Abstract
The sex-specific prevalence of adrenal diseases has been known for a long time. However, the reason for the high prevalence of these diseases in females is not completely understood. Mouse studies have shown that the adult adrenal gland is sexually dimorphic at different levels such as transcriptome, histology, and cell renewal. Here we used RNA-seq to show that in prepubertal mice, male and female adrenal glands were not only sexually dimorphic but also responded differently to the same external stimulus. We previously reported that thyroid hormone receptor β1 (TRβ1) in the adrenal gland is mainly expressed in the inner cortex and the fate of this TRβ1-expressing cell population can be changed by thyroid hormone (triiodothyronine; T3) treatment. In the present study, we found that adrenal glands in prepubertal mice were sexually dimorphic at the level of the transcriptome. Under T3 treatment, prepubertal females had 1162 genes differentially expressed between the saline and T3 groups, whereas in males of the same age, only 512 genes were T3-responsive. Immunostaining demonstrated that several top sexually dimorphic T3-responsive genes, including Cyp2f2 and Dhcr24, were specifically expressed in the adrenal inner cortex, precisely in an area partially overlapping with the X-zone. Under T3 treatment, a unique cortical layer that surrounds the adrenal X-zone expanded significantly, forming a distinct layer peculiar to females. Our findings identified novel marker genes for the inner adrenal cortex, indicating there are different sub-zones in the zona fasciculata. The results also highlight the sex-specific response to thyroid hormone in the mouse adrenal gland.
Keywords: adrenal inner cortex, RNA-seq, X-zone, thyroid hormone, sexual dimorphism
The adrenal gland is composed of 3 layers with distinct groups of cells: the capsule, cortex, and medulla. The cortex itself can be divided into different zones based on histological features and functions. Lineage tracing experiments demonstrated that the adrenal capsule and the outer cortex serve as a stem/progenitor cell pools, which continuously renew the adrenal cortex (1-3). Thus, the zonation of the adrenal cortex also reflects the age difference between the cells in each cortical zone: the younger cells are found in the outer cortex while the aged cells are in the inner cortex. There is a developmentally transient cortical zone between the cortex and the medulla in humans and mice called the fetal zone and X-zone, respectively (4). Lineage tracing experiments revealed that X-zone cells are different from other cortical cells. Cells in the X-zone originate from the early-stage fetal cortex (5, 6), while no cell coming from the differentiated outer cortex (the Cyp11b2-positive cells) contributes to the X-zone (3). In mice, the X-zone is positive for 20α-hydroxysteroid dehydrogenase (20αHSD, encoded by Akr1c18), which becomes histologically evident at 10 to 14 days of age. The 20αHSD-positive X-zone enlarges with increased gene expression of 20αHSD during the first 3 weeks of life, before it begins regressing (7, 8). In male mice, the X-zone regresses completely before postnatal day 40, whereas in females, the X-zone disappears during the first pregnancy (4).
While it is clear that sex hormones control the sexual dimorphism of X-zone regression, the link between the X-zone and the neighboring cortical cells in the zona fasciculata is not fully understood. Interestingly, in adult male mice, whose X-zone has already regressed, a secondary X-zone can be restored through castration (7). Moreover, studies on the constitutive PKA activation mouse model revealed that the adult cortex could transform into a “fetal-like” zone (9, 10). Data from the reporter mouse line of the thyroid hormone receptor β1 (Thrb1-LacZ mice) has indicated that the innermost “adult” cortex shares similar characteristics with the X-zone: cells in both areas are positive for TRβ1 and are responsive to thyroid hormone (triiodothyronine; T3) treatment, which leads to the 20αHSD-positive X-zone hypertrophy (8). Although the TRβ1 expression in the innermost adult cortex disappears with the regression of the X-zone in male mice, this TRβ1-positive zone persists in females after the X-zone has regressed. The sexually dimorphic TRβ1 expression after X-zone regression indicates that there might be a different group of cells in the adrenal cortex that contributes to the sexual dimorphism of the mouse adrenal gland.
In this paper, we used immunohistochemistry and RNA-seq to evaluate the difference in T3-mediated responses between male and female murine adrenal glands. Several marker genes were identified, indicating the existence of different sub-zones in adrenal gland inner cortex.
Materials and Methods
Animals
C57BL/6J mice were housed in 12:12-hour light-dark cycle with free access to regular rodent chow and water. For each stage/experimental group, 3 to 5 animals were examined. Male and female mice were examined separately. T3-treated mice were injected with 1 μg of T3 in 30 μL of saline subcutaneously once daily for 10 days as described (8, 11) starting from postnatal day (P)15 or P25. Control mice were injected with 30 μL of saline. To verify the physiological response of T3 treatment, quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) was used to examine the negative feedback of pituitary Tshb. There was a 170-fold reduction in males and a 33-fold reduction in females (T3 vs saline; P < 0.01). Cyp2f2-null mice shown in Supplementary Figures (12) were generated using C57BL/6-derived embryonic stem cells and were maintained on the C57BL/6 background (13). All procedures followed the protocols approved by the Institutional Animal Care and Use Committees at Auburn University and the University of Arizona.
Immunohistochemistry
The adrenal glands were fixed using in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C for 4 hours or overnight and rinsed in PBS for 3 × 10 minutes. Samples were embedded in paraffin for microtome sectioning at a thickness of 4 μm. Paraffin-embedded sections were dewaxed and rehydrated in 100%, 95%, and 70% ethanol, and then in PBS. Slides were pretreated in boiling 0.1 mM citric acid (pH 6.0) for 8 minutes in a microwave oven. After preincubating with blocking buffer (2% normal donkey serum in PBS with 0.1% Tween 20), sections were incubated with primary antibodies (3βHSD, RRID: AB_2722746 (14), 1:250; DHCR24, RRID: AB_2832944, 1:100 (15); Tyrosine Hydroxylase, RRID: AB_628422 (16), 1:1000; CYP2F2, RRID: AB_10987684 (17), 1:250; SPP1, RRID: AB_2194997 (18), 1:250; 20αHSD, RRID: AB_2832957 (19), 1:500; 20αHSD, RRID: AB_2832956 (20), 1:500). The specificity of CYP2F2 antibody was confirmed using Cyp2f2-null mice (Supplementary Figure A) (12). For double staining, 2 primary antibodies were co-incubated at 4 °C overnight followed by incubation with the proper secondary antibodies at room temperature for 1 hour. For CYP2F2,DHCR24, and SPP1 antibodies, sections were further incubated with avidin-biotin-peroxidase complex for 10 minutes and fluorescent tyramide was used to amplify the signal (TSA kit, PerkinElmer). For CYP2F2 double stained with DHCR24, shown in Fig. 3D, DHCR24 was used for the first round of staining, followed by the microwaving stripping step and then another round of staining with CYP2F2 (21). Fluorescent images were obtained using an ECHO Revolve4 microscope. ImageJ (http://rsb.info.nih.gov/ij/) was used to adjust the brightness, contrast, orientation, and channel merging. At least 3 sections that were 4 μm or farther apart were examined for each adrenal. For each group, at least 3 adrenals from 3 mice were analyzed.
