Abstract
The radiation treatment planning process includes contouring, planning, and reviewing the final plan, and each component requires substantial time and effort from multiple experts. Automation of treatment planning can save time and reduce the cost of radiation treatment, and potentially provides more consistent and better quality plans. With the recent breakthroughs in computer hardware and artificial intelligence technology, automation methods for radiation treatment planning have achieved a clinically acceptable level of performance in general. At the same time, the automation process should be developed and evaluated independently for different disease sites and treatment techniques as they are unique from each other. In this article, we will discuss the current status of automated radiation treatment planning for cervical cancer for simple and complex plans and corresponding automated quality assurance methods. Furthermore, we will introduce Radiation Planning Assistant (RPA), a web-based system designed to fully automate treatment planning for cervical cancer and other treatment sites.
Introduction
Treatment planning for radiation therapy is an extremely complex process that involves many different tasks performed by a team of highly trained and experienced people (Figure 1). Even simple tasks typically involve many button clicks by a radiation oncologist or treatment planner. As such, radiation therapy treatment planning is a time-consuming, inefficient, and expensive process. Furthermore, individual team members’ preferences and skills can lead to much variability in the performance of individual tasks (e.g., contouring, plan optimization).1–5 Fortunately, automation, which is the use of technology to perform a process or procedure with minimal human assistance, may significantly enhance the uniformity, efficiency, and speed of the radiation therapy planning process. In fact, almost all of the tasks listed in Figure 1 are candidates for automation except for taking a computed tomography (CT) scan and administering treatment.
Figure 1.
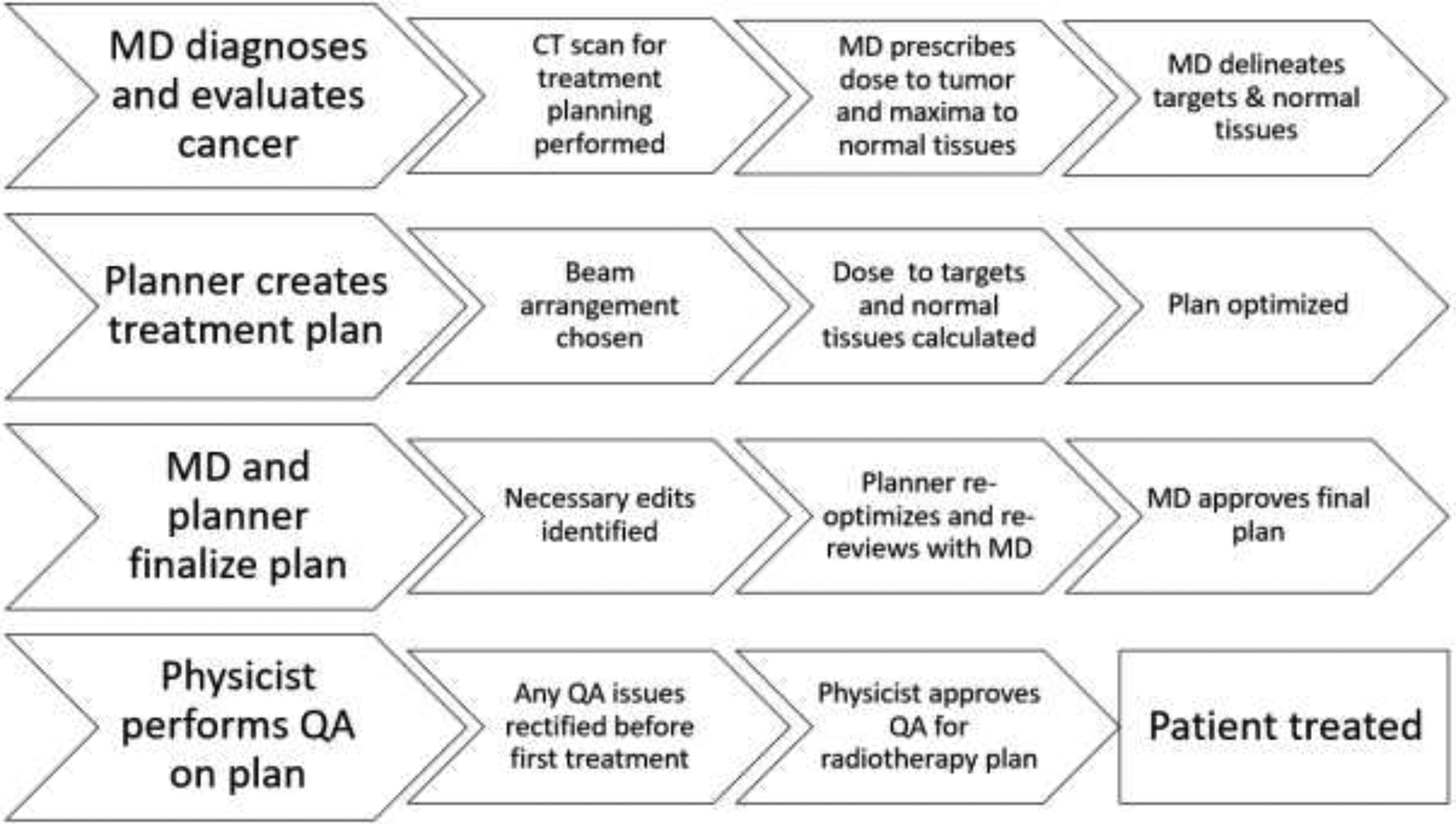
Flow chart of the radiation therapy planning process. CT, computed tomography; MD, doctor of medicine; QA, quality assurance.
