Abstract
Staphylococcus aureus (S. aureus) is one of the most common bacterial infections worldwide, and antibiotic resistant strains such as Methicillin-Resistant S. aureus (MRSA) are a major threat and burden to public health. MRSA not only infects immunocompromised patients but also healthy individuals and has rapidly spread from the healthcare setting to the outside community. However, all vaccines tested in clinical trials to date have failed. Immunocompromised individuals such as patients with HIV or decreased levels of CD4+ T cells are highly susceptible to S. aureus infections, and they are also at increased risk of developing fungal infections. We therefore wondered whether stimulation of antifungal immunity might promote the type of immune responses needed for effective host defense against S. aureus. Here we show that vaccination of mice with a fungal β-glucan particle (GP) loaded with S. aureus antigens provides protective immunity to S. aureus. We generated glucan particles loaded with the four S. aureus proteins ClfA, IsdA, MntC, and SdrE, creating the 4X-SA-GP vaccine. Vaccination of mice with three doses of 4X-SA-GP promoted protection in a systemic model of S. aureus infection with a significant reduction in the bacterial burden in the spleen and kidneys. 4X-SA-GP vaccination induced antigen-specific Th1 and Th17 CD4+ T cell and antibody responses and provided long-term protection. This work suggests that the GP vaccine system has potential as a novel approach to developing vaccines for S. aureus.
Author summary
Staphylococcus aureus (S. aureus) is a serious burden to public health as it is a leading cause of antibiotic-resistant infections worldwide. Vaccines aimed at targeting S. aureus have failed in clinical trials, and the reason for this lack of success remains unclear. As this pathogen continues to rapidly spread on a global scale, it is vital that new approaches to S. aureus vaccination are developed. In this study, we show that activation of antifungal immunity with a β-glucan particle vaccine can be harnessed to direct protection against S. aureus systemic infections in mice. This work broadens the use of the β-glucan particle vaccine system for potential use as a much-needed vaccine targeting S. aureus.
Introduction
Staphylococcus aureus is both a human commensal and a formidable opportunistic pathogen. As a commensal, S. aureus can be found in places such as the anterior nares, throat, skin, and gastrointestinal tract [1–3]. It is estimated that between 20–40% of individuals in the general population are colonized with S. aureus in the nasal mucosa [2, 4]. As an opportunistic pathogen, S. aureus can cause a range of diseases including skin and soft tissue infections (SSTIs), endocarditis, sepsis, pneumonia, osteomyelitis, bacteremia, and abscesses in organ tissues [5]. Moreover, S. aureus bacteremia mortality remains at a high rate of 15–50%, despite the use of novel antibiotics and emphasis on disease surveillance and prevention [6]. Antibiotic-resistant strains of S. aureus are becoming increasingly prevalent, with the most common, methicillin-resistant S. aureus (MRSA), emerging in hospitals and communities around the world. These strains infect healthy individuals and immunocompromised patients alike [1].
Unlike many bacterial infections, S. aureus does not usually promote robust protective immune responses, and individuals are not typically protected from subsequent S. aureus infections [7]. Hence, the development of a vaccine to target S. aureus is an urgent and crucial public health need. Unfortunately, vaccines tested in clinical trials thus far have proved unsuccessful in providing protection [8–11]. These vaccines focused primarily on generating antibody responses, and their failures, together with the observation that patients with defects in humoral immunity do not generally exhibit increased susceptibility to S. aureus infections [12, 13], suggest that cell-mediated immunity (CMI) may be more important than previously anticipated for vaccine-induced protection [7, 14].
Individuals that have defects in CMI such as HIV, defective IFNγ production, or treatment with high levels of glucocorticoids experience more S. aureus infections [7]. Patients with STAT3 mutations, which causes hyper-IgE (Job’s) syndrome, have dysfunctional or absent T-helper (Th)17 cells, and they are highly susceptible to S. aureus infections [15, 16]. Due to the involvement of Th17 cells in activating and recruiting neutrophils, it is not surprising that patients with neutrophil disorders are at more risk of becoming infected with S. aureus [7]. Intriguingly, many of the immune defects that predispose individuals to S. aureus also predispose individuals to fungal infections. Patients with HIV are more susceptible to fungal infections, especially chronic mucocutaneous candidiasis (CMC) [17]. Furthermore, patients with STAT3 deficiency and hyper-IgE syndrome often experience CMC along with severe S. aureus infections.
Th1 and Th17 subsets have been shown to be especially vital for antifungal responses in animal models and in humans [18–20]. Th1 and Th17 T cell subsets participate in promoting activation of phagocytes and recruitment of neutrophils via secretion of the cytokines IFNγ and IL-17 and they are also critical in clearing S. aureus infections [21]. Failure of these responses to elicit long term immunity, however, suggests that activation of other innate responses may be necessary to promote Th1 and Th17 responses during S. aureus infection. Antifungal immunity might be harnessed to activate such responses.
Innate pattern recognition receptors (PRRs), which recognize pathogen associated molecular patterns (PAMPs), are responsible for initial sensing of pathogens and tailor inflammatory immune responses to direct ensuing adaptive responses [22]. One such PAMP is β-glucan, which consists of carbohydrate polymers (β(1→3)- and β(1→6)-linked) abundantly found in most fungal cell walls [23]. β-glucans are recognized predominantly by the PRR Dectin-1 [24] as well as Complement Receptor 3 (CR3) via activation of the alternative pathway of complement [24, 25]. β-glucan particles (GPs) have been investigated as a potentially novel vaccine delivery platform [25–30]. β-glucan particles derived from the yeast Saccharomyces cerevisiae can act as an adjuvant by stimulating Dectin-1 and CR3. The hollow structure of GPs allows them to be loaded with payloads such as proteins or peptide antigens, DNA, siRNA, nanoparticles, or other small molecules for diverse purposes, including vaccine development [27]. Antigen-loaded glucan particles ultimately promote antigen presenting cell (APC) maturation and initiation of adaptive immune responses, with antibody production and polarization of CD4+ T cells to Th1 and Th17 subsets [26, 29, 31–37].
Given the connection between patients with specific immune defects and their increased risk for fungal and S. aureus infections, we hypothesized that a glucan particle vaccine could provide protective immunity to S. aureus by activating antifungal-tailored immunity though the β-glucan particle while delivering S. aureus antigens. Here we demonstrate that immunization of mice with a GP + S. aureus antigen vaccine protects mice against systemic infection with S. aureus. Multiple immune cell types including dendritic cells phagocytose the vaccine in vivo in the peritoneal cavity, and these professional antigen presenting cells are vital for stimulating strong adaptive T cell responses [38]. Moreover, this vaccination promotes polarization of antigen-specific CD4+ T cells to the Th1 and Th17 subsets and the production of antigen-specific antibodies, consistent with previous studies using ovalbumin-loaded GPs [26, 29]. While antibodies produced in response to the vaccine may provide some modest protection against infection, we determined that in our infection model, CD4+ T cells are critical for vaccine-induced protection. Finally, the GP + S. aureus antigen vaccine elicited long-term protection as mice had reduced bacterial burden in the spleen and kidneys and detectable antibody levels eight weeks after immunization.
Results
The ideal number of antigens that should be included in a S. aureus vaccine is still a matter of debate [7, 14, 39, 40]. Many investigators favor a multi-antigen vaccine with the idea that this approach can allow targeting of multiple S. aureus virulence factors and survival mechanisms that may be effective at stopping the bacteria at different stages and sites of infection [7]. We have thus opted to evaluate a multi-antigen GP vaccine. The Schneewind laboratory previously tested antigenicity of surface proteins conserved within eight different S. aureus strains and identified antigens promoting the best protection [41]. Among these antigens were the surface proteins clumping factor A (ClfA), iron-regulated surface determinant protein A (IsdA), and serine-aspartate repeat-containing protein E (SdrE). Each of these antigens has also been used by others in various mouse S. aureus vaccination studies providing protection from lethal i.v. challenge, abscess formation, arthritis, and necrotic wound infection ([41–45]). Additionally, many other groups have shown the importance of another surface protein, manganese transport protein C (MntC), in S. aureus virulence and the efficacy of incorporating it in vaccines for inducing protection [9, 46, 47]. As discussed further below, a couple of these antigens have progressed to study in human clinical trials. Together the data suggest that these are widely acceptable antigen targets.
We recombinantly produced and purified ClfA, IsdA, MntC, and SdrE and loaded them into our GPs to create “4X-SA-GP” for evaluation as a novel S. aureus vaccine in mice (S1 Fig). For some experiments, the four recombinant proteins were labeled with the fluorescent probe fluorescein isothiocyanate (FITC) and then incorporated into the GPs to create “FITC-4X-SA-GP”.
4X-SA-GP behave similarly to OVA-loaded GPs and are efficiently phagocytosed by dendritic cells and promote dendritic cell maturation and production of pro-inflammatory cytokines in vitro
In order to test antigen-loaded glucan particles (GPs) in the context of a S. aureus vaccine, we first validated the GP vaccine strategy using GPs loaded with ovalbumin (GP-OVA) as previously created and described by the Levitz and Ostroff groups [25, 26, 29]. GPs have immunostimulatory properties as an adjuvant, and they can also act as an antigen delivery system [27, 48–50]. We utilized a co-culture assay to confirm that the antigen we encapsulated within the GPs can be processed and presented by APCs to CD4+ T cells (S2 Fig). Mouse bone marrow-derived dendritic cells (BMDCs) were fed GPs loaded with varying concentrations of OVA and the BMDCs were then co-cultured with naïve OT-II CD4+ T cells, which recognize OVA peptide in the context of MHC-II. If the OVA antigen is eaten, processed, and presented to the OTII T cells as OVA peptide by the BMDCs, the T cells should be activated by their cognate antigen, proliferate, and polarize to specific T cell subsets based on the cytokine milieu. Assessment of the OT-II T cell cytokine production via flow cytometry revealed that the cells produced IL-17 and IFNγ, with an increase in IL-17 production corresponding with increasing concentrations of OVA in the GPs (S2A, S2C and S2D Fig). These results were also observed in the co-culture supernatants (S2F and S2G Fig). Moreover, GPs without antigen inside (GP-No OVA) did not elicit OT-II T cell proliferation, while the GPs encapsulated with OVA (0.5μg, 5 μg, 50 μg) promoted robust OT-II proliferation as determined by CFSE dilution (S2B and S2E Fig). These results establish that we were able to successfully generate antigen-loaded GPs similar to those that have been previously described [29, 51] and that these particles worked as an antigen delivery system to drive proliferation and cytokine production by OT-II CD4+ T cells. Moreover, the GP-OVA system specifically polarized naïve OT-II T cells into Th1 and Th17 subsets, which are the subsets most necessary to generate protection against S. aureus infection [21].
