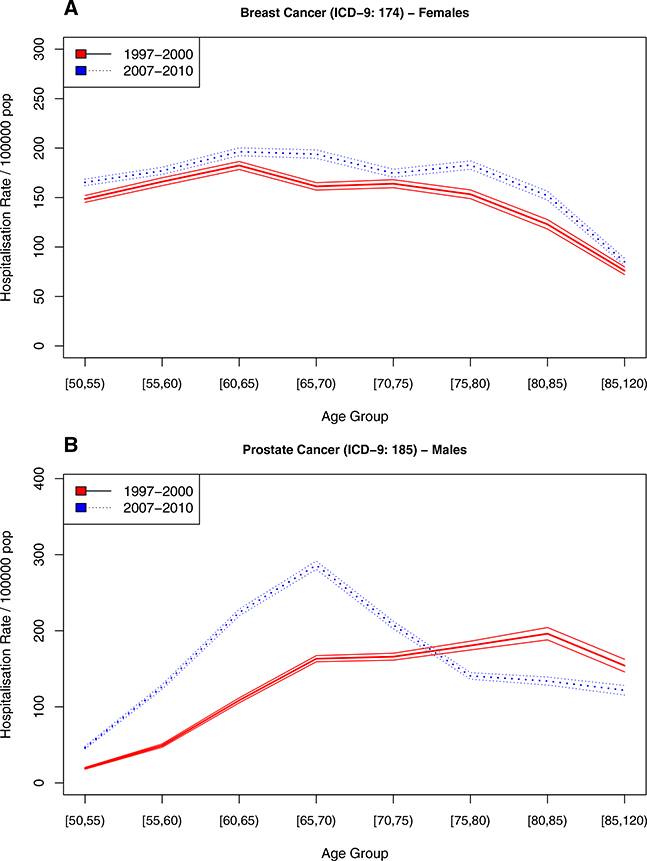
Figures 2, 3, and 4 show the incidence of hospitalizations for cardiovascular disease, cancer, and gender- specific cancers (e.g., breast and prostate), respectively, stratified by age, gender, and time period. The solid line represents the earlier time period 1997–2000 and the dotted line the period from 2007 to 2010, 10 years thereafter.
Fig. 2.
Hospitalization rate for cardiovascular disease in Spain stratified by sex and time period
Fig. 3.
Hospitalization rate for cancer in Spain, stratified by sex and time period
Fig. 4.
Hospitalization rate for gender specific cancers in Spain, stratified by period
For cardiovascular disease (Fig. 2a myocardial infarction, Fig. 2b heart failure, and Fig. 2c cerebrovascular disease), the age-specific rate of first hospitalizations increased continuously with age in men and women alike, with men having higher incidence of myocardial infarctions and cerebrovascular disease than women. For non-gender-specific cancerous diseases (Fig. 3a bladder cancer, Fig. 3b lung cancer, Fig. 3c colon cancer), the age-specific trends were similar to those of cardiovascular disease, albeit with lower incidence levels. The only exceptions were for lung cancer, where the age of highest incidence was 70–74 years— about 10 years earlier than the peak for CVDs, with men showing a much higher disease burden and faster increase in incidence with age.
There was a surprising contrast between the hospitalization rates for the two gender-specific cancers, i.e., breast (Fig. 4a) and prostate cancer (Fig. 4b). While the incidence of first hospitalization with breast cancer remained stable until the age of 80 years, there was a remarkable change in the trend in prostate cancer. In the 1997–2000 period, the incidence gradually increased with age, while in the 2007–2010 period, the age-specific incidence sharply increased to peak at age 65–69 years; it then declined equally sharply with age. Online Resources 2–11 show the exact numbers reporting the incidence of first hospitalization by period, gender, and age group, as well as the underlying population totals.
Comparing the age-specific incidence rates between the two time periods under study can provide evidence for compression or expansion of morbidity. For cardiovascular diseases (Fig. 2), there is some evidence of a reduction in age-specific incidence between the ages of 60–70 years. This reduction might be offset by an increase in younger ages, particularly for myocardial infarction (Fig. 2a). The age-specific incidence pattern and its change from 1997–2000 to 2007–2010 is very similar for men and women, albeit with higher incidence levels among men.