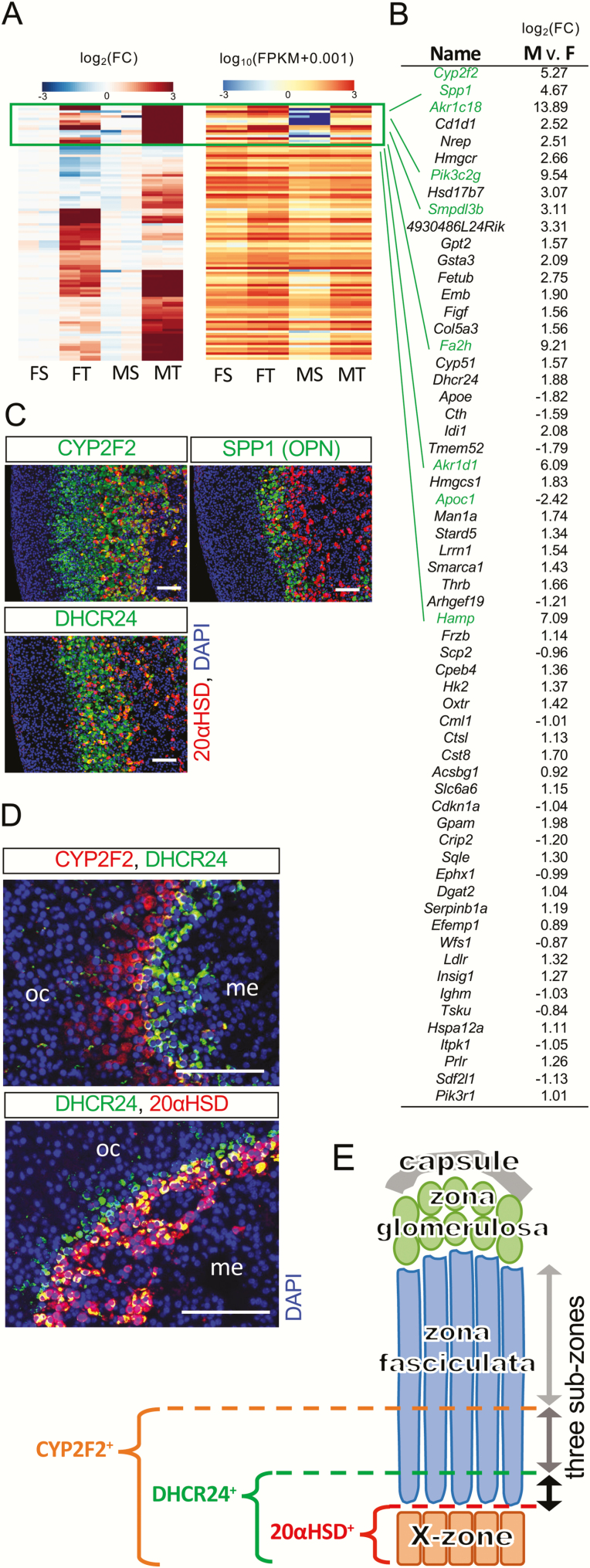
Figure 3.
Sexually dimorphic T3-responsive genes identifying sub-zones in zona fasciculata. (A) Heatmaps of sexually dimorphic T3-responsive genes (total 104 genes). Left panel: the clustered heatmap shows the fold change under T3 treatment within each sex. Note that most T3-responsive genes show a stronger response in males than in females. The heatmap on the right shows the corresponding FPKM of each gene on the clustered heatmap. Note that most genes that have low FPKM in saline-treated males are also grouped together (boxed in green). (B) The sexually dimorphic T3-responsive genes with an average FPKM value greater than 15 are listed. The fold change of log2(fold change under T3) between males and females are shown. The minus values represent genes that have stronger T3-responses in females than in males. Genes are ordered according to adjusted P values (p-adjust). Genes in the cluster boxed in 3A are highlighted in green. (C) CYP2F2, SPP1, and DHCR24 were specifically expressed in the adrenal inner cortex shown in P35 female mice (P25-P35 T3 treatment). (D) CYP2F2, DHCR24, and the X-zone maker 20αHSD were partially colocalized with each other as shown in nontreated nulliparous adult female mice. Similar pattern was also found in both males and females at P21 (data not shown). oc, outer cortex. me, medulla. DAPI (blue, cell nuclei). Scale bars, 100 μm. (E) Zona fasciculata can be divided into 3 concentric sub-zones.
RNA extraction, RNA-seq library preparation, and sequencing
Total RNA was extracted from whole adrenal glands using Monarch Total RNA Miniprep Kit (New England Biolabs) according to the manufacturer’s instructions. Each defined condition, treatment and sex, contained 2 biological replicates. Each biological replicate included adrenal glands from 3 mice. A total of 6 mice were included per condition. Briefly, adrenal glands from 3 mice were placed in the 1xRNA protection reagent, mechanically homogenized using a battery-powered homogenizer for 30 seconds and were treated as 1 biological replicate. Homogenates were treated with DNaseI provided in the kit. The total RNA was then eluted in 50 μL of elution buffer. The total RNA was sent to Novogene Life Sciences Co., Ltd. for its PE150 mRNA-seq service. All samples that passed quality control (concentration > 25 ng/µL, RIN ≥ 7.7) were used for library preparation and the following mRNA sequencing. Trimmed reads were aligned to the UCSC mm10 reference genome. Each sample had at least 43 million uniquely mapped reads.
RNA-seq bioinformatics analysis
The read count data were normalized using regularized logarithm (rlog) transformation in R/Bioconductor package DESeq2 (1.26.0) (22). The rlog normalized counts were then used for principal component analysis and hierarchical clustering. For differential expression analysis, the raw reads were adjusted for sequencing depth using size factors. The differential expression was performed using a negative binomial generalized linear model implemented in DESeq2. To compare the T3-induced gene expression in males and females, we performed 3 separate differential expression analyses using DESeq2. In the first 2 analyses, the effects of T3 versus saline treatment were compared in male and female mice, respectively. In the third analysis, the differences in response to T3 treatment between the sexes were captured by the interaction term in the DESeq2 model. Genes with a false discovery rate (FDR) of less than 0.05 were considered statistically significant. For the analysis of chromosomal linkage, the overrepresented chromosomes for the sexually dimorphic genes, female-biased genes, and male-biased genes compared to adrenal expressing genes were evaluated by Fisher exact test. Overrepresented chromosomes were determined by a nominal P value under 0.05 or a more conservative Bonferroni-based nominal threshold (adjusting for multiple chromosome comparisons) of 2.27 × 10–3. The 23 152 adrenal-expressing genes were used as the population gene set. GO enrichment analysis was analyzed using g:Profiler (23) and the enrichGO function in the clusterProfiler package (3.16.0) in R (24) with P < 0.05 as a cutoff value. Venn diagrams were created using the free online tool VENNY and Meta-Chart (25, 26). Original RNA-seq data (read count, Supplementary Table 1) are available on NIH Figshare (27).
Quantitative RT-PCR Analysis
Total RNA was isolated from whole adrenal glands using TRIzol reagent (Life Technologies). For quantitative RT-PCR (qRT-PCR), total RNA (1 μg) was reverse transcribed for a total volume of 20 μL using 200 U of Superscript IV reverse transcriptase (Invitrogen), 50 μM oligo (dT)20, 10 mM deoxy-NTP, and 40 U RNase inhibitor (Invitrogen). The resultant cDNA was diluted in a 30× dilution with nuclease-free water. Three μL of diluted cDNA was used in subsequent PCR reactions. All primers were designed and/or verified using nucleotide sequences and tools on NCBI (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Primers are listed in Table 1. Each qPCR reaction consisted of 2× Power SYBR Green PCR Master Mix (Thermo Fisher), 0.5 μM of forward and reverse primers, and cDNA to a total volume of 20 μL. qPCR reactions were carried out using a qTOWER3G (Analytik Jena AG) for 40 cycles (95 °C for 15 seconds, 60 °C for 30 seconds, and 72 °C for 30 seconds). The relative gene expressions were calculated using relative standard curves with Actb as a control. Each data point was an average of 3 or more biological replicates.
Table 1.