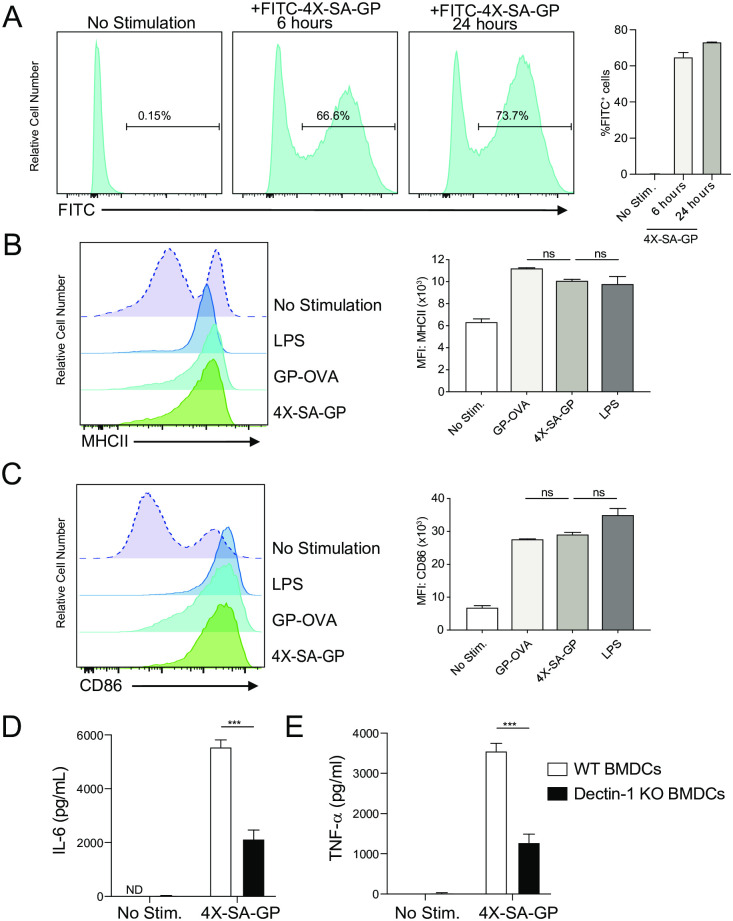
To establish that the GPs are efficiently phagocytosed, we fed BMDCs FITC-4X-SA-GP for 6 and 24 hours and analyzed them by flow cytometry (Fig 1A). The BMDCs efficiently phagocytosed the particles, with over 66% of the BMDCs being FITC+ at 6 hours and over 70% at 24 hours post-stimulation. 4X-SA-GP also induced maturation of BMDCs as seen by upregulation of surface MHC-II and CD86 (Fig 1B and 1C). The expression levels of these maturation markers were comparable to the levels seen in BMDCs stimulated with GP-OVA and LPS. Additionally, stimulation of wild-type BMDCs with 4X-SA-GP resulted in production of IL-6 (Fig 1D) and TNF-α (Fig 1E), while stimulation of Dectin-1 knockout BMDCs led to significant reduction in the production of these cytokines (Fig 1D and 1E). In the absence of serum and therefore complement in this in vitro system, Dectin-1 is the primary receptor for these GPs [25]. It is possible that the antigens (or co-purifying bacterial products) in the GPs may be sensed by other PPRs, resulting in detectable production of cytokines by the Dectin-1 knockout cells.
Fig 1. 4X-SA-GP are efficiently phagocytosed by dendritic cells and promote dendritic cell maturation and production of pro-inflammatory cytokines in vitro.
(A). Representative flow cytometry histograms showing the percentage of FITC+ bone marrow-derived dendritic cells (BMDCs) after being stimulated with FITC-labeled 4X-SA-GP for 6 and 24 hours. Percentages and standard deviation are representative of 2 replicates/stimulus. Flow cytometry analysis of surface MHC-II (B) and CD86 (C) on BMDCs stimulated for 24 hours with LPS, GP-OVA, and 4X-SA-GP. Data are quantified as the mean fluorescence intensity (MFI) for each marker. (D) Wild-type and (E) Dectin-1 knockout (Dectin-1 KO) BMDCs were stimulated with 4X-SA-GP and supernatants were harvested at 24 hours for cytokine determination by ELISA. Percentages and standard deviation are representative of 2 replicates/stimulus (A-C) and 3 replicates/stimulus (D-E). Data analysis was performed using ANOVA for (B) and (C) and Student’s t test for (D) and (E). ***p<0.0005; ns, not significant. Data are representative of two experiments.
Characterization of 4X-SA-GP distribution in vivo reveals uptake by multiple immune cell types
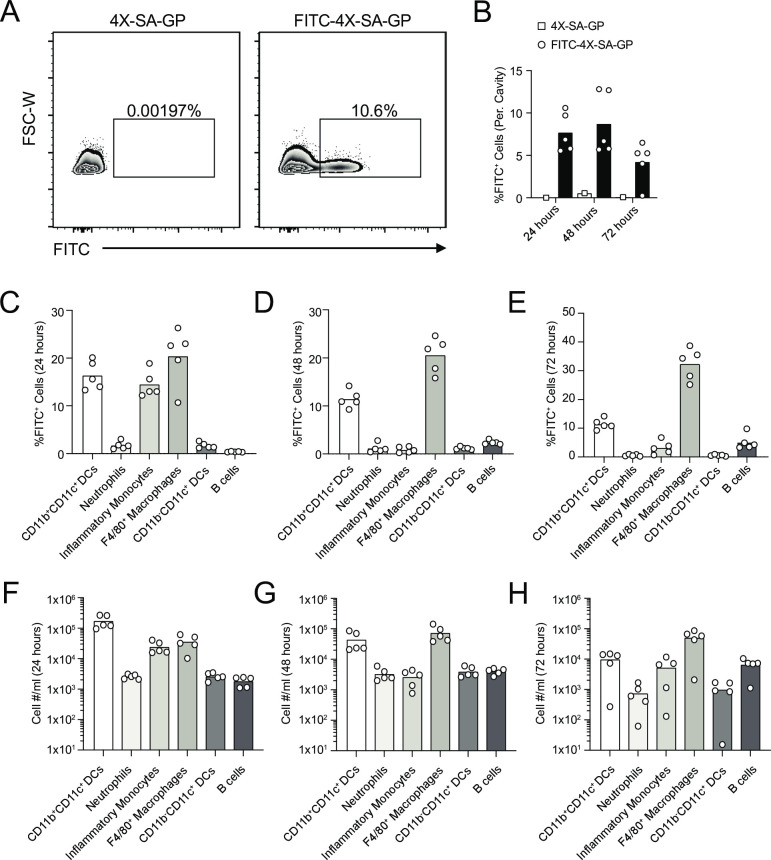
To determine which immune cell subsets phagocytose 4X-SA-GP in vivo, we injected mice once with an intraperitoneal (i.p.) dose of 5x107 FITC-4X-SA-GP or unlabeled 4X-SA-GP as a control. We collected cells by peritoneal lavages at 24, 48, and 72-hours post-injection and analyzed FITC+ immune cells by flow cytometry (Fig 2). The percent of FITC+ cells among the total peritoneal cavity ranged from an average of over 7.5% at 24 hours, to almost 9% at 48 hours and then dropped to a little over 4% at 72 hours post-injection (Fig 2A and 2B). Dissection of the immune subsets that were FITC+ revealed that myeloid DCs, neutrophils, inflammatory monocytes, F4/80+ macrophages, lymphoid DCs and B cells all ate the particles that were injected into the peritoneal cavity (Fig 2C–2H).
Fig 2. Multiple immune cell subsets phagocytose 4X-SA-GP in vivo.
(A) Representative flow cytometry plots and gating strategy of FITC+ cells from the peritoneal cavity of mice injected intraperitoneally (i.p.) with 4X-SA-GP (left plot) and FITC-labeled 4X-SA-GP (right plot) at 24 hours post-injection. (B) Percentage of FITC+ cells in the peritoneal cavity of mice that received an i.p. injection of 4X-SA-GP or FITC-labeled 4X-SA-GP at 24, 48, and 72 hours post-injection. Each data point represents an individual mouse (n = 1 for 4X-SA-GP per time point and n = 5 for FITC-4X-SA-GP per time point). (C-E) Flow cytometry bar graphs show the frequency of the indicated immune cell subsets that are FITC+ cells, indicative of phagocytosing the FITC-labeled 4X-SA-GP at 24 hours (C), 48 hours (D), and 72 hours (E) post i.p. injection of the particles. (F-H) Flow cytometry bar graphs show the numbers of immune cell subsets from (C-E) that are FITC+ at the indicated time points, 24 hours (F), 48 hours (G), and 72 hours (H) post-injection isolated from the peritoneal cavity. Each data point represents percentages or total numbers from an individual mouse. Percentages data are representative of two experiments and total numbers data are from a single experiment.
Initially, at 24 hours post-injection, myeloid DCs, inflammatory monocytes, and F4/80+ macrophages were the main cell types phagocytosing the 4X-SA-GPs based on FITC positivity (Fig 2C and 2F). After 48 hours, F4/80+ macrophages were the major cells that were FITC+, followed by the myeloid DCs; the percentage of FITC+ inflammatory monocytes had decreased substantially (Fig 2D and 2G). Interestingly, at this timepoint, the total number and percentage of FITC+ B cells increased (Fig 2D and 2G). Murine peritoneal cavity B cells (B-1 B cells) have been reported to have anti-microbial capabilities and are able phagocytose particulate antigen and present antigen to CD4+ T cells [52]. This type of murine B cell expresses CR3 [53] suggesting a plausible mechanism by which to imagine these B cells recognize and uptake the 4X-SA-GP vaccine. After 72 hours, F4/80+ macrophages remained the dominant FITC+ cell type, followed by myeloid DCs and B cells (Fig 2E and 2H).
4X-SA-GP vaccination induces protection from S. aureus
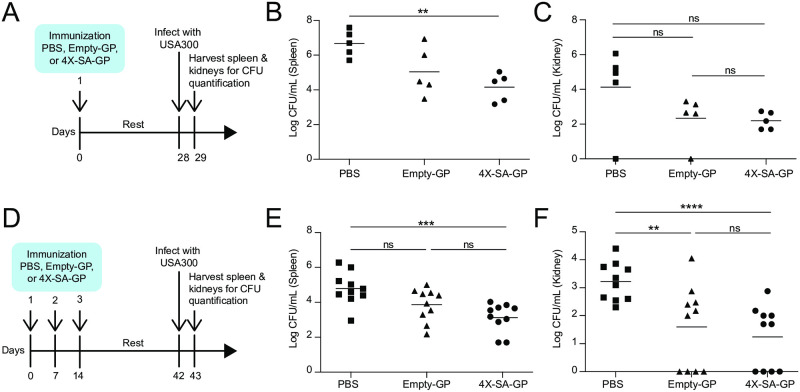
We tested our S. aureus vaccine in vivo using a systemic infection model induced by S. aureus peritoneal challenge. We vaccinated wild-type female mice with a single dose of 5x107 4X-SA-GPs (i.p.) followed by a four-week resting period (Fig 3A). Control animals were “vaccinated” with PBS or with empty-GPs. We then challenged all three groups of vaccinated mice with S. aureus (USA300) by i.p. injection of 2x107 colony forming units (CFUs), and we measured bacterial burdens in the spleen and kidney after 24 hours. Mice vaccinated with 4X-SA-GP had significantly reduced bacterial burden in the spleen compared to mice vaccinated with the vehicle control (Fig 3B). The bacterial burden was also decreased in the kidneys of 4X-SA-GP immunized mice relative to the PBS control mice (Fig 3C). Although not significant, mice that received the empty-GP vaccination appeared to have diminished spleen and kidney CFUs compared to PBS control mice (Fig 3B and 3C). This could be due to the adjuvant effect of the GPs, resulting in inflammation that is still present in the peritoneal cavity at the time of infection. Alternatively, this protection may be the result of trained immunity [54–56].