The potential benefits of automating the radiation therapy treatment planning process are:
Improved efficiency. After patients receive their radiation therapy-planning CT scan, they often have to wait a week or more before starting treatment. Automation of the treatment planning workflow could enable patients to start treatment shortly after their CT scan. This would bring many benefits, including significant cost savings for the patient.
Improved quality and consistency of treatment plans. Researchers have shown that plans of poor quality can negatively impact patient outcomes.3,6–8
Improved safety. Hand-offs between staff are known to be a risk point, with miscommunication between staff members potentially impacting the safety of radiation therapy.9–13 Automation of multiple tasks (rather than individual tasks) can reduce the number of hand-offs between staff.
Increased access to high-quality radiation therapy across the world. Access to radiation therapy is severely lacking across the world, partially because of a lack of appropriate staff.14 Automation can make planning easier, thus enabling existing staff to spend more time on other important tasks.
In this review, we describe how automation has been used to develop simple and complex external beam radiation treatment plans for cervical cancer.
Automation of simple plans (four-field box treatments)
Four-field box treatments use four orthogonal radiation fields. Each field shape is based on the location of bony landmarks or soft tissue structures. This treatment approach is simple and effective and is recommended for treatment of invasive cervical cancer in low-resource settings.15,16 Kisling et al.17 developed an automated approach to determining beam apertures based on bony landmarks. First, the bony pelvis, femoral heads, sacrum, and fourth and fifth lumbar vertebral bodies (L4 and L5, respectively) are automatically contoured. The bony structures are then projected into each beam’s eye view; several landmarks, such as the widest extent of the pelvic inlet, are identified; and the beam apertures are determined according to a set of predefined rules. More recently, the same research group replaced the multi-atlas segmentation approach with a deep learning approach, which increased the success rate for auto-planning from 90% to above 95%.
Alternative approaches to the method developed by Kisling and colleagues are proposed in the literature. For example, Cardenas et al.18 recently described the use of a convolutional neural network (CNN) approach to predicting field apertures. They used digitally reconstructed radiographs as inputs and physician-approved beam apertures as the ground truth. In this work, they found that using the projection images alone was prone to error in some uncommon situations, such as patients with metal hardware (e.g., from spine reconstruction) or excessive contrast in the bowel.
The manual tasks involved in planning a simple four-field box are all straightforward. However, the challenge is that the tasks are many, and they are performed by various staff members, meaning that the entire process is subject to delays caused by hand-offs between staff. Thus, although the automation of the field shape has only modest potential for time savings (given the simplicity of the field shapes), the real benefit comes when this task is combined with other automated tasks such as dose calculation and the optimization of field weights to achieve homogeneous dose distributions. Kisling et al.17 described such an approach, which included optimizing beam weights to minimize dose heterogeneity. Full automation means that treatment plans can be ready for final physician review within a few minutes (rather than a few days, which is currently typical without automation), potentially enabling patients to start treatment the same day that they receive their CT scans.
Automation of complex plans
Volumetric modulated arc therapy (VMAT) is the most advanced beam-delivery technique for treating invasive cervical cancer. Unlike the optimization process for the simple plan described above, the optimization process for VMAT requires precisely defined soft tissue contours. Therefore, development of a fully automated contouring system is essential for an automated process. These contours can then be used as input to advanced automated inverse treatment planning approaches.