List of Primers for Quantitative RT-PCR
| Gene name | Accession Number | Amplicon (bp) | Primer set (forward and reverse) | Exon Target (forward/reverse) |
|---|---|---|---|---|
| Actb | NM_007393 | 149 | ATGGAGGGGAATACAGCCC TTCTTTGCAGCTCCTTCGTT |
1/2 |
| Akr1c18 | NM_134066.3 | 200 | GATAGGCCAGGCCATTCTAAGC CATTCCCTGGCTTCAGAGACAC |
2/3,4 |
| Pik3c2g | NM_207683.3 | 221 | CCATTTGTGGACCCAGGTGA GGGTCAGTGCATTTTGGAACA |
21/23 |
| Cyp2f2 | NM_007817.2 | 162 | AAGTGCAACGCTTTGCTGAC TGAACTCCTGAGGCGTCTTG |
8/9 |
| Tshb | NM_001165939.1 | 187 | CCTGACCATCAACACCACCA | 3/4 |
| TATGGCGACAGGGAAGGAGA |
Results
The sexually dimorphic adrenal gland in prepubertal mice
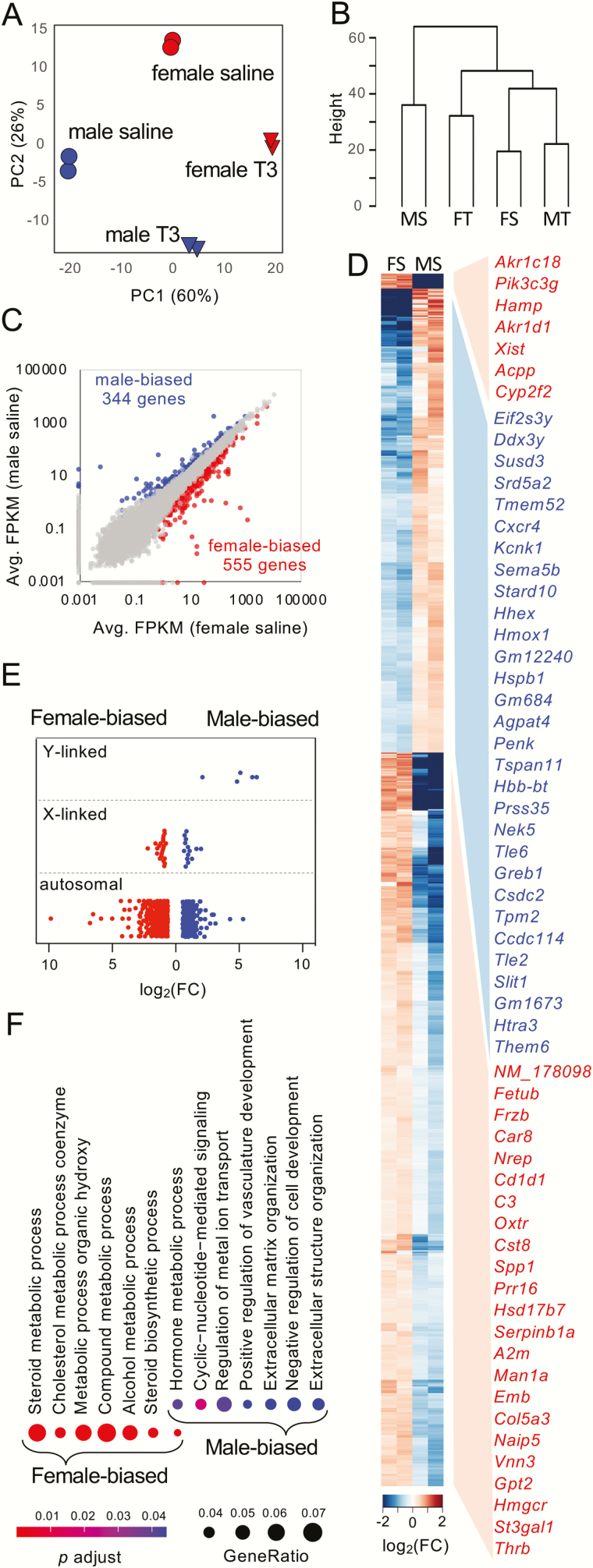
Although it has been reported that the adrenal glands of rodents are sexually dimorphic at the transcriptome level, most studies have focused on adult animals (28-30). The sexual dimorphism of mouse adrenal glands at the age before puberty at the transcriptome level is still unclear. Here, paired-end RNA-seq was used to sequence the transcriptome of adrenals from mice at P35. The mice were treated with saline or T3 from P25 to P35. The principal component analysis showed that all replicates were closely grouped, and different groups were well-separated (Fig. 1A). Unsupervised clustering showed that saline-treated females were clustered with T3-treated males and then T3-treated females, apart from the saline-treated males (Fig. 1B). Within the saline-treated groups, differential expression analyses identified 344 genes with greater transcript abundance in male adrenal glands (male-biased genes), while 555 genes were identified as female-biased genes (Fig. 1C and Supplementary Table 2 (31)). The clustered heatmap (Fig. 1D) showed that female-biased genes could be further clustered into 2 groups. The X-zone maker genes Akr1c18 and Pik3c2g were the top 2 genes clustered in the same group. The chromosomal distribution of these sex-enriched genes showed that the sexually dimorphic genes were mainly located on autosomal chromosomes (Fig. 1E). In all, there were 19 male-biased genes (5.52% of 344 male-biased genes) and 22 female-biased genes (3.96% of 555 female-biased genes) located on sex chromosomes, whereas of all the total adrenal expressing genes, 923 of them were sex chromosome-linked (3.99% of 23 152 adrenal expressing genes). When considering the highly overrepresented chromosomes, the sexually dimorphic genes, especially male-biased genes, were significantly enriched on the Y chromosome with additional overrepresented loci on chromosome 9 (Table 2). An analysis of female-biased genes revealed an enrichment on chromosome 16, confirming that the concentration of sexually dimorphic transcripts can be both on sex chromosomes and autosomes (32). Gene ontology enrichment analysis indicated that male-biased genes and female-biased genes showed diverse GO enrichment (Fig. 1F), with only 1 of the top 10 significant GO terms in common.
Figure 1.
Sexually dimorphic adrenal gland transcriptomes before puberty. (A) Principal-component analysis of expression patterns for T3/saline treatment in males and females. (B) Hierarchical clustering of all 8 samples. Abbreviations: MS, male saline; FT, female T3; FS, female saline; MT, male T3. (C) Scatter plots show differential expressed genes between sexes within saline-treated groups. (D) Clustered heatmap of male-biased genes (344 genes) and female-biased genes (555 genes) in saline-treated groups. The top 30 sex-biased genes of each sex (in red, female-biased genes; in blue, male-biased genes) are listed on the right and are ordered according to the fold change (FC). Only genes with average FPKM greater than 10 are listed. Note that female-biased genes were clustered into 2 groups. (E) Chromosome distribution of the 344 male-biased genes and the 555 female-biased genes. (F) GO enrichment analysis of top biological processes of male-biased genes and female-biased genes.
Table 2.