Fig 3. Mice immunized with 4X-SA-GP have enhanced protection against S. aureus infection.
(A) Experimental timeline of 1 vaccination and S. aureus infection model. (B and C) Wild-type female mice (n = 5 per immunization group: PBS, Empty-GP, 4X-SA-GP) were infected i.p. with 2x107 CFUs of S. aureus (LAC USA300) 4 weeks after the one vaccination. Bacteria from the spleen (B) and kidneys (C) were recovered after 24 hours and CFUs were enumerated. (D) Experimental timeline of 3 vaccinations and S. aureus infection model. (E and F) Wild-type female mice (n = 10 per immunization group: PBS, Empty-GP, 4X-SA-GP) were infected i.p. with 2x107 CFUs of S. aureus (LAC USA300) 4 weeks after the third and final vaccination. Bacteria from the spleen (E) and kidneys (F) were recovered after 24 hours and CFUs were enumerated. Each data point represents an individual mouse. Data analysis was performed using Kruskal-Wallis test ((B) = 0.02, (C) = 0.14, (E) = 0.003, (F) = 0.001) and Mann-Whitney U test. **p<0.01, ***p<0.001, ****p<0.0005; ns, not significant. Data in (B) and (C) are representative of two experiments, data in (E) and (F) are representative of three experiments.
We next explored the effects of increasing the vaccination schedule to three total vaccinations (Fig 3D). This approach has been suggested to provide the best adaptive memory response in mouse studies characterizing the OVA-loaded glucan particle system [29]. We vaccinated (i.p.) wild-type female mice once a week for three weeks. Four weeks after the final vaccination, we challenged the mice (i.p.) with S. aureus and analyzed bacterial burdens in the spleen and kidneys after 24 hours (Fig 3D). 4X-SA-GP vaccinated mice had significantly reduced bacterial burden in the spleen and kidneys when compared to mice that received a PBS control vaccination (Fig 3E and 3F). While the average CFU counts from the 4X-SA-GP vaccinated mice trended lower than those from the empty-GP vaccinated mice in the spleen and kidneys, they were not significantly different (Fig 3E and 3F). In fact, the empty-GP vaccination resulted in reduced bacterial burden in the kidneys that was significantly lower than that of the PBS control group (Fig 3F). These results once again point to the adjuvant quality of the glucan particles on their own and possibly of short-lived, non-antigen-specific, protective inflammatory immunity.
4X-SA-GP promotes antigen-specific Th1 and Th17 CD4+ T cell responses and strong antibody responses
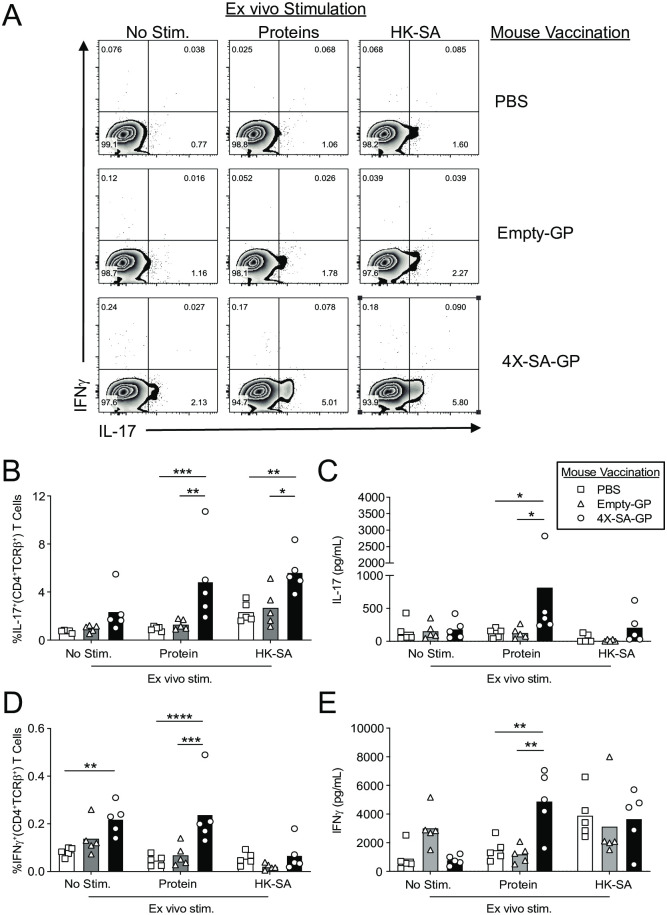
To determine if three vaccinations promoted adaptive immune responses, we assessed antigen-specific T cell responses (Fig 4). We vaccinated mice for three weeks, and five days after the final vaccination, we isolated cells from the spleens and stimulated them with a mixture of the four purified, recombinant S. aureus proteins or with heat-killed S. aureus (HK-SA). We collected supernatants after three days of culture for analysis by ELISA, and we restimulated the cells with PMA and ionomycin for analysis by intracellular cytokine staining for IFNγ and IL-17. Flow cytometry revealed a significant increase in the frequency of IL-17+ CD4+ T cells from the spleen of 4X-SA-GP vaccinated mice when stimulated with the protein mixture and with HK-SA in comparison to the CD4+ T cells of mice vaccinated with empty-GPs or PBS control (Fig 4A and 4B). We confirmed the increased production of IL-17 from the splenocyte culture of 4X-SA-GP vaccinated mice after stimulation with the proteins by ELISA, observing that the secreted IL-17 levels were significantly higher compared to mice immunized with empty-GPs or the PBS control (Fig 4C). Stimulation with HK-SA promoted a rise in IL-17 levels in some of the vaccinated mice, though it did not reach significance (Fig 4C).
Fig 4. 4X-SA-GP promotes antigen-specific Th1 and Th17 CD4+ T cell responses in mice after three immunizations.
(A-E) Wild-type female mice were immunized once a week for 3 weeks with PBS (n = 5), Empty-GPs (n = 5), or 4X-SA-GP (n = 5). Five days after the final vaccination, splenocytes from each mouse were stimulated with a mixture of all 4 S. aureus purified recombinant proteins (proteins) and heat-killed S. aureus (HK-SA). After 3 days, the splenocytes were stimulated with phorbol myristate acetate (PMA) and ionomycin and then analyzed by flow cytometry after intracellular cytokine staining. Representative flow cytometry plots demonstrate the degree of IFNγ and IL-17 production from CD4+ T cells (gated on TCRβ+CD4+) in response to the stimuli (proteins, HK-SA) from the spleen (A). Bar graphs show the frequency of IL-17 (B) and IFNγ (D) cytokine positive cells in the spleen from the mice. Each data point represents an individual mouse in all the bar graphs. (C and E) Supernatants were harvested at day 3 from the splenocyte stimulation and the cytokines IL-17 (C) and IFNγ (E) were determined by ELISA. Data are representative of two experiments. Data analysis was performed using ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Analysis of CD4+ T cells from each of the groups via flow cytometry revealed an elevated frequency of IFNγ+CD4+ T cells in the splenocyte cultures from the 4X-SA-GP vaccinated mice when stimulated with the four S. aureus recombinant proteins that was significant relative to the frequency of IFNγ+CD4+ T cells from the empty-GP vaccinated mice and PBS control mice (Fig 4A and 4D). IFNγ levels in the splenocyte supernatants were also elevated in cultures from mice vaccinated with 4X-SA-GP relative to cultures of the other two vaccination groups when stimulated with the proteins (Fig 4E). Ultimately, this assay demonstrated that immunization of mice three times with the 4X-SA-GP vaccine promoted antigen-specific Th1 and Th17 CD4+ T cell responses that are not seen in the empty-GP vaccination group (or the PBS control group). By comparison, single dose vaccination with 4X-SA-GP also induced antigen-specific CD4+ T cell responses, though the Th17 responses were not as strong (S3 Fig).
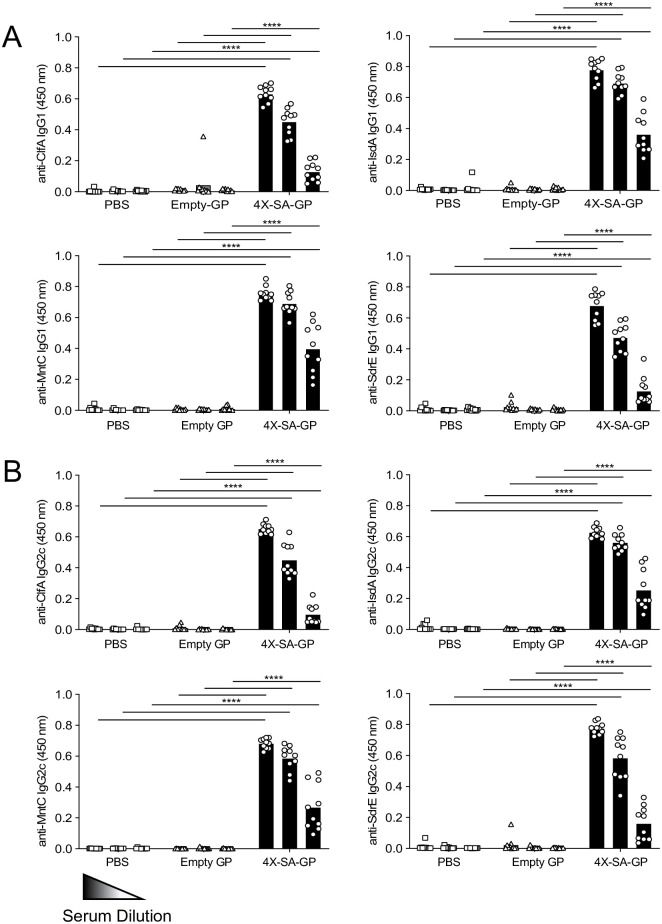
To further characterize the adaptive response elicited by three vaccinations with 4X-SA-GP, we investigated production of antigen-specific antibodies, specifically the IgG subclasses IgG1 and IgG2c. B cells often require help from CD4+ T cells to allow for isotype switching, affinity maturation, and memory [57], and therefore looking at antigen-specific antibody production can also provide some understanding into the CD4+ T cell response elicited by the vaccination. We detected high levels of IgG1 (Fig 5A) and IgG2c (Fig 5B) antibodies against recombinant (r)ClfA, rIsdA, rMntC, and rSdrE in the serum of all the mice vaccinated with 4X-SA-GP. All three of the serum dilutions tested for antibodies against each protein were significantly elevated compared to the serum tested from PBS or empty-GP vaccinated mice (Fig 5A and 5B). These responses are a sharp contrast to the relatively low antibody levels present in mice vaccinated with only one dose of 4X-SA-GP (S4A and S4B Fig).