Automated contouring
Over the past few decades, atlas-based auto-contouring methods have been among the most advanced such methods,19–23 and researchers have successfully used them in the development of auto-contouring tools for some of the critical organs in the female pelvis. As described above, Kisling et al.17 developed a deformable, multi-atlas technique for automatic segmentation that can auto-contour the bony pelvis, femoral heads, sacrum, and fourth and fifth lumbar vertebral bodies. Young et al.24 used atlas-based segmentation to automatically generate endometrial cancer nodal clinical target volumes (CTVs); this led to a 26% time savings for the clinicians and increased the accuracy of the nodal CTV contours by 2% as per Dice similarity coefficient calculations. Furthermore, Bondar et al.25 automatically generated cervix-uterus contours on daily CT scans acquired with a CT-on-rails system. This involved deformable registration and manually drawing of contours on patients’ pretreatment CT scans.
On the other hand, atlas-based auto-contouring methods may be suboptimal for contouring soft-tissue organs in the female pelvis because the shape and relative positions of the organs differ substantially among individuals and are therefore unpredictable. However, recent developments in deep learning techniques—specifically, CNN-based image segmentation techniques26–29—overcame this limitation of atlas-based auto-contouring methods. The performance of CNN-based models improves as the number of training data sets increases;20 in contrast, atlas-based models are optimized with 10–20 training data sets23,24,30,31. Training the CNN-based models with various data sets enables the models to “understand” the general features of the soft-tissue organs in the female pelvis; thus, the models become more suitable for identifying patterns in patient-specific features.
Because of these advantages, researchers have investigated the possibility of auto-contouring organs using CNN-based segmentation models for multiple body sites. The auto-contouring studies of patients with prostate or rectal cancer that used CNN-based models showed that automatically generated bladder, rectal, and femur contours on CT images have an accuracy equivalent to the inter-observer variabilities among different radiation oncologists.32,33 Liu et al.34 developed the CNN-based auto-segmentation tool to segment 7 organs-at-risk (bladder, bone marrow, left and right femurs, small intestine, and spinal cord) in cervical cancer CT images and achieved clinically acceptable outcomes. Our group has been developing a CNN-based auto-contouring method for primary and nodal CTVs and six normal structures (bladder, bowel space, left and right femurs, rectum, and spinal cord) that will automate cervical VMAT planning as shown in Figure 3. Most of the contours were clinically acceptable on test data.
Figure 3.

Unpublished recent results from our work on auto-contouring. The images are of two patients. In the upper images, the primary CTV (red), bladder (yellow), rectum (green), and femurs (pink) are shown. In the lower images, the primary CTV (red), nodal CTV (blue), and bowel space (brown) are shown.
Automated planning
Knowledge-based planning (KBP) can automate both IMRT and VMAT-planning processes. KBP software programs, such as RapidPlan (Varian Medical Systems, Palo Alto, CA) and Erasmus-iCycle (Elekta AB, Stockholm, Sweden), are commercially available, and the performance of KBP models created using the software has been validated in many research studies. In regard to cervical cancer KBP models, Ma et al.35 tested an IMRT RapidPlan model for postoperative cervical cancer patients and showed that planning target volume coverage was within 1% and critical organ dose metrics were within 4% of manual plan results. Also, Li et al.36 and Tinoco et al.37 showed that IMRT and VMAT RapidPlan models for cervical cancer patients are better than or equal to clinical plans. Sharfo et al.38 showed that, for patients with cervical cancer, their dual-arc VMAT Erasmus-iCycle model created plans that were equivalent to or better than manually generated dual-arc VMAT and nine-beam IMRT. Thus, an automatically generated IMRT or VMAT plan for cervical cancer made using KBP techniques will be clinically acceptable if the user can provide high-quality plans for model training.
Automated quality assurance
Once the treatment plan is complete, standard-of-care requires that it is carefully reviewed prior to treatment. This treatment plan review process is an important part of radiation therapy planning. It has several different components, all of which help maintain quality, consistency, and safety:
Peer review. This is a review of the proposed treatment approach by radiation oncologists and other clinical staff. It includes a review of the treatment plan and may include a review of the contours used in the plan.
Physics plan check. This is primarily a review of the technical aspects of the plan, such as the dose-calculation accuracy, but the check can also include a second review of the clinical aspects of the plan.
Therapists’ check. Therapists typically review the plan for completeness and “treatability.”
Aspects of the physics plan check and therapists’ check, such as recalculation of the radiation dose, detection of elements of the plans which cannot be carried through, and verification of correct data transfer from the planning system to the oncology information system, have been automated for many years. However, less attention has been paid to automation of the quality assurance process for simple and complex treatment plans for cervical cancer. In particular, automating tasks that are part of the peer-review process has received less attention than has automating other aspects of treatment planning.
Automated quality assurance for simple plans
For simple cervical cancer treatment plans, two quality assurance tasks determine the quality of a patient’s treatment: confirmation of the shapes of the treatment apertures and verification of the radiation dose. Verifying the dose calculation in a treatment plan by recalculating the same plan using independent software is a routine clinical practice. Although older software required extensive manual entry, this is no longer the case, and many clinics have implemented automated dose-calculation verification.