Overrepresented Chromosomes for Sexually Dimorphic Genes, Male-Biased Genes and Female-Biased Genes at 1- to 3-Fold-Change Thresholds With the Corresponding P Values Determined by Fisher’s Exact test (P < 0.05)
| Sexually dimorphic genes (all) | Male-biased genes | Female-biased genes | ||||
|---|---|---|---|---|---|---|
| Fold change | Chromosome | P value | Chromosome | P value | Chromosome | P value |
| >1 | chrY | 2.64E-04 | chrY | 2.65E-06 | chr16 | 1.18E-03 |
| chr16 | 1.75E-02 | chr9 | 2.91E-02 | |||
| >2 | chrY | 2.64E-04 | chrY | 2.65E-06 | - | |
| >3 | chrY | 2.64E-04 | chrY | 2.65E-06 | - |
Differential expression analysis of male and female mice under T3 treatment
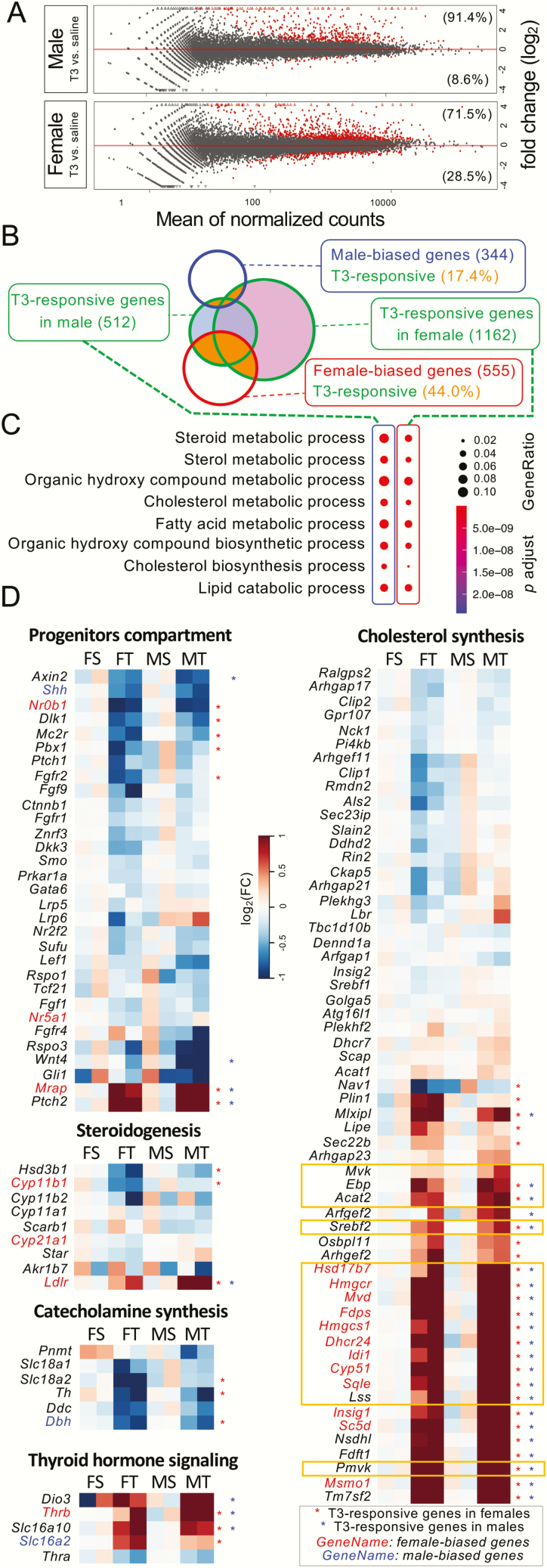
We then compared the T3-mediated effects in males and females. Numerically speaking, female mice had significantly more T3-responsive genes than male mice (1162 genes vs 512 genes, Fig. 2A and 2B). In both sexes, most T3-responsive genes were upregulated under T3 treatment (91.4% in males and 71.5% in females). As for the female-biased genes (shown in the red circle, Fig. 2B), 44.0% of them were T3 responsive, whereas only 17.4% of male-biased genes (shown in the blue circle) responded to T3 (Fig. 2B). GO analysis showed that T3-responisve genes in males and in females shared similar top biological processes, including the organic hydroxy compound metabolic process, fatty acid metabolic process, and pathways involved in the metabolism of steroid hormones (Fig. 2C).
Figure 2.
T3 effects on adrenal gland transcriptomes. (A) Scatter plots of T3-responsive genes in males and in females. Red dots indicate genes with statistical significance at a false discovery rate <5%. In both sexes, most T3-responsive genes are upregulated under T3 treatment (91.4% in males and 71.5% in females). (B) Venn diagram comparing the number of male-biased genes, female-biased genes, and T3 responsive genes in males and in females. The areas in orange represent genes that are both T3-responsive and sex-biased. (C) GO analysis of T3-responsive genes in males and in females. (D) Clustered heatmaps representing the fold change of gene expression under T3 treatment within each sex (comparing to the saline-treated group of the same sex). Genes were identified as T3-responsive genes in males (asterisks in blue) or in females (asterisks in red) have asterisks on the right. Male-biased and female-biased genes are colored in blue and red, respectively. Note that genes with FPKM below 1 in all 8 samples are considered low-expressing genes and are not listed on the heatmap. Key enzymes and regulators of the mevalonate pathway are boxed in orange rectangles. Abbreviations: MS, male saline; FT, female T3; FS, female saline; MT, male T3.
Clustered heatmaps showed that genes in different gene sets (9) had unique T3 response trends (Fig. 2D). For example, 7 genes linked with progenitor cell growth/development such as Nr0b1 and Wnt4 were suppressed by T3 treatment, whereas Mrap and Ptch2 were induced under T3 treatment. Genes involved in steroidogenesis were mostly either unchanged or downregulated in females, except Ldlr, which was upregulated by T3 in both sexes. Three genes involved in catecholamine synthesis were downregulated under T3 treatment, indicating medullary chromaffin cells may directly respond to T3, which could be supported by the expression of TRβ1 in the adrenal medulla (8). Thyroid hormone signaling-related genes were all upregulated under T3 treatment, except Thra, which remained unchanged. Interestingly, within the cholesterol synthesis gene set, almost all key enzymes and regulators involved in the mevalonate pathway (shown in orange boxes in Fig. 2D) were clustered and strongly upregulated after T3 treatment.
Many sexually dimorphic T3-responsive genes were expressed in the adrenal gland inner cortex
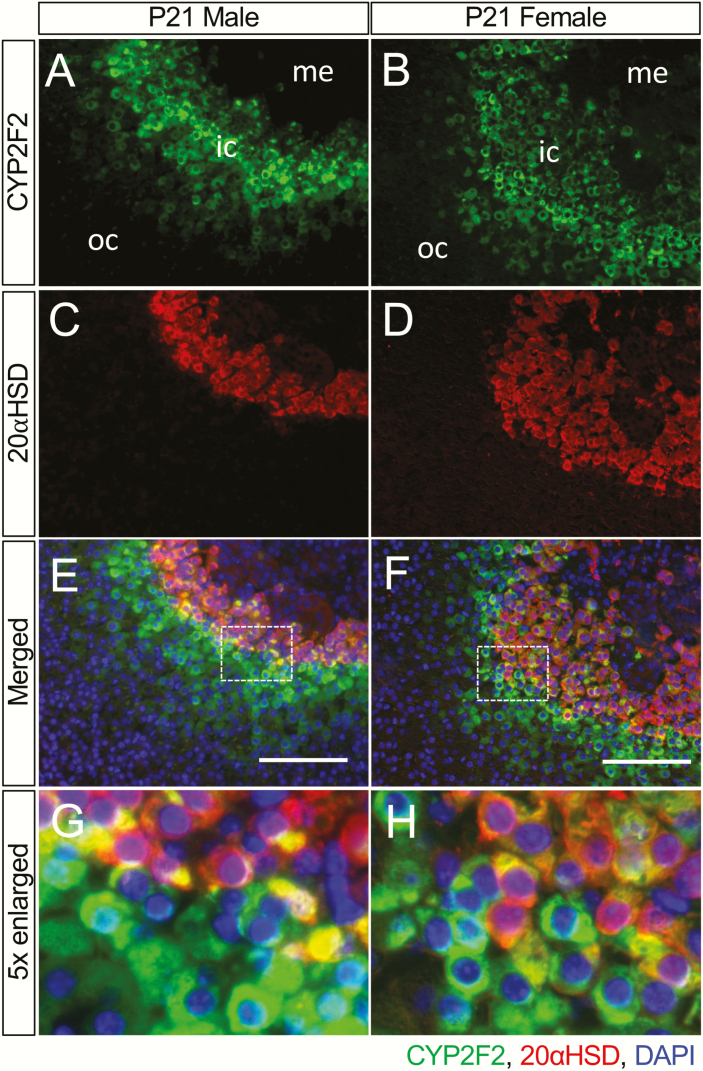
To further understand how sex affects T3-mediated action in the adrenals, we analyzed the interaction between the sex of the mice and the T3-mediated effect by incorporating the interaction effects into the DESeq2 analysis (22). A total of 104 genes (Supplementary Table 2 (31)) showed a sexually dimorphic response between males and females upon T3 treatment. Of these sexually dimorphic T3-responsive genes, 77 responded more vigorously in males than in females. Nine genes within a cluster had a very low expression in saline-treated males with a significant induction (>15-fold) under T3 treatment (Fig. 3A). Two previously published X-zone marker genes, Akr1c18 and Pik3c2g (7, 33), were clustered in this group and were among the top 10 genes of the entire list (Fig. 3B). The recently identified inner cortex marker gene, Thrb (8), was also on the list but not in the same cluster with Akr1c18 (encodes 20αHSD) and Pik3c2g. Since TRβ1 is only partially colocalized with the X-zone marker 20αHSD (8), we used immunostaining to test some top candidate genes both within and outside of the Akr1c18–Pik3c2g cluster to determine their cellular expression pattern. Interestingly, many top candidate genes outside the Akr1c18–Pik3c2g cluster were also specifically expressed in the adrenal inner cortex. However, the spatial expression patterns varied from one marker gene to another. For example, CYP2F2, SPP1, and DHCR24 were expressed in the inner cortex partially colocalized with the X-zone marker gene 20αHSD (Fig. 3C). The double staining of CYP2F2 and DHCR24 showed that these 2 newly identified marker genes were only partially colocalized with each other (Fig. 3D). Under normal conditions, without T3 treatment, the CYP2F2(+) zone was thicker than the DHCR24(+) zone and there was a DHCR24(+); 20αHSD(-) zone surrounding the 20αHSD(+) X-zone. This finding supports the idea that there are different types of adrenal cortical cells in the inner cortex (34), leading to at least 3 concentric sub-zones in the zona fasciculata (Fig. 3E).