Fig 5. Three immunizations of mice with 4X-SA-GP induces robust antibody responses.
(A and B) Serum was collected from each group of vaccinated mice 2 weeks after the third and final immunization with PBS, Empty-GP, or 4X-SA-GP (n = 10 mice per group). The serum was diluted 10X 3 times starting at 1:1000 and was then tested for antibodies specific for each of the 4 S. aureus proteins encapsulated in 4X-SA-GP by ELISA. The subclasses IgG1 (A) and IgG2c (B) were analyzed for specificity towards rClfA, rIsdA, rMntC, and rSdrE. The read-out of the assay is the optical density (OD) at 450 nm for each serum sample. Each data point represents an individual mouse. Data are representative of three experiments and were analyzed using ANOVA at each dilution. ****p<0.0001.
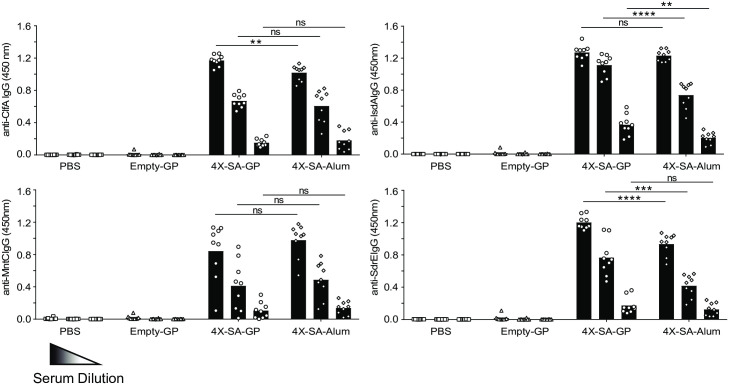
We also directly compared antibody production induced by 4X-SA-GP to antibodies produced when the widely used antibody-promoting adjuvant alum (4X-SA-Alum) was used. Mice were immunized three times as above, and an additional cohort of mice was immunized by the same route and schedule with matching amounts of the four S. aureus antigens mixed with alum. The 4X-SA-GP vaccine induced antibodies comparably, if not better than, the alum adjuvanted approach (Fig 6). Taken together, the data indicate that vaccination of mice three times with 4X-SA-GP promotes protection in mice against S. aureus infection and induces strong antigen-specific CD4+ T cell and antibody responses.
Fig 6. 4X-SA-GP induced antibody responses are similar to those induced by the common adjuvant alum.
Wild-type female mice were immunized once a week for 3 weeks with PBS (n = 10), Empty-GPs (n = 10), 4X-SA-GP (n = 9) or the same amount of S. aureus antigens mixed with alum (n = 9). Serum was collected from each group 2 weeks after the third and final immunization. The serum was diluted 10X 3 times starting at 1:1000 and was then tested for total IgG antibodies specific for each of the 4 S. aureus proteins included in the vaccine by ELISA. The read-out of the assay is the optical density (OD) at 450 nm for each serum sample. Data are representative of three experiments and were analyzed using ANOVA and t test at each dilution. **p<0.01, ***p<0.001, ****p<0.0001; ns, not significant.
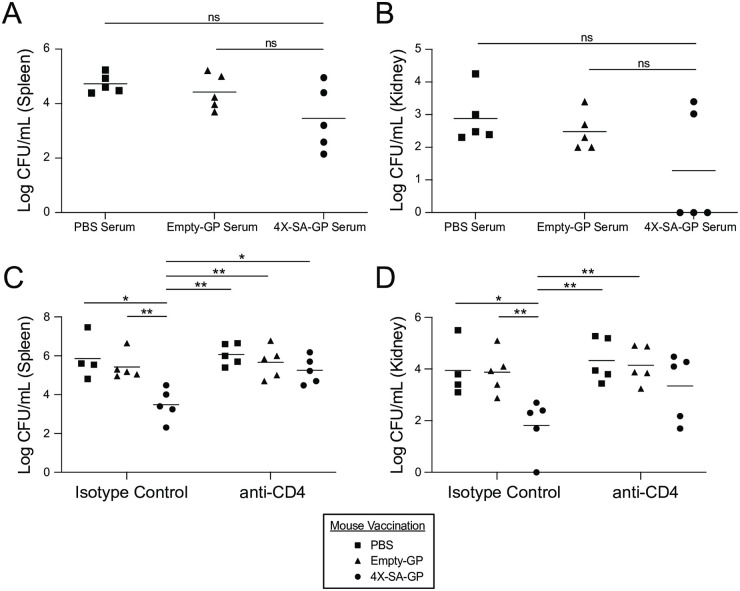
Transferred antibodies do not elicit protection and CD4+ T cells are needed for the efficacy of the 4X-SA-GP vaccine in mice
A successful S. aureus vaccine should most likely elicit both antibody and CD4+ T cell responses [7, 14]. To test whether the antibodies produced in response to 4X-SA-GP are enough to provide protection on their own, we performed a serum transfer. We collected serum from mice vaccinated with 4X-SA-GP, empty-GPs, or PBS and transferred the serum to naïve recipient mice via i.p. injection. The following day, we infected the recipient mice with S. aureus and assessed spleen and kidney CFUs after 24 hours. There were no differences in the bacterial burdens in the spleen (Fig 7A) or kidneys (Fig 7B) of mice that received the 4X-SA-GP serum compared to mice that received PBS or empty-GP serum. Mice that were given the 4X-SA-GP serum had lower CFU count averages in the spleen and kidneys (Fig 7A and 7B) in comparison to the PBS and empty-GP serum groups suggesting that the antigen-specific antibodies from the 4X-SA-GP serum may have a modest protective effect, though not enough to formally provide significant protection.
Fig 7. Antibodies alone are not sufficient to provide 4X-SA-GP-induced protection against systemic S. aureus infection, while CD4+ T cells are indispensable.
(A and B) Wild-type female mice were immunized once a week for 3 weeks with PBS, Empty-GP, or 4X-SA-GP and serum was collected from each group of mice 2 weeks after the final vaccination. The serum from each group of mice was pooled and injected i.p. into wild-type female naïve recipient mice (n = 5 mice/group, PBS serum, Empty-GP serum, 4X-SA-GP serum). The recipient mice were infected i.p. with 2x107 CFUs of S. aureus (LAC USA300) the following day. Bacteria from the spleen (A) and kidneys (B) were recovered after 24 hours and CFUs were enumerated. (C and D) Wild-type female mice were immunized once a week for 3 weeks with PBS (n = 9), Empty-GPs (n = 10), or 4X-SA-GP (n = 10). Four weeks after the final vaccination 4–5 mice per vaccination group were treated i.p. with anti-CD4+ antibody or the corresponding isotype control antibody on day -1 and day 0. On day 0, all groups of the mice were infected i.p. with 2x107 CFUs of S. aureus (LAC USA300). Bacteria from the spleen (C) and kidneys (D) were recovered after 24 hours after the S. aureus infection and CFUs were enumerated. Each data point represents an individual mouse. Data from the serum transfer is representative of two experiments. Data from the CD4+ T cell depletion is from a single experiment. Data analysis was performed using Kruskal-Wallis test ((A) = 0.18, (B) = 0.46, (C) = 0.0.02, (D) = 0.05) and Mann-Whitney U test. *p<0.05, **p<0.01; ns, not significant.
To evaluate the role of antigen-specific CD4+ T cells in vaccine-induced protection, we depleted CD4+ T cells prior to infection. Vaccinated mice from each group (PBS, empty-GP, 4X-SA-GP) received an i.p. injection of either anti-CD4+ antibody or the corresponding isotype control antibody four weeks post-vaccination. To ensure depletion of the CD4+ T cells, we measured CD4+ T cell percentages prior to infection in peripheral blood mononuclear cells (PBMCs) isolated from each group of mice and post-infection in the MLNs by flow cytometry (S5 Fig). Upon infection with S. aureus, we observed that depletion of T cells blocked the protection offered by the 4X-SA-GP vaccine (Fig 7C and 7D).
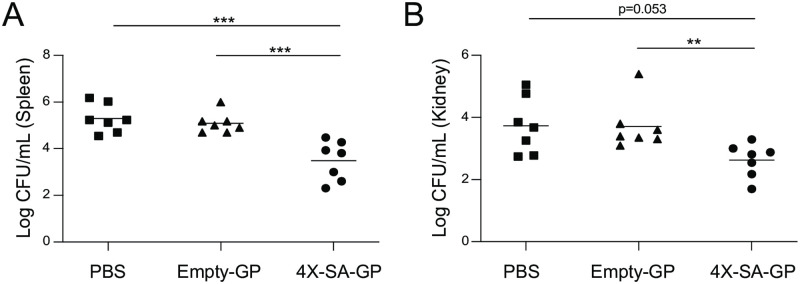
Mice are protected from systemic S. aureus infection eight weeks after vaccination with 4X-SA-GP
We next sought to establish if the protective immunity induced by 4X-SA-GP vaccination could provide protection after a time period longer than four weeks. We vaccinated animals three times as before, but we rested them for eight weeks prior to infection. 4X-SA-GP vaccinated mice were still significantly protected after 8 weeks (Fig 8). Intriguingly, at this later time point, the protection provided by the empty-GP vaccinated mice appears to have faded entirely (Fig 8A and 8B). Moreover, eight weeks after the final vaccination, the relative antibody levels of IgG1 and IgG2c subclasses specific for each protein were strong, detectable and often comparable to the levels seen in serum collected from mice two weeks post-vaccination (S6A and S6B Fig). These results suggest that vaccination of mice with three doses of 4X-SA-GP induces long-lasting protective immunity against S. aureus with antigen-specific adaptive cellular and humoral immune responses.
Fig 8. Protective immunity from 4X-SA-GP against S. aureus infection is long lasting.
(A and B) Wild-type female mice were immunized once a week for 3 weeks with PBS (n = 7), Empty-GPs (n = 7), or 4X-SA-GP (n = 7). Eight weeks after the final vaccination, mice were infected i.p. with 2x107 CFUs of S. aureus (LAC USA300). Bacteria from the spleen (A) and kidneys (B) were recovered after 24 hours and CFUs were enumerated. Each data point represents an individual mouse. Data analysis was performed using Kruskal-Wallis test ((A) = 0.0001 (B) = 0.008, and Mann-Whitney U test. **p<0.01, ***p<0.005; ns, not significant. Data are from a single experiment.
Discussion
Staphylococcus aureus is the most frequent cause of infections in the United States. Antibiotic resistant S. aureus strains, such as Methicillin-Resistant S. aureus (MRSA), have rapidly emerged worldwide, infecting immunocompromised and healthy individuals alike, making it a major public health threat [8]. Vaccines aimed at targeting S. aureus have failed in clinical trials [9] and the reason for this lack of success remains unknown. As this pathogen continues to rapidly spread on a global scale, it is vital that new approaches towards a S. aureus vaccine emerge. The observation that conditions that predispose a person to S. aureus infections often also predispose to fungal infections suggests that the elements of immunity that are effective against S. aureus share much in common with the elements of immunity that promote defense against fungal infections. We therefore hypothesized that a vaccine based on activating antifungal innate immune responses in the context of delivery of S. aureus antigens might be productive. In this study, we developed and tested in mice a vaccine candidate consisting of fungal β-glucan particles loaded with four S. aureus protein antigens (4X-SA-GP).