As with dose verification, the automated beam aperture quality assurance is possible using two independent beam aperture prediction algorithms. This was first demonstrated by Kisling et al.,39 who used the two methods summarized above—a deep learning approach and an automatic algorithm from automatically-generated bone contours. The comparison of two algorithms can be used to verify the field apertures. For most patients, both algorithms agree (generally meaning that the aperture is clinically acceptable). On occasion, however, one algorithm fails. In such instances, the cases are flagged for the algorithm user to indicate that additional review by a physician is needed.
Figure 4 shows how two independent beam aperture-prediction algorithms can be compared to verify field apertures. The histogram in Figure 4 shows the mean surface distance between the two algorithms for a set of apertures that had been scored by a radiation oncologist as acceptable or unacceptable. This example illustrates that this approach can identify the majority of patients for whom the automatically generated apertures would have been inappropriate. The main advantage of these automated quality assurance techniques is that the radiation oncologist may not have to review the plan until the final plan is ready – rather than the more usual situation where they have to be involved to draw the initial field apertures, and then again to review the final plan.
Figure 4.

Automatic quality assurance process for simple plans using the two independent beam aperture prediction algorithms. Red solid lines and yellow dashed lines indicate the primary and verification techniques, respectively.
Automated quality assurance for complex plans
Contour quality assurance
Although manual reviews of automatically generated contours should be conducted before the contours are used for clinical purposes, automated contouring quality assurance tools can still be beneficial as a means of avoiding potential mistakes. Most automatic contouring error-detection techniques use machine-learning algorithms to identify irregularities in extracted features and/or geometric locations of contours. McIntosh et al.40 identified errors in contours by extracting the geometric and intensity features of contours and analyzing the features with a conditional random forests model. Chen et al.41 developed a geometric attribute distribution model that uses relative geometric positions between organs to detect contouring errors. Most of these feature- and location-based algorithms assume that the tested organs always have similar features and relative geometries. These assumptions are valid for bony anatomies or for the organs in static region, such as the head and neck. However, because most of the critical organs in the female pelvis vary in size, shape, and position—even in the same patient at different time points—most feature- and location-based algorithms are not suitable for patients with cervical cancer. In contrast to this, Rhee et al.’s approach42 involves calculating the volume overlap between two contours created from two independent auto-contouring algorithms to identify errors in the reference contours. Because no prior assumptions are made when identifying contouring errors, this approach would be the more appropriate means of detecting contouring errors for the organs in the female pelvis.
Plan quality assurance
Automatic verification of the accuracy, quality, and safety of planned dose distributions can be achieved in a variety of ways. Firstly, the dose-calculation accuracy can be verified using independent software, as discussed above. The overall plan quality can be verified in a peer-review process in which each treatment plan is reviewed by other radiation oncologists and clinical staff. This is the verification procedure followed in many clinical practices and clinical trials. It involves not a review of the details of the dose calculation or other plan parameters, which are checked as part of physics checks, but rather a review of the overall suitability of the plan for a specific patient.
This peer-review process is extremely time-consuming, and therefore researchers have invested much work in the development of automated peer-review processes. These include the use of scorecards to assess whether the plan meets expected dose metrics and the prediction of dose distributions (or dose-volume histograms) by matching a patient’s anatomy with anatomical data from a library of patients or with machine-learning data based on the geometry and dose prescriptions of previous patients.43–48 More recently, groups of researchers have extended these ideas to predict the likely dose distribution for a patient using deep learning approaches49,50. Although not yet in widespread clinical use, these automated plan checks all have the potential to help clinical team members, especially dosimetrists, determine whether they have achieved the optimal plan for their patients. These automated plan quality assurance techniques are probably of particular use to clinical teams at centers that are transitioning to complex plans and have limited experience in assessing the quality of individual treatment plans.
Safe clinical use of automated treatment planning
Automated contouring and treatment planning will likely bring increased consistency and improved efficiency to radiation therapy treatment planning. An important point to realize, however, is that even with automated techniques that appear to be very robust, errors will occasionally happen. These may be caused by algorithm errors (e.g., incorrect automatic contouring) or by human error (e.g., entering an inappropriate prescription). Also, errors that are less likely to be detected with automated processes than with manual planning may occur. One example of this is the use of an incorrect CT field of view, which gives a circular edge to the patient in the CT images. This circular edge is immediately obvious to a human planner but may not be identified in an automated process (unless the program is specifically trained to identify such scenarios). Thus, although automation has many potential advantages, the risks must be carefully considered and mitigated when introducing automation to clinical practice.