Sexually dimorphic expression of CYP2F2 in the adrenal inner cortex
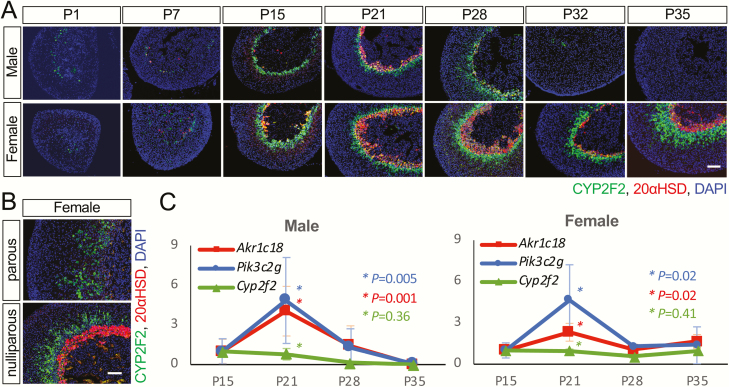
We then did a thorough expression profiling of the top sexually dimorphic T3-responsive gene, CYP2F2, the gene with the most significant adjusted P value on the list of 104 sexually dimorphic T3-responsive genes. At the early postnatal stages, the cellular distribution of CYP2F2 in the adrenal gland was similar in both males and females (Fig. 4). As early as at postnatal day (P) 1, CYP2F2(+) cells were found in the inner cortex around the adrenal medulla in both sexes. The CYP2F2(+) domain then became sharply defined and formed a clear “zone” as early as at P15, before a clear 20αHSD(+) X-zone was established (Fig. 4A). The sexually dimorphic pattern of the CYP2F2(+) population became obvious at P32. In males, the number of CYP2F2(+) cells declined between P28 and P32 and then became undetectable by P35 (Fig. 4A). In females, the expression of CYP2F2 persisted into adulthood even after pregnancy (Fig. 4B). Although the CYP2F2(+) domain was in the inner cortex and its decline in males occurred soon after the disappearance of the 20αHSD(+) X-zone, the overall expression timeline of CYP2F2 was different from 20αHSD. The qRT-PCR results further supported the fact that the developmental change in Cyp2f2 expression was different from the 2 X-zone marker genes, Akr1c18 and Pik3c2g, which were both elevated at P21 (Fig. 4C) compared with the nearby age groups of P15 and P28. Double immunostaining demonstrated a thick CYP2F2(+) domain across the 20αHSD(+) X-zone, leading to a CYP2F2(+);20αHSD(-) zone surrounding the CYP2F2(+);20αHSD(+) X-zone (Fig. 5). This unique CYP2F2 expression pattern was found in all adrenals containing an X-zone, further confirming that there is a unique cortical cell population in the zona fasciculata sharing some similarities with the X-zone. Similar to the normal adrenal gland found in the Akr1c18 null mice (7), the X-zone in Cyp2f2 null mice formed and regressed the same as in wild-type mice (Supplementary Figure A) (12). The overall histological structure of the mutant adrenals, examined by the cortical marker gene 3βHSD and the medullary marker gene tyrosine hydroxylase, was also similar to the wild type both at P21 and P35 in both sexes (Supplementary Figure B) (12).
Figure 4.
The sexually dimorphic expression of the novel inner cortex marker gene CYP2F2. (A) Adrenal glands from P1, P7, P15, P21, P28, P32, and P35 were immunostained with antibodies for CYP2F2 (green) and 20αHSD (red). DAPI (blue, cell nuclei). Scale bars, 100 μm. (B) Parous and nulliparous mice (between 2.5 to 3 months old) were also analyzed. C, qRT-PCR results showing the relative expression levels of Cyp2f2 and the X-zone marker genes (Akr1c18 and Pik3c2g). Asterisks indicate the P value of the comparison of P21 with 2 nearby age groups P15 and P28.
Figure 5.
The partial colocalization of CYP2F2 and 20αHSD. (A and B) Expression of CYP2F2 (green). (C and D) Expression of 20αHSD (red). (E and F) Merged images of CYP2F2 and 20αHSD with DAPI (blue, cell nuclei). (G and H) Enlarged images showing the CYP2F2(+);20αHSD(-) zone and the CYP2F2(+); 20αHSD(+) X-zone; Abbreviations: ic, inner cortex; me, medulla; oc, outer cortex. Scale bars, 100 μm.