Of the four S. aureus protein antigens we used, ClfA and MntC have and are being used (together with capsular polysaccharides) in vaccines that have gone on to clinical trials. In this case, the vaccine employs a non-toxic mutant of diphtheria toxin cross-reacting material 197 (CRM197) as an adjuvant, but it has so far not proven effective at preventing infection. The vaccine generates antibodies to ClfA and MntC that would appear to be effective in vitro [58]. A variety of other antigens have moved to clinical trials as well, but none yet have proven effective [59]. A working hypothesis is that a successful vaccine will need to generate a protective T cell response instead of or in addition to an antibody response [60].
Vaccinating mice with 3 doses of 4X-SA-GP induced long lasting (at least 8 weeks) cellular and humoral antigen specific immune responses. Antigen-specific T cell responses were polarized towards Th1 and Th17 as would be anticipated for the adjuvant activity of β-glucan, and these T cell responses were essential for vaccine-induced protection from systemic infection with S. aureus. The vaccine also induced strong IgG1 and IgG2c antibody responses to the S. aureus proteins, although it was not clear that these antibody responses were sufficient to mediate protection from infection.
Four weeks after completing the vaccination, animals retained some variable non-specific enhanced resistance to S. aureus infection that was induced by β-glucan particles having no S. aureus antigens. This immunity wore off after 8 weeks, but the antigen specific immunity induced by antigen-loaded particles persisted. It is therefore possible that the β-glucan particles alone elicit some short-lived non-antigen-specific inflammatory immunity by which innate immune cells show enhanced responsiveness. Future studies could be designed to characterize the cellular and molecular signatures associated with this temporary protection, but the antigen-specific responses are longer-lived.
Some years ago, a group of investigators working on development of a Candida albicans vaccine discovered that their vaccine provided cross-protection from S. aureus [61]. The vaccine employs recombinant Candida agglutinin-like sequence 3 (Als3) adhesin/invasin as the antigen and is mixed with alum as an adjuvant. While the vaccine induces good antibody production against Als3 that cross react with S. aureus cell walls, protection in mouse models of infection required T cell mediated immunity (especially Th1 and Th17 polarized responses), not B cell immunity [62, 63]. Polarization of CD4+ T cells to both the Th1 and Th17 subsets in response to the 4X-SA-GP may make this approach more desirable compared to vaccines that polarize T cells to only one main subset. Th17 cells appear to play a major role in eliciting protection for skin, respiratory, and mucocutaneous infections [64, 65], while Th1 cells play a protective role in bloodstream infection [62]. Therefore, differentiation of CD4+ T cells to both these subsets by 4X-SA-GP immunization may help ensure that an effective T cell-mediated downstream response will occur upon infection. Previous studies have indicated that S. aureus-specific T cells that stimulate phagocyte recruitment and activation through the production of the proinflammatory cytokines IFNγ and/or IL-17 are important for immunity to S. aureus [61, 66].
The GP vaccine platform has been previously investigated primarily in the context of developing fungal vaccines [28, 67] due to the lack of effective vaccines for fungal infections. Fungi resist lysis by the complement system, but the deposition of iC3b on a fungus prompts opsonization and phagocytosis [68]. In many cases, antibodies are not protective against fungal infections [69], and some vaccines are now focusing on producing cellular immune responses as well [28] given that T cells are major players in fighting fungal infections. Studies focusing on candidiasis and aspergillosis show that Th1 and Th17 responses are more important than generation of neutralizing antibodies [70]. Vaccination of mice with glucan particles loaded with Cryptococcus-derived alkaline extracts protected animals from a lethal challenge with Cryptococcus neoformans and Cryptococcus gattii [28]. Glucan particles alone as a vaccine also afforded mice protection against Aspergillus fumigatus infection [67]. Thus, studies evaluating GPs as vaccine platforms for inducing antifungal immunity are farther along than evaluation of GPs as a vaccine for S. aureus, and they demonstrate the potential GPs have as a vaccine to induce protective cellular immunity. The similarities between the immune responses needed for antifungal and anti-staphylococcal immunity suggest that similar vaccination strategies might be effective, and further studies will be required to more completely optimize a GP-based S. aureus vaccine.
Materials and methods
Ethics statement
This study was performed under strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. This protocol was approved by the institutional animal use and care committee of the Cedars-Sinai Medical Center (IACUC 7174 and 8836).
Microbes
For all S. aureus infection experiments, the dominant CA-MRSA strain in the United States, USA300, specifically the Los Angeles County clone (LAC) was used [71]. S. aureus LAC was routinely cultured in Tryptic Soy Broth. Mid-log phase bacteria sub-cultured from overnight cultures were used for experiments. Bacteria were washed in PBS twice prior to use and inocula were confirmed by CFU determination on blood agar plates. Heat-killed S. aureus was prepared by incubating a washed overnight culture of S. aureus for 45 minutes at 90°C. The bacteria were then washed to remove secreted proteins. The sterility of killed bacteria was confirmed by enumeration of CFU on an agar plate.
Mice
C57BL/6 mice were purchased from Jackson Laboratories and Rag2/OT-II transgenic mice were purchased from Taconic. OT-II TCR transgenic mice and Dectin-1 knockout mice were bred and housed under specific pathogen-free conditions in the Cedars-Sinai Medical Center animal facility. Aged-matched female mice aged 8–12 weeks were used for in vitro and in vivo experiments. Due to sex-related differences in susceptibility to S. aureus [72], only female mice were used in this study.
Primary Cells
Bone marrow-derived dendritic cells (BMDCs) from C57BL/6 and Dectin-1 knockout mice were grown as previously described [73] in RPMI (Corning) supplemented with 10% fetal bovine serum (FBS) and 10 ng/mL recombinant murine granulocyte-monocyte colony-stimulating factor (GM-CSF) (Peprotech). Between days 7–10, the differentiated cells were used for in vitro stimulation assays. CD4+ T cells were isolated from pooled spleens and lymph nodes of 8-12-week-old female OT-II or Rag2/OT-II mice using an EasySep mouse naïve CD4+ T cell Isolation kit (STEMCELL Technologies).
BMDC stimulation
BMDCs were plated at 5x105 cells/well in 24-well TC plates (for flow cytometry analysis) or 2x105 cells/well in 96 well round-bottom TC plates (for T cell co-culture). The following day after re-plating, BMDCs were stimulated for 24 hours with GP-OVA (10 particles/cell), 4X-SA-GP (10 particles/cell), or 100 ng/mL LPS (Invivogen). For cytokine analysis of BMDCs, supernatants were harvested 24 hours after stimulation, and for analysis of maturation markers, BMDCs were lifted after overnight stimulation with PBS containing 2mM EDTA, followed by immunofluorescent staining and flow cytometry.
ELISA and flow cytometry
Enzyme linked immunosorbent assays (ELISA) were performed according to the manufacturer’s instructions for IL-6, TNFα, IL-17A, and IFNγ (BioLegend). For antibody analysis in the serum of mice, ELISA plates (Corning Costar) were coated with 5 μg per well of rClfA, rIsdA, rMntC, rSdrE at 4°C overnight. The plates were blocked with ELISA assay diluent (BioLegend) followed by incubation with the sera from vaccinated mice at dilutions of 1:1000, 1:10,000, and 1:100,000 in the assay diluent. The plates were then incubated with biotin-conjugated anti-mouse IgG1 (BioLegend) followed by streptavidin-conjugated anti-mouse IgG-HRP (BioLegend). For IgG2c, sera were incubated with anti-mouse IgG2c-HRP (SouthernBiotec). The plates were then incubated with TMB substrate solution (BD OptEIA) and the reactions were stopped with 2N H2SO4 and the plates were read at 450 nm.
Fluorophore-conjugated anti-mouse antibodies directed against the following molecules were used to stain cells: CD11b (M1/70), CD11c (N418), Ly6C (HK1.4), Ly6G (1A8), F4/80 (BM8), CD19 (1D3/CD19), I-A/I-E (M5/114.15.2, CD80 (16-10A1), CD86 (GL-1), IL-17A (TC11-8H10.1), IFNγ (XMG1.2), CD3 (145-2C11), TCRβ (H57-597), CD4 (GK1.5), TCR Vβ5.1/5.2 (MR9-4), TCR Vα2 (B20.1) (all from BioLegend). All samples were prestained with anti-CD16/CD32 (eBioscience) to block FC receptors, and Zombie fixable viability dye (BioLegend) to identify dead cells. For intracellular cytokine staining, cells were stimulated for 4 hours with Cell Activation Cocktail (BioLegend) in the presence of GolgiStop (BD Biosciences) for the last 3 hours of stimulation. Following the addition of Fc-block, viability dye and surface staining, intracellular cytokine staining was performed using the cytofix/cytoperm staining kit (BD Biosciences) according to the manufacturer’s instructions. Samples were acquired with a LSRII (BD Biosciences) and data were analyzed with FlowJo software (Tree star).
Western Blotting
For assessing ovalbumin or the recombinant proteins in the glucan particles, 2x106 particles were loaded onto a gradient gel and subjected to gel electrophoresis using the Novex NuPAGE Gel electrophoresis system (Invitrogen), and proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore). GP-OVA blots were probed with an anti-ovalbumin antibody (anti-albumin, Calbiochem), followed by an HRP-conjugated secondary antibody (Jackson ImmunoResearch). Blots were incubated with ECL substrate (Pierce) and then exposed to X-ray film. 4X-SA-GP blots were probed with an anti-6xHN antibody (Clontech Laboratories) to detect the polyhistidine tag on the N-terminus of each of the recombinant proteins followed by infrared dye-conjugated secondary antibodies (LI-COR). Bands were visualized on an Odyssey imaging system (LI-COR).
DC: OT-II T cell co-culture
BMDCs were plated in 96 well round-bottom TC plates at 2x105 cells/well and were stimulated overnight with GP-No OVA or GP-OVA (10 particles/cell). The following day, the BMDCs were washed thoroughly and CFSE-labeled [74] naïve OT-II CD4+ T cells were added at a 1:5 ratio (2x105 DC: 1x106 T cells) and cells were cultured with RPMI supplemented with 10% FBS, 1 mM sodium pyruvate and 55 μM 2-mercaptoethanol. Following 4–5 days of co-culture, supernatants were harvested for cytokine analysis by ELISA and T cells were restimulated with Cell Activation Cocktail (BioLegend) and Golgistop (BD Biosciences) and stained intracellularly with fluorophore-conjugated antibodies against IL-17A and IFNγ.