For cancerous diseases (Fig. 3), there is evidence for an increase in age-specific incidence for both genders from 1997–2000 to 2007–2010. Lung cancer among men (Fig. 3b1) is a notable exception. It exhibited a decrease in hospitalizations at younger ages and an increase at older ages over time, which is an indication of postponement of disease onset. A similar pattern can be observed for myocardial infarction among men (Fig. 2a1) and cerebrovascular disease (Fig. 2c), although less well defined and with some evidence of an increase in incidence at younger ages, a reduction between the ages of 60 and 75, and a subsequent increase in incidence at older ages as compared to the earlier period. Prostate cancer (Fig. 4), as mentioned above, shows a steep increase in hospitalizations at younger ages in the later period as compared to the earlier one, paired with lower incidence of first hospitalizations at older ages.
Following the methodology developed by Brinks, Landwehr, and Waldeyer (Brinks et al. 2013), we estimated the age of onset of the chronic conditions. For all categories of cardiovascular disease and cancer, the age of onset showed a shift toward younger ages from 1999 to 2009. The only exception was cerebrovascular disease that showed a slight increase in the age of onset among males, but not among females (see Table 1).
Table 1.
Age of onset of major chronic disease stratified by sex and period: Spain 1997-2000 and 2007-2010
| Male | Female | |||
|---|---|---|---|---|
| 1999 | 2009 | 1999 | 2009 | |
| Cardiovascular diseases | ||||
| Myocardial infarction | 54.69 (3.64) | 5429 (3.29) | 64.85 (7.16) | 63.11 (8.02) |
| Heart failure | 59.67 (5.86) | 59.25 (5.63) | 64.61 (6.42) | 63.60 (6.70) |
| Cerebrovascular disease | 54.88(3.51) | 54.94 (3.54) | 58.74 (5.52) | 58.25 (5.54) |
| Cancers | ||||
| Bladder cancer | 60.22 (6.34) | 5850 (5.37) | 71.51 (9.47) | 70.54(10.17) |
| Prostate cancer | 63.45 (6.36) | 59.00 (4.87) | ||
| Breast cancer | 56.20 (5.49) | 55.62 (4.92) | ||
| Lung cancer | 56.92 (5.00) | 57.24 (4.89) | 67.85 (9.55) | 64.61 (10.31) |
| Colon cancer | 63.88 (7.48) | 6150 (6.75) | 66.30 (9.98) | 65.07 (8.90) |
Mean age of onset and standard deviation
To further illustrate the influence of the age-specific incidence on disease- and disease-free life expectancy we calculated the percentage of life expectancy spent with these specific conditions (Table 2). First, the overall survival in the Spanish population increased over the period of study by about 2 years of life at age 50 years for males and females between 2007–2010 and 1997–2000 (Table 2, last row). Second, because overall survival in the Spanish population increased, compression of morbidity would predict that people spend a lower fraction of their remaining life with a disease in 2007–2010 than in 1997–2000, thus effectively compressing their morbidity in more recent times. Results indicate otherwise, for almost all diseases, we observed an increase in the relative time spent with disease from the 1997–2000 period to the 2007–2010 period. There were only two exceptions: lung cancer (1997–2000: 4.94 %, 2007–2010: 4.89 % of life expectancy, relative decrease of 0.9 %) among men and cerebrovascular disease (1997–2000: 6.09 %, 2007–2010: 6.08 % of life expectancy, relative decrease of 0.2 %) among women.
Table 2.