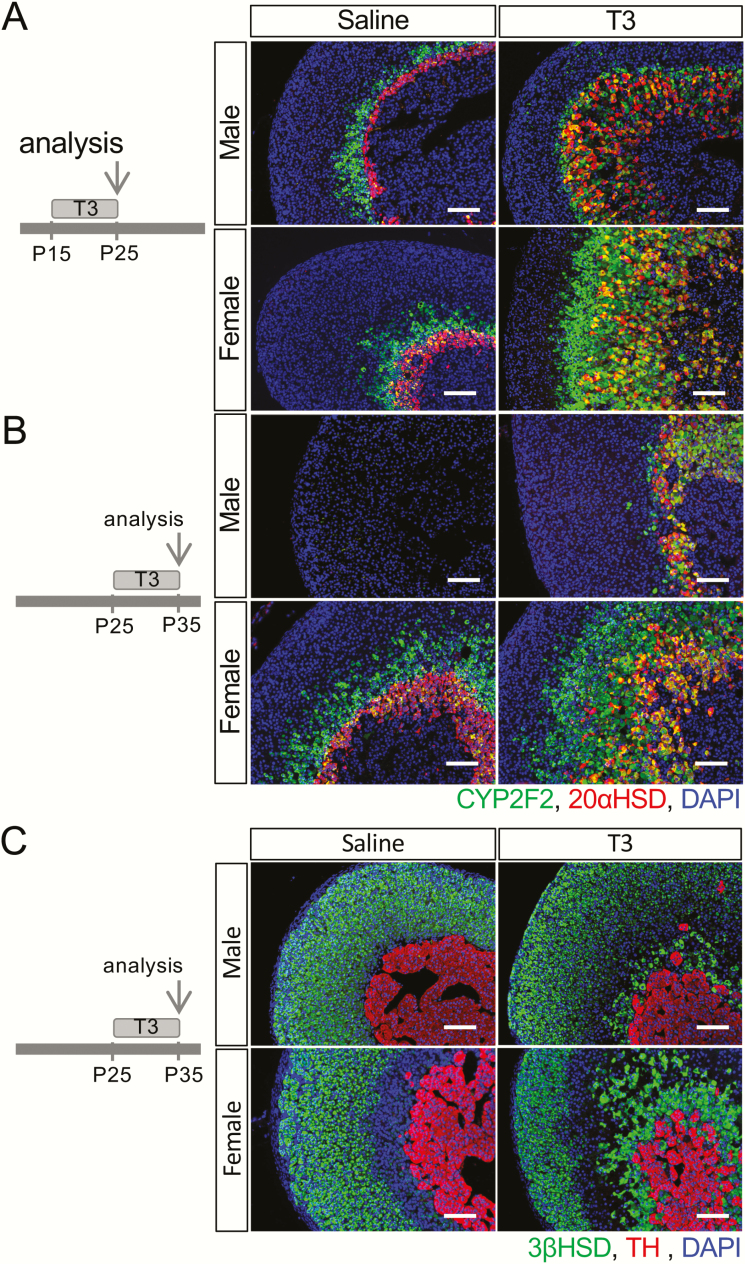
Sexually dimorphic T3-induced hypertrophy of the innermost adult zone
To understand how the newly identified CYP2F2(+) cell population responds to T3 treatment, T3 was given to mice daily for 10 days in both sexes from either P15 or P25. After T3 treatment, the 20αHSD(+) X-zone as well as the CYP2F2(+) zone were expanded in both males and females in the P15-P25 treatment group (Fig. 6A). Similar to reports that T3 treatment prevents the X-zone regression (8), the CYP2F2(+) domain was also maintained in P35 males after a 10-day T3 treatment (Fig. 6B). Although the CYP2F2(+) domain responded to T3 treatment in the adrenals in both sexes, it expanded much more in females than in males, leading to a different pattern when double-stained with 20αHSD. Notably, in female adrenals, both at P25 and P35, there was always a CYP2F2(+);20αHSD(-) zone surrounding the CYP2F2(+);20αHSD(+) area (Fig. 6A and 6B). However, in males, the T3-mediated effect on the adrenal inner cortex resulted in only a single CYP2F2(+);20αHSD(+) domain. This sexually dimorphic T3 response was not easily recognized in the adrenals examined using traditional adrenal cortical marker genes including 3βHSD, SF1, and AKR1B7 (Fig. 6C; only the data of 3βHSD were shown).
Figure 6.
T3 influence on the fate of the inner cortex. Periods of treatment of mice with T3 or saline and day of analysis are shown on the left. (A) At P25, a 10-day T3 treatment led to an expanded inner cortex. (B) At P35, a 10-day T3 treatment prolonged the presence of the inner cortex in males and caused the expansion of the inner cortex in both sexes. (C) The sex difference of the T3-mediated response is less significant if examining the histological distribution of 3βHSD(+) cells and tyrosine hydroxylase(+) cells. DAPI (blue) marks cell nuclei. Scale bars, 100 μm.
Discussion
In this study, we demonstrated that the adrenal gland is sexually dimorphic at the transcriptome level even before puberty. We then identified sexually dimorphic T3-responsive genes in the mouse adrenal gland. All 3 genes known to be specifically expressed in the adrenal gland inner cortex (Akr1c18, Pik3c2g, and Thrb) are on the list. Several top sexually dimorphic T3-responsive genes are also specifically expressed in the inner cortex. The partial colocalization of these newly identified inner cortical marker genes suggests that the zona fasciculata can be further divided into different concentric sub-zones. Results from the T3 treatment indicate that the adrenal inner cortex could respond to the same external cue in a sex-specific manner.
In mice, the existence of extragenital sexual dimorphism has been reported in several organs including the brain, liver, kidney, skeletal muscle, and even the adrenal gland (32, 35-39). In a recent review, Levasseur et al summarized the state of art regarding mechanisms influencing the adrenal gland sexual dimorphism (40). The current understanding of the sexual dimorphism of the adrenal gland is focused on the androgenic control and the mechanisms downstream of the androgen receptors. A genome-wide study was done by El Wakli and colleagues using microarrays to compare gene expression profiles in adult mouse adrenal glands between the two sexes (29). The effects of castration and testosterone treatment were also examined in that study. Out of all the 4 conditions they analyzed (sham male, sham female, castrated male, testosterone treated female), 71 genes were identified as differentially regulated. Only 13 of those genes are also found among the 104 genes we identified as the sexually dimorphic T3-responsive genes. This difference suggests that besides gonadal hormones, other factors, such as age and T3, could also lead to a sexually dimorphic adrenal gland. From our study, the unique CYP2F2(+);20αHSD(-) zone in females under T3 treatment further indicates that the existence of the X-zone may not be the only factor responsible for the sexual dimorphism of murine adrenal glands. Indeed, as early as at embryonic day 14.5, a stage before the X-zone is formed, Cyp17 is weakly expressed in male adrenal glands but is highly expressed in female adrenal glands (41). Moreover, in species such as rats that do not have an X-zone in their adrenal glands, gonadectomy with/without sex hormone replacement could lead to obvious sex-linked differences in the adrenal glands at the transcriptome level (30).
As it is not solely controlled by the plasma T3 concentration, the cellular control or the local regulation of thyroid hormone signaling can also be modified by many factors including thyroid hormone receptors, transporters, and deiodinases (42). Many studies have shown that the local activation or inactivation of thyroid hormones can lead to structural and/or functional changes during development (43). The sexually dimorphic T3 action may be due to the local control of the T3 content, even though no significant differences in serum total T3, T4, and TSH between males and females are found in prepubertal rodents (44). The sex-specific response to T3 has been noticed at different levels in many species (44-46). Despite requiring further studies to fully elucidate the underlying mechanism, it has been reported that the expression of type 1 deiodinase (Dio1), which converts thyroid hormones into active or inactive forms, can be regulated by sex hormones in many tissues (47, 48). Although our data showed that Dio1 and type 2 deiodinases (Dio2) are not actively expressed in the adrenal glands we analyzed (FPKM <1 in both sexes, both saline and T3 treatment), the type 3 deiodinase (Dio3) was identified as showing adrenal expression (Fig. 2C) especially under T3 treatment (average FPKM 10.67 in T3-treated males vs FPKM 1.41 in T3-treated females). It is unclear whether the sexually dimorphic T3 response in the adrenal gland is due to the differentially expressed Dio3 and local inactivation of T3. However, it is interesting that many molecules involved in thyroid hormone signaling, including the thyroid hormone receptor (TRβ1) and the transporter (MCT8), are also expressed in the inner cortex of the adrenal gland (8, 49). Since the expression of Thrb is sexually dimorphic (Fig. 1D) and T3-responsive (Fig. 3B), it is possible that the thyroid hormone receptor not only directly mediates the T3 response locally (50) but also drives the sexually dimorphic T3 response with the deiodinase on site. Our data, which include the findings of differentially expressed genes involved in the cholesterol synthesis and steroidogenesis under T3 treatment, also suggest that the thyroid hormone signaling may contribute to the sexually dimorphic levels of adrenal hormones reported under some circumstances (51).
The finding of the sex-specific T3 response of the CYP2F2(+);20αHSD(-) zone suggests that a particular group of cells in the adrenal cortex responds to the same external cue in a sex-specific manner. A sexually dimorphic response of the adrenocortical cells was recently reported by Grabek et al who showed that cell proliferation and cell recruitment from the adrenal capsule is active in females but suppressed in males (52). They reported that this difference is driven by male hormones that suppress the Gli1(+) stem cells in the capsule, leaving only one progenitor compartment in the male adrenals. Whereas female adrenals still have 2 different cell populations, thus have more rapid tissue renewal. In our RNA-seq study, mice were treated with T3 from P25 to P35. Since the onset of puberty in B6 mice occurred around P32 (53), there is a possibility of influence from sexual maturity. However, testosterone levels remain similar in both sexes during P15 to P25 (54), thus the immunostaining results from the P15-P25 group suggest that there may be a factor(s) besides testosterone contributing to the sexually dimorphic T3 response.