A failure mode and effects analysis of the deployment of fully automated treatment planning for cervical cancer identified three components required for patient safety51:
User training. Carefully designed user training is essential, not only for the planners (to prevent error modes in automatically generated plans), but also for the staff involved in plan quality assurance (as new error modes that they are not used to checking for may appear).
Manual plan checks by radiation oncologists, physicists, and other clinical team members. The active participation of experienced clinical staff is essential to the safe deployment of automated planning approaches.
Automated plan verification (quality assurance). Wherever possible, automated solutions should be incorporated into plan verification.
The Radiation Planning Assistant project
There are many examples of the development and clinical use of partially automated tasks in radiation therapy, but full automation has, until recently, been reasonably rare. The University of Texas M.D. Anderson Cancer Center’s Radiation Planning Assistant (RPA) project is an early example of a system designed to fully automate the contouring and treatment-planning processes. The RPA, which is not yet in clinical use, was developed as a web-based service (http://rpa.mdanderson.org) and was started specifically to serve clinics in low- and middle-income countries where staffing is insufficient. The local user will upload a CT scan of a patient and a detailed plan order. Next, the RPA will automatically generate contours and/or a treatment plan that the user will then download to their own treatment-planning system. Finally, the user will recalculate the radiation dose (for their own local treatment linear accelerator) before making edits to and approving the final plan.
Initial efforts regarding the RPA have focused on treatment plans for cervical cancer (four-field box), breast cancer (post-mastectomy, tangents, and supraclavicular fields), and head and neck cancer (VMAT), although further development for other anatomies is ongoing. The RPA is likely to be one of the first fully automated systems in clinical use, and additional fully automated tools soon will be available for use with common commercial treatment-planning systems or though other hospital-led development efforts.
Conclusions
Automation of radiotherapy treatment planning can provide improvements in efficiency, safety, and quality. The majority of tasks for external beam radiation therapy treatment planning for patients with cervical cancer, including the determination of field borders (four-field box), contouring, and complex planning (VMAT), have been automated. Although these tools are not all available clinically at this point, they likely will be available within the next year and widely available within 3–5 years.
Figure 2.
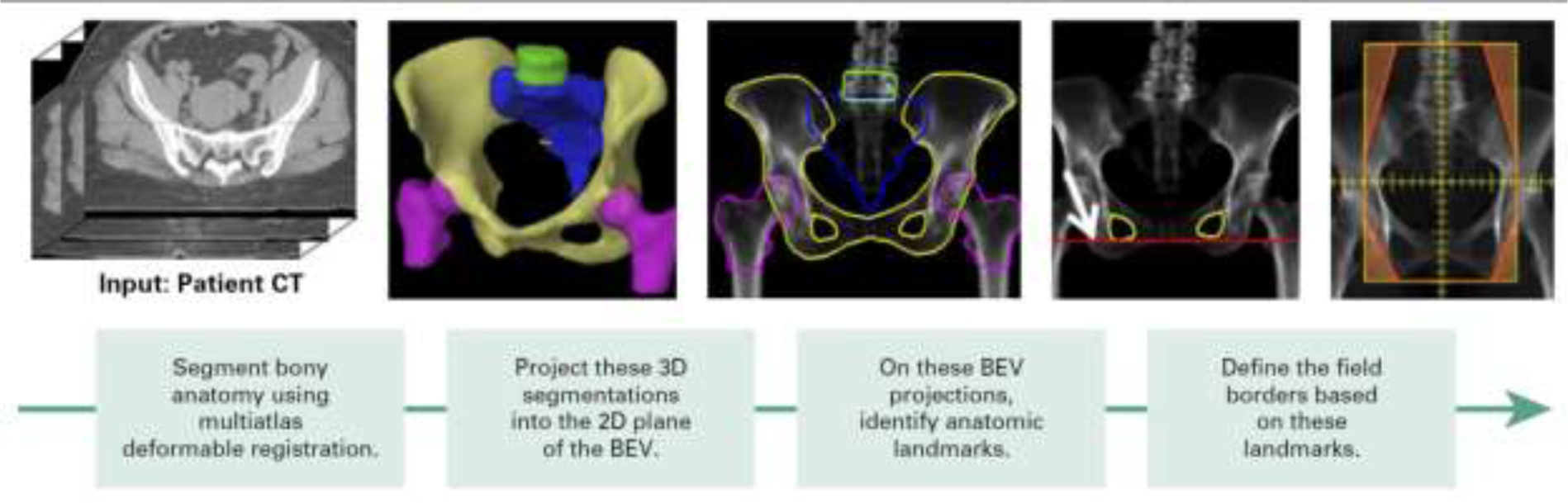
An automated approach to determining the field shapes for simple cervical cancer treatment. 3D, three-dimensional; 2D, two-dimensional; BEV, beam’s eye view. (from Kisling et al.17)
Acknowledgment
This work was supported by National Institutes of Health/National Cancer Institute grants UH2-CA202665, UH3-CA202665, and P30CA016672 (Clinical Trials Support Resource). The authors also thank Laura Russell from the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editing this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry SL, Boczkowski A, Ma R, Mechalakos J, Hunt M. Interobserver variability in radiation therapy plan output: Results of a single-institution study. Pract Radiat Oncol. 2016;6(6):442–449. doi: 10.1016/j.prro.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinod SK, Jameson MG, Min M, Holloway LC. Uncertainties in volume delineation in radiation oncology: A systematic review and recommendations for future studies. Radiother Oncol. 2016;121(2):169–179. doi: 10.1016/j.radonc.