Percentage of life expectancy at age 50 years spent with specific diseases and relative increase over the decade in Spain, 1997-2000 (centered in 1999) and 2007-2010 (centered in 2009)
| Condition | Females at age 50 years | Males at age 50 years | ||||
|---|---|---|---|---|---|---|
| 1999 | 2009 | Relative rise ± (%) | 1999 | 2009 | Relative rise ± (%) | |
| Cardiovascular diseases | ||||||
| Myocardial infarction | 2.06 | 2.31 | 12.2 | 7.66 | 8.54 | 11.5 |
| Heart failure | 3.72 | 4.50 | 21.1 | 5.00 | 6.24 | 24.7 |
| Cerebrovascular disease | 6.09 | 6.08 | −0.2 | 10.82 | 11.29 | 4.3 |
| Cancers | ||||||
| Bladder cancer | 0.40 | 0.58 | 45.1 | 3.63 | 4.76 | 31.0 |
| Prostate cancer | 2.13 | 3.87 | 81.8 | |||
| Breast cancer | 3.43 | 3.82 | 11.6 | |||
| Lung cancer | 0.35 | 0.65 | 84.7 | 4.94 | 4.89 | −0.9 |
| Colon cancer | 1.10 | 1.42 | 29.7 | 1.17 | 276 | 136.4 |
| Total life expectancy | 33.98 | 35.85 | 5.5 | 28.32 | 30.51 | 7.7 |
Discussion
In this retrospective study in Spain, we found evidence that if morbidity is determined on the basis of age at onset of major chronic diseases, compression of morbidity was not the norm between 1997–2000 and 2007–2010. Compression of morbidity requires a delay in the onset of major chronic disease, with a corresponding reduction in the remaining life spent with the disease, and hence a lower age-specific incidence at younger ages when life expectancy is increasing as is the case for Western Europe and Spain in particular. Contrary to this hypothesis, we found that the age-specific incidence levels of major diseases had remained similar over time, and that, for some diseases, it had increased in recent years rather than decreasing. Using compression of morbidity as a paradigm might be appropriate for debating the increase in retirement age, particularly if technological progress delays the onset of mobility limitations. Generally, however, technological progress more than equalizes these benefits through increased health-care spending, particularly for chronic conditions such as cardiovascular disease—where we observed hardly any evidence of delay in onset, much less real compression.
For compression of morbidity (Fig. 1b) to be evident, we would expect (a) the dotted line (i.e., the later period) to lie consistently below the solid line (i.e., the earlier period), as for example for cerebrovascular disease among women (Fig. 2c2), where the age-specific incidence has been lower in recent years; or (b) the dotted line to lie below the solid line at younger ages and crossing the solid line at older ages, as for example for lung cancer among men (Fig. 3b1). The results of the age of onset and life table calculations also corroborate our findings based on the age-specific incidence curves: an earlier age of onset for most chronic conditions and an increase, rather than a reduction, in the fraction of the remaining life spent with a chronic disease.
Given that some of the risk factors for these diseases are well known, we can try to associate these patterns with changes in obesity and smoking. For instance, in Spain, breast cancer and prostate cancer screening programs are part of the social health-care system, which might explain some of these patterns. Over the observational period, obesity as measured by body mass index (kg/m2) increased in Spain (Grau et al. 2011; Gutierrez-Fisac et al. 2000). Although awareness of obesity has increased and its prevention is on the agenda of public health officials, its hazardous impact on cardiovascular disease is believed to accumulate throughout life, and any changes in policy will not have affected the observational period.
Smoking, on the other hand, has a much more immediate impact on cardiovascular disease. In Spain, the introduction of anti-tobacco policies started in December 2005, with an obligation to physically separate a designated smoking area occupying 30 % of the total area of the venue. Venues smaller than 100 m2 were allowed to choose between smoking or non-smoking (Muggli et al. 2010) In 2011, the policy became stricter when smoking was banned in all hospitality venues (Lopez et al. 2012). This legislation was accompanied by public awareness campaigns. The 2011 restriction did not affect the period under study. But the changes implemented in 2005 fell within the observational period, and there is evidence that the prevalence of smoking decreased (Guerrero et al. 2011). This change in smoking patterns might well be reflected in the reduction in the age-specific incidence of CVD observed between the ages of 60 and 75.
Spain has screening programs for breast and prostate cancer, and the most dramatic change in age-specific incidence has been seen with regard to hospitalizations due to prostate cancer. Given that age and family history are the most important risk factors for prostate cancer in Spain, the changes seen between 1997–2000 and 2007–2010 cannot be attributed to differences in risk factor exposure, but to the introduction into clinical practice of the prostate- specific antigen (PSA) test and other screening techniques.