Antigen-loaded glucan particles
β-glucan particles were prepared by treating Saccharomyces cerevisiae zymosan A (Sigma) with a hot-alkali treatment to remove TLR agonists as previously described [75]. Briefly, a suspension of zymosan particles were initially prepared and the particles were then boiled in 10 M NaOH for 1 hour followed by 5 washes in sterile PBS. The glucan particles were then dried in a Savant SpeedVac vacuum concentrator. Dried glucan particles were loaded with various concentrations of ovalbumin (OVA) or the recombinant S. aureus proteins (with or without TRITC or FITC-labeling) as previously described [29, 51]. Glucan particles (GPs) were kept as frozen aliquots and were sonicated in an ultrasonic water bath for 15 minutes prior to their in vitro or in vivo use. Empty-GPs were prepared the same as the loaded-GPs minus the addition of any protein antigen.
Cloning and purification of recombinant proteins
Coding sequences for ClfA, IsdA, MntC, and SdrE were PCR amplified without the signal peptide or sorting signal (see Table 1 for a list of primer sequences) as previously described [41]. PCR products were cloned into pET6xHN Expression Vector using the In-Fusion Cloning Kit (Clontech Laboratories) to express recombinant proteins that have a N-terminal 6xHN fusion tag. The recombinant proteins were purified from bacterial-clarified lysates by using His60 Ni Superflow Resin (Ni-IDA resin), His60 Ni Buffers and gravity columns (ClonTech Laboratories) per the manufacturer’s instructions. Purified proteins underwent endotoxin removal via endotoxin removal spin columns (Pierce), were concentrated with Amicon Ultra centrifugal filter units (Millipore), and the final protein concentration was determined by BCA protein assay (Pierce).
Table 1. List of primer sequences for In-Fusion Cloning, 5’ to 3’.
| Primer | Sequence 5’ to 3’ |
|---|---|
| ClfA forward | TAAGGCCTCTGTCGACAGTGAAAATAGTGTTACGCAA |
| ClfA reverse | CAGAATTCGCAAGCTTTGTATCTGGTAATGGTTCTTT |
| IsdA forward | TAAGGCCTCTGTCGACGCAACAGAAGCTACGAACG |
| IsdA reverse | CAGAATTCGCAAGCTTAGTTTTTGGTAATTCTTTAGCT |
| MntC forward | TAAGGCCTCTGTCGACAGTGATAAGTCAAATGGCAAA |
| MntC reverse | CAGAATTCGCAAGCTTTTATTTCATGCTTCCGTGTAC |
| SdrE forward | TAAGGCCTCTGTCGACGCTGAAAACACTAGTACAGA |
| SdrE reverse | CAGAATTCGCAAGCTTTGTTTCTGGTAATGCTTTTGC |
Intraperitoneal injections of FITC-labeled 4X-SA-GP
Mice were injected intraperitoneally with 5x107 FITC-labeled or unlabeled 4X-SA-GP and allowed to rest for 24, 48, or 72 hours before being euthanized. The peritoneal cavity was lavaged with 10 ml of cold PBS with 2 mM EDTA. The cells there then counted and stained for the surface markers CD11b, CD11c, Ly6C, Ly6G, F4/80, and CD19 and analyzed by flow cytometry. The cell number per mL (gated first on FITC+ cells) was determined via CountBright Absolute Counting Beads (Invitrogen).
Ex vivo stimulation of splenocytes
Spleens were isolated 5 days after the final vaccination of mice with PBS, empty-GP, or 4X-SA-GP. Red blood cells were lysed and cells were resuspended in RPMI supplemented with 10% FBS, 1 mM sodium pyruvate, and 55 μM 2-mercaptoethanol and plated at 3x106 cells/well. For specific stimulation, cells were cultured with a mixture of the 4 recombinant proteins (10 μg/protein per mL) or heat-killed S. aureus at an MOI of 5. Supernatants were collected after 72 hours for analysis of cytokines by ELISA. For intracellular cytokine analysis, the cells were stimulated with Cell Activation Cocktail (BioLegend) for 4 hours and Golgistop (BD Biosciences) for the last 3 hours of stimulation. Intracellular cytokines (IL-17A and IFNγ) were then analyzed by flow cytometry.
Vaccination and infection model
Single vaccination model: Mice were vaccinated once with PBS as a control, empty-GPs, or 4X-SA-GP (5x107 GPs). Mice were rested for 5 days before the spleen and MLNs were harvested for the T cell restimulation assay. For the infection model, mice were rested for 4 weeks after the one vaccination and were then inoculated with 2x107 CFU of S. aureus (LAC USA300) via i.p. injection. Mice were euthanized 24 hours after the infection and spleen and kidneys were harvested, homogenized, and plated on blood agar plates. CFUs were enumerated after overnight incubation at 37°C.
Triple vaccination model: Mice were vaccinated once a week for 3 weeks with PBS as a control, empty-GPs, or 4X-SA-GP (5x107 GPs/dose). For comparison to alum, mice were immunized with payload-matched amounts of S. aureus antigens (50 μg/injection) mixed with alum (1 mg/injection). The quantities of the glucan particle-loaded recombinant proteins were estimated by knowing the amount of protein added to a particle preparation and subtracting the amount left over in supernatants. Mice were rested for 5 days before the spleen and MLNs were harvested for the T cell restimulation assay. For the infection model, mice were rested for 4 or 8 weeks (where indicated) after the final vaccination and were then inoculated with 2x107 CFU of S. aureus (LAC USA300). Mice were euthanized 24 hours after the infection and spleen and kidneys were harvested, homogenized, and plated on blood agar plates. CFUs were enumerated after overnight incubation at 37°C.
Serum transfer
Mice were vaccinated once a week for 3 weeks with PBS as a control, empty-GPs, or 4X-SA-GP (5x107 GPs/dose). Two weeks after the final vaccination, serum was collected from all of the mice and pooled according to the vaccination group. Recipient mice received an i.p. injection of 150 μL of the pooled serum on day -1 and were then inoculated with 2x107 CFU of S. aureus (LAC USA300) via i.p. injection the following morning on day 0. Mice were euthanized 24 hours after the infection and spleen and kidneys were harvested, homogenized, and plated on blood agar plates. CFUs were enumerated after overnight incubation at 37°C.
CD4+ T cell depletion
Mice were vaccinated once a week for 3 weeks with PBS as a control, empty-GPs, or 4X-SA-GP (5x107 GPs/dose). Mice were rested for 4 weeks and then received an i.p. injection with an anti-CD4+ antibody (clone Gk1.5, BioXCell) 300 μg in 500 μl PBS on day -1 and 100 μg in 500 μl PBS on day 0 as previously described [76]. Control mice were treated with an i.p. injection of the corresponding isotype control antibody (BioXCell) at the same dosage and volume. On day 0, all mice were then inoculated with 2x107 CFU of S. aureus (LAC USA300) via i.p. injection. Mice were euthanized 24 hours after the infection and spleen and kidneys were harvested, homogenized, and plated on blood agar plates. CFUs were enumerated after overnight incubation at 37°C. PBMCs were isolated from the blood of mice and pooled from all groups of mice on day 0 prior to infection and were stained with the surface markers CD3, CD4, and TCRβ and analyzed by flow cytometry to assess the efficacy of the CD4+ T cell depletion. MLNs were harvested on day 1, after the infection, and the isolated cells were stained for the surface markers CD3, CD4, and TCRβ and analyzed via flow cytometry to assess that the CD4+ T cells were still depleted post-infection.
Statistical analysis
Statistical analysis was determined by Student’s t test, two-way ANOVA with the Tukey multiple comparisons post-hoc test (denoted ANOVA in figure legends), or Mann-Whitney U test using GraphPad Prism software. All statistical details of experiments such as the number of replicates can be found in the figure legends. Where present, error bars indicate the mean +SD. p values less than 0.05 are considered significant.
Schematics
S1A Fig schematic was created using BioRender.com.
Supporting information
(A) Schematic for the generation of 4X-SA-GP. Glucan particles were loaded with the purified, his-tagged, recombinant proteins: rClfA, rIsdA, rMntC, and rSdrE. (B) Coomassie stained SDS-PAGE gel of the 4 purified recombinant proteins: Lane 1, rClfA (98.3 kDa), Lane 2, rMntC (36.2 kDa), Lane 3, rIsdA (34.1 kDa), Lane 4, rSdrE (118.6 kDa). The approximate molecular weight of each protein is shown in parentheses. All lanes contain approximately 10 μg of protein. (C). Western blot analysis of 4X-SA-GP using an anti-6xHN antibody to probe for the his-tagged proteins within the GPs. 2x107 4X-SA-GPs were loaded into the lane.
(TIF)
(A-G) BMDCs stimulated with GP-No OVA or GP-OVA (loaded with various concentrations of OVA) were used to activate naïve CFSE-labeled OT-II CD4+ T cells under non-polarizing conditions. (A) Representative flow cytometry plots show the degree of Th1 (IFNγ+) and Th17 (IL-17+) T cell differentiation under each condition of BMDC activation. (B) Representative flow cytometry histograms demonstrate the degree of T cell polarization corresponding to the data above with the pink histogram from the co-culture with no stimulation used as a reference point for the percentage of proliferation. (C-D) Bar graphs quantifying the percentage of IL-17+ (C) and IFNγ+ (D) OT-II cells and the percentage of CFSE proliferation (E). (F-G) Supernatants from the co-culture were collected on day 5 and analyzed for production of IL-17 (F) and IFNγ (G) by ELISA. Flow cytometry percentages and ELISA data and their standard deviation are representative of two replicates/condition. Data are representative of two experiments.
(TIF)
(A-E) Wild-type female mice were immunized one time with PBS (n = 5), Empty-GPs (n = 5), or 4X-SA-GP (n = 5). Five days after the vaccination, splenocytes from each mouse were stimulated with a mixture of all 4 S. aureus purified recombinant proteins (proteins) and heat-killed S. aureus (HK-SA) for 3 days. (No stim., no stimulation). After 3 days, the splenocytes were stimulated with phorbol myristate acetate (PMA) and ionomycin and then analyzed by flow cytometry after intracellular cytokine staining. Representative flow cytometry plots demonstrate the degree of IL-17 and IFNγ production from CD4+ T cells (gated on TCRβ+CD4+) in response to the stimuli (proteins, HK-SA) from the spleen (A). (B and D) Bar graphs show the frequency of IL-17 (B) and IFNγ positive cells in the spleen from the mice. Each data point represents an individual mouse in all of the bar graphs. (C and E) Supernatants were harvested at day 3 from the splenocyte (A) stimulation and the cytokines IL-17 (C) and IFNγ (E) were determined by ELISA. Data analysis was performed using ANOVA. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001. Data are representative of a single experiment.
(TIF)
(A-B) Serum was collected from each group of vaccinated mice 2 weeks after one immunization with PBS, Empty-GP, or 4X-SA-GP (n = 5 mice per group). The serum was diluted 3 times at 1:1000, 1:10,000 and 1;100,000 and was then tested for antibodies specific for each of the 4 S. aureus proteins encapsulated in 4X-SA-GP by ELISA. The subclasses IgG1 (A) and IgG2c (B) were analyzed for specificity towards rClfA, rIsdA, rMntC, and SdrE. The read-out of the assay is the optical density (OD) at 450 nm for each serum sample. Each data point represents an individual mouse. Data analysis was performed using ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Data are representative of a single experiment.