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 3.Zhong H, Men K, Wang J, et al. The Impact of Clinical Trial Quality Assurance on Outcome in Head and Neck Radiotherapy Treatment. Front Oncol. 2019;9:792. doi: 10.3389/fonc.2019.00792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelms BE, Robinson G, Markham J, et al. Variation in external beam treatment plan quality: An inter-institutional study of planners and planning systems. Pract Radiat Oncol. 2012;2(4):296–305. doi: 10.1016/j.prro.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 5.Batumalai V, Jameson MG, Forstner DF, Vial P, Holloway LC. How important is dosimetrist experience for intensity modulated radiation therapy? A comparative analysis of a head and neck case. Pract Radiat Oncol. 2013;3(3):e99–e106. doi: 10.1016/j.prro.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 6.Moore KL, Schmidt R, Moiseenko V, et al. Quantifying Unnecessary Normal Tissue Complication Risks due to Suboptimal Planning: A Secondary Study of RTOG 0126. Int J Radiat Oncol Biol Phys. 2015;92(2):228–235. doi: 10.1016/j.ijrobp.2015.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters LJ, O’Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. 2010;28(18):2996–3001. doi: 10.1200/JCO.2009.27.4498 [DOI] [PubMed] [Google Scholar]
- 8.Fairchild A, Straube W, Laurie F, Followill D. Does quality of radiation therapy predict outcomes of multicenter cooperative group trials? A literature review. Int J Radiat Oncol Biol Phys. 2013;87(2):246–260. doi: 10.1016/j.ijrobp.2013.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asnaashari K, Gholami S, Khosravi HR. Lessons learnt from errors in radiotherapy centers TT −. Int-J-Radiat-Res. 2014;12(4):361–367. http://ijrr.com/article-1-1356-en.html. [Google Scholar]
- 10.World Health Organization: Radiotherapy Risk Profile. Technical Manual Geneva; 2008. [Google Scholar]
- 11.Clark BG, Brown RJ, Ploquin J, Dunscombe P. Patient safety improvements in radiation treatment through 5 years of incident learning. Pract Radiat Oncol. 2013;3(3):157–163. doi: 10.1016/j.prro.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 12.Leonard S, O’Donovan A. Measuring safety culture: Application of the Hospital Survey on Patient Safety Culture to radiation therapy departments worldwide. Pract Radiat Oncol. 2018;8(1):e17–e26. doi: 10.1016/j.prro.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 13.Boadu M, Rehani MM. Unintended exposure in radiotherapy: identification of prominent causes. Radiother Oncol. 2009;93(3):609–617. doi: 10.1016/j.radonc.2009.08.044 [DOI] [PubMed] [Google Scholar]
- 14.Datta NR, Samiei M, Bodis S. Radiation Therapy Infrastructure and Human Resources in Low- and Middle-Income Countries: Present Status and Projections for 2020. Int J Radiat Oncol • Biol • Phys. 2014;89(3):448–457. doi: 10.1016/j.ijrobp.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 15.Management of Cervical Cancer: Strategies for Limited-Resource Centres - A Guide for Radiation Oncologists. Vienna: INTERNATIONAL ATOMIC ENERGY AGENCY; 2013. https://www.iaea.org/publications/8738/management-of-cervical-cancer-strategies-for-limited-resource-centres-a-guide-for-radiation-oncologists. [Google Scholar]
- 16.Chuang LT, Feldman S, Nakisige C, Temin S, Berek JS. Management and Care of Women With Invasive Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. J Clin Oncol. 2016;34(27):3354–3355. doi: 10.1200/JCO.2016.68.3789 [DOI] [PubMed] [Google Scholar]
- 17.Kisling K, Zhang L, Simonds H, et al. Fully Automatic Treatment Planning for External-Beam Radiation Therapy of Locally Advanced Cervical Cancer: A Tool for Low-Resource Clinics. J Glob Oncol. 2019;(5):1–9. doi: 10.1200/JGO.18.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardenas CE, Anderson BM, Zhang L, et al. A Comparison of Two Deep Learning Architectures to Automatically Define Patient-Specific Beam Apertures. Med Phys. 2018;45(6):e120–e706. doi: 10.1002/mp.12938 [DOI] [Google Scholar]