Limitations
Among the limitations of using hospital discharge data is that compression of morbidity can only be studied using diseases whose incidence has been (and still is) registered in the hospital setting during the study period. The dynamic equilibrium hypothesis cannot be assessed because the hospital discharge data lack measurements of disease severity. For this reason, we included major chronic conditions whose treatment requires hospitalizations either because they are of a sudden onset such as myocardial infarction and stroke or because they require chemotherapy, radiation therapy, or surgical interventions such as major cancers. This also means that individuals who die of any of these conditions before they reach the hospital are not included in the analysis. A case can be made that heart failure is not a sudden onset condition and often preceded by other cardiovascular conditions. A similar argument can be made for cancers that are treated using chemotherapy in the outpatient setting, because they have been diagnosed at an early stage of the disease progress. Nevertheless, we would expect a change in clinical practice toward treatment in the outpatient setting to reduce the age-specific hospitalization incidence over time, something that we do not observe in our data, where the general trend points to an increase in the age-specific incidence.
The case of prostate cancer highlights one of the limitations of our study data. While hospitalization registries give an accurate picture of the burden of diseases treated in a particular country, they cannot provide information on changes in the underlying biology and disease processes. The dramatic change in the age-specific incidence of prostate cancer and the estimated reduction in age of onset by almost 4.5 years over a 10-year period cannot be explained by the changes attributable to the disease and its risk factors, but rather by technological changes that allow it to be detected at an early stage. We are unaware of similar major changes in clinical practice over the observational period. But in general, early detection of disease can prevent future disability; then detection can be useful and lead to compression of morbidity under the disability framework of morbidity. After establishing whether this is the case for prostate cancer, it will then be possible to determine whether prostate cancer morbidity was compressed.
Our results for the 85+ age category should be interpreted cautiously, as life expectancy in Spain has been increasing at an average of one additional year for every 4 years that have passed. As a consequence, this age category now includes a much larger population at risk with a higher mean age and age is strongly associated with an increase in hospitalizations. This does not influence the age of onset or life table calculations given that the only transition possible from age 85+ years is death and an increase in incidence in the 85+ years age group due to a higher population age is not considered.
In conclusion, measuring morbidity by age at onset of major chronic disease provides no clear evidence for compression of morbidity in Spain. Our evidence suggests that if age at the first diagnosis of a major chronic disease is taken as the onset of morbidity, expansion of morbidity is the norm. The morbidity expansion is likely accompanied by high disease burden even if functional limitation/dis- ability is not yet occurring (Beltran-Sanchez et al. 2014). It remains to be established whether the notable exceptions for lung cancer among men and the slight delay in the onset of CVD can be attributed to changes in smoking behavior. Nationally representative studies incorporating measures of disease severity to test the dynamic equilibrium hypothesis could greatly improve our understanding of disease processes in the population.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00038-016-0829-5) contains supplementary material, which is available to authorized users.
Contributor Information
Stefan Walter, Department of Epidemiology and Biostatics, University of California San Francisco, 550 16th Street, 2nd Floor, Campus Mail Box 0560, San Francisco, CA 94158-2549, USA.
Hiram Beltrán-Sánchez, Department of Community Health Sciences, Fielding School of Public Health, and California Center for Population Research, University of California, Los Angeles, USA.
Enrique Regidor, Department of Preventive Medicine and Public Health, Universidad Complutense de Madrid, Madrid, Spain.
Carlos Gomez-Martin, Gastrointestinal Cancer and Early Clinical and Translational Research Units, Medical Oncology Division, 12 de Octubre University Hospital, Madrid, Spain.
Jose Luis del-Barrio, Area of Preventive Medicine and Public Health, Rey Juan Carlos University, Madrid, Spain.
Angel Gil-de-Miguel, Area of Preventive Medicine and Public Health, Rey Juan Carlos University, Madrid, Spain.
S. V. Subramanian, Department of Social and Behavioral Sciences, Harvard T.H. Chan School of Public Health, Boston, USA
Ruth Gil-Prieto, Area of Preventive Medicine and Public Health, Rey Juan Carlos University, Madrid, Spain; Department of Population Medicine, Harvard Medical School, Boston, MA, USA.