Other than the adrenal gland, Cyp2f2 is highly expressed in the lung, liver and olfactory mucosa (55, 56). Studies using knockout mouse models showed that the Cyp2f2-mediated oxidative metabolism is a key event in the mechanism of cytotoxicity and tumorigenicity (13, 57, 58). Cyp2f2 deficiency attenuates lung cell proliferation in mice challenged with toxic substrates of CYP2F2 (57, 59). Even though Cyp2f2 is identified as the top sexually dimorphic T3-responsive gene, it is unlikely that Cyp2f2 is a primary regulator driving neither the sexual dimorphism nor the cell fate during adrenal development as Cyp2f2 global knockout mice grow and develop normally (13). Indeed, we found that the lack of Cyp2f2 does not affect the X-zone formation and regression. The normal adrenal gland in Cyp2f2 global knockout mice suggest that Cyp2f2 may only be a downstream gene similar to Akr1c18 that reflects the consequence of the T3-mediated histological changes. Interestingly, Cyp2f2 has been reported as one of the differentially expressed genes in the adrenal gland under a variety of conditions (29, 60-62). Based on the centripetal renewal of the adrenal cortex and the unique expression of Cyp2f2 in the inner cortex, we hypothesized that Cyp2f2 may be used as a marker gene for senescence of the adrenocortical cells.
Our data showed that many progenitor compartments, including Dlk1, Rspo3, Wnt4, and the canonical Wnt-responsive gene Axin2, were downregulated under T3 treatment. The nuclear receptor Nr0b1 (encodes DAX-1), which is highly expressed in the female adrenal glands (29, 63) and is known to be a critical transcription factor in adrenal gland development (64, 65), is also downregulated under T3 treatment. Similar to Nr0b1, other factors including Nr5a1, Mrap, and Shh were also identified as sex-biased genes in our study. Although none of these factors exceeded the statistical threshold and was identified as a sexually dimorphic T3-responsive gene, it is still possible that those T3-responsive, sex-biased progenitor compartments are upstream factors that drive the T3-mediated sexual dimorphism.
In conclusion, we have demonstrated that adrenal glands in prepubertal mice were sexually dimorphic at the transcriptome level and have shown that factors other than sex hormones and the X-zone can contribute to the sexual dimorphism of the murine adrenal gland. The sexually dimorphic T3 response of the adrenal inner cortex supports the idea that the male and female adrenal glands could respond differently to the same external cue. Our data also identified several inner cortex marker genes indicating that not all adrenocortical cells in the adrenal inner cortex are the same. The zona fasciculata in murine adrenals can be further divided into unique sub-zones. It is interesting to note that the expression patterns of these marker genes are partially colocalized. The transitional expression pattern of these genes supports the idea that adrenocortical cells are differentiating while migrating from the outer cortex to the inner cortex.
Acknowledgments
Financial Support: This work was supported in part by National Institutes of Health grants R00 HD032636, R00 HD082686 (to C.J.H.) and R01 CA092596 (to X.D.). Q.L. and H.W. were supported by the Chinese government scholarship (from China Scholarship Council) and were visiting scholars to the lab. Some of the equipment used in this study was supported by the Auburn University new faculty start-up funds to C.J.H.
Author Contributions: Q.L. conducted experiments, performed analyses and wrote the manuscript. H.W. performed analyses and wrote the manuscript. Y.K. and H.S.Z. conducted experiments. K.L and K.J. conducted experiments and edited the manuscript. X.W. and X.D. designed and conducted experiments. C.J.H. designed the study, conducted experiments, performed analyses, and wrote the manuscript.
Glossary
Abbreviations
- 20αHSD
20α-hydroxysteroid dehydrogenase
- FPKM
fragments per kilobase of exon per million reads
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative reverse transcriptase–polymerase chain reaction
- T3
triiodothyronine (thyroid hormone)
- TRβ1
thyroid hormone receptor β1
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Huang CC, Miyagawa S, Matsumaru D, Parker KL, Yao HH. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology. 2010;151(3):1119-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. King P, Paul A, Laufer E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc Natl Acad Sci U S A. 2009;106(50):21185-21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freedman BD, Kempna PB, Carlone DL, et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev Cell. 2013;26(6):666-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang CC, Kang Y. The transient cortical zone in the adrenal gland: the mystery of the adrenal X-zone. J Endocrinol. 2019;241(1):R51-R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26(11):4111-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28(23):7030-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148(3):976-988. [DOI] [PubMed] [Google Scholar]
- 8. Huang CC, Kraft C, Moy N, Ng L, Forrest D. A Novel Population of Inner Cortical Cells in the Adrenal Gland That Displays Sexually Dimorphic Expression of Thyroid Hormone Receptor-β1. Endocrinology. 2015;156(6):2338-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumontet T, Sahut-Barnola I, Septier A, et al. PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal. JCI Insight. 2018;3(2):e98394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahut-Barnola I, de Joussineau C, Val P, et al. Cushing’s syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. Plos Genet. 2010;6(6):e1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharlin DS, Ng L, Verrey F, et al. Deafness and loss of cochlear hair cells in the absence of thyroid hormone transporters Slc16a2 (Mct8) and Slc16a10 (Mct10). Sci Rep. 2018;8(1):4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang, C-CJ. Supplementary Figure on the influence of Cyp2f2 deficiency on adrenal phenotype for “RNA-seq reveals sub-zones in mouse adrenal zona fasciculata and the sexually dimorphic responses to thyroid hormone” Figshare, Deposited 15 July 2020. https://DOI.org/10.35092/yhjc.12288272 [DOI] [PMC free article] [PubMed]
- 13. Li L, Wei Y, Van Winkle L, et al. Generation and characterization of a Cyp2f2-null mouse and studies on the role of CYP2F2 in naphthalene-induced toxicity in the lung and nasal olfactory mucosa. J Pharmacol Exp Ther. 2011;339(1):62-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RRID:AB_2722746, https://antibodyregistry.org/AB_2722746
- 15.RRID:AB_2832944, https://antibodyregistry.org/AB_2832944
- 16.RRID:AB_628422, https://antibodyregistry.org/AB_628422
- 17.RRID:AB_10987684, https://antibodyregistry.org/AB_10987684
- 18.RRID:AB_2194997, https://antibodyregistry.org/AB_2194997
- 19.RRID:AB_2832957, https://antibodyregistry.org/AB_2832957
- 20.RRID:AB_2832956, https://antibodyregistry.org/AB_2832956
- 21. Lyu Q, Zheng HS, Laprocina K, Huang CC. Microwaving and fluorophore-tyramide for multiplex immunostaining on mouse adrenals - using unconjugated primary antibodies from the same host species. J Vis Exp. 2020(156): 10.3791/60868. doi: 10.3791/60868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raudvere U, Kolberg L, Kuzmin I, et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47(W1):W191-W198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliveros JC. Venny. An interactive tool for comparing lists with Venn’s diagrams. 2007-2015. http://bioinfogp.cnb.csic.es/tools/venny/index.html. Accessed 19 May 2020. [Google Scholar]
- 26.Meta-Chart. https://www.meta-chart.com/venn. Accessed 19 May 2020.