- 19.Yang J, Haas B, Fang R, et al. Atlas ranking and selection for automatic segmentation of the esophagus from CT scans. Phys Med Biol. 2017;62(23):9140–9158. doi: 10.1088/1361-6560/aa94ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardenas CE, Yang J, Anderson BM, Court LE, Brock KB. Advances in Auto-Segmentation. Semin Radiat Oncol. 2019;29(3):185–197. doi: 10.1016/j.semradonc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 21.Pekar V, Allaire S, Qazi A, Kim J, Jaffray D. Head and Neck Auto-segmentation Challenge: Segmentation of the Parotid Glands. MICCAI. September 2010. [Google Scholar]
- 22.Raudaschl PF, Zaffino P, Sharp GC, et al. Evaluation of segmentation methods on head and neck CT: Auto-segmentation challenge 2015. Med Phys. 2017;44(5):2020–2036. doi: 10.1002/mp.12197 [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Zhang Y, Zhang L, Dong L. Automatic Segmentation of Parotids from CT Scans Using Multiple Atlases. Med Image Anal Clin A Gd Chall. 2010. [Google Scholar]
- 24.Young AV, Wortham A, Wernick I, Evans A, Ennis RD. Atlas-based segmentation improves consistency and decreases time required for contouring postoperative endometrial cancer nodal volumes. Int J Radiat Oncol Biol Phys. 2011;79(3):943–947. doi: 10.1016/j.ijrobp.2010.04.063 [DOI] [PubMed] [Google Scholar]
- 25.Bondar ML, Hoogeman M, Schillemans W, Heijmen B. Intra-patient semi-automated segmentation of the cervix-uterus in CT-images for adaptive radiotherapy of cervical cancer. Phys Med Biol. 2013;58(15):5317–5332. doi: 10.1088/0031-9155/58/15/5317 [DOI] [PubMed] [Google Scholar]
- 26.Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O. 3D U-Net: Learning Dense Volumetric Segmentation from Sparse Annotation. June 2016. http://arxiv.org/abs/1606.06650. Accessed October 21, 2019.
- 27.Milletari F, Navab N, Ahmadi SA. V-Net: Fully convolutional neural networks for volumetric medical image segmentation. Proc - 2016 4th Int Conf 3D Vision, 3DV 2016. 2016:565–571. doi: 10.1109/3DV.2016.79 [DOI] [Google Scholar]
- 28.Shelhamer E, Long J, Darrell T. Fully Convolutional Networks for Semantic Segmentation. IEEE Trans Pattern Anal Mach Intell. 2017;39(4):640–651. doi: 10.1109/TPAMI.2016.2572683 [DOI] [PubMed] [Google Scholar]
- 29.Chen L-C, Zhu Y, Papandreou G, Schroff F, Adam H. Encoder-Decoder with Atrous Separable Convolution for Semantic Image Segmentation. February 2018. http://arxiv.org/abs/1802.02611. Accessed October 21, 2019. [Google Scholar]
- 30.Aljabar P, Heckemann RA, Hammers A, Hajnal JV, Rueckert D. Multi-atlas based segmentation of brain images: Atlas selection and its effect on accuracy. Neuroimage. 2009;46(3):726–738. doi: 10.1016/j.neuroimage.2009.02.018 [DOI] [PubMed] [Google Scholar]
- 31.Zhou R, Liao Z, Pan T, et al. Cardiac atlas development and validation for automatic segmentation of cardiac substructures. Radiother Oncol. 2017;122(1):66–71. doi: 10.1016/j.radonc.2016.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Men K, Dai J, Li Y. Automatic segmentation of the clinical target volume and organs at risk in the planning CT for rectal cancer using deep dilated convolutional neural networks. Med Phys. 2017;44(12):6377–6389. doi: 10.1002/mp.12602 [DOI] [PubMed] [Google Scholar]
- 33.Macomber MW, Phillips M, Tarapov I, et al. Auto-segmentation of prostate anatomy for radiation treatment planning using deep decision forests of radiomic features. Phys Med Biol. 2018;63(23):235002. doi: 10.1088/1361-6560/aaeaa4 [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Liu X, Xiao B, et al. Segmentation of organs-at-risk in cervical cancer CT images with a convolutional neural network. Phys Medica. 2020;69:184–191. doi: 10.1016/j.ejmp.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Ma C, Huang F. Assessment of a knowledge-based RapidPlan model for patients with postoperative cervical cancer. Precis Radiat Oncol. 1(3):102–107. doi: 10.1002/pro6.23 [DOI] [Google Scholar]