References
- Beltran-Sanchez H, Razak F, Subramanian SV (2014) Going beyond the disability-based morbidity definition in the compression of morbidity framework. Glob Health Action 7:24766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom DE, Canning D, Mansfield RK, Moore M (2007) Demographic change, social security systems, and savings. J Monet Econ 54:92–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinks R, Landwehr S, Waldeyer R (2013) Age of onset in chronic diseases: new method and application to dementia in Germany. Popul Health Metr 11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandang R, Seshadri S, Beiser A et al. (2006) Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 296:2939–2946 [DOI] [PubMed] [Google Scholar]
- Chatterji S, Byles J, Cutler D, Seeman T, Verdes E (2015) Health, functioning, and disability in older adults—present status and future implications. Lancet 385(9967):563–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergin A, Muntner P, Sherwin R, He J (2004) Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med 117:219–227 [DOI] [PubMed] [Google Scholar]
- Freedman VA, Agree EM, Martin LG, Cornman J (2006) Trends in the use of assistive technology and personal care for late-life disability, 1992–2001. Gerontol 46:124–127 [DOI] [PubMed] [Google Scholar]
- Freedman VA, Spillman BC, Andreski PM et al. (2013) Trends in late- life activity limitations in the United States: an update from five national surveys. Demography 50:661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF (1980) Aging, natural death, and the compression of morbidity. N Engl J Med 303:130–135 [DOI] [PubMed] [Google Scholar]
- Fries JF, Bruce B, Chakravarty E (2011) Compression of morbidity 1980–2011: a focused review of paradigms and progress. J Aging Res 2011:261702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau M, Elosua R, Cabrera de León A et al. (2011) Factores de riesgo cardiovascular en Espaüa en la primera decada del siglo xxi: analisis agrupado con datos individuales de 11 estudios de base poblacional, estudio DARIOS. Rev Esp Cardiol 64:295–304 [DOI] [PubMed] [Google Scholar]
- Guerrero F, Santonja F, Villanueva R (2011) Analysing the Spanish smoke-free legislation of 2006: a new method to quantify its impact using a dynamic model. Int J Drug Policy 22:247–251 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Fisac J, Banegas BJ, Artalejo FR, Regidor E (2000) Increasing prevalence of overweight and obesity among Spanish adults, 1987–1997. Int J Obes Relat Metab Disord J Int Assoc Study Obes 24:1677–1682 [DOI] [PubMed] [Google Scholar]
- Human Mortality Database. University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany) (2013). Available at http://www.mortality.org, http://www.humanmortality.de [Google Scholar]
- Lopez MJ, Nebot M, Schiaffino A et al. (2012) Two-year impact of the Spanish smoking law on exposure to secondhand smoke: evidence of the failure of the ‘Spanish model’. Tob Control 21:407–411 [DOI] [PubMed] [Google Scholar]
- Manton KG (1982) Changing concepts of morbidity and mortality in the elderly population. Milbank Mem Fund Q Health Soc 60:183–244 [PubMed] [Google Scholar]
- Muggli ME, Lockhart NJ, Ebbert JO, Jimenez-Ruiz CA, Riesco Miranda JA, Hurt RD (2010) Legislating tolerance: Spain’s national public smoking law. Tob Control 19:24–30 [DOI] [PubMed] [Google Scholar]
- Murray CJL, Barber RM, Foreman KJ, et al. (2015) Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 386(10009):2145–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh NI, Gona P, Larson MG et al. (2009) Long-term trends in myocardial infarction incidence and case fatality in the National Heart, Lung, and Blood Institute’s Framingham Heart study. Circulation 119:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiro S, Librero J (1999) The quality assessment from the minimum basic hospital discharge data set. Rev Neurol 29:651–661 [PubMed] [Google Scholar]
- Rivero-Cuadrado A (2001) El conjunto mínimo básico de datos en el SNS: inicios y desarrollo actual. Rev Fuentes Estadísticas 49:18–19 [Google Scholar]
- Romeu Gordo L (2011) Compression of morbidity and the labour supply of older people. Appl Econ 43:503–513 [Google Scholar]
- Sullivan DF (1971) A single index of mortality and morbidity. HSMHA Health Rep 86:347–354 [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM (1994) The disablement process. Soc Sci Med 38:1–14 [DOI] [PubMed] [Google Scholar]
- Vos T, Barber RM, Bell B et al. (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:743–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.