(TIF)
(A-B) Wild-type female mice were immunized once a week for 3 weeks with PBS (n = 9), Empty-GPs (n = 10), or 4X-SA-GP (n = 10). Four weeks after the final vaccination 4–5 mice per vaccination group were treated i.p. with anti-CD4+ antibody or the corresponding isotype control antibody on day -1 and day 0. On day 0, all groups of the mice were infected i.p. with 2x107 CFUs of S. aureus (LAC USA300). (A) flow cytometry plots demonstrating the degree of CD4+ T cell depletion from pooled peripheral blood mononuclear cells (PBMCs) from each vaccination group of mice on day 0 before the mice were infected with S. aureus. (B) flow cytometry plots demonstrating the degree of CD4+ T cell depletion from pooled MLNs from each vaccination group of mice 24 hours after the mice were infected with S. aureus (day 1). Cells from (A) and (B) were gated on CD3+ cells.
(TIF)
(A-B) Two sets of wild-type female mice were immunized once a week for 3 weeks with PBS (n = 5), Empty-GPs (n = 5), or 4X-SA-GP (n = 5). Serum was collected from one set of mice (PBS, Empty-GP, 4X-SA-GP; n = 5 mice/group) 2 weeks after the final vaccination and 8 weeks after the final immunization for the other set of mice (PBS, Empty-GP, 4X-SA-GP; n = 5 mice/group). The serum was diluted 3 times at 1:1000, 1:10,000 and 1;100,000 and was then tested for antibodies specific for each of the 4 S. aureus proteins encapsulated in 4X-SA-GP by ELISA. The subclasses IgG1(A) and IgG2c (B) were analyzed for specificity towards rClfA, rIsdA, rMntC, and rSdrE. The read-out of the assay is the optical density (OD) at 450 nm for each serum sample. Each data point represents an individual mouse. Data analysis was performed using ANOVA. *p<0.05, **p<0.005. Data are representative of at least two experiments for serum at two weeks and a single experiment for serum at 8 weeks.
(TIF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The study was supported by the National Institutes of Health (https://www.nih.gov/) grants R01 DK093426 (to D.M.U.) and R01 AI127406 (to D.M.U, G.Y.L., and G.A.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu GY. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr Res. 2009;65(5 Pt 2):71R–7R. 10.1203/PDR.0b013e31819dc44d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751–62. 10.1016/S1473-3099(05)70295-4 . [DOI] [PubMed] [Google Scholar]
- 3.Crossley K, Solliday J. Comparison of rectal swabs and stool cultures for the detection of gastrointestinal carriage of Staphylococcus aureus. J Clin Microbiol. 1980;11(4):433–4. 10.1128/JCM.11.4.433-434.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker K, Schaumburg F, Fegeler C, Friedrich AW, Köck R, Study PoMMP. Staphylococcus aureus from the German general population is highly diverse. Int J Med Microbiol. 2017;307(1):21–7. Epub 2016/11/30. 10.1016/j.ijmm.2016.11.007 . [DOI] [PubMed] [Google Scholar]
- 5.Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17(4):203–18. 10.1038/s41579-018-0147-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev. 2012;25(2):362–86. 10.1128/CMR.05022-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol. 2012;34(2):335–48. Epub 2011/11/14. 10.1007/s00281-011-0293-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019. Epub 2019/02/08. 10.1038/s41579-018-0147-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redi D, Raffaelli CS, Rossetti B, De Luca A, Montagnani F. Staphylococcus aureus vaccine preclinical and clinical development: current state of the art. New Microbiol. 2018;41(3):208–13. Epub 2018/06/06. . [PubMed] [Google Scholar]
- 10.Missiakas D, Schneewind O. Staphylococcus aureus vaccines: Deviating from the carol. J Exp Med. 2016;213(9):1645–53. Epub 2016/08/15. 10.1084/jem.20160569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: "Staphyloccocus aureus vaccines: problems and prospects". Vaccine. 2013;31(25):2723–30. Epub 2013/04/23. 10.1016/j.vaccine.2013.04.002 . [DOI] [PubMed] [Google Scholar]
- 12.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38(1):3–10. 10.1007/s12016-009-8136-z . [DOI] [PubMed] [Google Scholar]
- 13.Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol Dis. 2010;45(3):246–65. Epub 2010/08/21. 10.1016/j.bcmd.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54(8):1179–86. Epub 2012/02/21. 10.1093/cid/cis033 . [DOI] [PubMed] [Google Scholar]
- 15.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–6. Epub 2008/03/12. 10.1038/nature06764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heimall J, Freeman A, Holland SM. Pathogenesis of hyper IgE syndrome. Clin Rev Allergy Immunol. 2010;38(1):32–8. 10.1007/s12016-009-8134-1 . [DOI] [PubMed] [Google Scholar]
- 17.Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, et al. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr. 2013;25(6):736–47. 10.1097/MOP.0000000000000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma A, Wüthrich M, Deepe G, Klein B. Adaptive immunity to fungi. Cold Spring Harb Perspect Med. 2014;5(3):a019612 Epub 2014/11/06. 10.1101/cshperspect.a019612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vautier S, Sousa MaG, Brown GD. C-type lectins, fungi and Th17 responses. Cytokine Growth Factor Rev. 2010;21(6):405–12. Epub 2010/11/12. 10.1016/j.cytogfr.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275–88. Epub 2011/03/11. 10.1038/nri2939 . [DOI] [PubMed] [Google Scholar]
- 21.Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol. 2012;34(2):261–80. Epub 2011/11/06. 10.1007/s00281-011-0292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plato A, Willment JA, Brown GD. C-type lectin-like receptors of the dectin-1 cluster: ligands and signaling pathways. Int Rev Immunol. 2013;32(2):134–56. 10.3109/08830185.2013.777065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6(1):33–43. 10.1038/nri1745 . [DOI] [PubMed] [Google Scholar]
- 24.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15(10):630–42. Epub 2015/09/21. 10.1038/nri3897 . [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Ostroff GR, Lee CK, Agarwal S, Ram S, Rice PA, et al. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J Immunol. 2012;189(1):312–7. Epub 2012/05/30. 10.4049/jimmunol.1200603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded beta-glucan particles. MBio. 2010;1(3). Epub 2010/07/20. 10.1128/mBio.00164-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitz SM, Huang H, Ostroff GR, Specht CA. Exploiting fungal cell wall components in vaccines. Semin Immunopathol. 2015;37(2):199–207. Epub 2014/11/18. 10.1007/s00281-014-0460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Specht CA, Lee CK, Huang H, Tipper DJ, Shen ZT, Lodge JK, et al. Protection against Experimental Cryptococcosis following Vaccination with Glucan Particles Containing Cryptococcus Alkaline Extracts. MBio. 2015;6(6):e01905–15. Epub 2015/12/22. 10.1128/mBio.01905-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H, Ostroff GR, Lee CK, Specht CA, Levitz SM. Characterization and optimization of the glucan particle-based vaccine platform. Clin Vaccine Immunol. 2013;20(10):1585–91. Epub 2013/08/14. 10.1128/CVI.00463-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Smet R, Demoor T, Verschuere S, Dullaers M, Ostroff GR, Leclercq G, et al. β-Glucan microparticles are good candidates for mucosal antigen delivery in oral vaccination. J Control Release. 2013;172(3):671–8. Epub 2013/09/14. 10.1016/j.jconrel.2013.09.007 . [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11(5):425–35. 10.1016/j.chom.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reid DM, Gow NA, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21(1):30–7. Epub 2009/02/14. 10.1016/j.coi.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8(6):630–8. Epub 2007/04/22. 10.1038/ni1460 . [DOI] [PubMed] [Google Scholar]
- 34.Tsoni SV, Kerrigan AM, Marakalala MJ, Srinivasan N, Duffield M, Taylor PR, et al. Complement C3 plays an essential role in the control of opportunistic fungal infections. Infect Immun. 2009;77(9):3679–85. Epub 2009/07/06. 10.1128/IAI.00233-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Q, Li D, Nurieva R, Patenia R, Bassett R, Cao W, et al. Reduced graft-versus-host disease in C3-deficient mice is associated with decreased donor Th1/Th17 differentiation. Biol Blood Marrow Transplant. 2012;18(8):1174–81. Epub 2012/06/01. 10.1016/j.bbmt.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunkelberger JR, Song WC. Role and mechanism of action of complement in regulating T cell immunity. Mol Immunol. 2010;47(13):2176–86. Epub 2010/06/18. 10.1016/j.molimm.2010.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemper C, Köhl J. Novel roles for complement receptors in T cell regulation and beyond. Mol Immunol. 2013;56(3):181–90. Epub 2013/06/22. 10.1016/j.molimm.2013.05.223 . [DOI] [PubMed] [Google Scholar]
- 38.Tai Y, Wang Q, Korner H, Zhang L, Wei W. Molecular Mechanisms of T Cells Activation by Dendritic Cells in Autoimmune Diseases. Front Pharmacol. 2018;9:642 Epub 2018/06/26. 10.3389/fphar.2018.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowler VG, Proctor RA. Where does a Staphylococcus aureus vaccine stand? Clin Microbiol Infect. 2014;20 Suppl 5:66–75. 10.1111/1469-0691.12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proctor RA. Is there a future for a Staphylococcus aureus vaccine? Vaccine. 2012;30(19):2921–7. Epub 2011/11/21. 10.1016/j.vaccine.2011.11.006 . [DOI] [PubMed] [Google Scholar]
- 41.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103(45):16942–7. Epub 2006/10/30. 10.1073/pnas.0606863103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawkins J, Kodali S, Matsuka YV, McNeil LK, Mininni T, Scully IL, et al. A recombinant clumping factor A-containing vaccine induces functional antibodies to Staphylococcus aureus that are not observed after natural exposure. Clin Vaccine Immunol. 2012;19(10):1641–50. Epub 2012/08/15. 10.1128/CVI.00354-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Josefsson E, Hartford O, O’Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184(12):1572–80. Epub 2001/12/12. 10.1086/324430 . [DOI] [PubMed] [Google Scholar]
- 44.Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, et al. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine. 2010;28(38):6382–92. Epub 2010/03/10. 10.1016/j.vaccine.2010.02.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becherelli M, Prachi P, Viciani E, Biagini M, Fiaschi L, Chiarot E, et al. Protective activity of the CnaBE3 domain conserved among Staphylococcus aureus Sdr proteins. PLoS One. 2013;8(9):e74718 Epub 2013/09/17. 10.1371/journal.pone.0074718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, et al. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis. 2012;205(11):1688–96. Epub 2012/04/02. 10.1093/infdis/jis272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng H, Yang F, Feng Q, Zhang J, Gu J, Jing H, et al. Rapid and Broad Immune Efficacy of a Recombinant Five-Antigen Vaccine against Staphylococcus Aureus Infection in Animal Models. Vaccines (Basel). 2020;8(1). Epub 2020/03/22. 10.3390/vaccines8010134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmerman JW, Lindermuth J, Fish PA, Palace GP, Stevenson TT, DeMong DE. A novel carbohydrate-glycosphingolipid interaction between a beta-(1–3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J Biol Chem. 1998;273(34):22014–20. 10.1074/jbc.273.34.22014 [DOI] [PubMed] [Google Scholar]