- 36.Li N, Carmona R, Sirak I, et al. Highly Efficient Training, Refinement, and Validation of a Knowledge-based Planning Quality-Control System for Radiation Therapy Clinical Trials. Int J Radiat Oncol Biol Phys. 2017;97(1):164–172. doi: 10.1016/j.ijrobp.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tinoco M, Waga E, Tran K, et al. RapidPlan development of VMAT plans for cervical cancer patients in low- and middle-income countries. Med Dosim. 2019:5086–8097. doi: 10.1016/j.meddos.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 38.Sharfo AWM, Breedveld S, Voet PWJ, et al. Validation of Fully Automated VMAT Plan Generation for Library-Based Plan-of-the-Day Cervical Cancer Radiotherapy. PLoS One. 2016;11(12):e0169202. doi: 10.1371/journal.pone.0169202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kisling K DEVELOPMENT OF AUTOMATED RADIOTHERAPY TREATMENT PLANNING FOR CERVICAL AND BREAST CANCER FOR RESOURCE-CONSTRAINED CLINICS. PhD diss, Univ Texas MD Anderson Cancer Cent UT Heal Grad Sch Biomed Sci. May 2019. [Google Scholar]
- 40.McIntosh C, Svistoun I, Purdie TG. Groupwise Conditional Random Forests for Automatic Shape Classification and Contour Quality Assessment in Radiotherapy Planning. IEEE Trans Med Imaging. 2013;32(6):1043–1057. doi: 10.1109/TMI.2013.2251421 [DOI] [PubMed] [Google Scholar]
- 41.Chen H-C, Tan J, Dolly S, et al. Automated contouring error detection based on supervised geometric attribute distribution models for radiation therapy: A general strategy. Med Phys. 2015;42(2):1048–1059. doi: 10.1118/1.4906197 [DOI] [PubMed] [Google Scholar]
- 42.Rhee DJ, Cardenas CE, Elhalawani H, et al. Automatic detection of contouring errors using convolutional neural networks. Med Phys. 2019;46(11):5089–5097. doi: 10.1002/mp.13814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsen LA, Robinson CG, He GR, et al. Automated radiation therapy treatment plan workflow using a commercial application programming interface. Pract Radiat Oncol. 2014;4(6):358–367. doi: 10.1016/j.prro.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 44.Moore KL, Brame RS, Low DA, Mutic S. Experience-based quality control of clinical intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;81(2):545–551. doi: 10.1016/j.ijrobp.2010.11.030 [DOI] [PubMed] [Google Scholar]
- 45.Wu B, Ricchetti F, Sanguineti G, et al. Patient geometry-driven information retrieval for IMRT treatment plan quality control. Med Phys. 2009;36(12):5497–5505. doi: 10.1118/1.3253464 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Heijmen BJM, Petit SF. Prospective clinical validation of independent DVH prediction for plan QA in automatic treatment planning for prostate cancer patients. Radiother Oncol. 2017;125(3):500–506. doi: 10.1016/j.radonc.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 47.Appenzoller LM, Michalski JM, Thorstad WL, Mutic S, Moore KL. Predicting dose-volume histograms for organs-at-risk in IMRT planning. Med Phys. 2012;39(12):7446–7461. doi: 10.1118/1.4761864 [DOI] [PubMed] [Google Scholar]
- 48.Wu B, Ricchetti F, Sanguineti G, et al. Data-driven approach to generating achievable dose-volume histogram objectives in intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;79(4):1241–1247. doi: 10.1016/j.ijrobp.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 49.Nguyen D, Jia X, Sher D, et al. 3D radiotherapy dose prediction on head and neck cancer patients with a hierarchically densely connected U-net deep learning architecture. Phys Med Biol. 2019;64(6):65020. doi: 10.1088/1361-6560/ab039b [DOI] [PubMed] [Google Scholar]
- 50.Nguyen D, Long T, Jia X, et al. A feasibility study for predicting optimal radiation therapy dose distributions of prostate cancer patients from patient anatomy using deep learning. Sci Rep. 2019;9(1):1076. doi: 10.1038/s41598-018-37741-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kisling K, Johnson JL, Simonds H, et al. A risk assessment of automated treatment planning and recommendations for clinical deployment. Med Phys. 2019;46(6):2567–2574. doi: 10.1002/mp.13552 [DOI] [PMC free article] [PubMed] [Google Scholar]