- 27.Huang, C-CJ. Supplementary Table 1 RNAseq read count file for “RNA-seq reveals sub-zones in mouse adrenal zona fasciculata and the sexually dimorphic responses to thyroid hormone”. Figshare, Deposited 15 July 2020. https://DOI.org/10.35092/yhjc.12148467. [DOI] [PMC free article] [PubMed]
- 28. Trejter M, Hochol A, Tyczewska M, et al. Sex-related gene expression profiles in the adrenal cortex in the mature rat: microarray analysis with emphasis on genes involved in steroidogenesis. Int J Mol Med. 2015;35(3):702-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El Wakil A, Mari B, Barhanin J, Lalli E. Genomic analysis of sexual dimorphism of gene expression in the mouse adrenal gland. Horm Metab Res. 2013;45(12):870-873. [DOI] [PubMed] [Google Scholar]
- 30. Jopek K, Celichowski P, Szyszka M, et al. Transcriptome Profile of Rat Adrenal Evoked by Gonadectomy and Testosterone or Estradiol Replacement. Front Endocrinol (Lausanne). 2017;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, C-CJ. Supplementary Table 2 RNAseq read count file for “RNA-seq reveals sub-zones in mouse adrenal zona fasciculata and the sexually dimorphic responses to thyroid hormone”. Figshare, Deposited 15 July 2020. https://DOI.org/10.35092/yhjc.12148428. [DOI] [PMC free article] [PubMed]
- 32. Yang X, Schadt EE, Wang S, et al. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16(8):995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pihlajoki M, Gretzinger E, Cochran R, et al. Conditional mutagenesis of Gata6 in SF1-positive cells causes gonadal-like differentiation in the adrenal cortex of mice. Endocrinology. 2013;154(5):1754-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pihlajoki M, Dörner J, Cochran RS, Heikinheimo M, Wilson DB. Adrenocortical zonation, renewal, and remodeling. Front Endocrinol (Lausanne). 2015;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bardin CW, Catterall JF. Testosterone: a major determinant of extragenital sexual dimorphism. Science. 1981;211(4488):1285-1294. [DOI] [PubMed] [Google Scholar]
- 36. Coleman MA, Garland T Jr, Marler CA, Newton SS, Swallow JG, Carter PA. Glucocorticoid response to forced exercise in laboratory house mice (Mus domesticus). Physiol Behav. 1998;63(2):279-285. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka S, Matsuzawa A. Comparison of adrenocortical zonation in C57BL/6J and DDD mice. Exp Anim. 1995;44(4): 285-291. [DOI] [PubMed] [Google Scholar]
- 38. Waxman DJ, Celenza JL. Sexual dimorphism of hepatic gene expression: novel biological role of KRAB zinc finger repressors revealed. Genes Dev. 2003;17(21):2607-2613. [DOI] [PubMed] [Google Scholar]
- 39. Kloehn I, Pillai SB, Officer L, Klement C, Gasser PJ, Evans JA. Sexual Differentiation of Circadian Clock Function in the Adrenal Gland. Endocrinology. 2016;157(5):1895-1904. [DOI] [PubMed] [Google Scholar]
- 40. Levasseur A, Typhanie D, Antoine M.. Sexual dimorphism in adrenal gland development and tumorigenesis. Curr Opinion Endocr Metab Res. 2019;8:60-65. [Google Scholar]
- 41. Heikkilä M, Peltoketo H, Leppäluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143(11):4358-4365. [DOI] [PubMed] [Google Scholar]
- 42. Gereben B, Zavacki AM, Ribich S, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29(7):898-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116(10):2571-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marassi MP, Fortunato RS, da Silva AC, et al. Sexual dimorphism in thyroid function and type 1 iodothyronine deiodinase activity in pre-pubertal and adult rats. J Endocrinol. 2007;192(1):121-130. [DOI] [PubMed] [Google Scholar]
- 45. Suzuki S, Nishio S, Takeda T, Komatsu M. Gender-specific regulation of response to thyroid hormone in aging. Thyroid Res. 2012;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Romero GS, Stephan DA, Sperling MA, Menon RK. Distinct sexual dimorphism in the effect of hypothyroidism on the expression of the growth hormone receptor and growth hormone-binding protein gene in rat liver. Horm Res. 1996;45(6):273-278. [DOI] [PubMed] [Google Scholar]
- 47. Miyashita K, Murakami M, Iriuchijima T, Takeuchi T, Mori M. Regulation of rat liver type 1 iodothyronine deiodinase mRNA levels by testosterone. Mol Cell Endocrinol. 1995;115(2):161-167. [DOI] [PubMed] [Google Scholar]
- 48. Šošić-Jurjević B, Filipović B, Renko K, et al. Testosterone and estradiol treatments differently affect pituitary-thyroid axis and liver deiodinase 1 activity in orchidectomized middle-aged rats. Exp Gerontol. 2015;72:85-98. [DOI] [PubMed] [Google Scholar]
- 49. Cleary C, Peterson S, Junghans K, et al. Thyroid Hormone Transporter Mct8 is specially expressed in the adrenal gland inner cortex and partially mediates the thyroid hormone action in the adrenal cortex. J Endocr Soc. 2019;3(Suppl 1):SAT-562. [Google Scholar]
- 50. Wondisford FE. A direct role for thyroid hormone in development of the adrenal cortex. Endocrinology. 2015;156(6):1939-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laughlin GA, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(10):3561-3568. [DOI] [PubMed] [Google Scholar]
- 52. Grabek A, Dolfi B, Klein B, Jian-Motamedi F, Chaboissier MC, Schedl A. The adult adrenal cortex undergoes rapid tissue renewal in a sex-specific manner. Cell Stem Cell. 2019;25(2):290-296.e2. [DOI] [PubMed] [Google Scholar]
- 53. MacLennan MB, Anderson BM, Ma DW. Differential mammary gland development in FVB and C57Bl/6 mice: implications for breast cancer research. Nutrients. 2011;3(11):929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bell MR. Comparing postnatal development of gonadal hormones and associated social behaviors in rats, mice, and humans. Endocrinology. 2018;159(7):2596-2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Renaud HJ, Cui JY, Khan M, Klaassen CD. Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice. Toxicol Sci. 2011;124(2):261-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baldwin RM, Jewell WT, Fanucchi MV, Plopper CG, Buckpitt AR. Comparison of pulmonary/nasal CYP2F expression levels in rodents and rhesus macaque. J Pharmacol Exp Ther. 2004;309(1):127-136. [DOI] [PubMed] [Google Scholar]
- 57. Cruzan G, Bus JS, Banton MI, et al. Editor’s Highlight: Complete Attenuation of Mouse Lung Cell Proliferation and Tumorigenicity in CYP2F2 Knockout and CYP2F1 Humanized Mice Exposed to Inhaled Styrene for up to 2 Years Supports a Lack of Human Relevance. Toxicol Sci. 2017;159(2):413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cruzan G, Bus J, Hotchkiss J, Harkema J, Banton M, Sarang S. CYP2F2-generated metabolites, not styrene oxide, are a key event mediating the mode of action of styrene-induced mouse lung tumors. Regul Toxicol Pharmacol. 2012;62(1):214-220. [DOI] [PubMed] [Google Scholar]
- 59. Cruzan G, Bus J, Hotchkiss J, et al. Studies of styrene, styrene oxide and 4-hydroxystyrene toxicity in CYP2F2 knockout and CYP2F1 humanized mice support lack of human relevance for mouse lung tumors. Regul Toxicol Pharmacol. 2013;66(1):24-29. [DOI] [PubMed] [Google Scholar]
- 60. Kim Y-O, Lee SW. Microarray Analysis of Gene Expression by Ginseng Water Extracts in a Mouse Adrenal Cortex after Immobilization Stress. J Ginseng Res. 2011;35(1):111-113. [Google Scholar]
- 61. Krill KT, Gurdziel K, Heaton JH, Simon DP, Hammer GD. Dicer deficiency reveals microRNAs predicted to control gene expression in the developing adrenal cortex. Mol Endocrinol. 2013;27(5):754-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takamiya M, Saigusa K, Dewa K. DNA microarray analysis of the mouse adrenal gland for the detection of hypothermia biomarkers: potential usefulness for forensic investigation. Ther Hypothermia Temp Manag. 2013;3(2):63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mukai T, Kusaka M, Kawabe K, et al. Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells. 2002;7(7):717-729. [DOI] [PubMed] [Google Scholar]
- 64. Scheys JO, Heaton JH, Hammer GD. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology. 2011;152(9):3430-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xing Y, Morohashi KI, Ingraham HA, Hammer GD. Timing of adrenal regression controlled by synergistic interaction between Sf1 SUMOylation and Dax1. Development. 2017;144(20):3798-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]