- 49.Vetvicka V. Glucan-immunostimulant, adjuvant, potential drug. World J Clin Oncol. 2011;2(2):115–9. 10.5306/wjco.v2.i2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin Y, Li P, Wang F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine. 2018;36(35):5235–44. Epub 2018/07/23. 10.1016/j.vaccine.2018.07.038 . [DOI] [PubMed] [Google Scholar]
- 51.Soto ER, Ostroff GR. Characterization of multilayered nanoparticles encapsulated in yeast cell wall particles for DNA delivery. Bioconjug Chem. 2008;19(4):840–8. Epub 2008/04/01. 10.1021/bc700329p . [DOI] [PubMed] [Google Scholar]
- 52.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, et al. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2012;91(4):525–36. Epub 2011/11/04. 10.1189/jlb.0711372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobson AC, Roundy KM, Weis JJ, Weis JH. Regulation of murine splenic B cell CR3 expression by complement component 3. J Immunol. 2009;183(6):3963–70. Epub 2009/08/26. 10.4049/jimmunol.0900038 . [DOI] [PubMed] [Google Scholar]
- 54.Bistoni F, Vecchiarelli A, Cenci E, Puccetti P, Marconi P, Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986;51(2):668–74. 10.1128/IAI.51.2.668-674.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Valtanen P, Guzman-Genuino RM, Williams DL, Hayball JD, Diener KR. Evaluation of trained immunity by β-1, 3 (d)-glucan on murine monocytes in vitro and duration of response in vivo. Immunol Cell Biol. 2017;95(7):601–10. Epub 2017/02/23. 10.1038/icb.2017.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098 Epub 2016/04/23. 10.1126/science.aaf1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74(11):4999–5005. 10.1128/jvi.74.11.4999-5005.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Begier E, Seiden DJ, Patton M, Zito E, Severs J, Cooper D, et al. SA4Ag, a 4-antigen Staphylococcus aureus vaccine, rapidly induces high levels of bacteria-killing antibodies. Vaccine. 2017;35(8):1132–9. Epub 2017/02/02. 10.1016/j.vaccine.2017.01.024 . [DOI] [PubMed] [Google Scholar]
- 59.Clowry J, Irvine AD, McLoughlin RM. Next-generation anti-Staphylococcus aureus vaccines: A potential new therapeutic option for atopic dermatitis? J Allergy Clin Immunol. 2019;143(1):78–81. Epub 2018/09/16. 10.1016/j.jaci.2018.08.038 . [DOI] [PubMed] [Google Scholar]
- 60.O’Brien EC, McLoughlin RM. Considering the ‘Alternatives’ for Next-Generation Anti-Staphylococcus aureus Vaccine Development. Trends Mol Med. 2019;25(3):171–84. Epub 2019/02/05. 10.1016/j.molmed.2018.12.010 . [DOI] [PubMed] [Google Scholar]
- 61.Spellberg B, Ibrahim AS, Yeaman MR, Lin L, Fu Y, Avanesian V, et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun. 2008;76(10):4574–80. Epub 2008/07/21. 10.1128/IAI.00700-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5(12):e1000703 Epub 2009/12/24. 10.1371/journal.ppat.1000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeaman MR, Filler SG, Chaili S, Barr K, Wang H, Kupferwasser D, et al. Mechanisms of NDV-3 vaccine efficacy in MRSA skin versus invasive infection. Proc Natl Acad Sci U S A. 2014;111(51):E5555–63. Epub 2014/12/10. 10.1073/pnas.1415610111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206(6):1291–301. Epub 2009/06/01. 10.1084/jem.20082767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun. 2014;82(5):2125–34. Epub 2014/03/10. 10.1128/IAI.01491-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Misstear K, McNeela EA, Murphy AG, Geoghegan JA, O’Keeffe KM, Fox J, et al. Targeted nasal vaccination provides antibody-independent protection against Staphylococcus aureus. J Infect Dis. 2014;209(9):1479–84. Epub 2013/11/22. 10.1093/infdis/jit636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clemons KV, Danielson ME, Michel KS, Liu M, Ottoson NC, Leonardo SM, et al. Whole glucan particles as a vaccine against murine aspergillosis. J Med Microbiol. 2014;63(Pt 12):1750–9. Epub 2014/10/06. 10.1099/jmm.0.079681-0 . [DOI] [PubMed] [Google Scholar]
- 68.Santos E, Levitz SM. Fungal vaccines and immunotherapeutics. Cold Spring Harb Perspect Med. 2014;4(11):a019711 Epub 2014/11/03. 10.1101/cshperspect.a019711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lopes LC, Guimarães AJ, de Cerqueira MD, Gómez BL, Nosanchuk JD. A histoplasma capsulatum-specific IgG1 isotype monoclonal antibody, H1C, to a 70-kilodalton cell surface protein is not protective in murine histoplasmosis. Clin Vaccine Immunol. 2010;17(7):1155–8. Epub 2010/05/19. 10.1128/CVI.00033-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poggi A, Catellani S, Musso A, Zocchi MR. Gammadelta T lymphocytes producing IFNgamma and IL-17 in response to Candida albicans or mycobacterial antigens: possible implications for acute and chronic inflammation. Curr Med Chem. 2009;16(35):4743–9. 10.2174/092986709789878238 . [DOI] [PubMed] [Google Scholar]
- 71.Junie LM, Jeican II, Matroş L, Pandrea SL. Molecular epidemiology of the community-associated methicillin-resistant staphylococcus aureus clones: a synthetic review. Clujul Med. 2018;91(1):7–11. Epub 2018/01/15. 10.15386/cjmed-807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yanke SJ, Olson ME, Davies HD, Hart DA. A CD-1 mouse model of infection with Staphylococcus aureus: influence of gender on infection with MRSA and MSSA isolates. Can J Microbiol. 2000;46(10):920–6. . [PubMed] [Google Scholar]
- 73.Goodridge HS, Shimada T, Wolf AJ, Hsu YM, Becker CA, Lin X, et al. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J Immunol. 2009;182(2):1146–54. 10.4049/jimmunol.182.2.1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2(9):2049–56. 10.1038/nprot.2007.296 . [DOI] [PubMed] [Google Scholar]
- 75.Underhill DM. Macrophage recognition of zymosan particles. J Endotoxin Res. 2003;9(3):176–80. 10.1179/096805103125001586 . [DOI] [PubMed] [Google Scholar]
- 76.Dillen CA, Pinsker BL, Marusina AI, Merleev AA, Farber ON, Liu H, et al. Clonally expanded γδ T cells protect against Staphylococcus aureus skin reinfection. J Clin Invest. 2018;128(3):1026–42. Epub 2018/02/05. 10.1172/JCI96481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Schematic for the generation of 4X-SA-GP. Glucan particles were loaded with the purified, his-tagged, recombinant proteins: rClfA, rIsdA, rMntC, and rSdrE. (B) Coomassie stained SDS-PAGE gel of the 4 purified recombinant proteins: Lane 1, rClfA (98.3 kDa), Lane 2, rMntC (36.2 kDa), Lane 3, rIsdA (34.1 kDa), Lane 4, rSdrE (118.6 kDa). The approximate molecular weight of each protein is shown in parentheses. All lanes contain approximately 10 μg of protein. (C). Western blot analysis of 4X-SA-GP using an anti-6xHN antibody to probe for the his-tagged proteins within the GPs. 2x107 4X-SA-GPs were loaded into the lane.
(TIF)
(A-G) BMDCs stimulated with GP-No OVA or GP-OVA (loaded with various concentrations of OVA) were used to activate naïve CFSE-labeled OT-II CD4+ T cells under non-polarizing conditions. (A) Representative flow cytometry plots show the degree of Th1 (IFNγ+) and Th17 (IL-17+) T cell differentiation under each condition of BMDC activation. (B) Representative flow cytometry histograms demonstrate the degree of T cell polarization corresponding to the data above with the pink histogram from the co-culture with no stimulation used as a reference point for the percentage of proliferation. (C-D) Bar graphs quantifying the percentage of IL-17+ (C) and IFNγ+ (D) OT-II cells and the percentage of CFSE proliferation (E). (F-G) Supernatants from the co-culture were collected on day 5 and analyzed for production of IL-17 (F) and IFNγ (G) by ELISA. Flow cytometry percentages and ELISA data and their standard deviation are representative of two replicates/condition. Data are representative of two experiments.
(TIF)
(A-E) Wild-type female mice were immunized one time with PBS (n = 5), Empty-GPs (n = 5), or 4X-SA-GP (n = 5). Five days after the vaccination, splenocytes from each mouse were stimulated with a mixture of all 4 S. aureus purified recombinant proteins (proteins) and heat-killed S. aureus (HK-SA) for 3 days. (No stim., no stimulation). After 3 days, the splenocytes were stimulated with phorbol myristate acetate (PMA) and ionomycin and then analyzed by flow cytometry after intracellular cytokine staining. Representative flow cytometry plots demonstrate the degree of IL-17 and IFNγ production from CD4+ T cells (gated on TCRβ+CD4+) in response to the stimuli (proteins, HK-SA) from the spleen (A). (B and D) Bar graphs show the frequency of IL-17 (B) and IFNγ positive cells in the spleen from the mice. Each data point represents an individual mouse in all of the bar graphs. (C and E) Supernatants were harvested at day 3 from the splenocyte (A) stimulation and the cytokines IL-17 (C) and IFNγ (E) were determined by ELISA. Data analysis was performed using ANOVA. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001. Data are representative of a single experiment.
(TIF)
(A-B) Serum was collected from each group of vaccinated mice 2 weeks after one immunization with PBS, Empty-GP, or 4X-SA-GP (n = 5 mice per group). The serum was diluted 3 times at 1:1000, 1:10,000 and 1;100,000 and was then tested for antibodies specific for each of the 4 S. aureus proteins encapsulated in 4X-SA-GP by ELISA. The subclasses IgG1 (A) and IgG2c (B) were analyzed for specificity towards rClfA, rIsdA, rMntC, and SdrE. The read-out of the assay is the optical density (OD) at 450 nm for each serum sample. Each data point represents an individual mouse. Data analysis was performed using ANOVA. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Data are representative of a single experiment.
(TIF)
(A-B) Wild-type female mice were immunized once a week for 3 weeks with PBS (n = 9), Empty-GPs (n = 10), or 4X-SA-GP (n = 10). Four weeks after the final vaccination 4–5 mice per vaccination group were treated i.p. with anti-CD4+ antibody or the corresponding isotype control antibody on day -1 and day 0. On day 0, all groups of the mice were infected i.p. with 2x107 CFUs of S. aureus (LAC USA300). (A) flow cytometry plots demonstrating the degree of CD4+ T cell depletion from pooled peripheral blood mononuclear cells (PBMCs) from each vaccination group of mice on day 0 before the mice were infected with S. aureus. (B) flow cytometry plots demonstrating the degree of CD4+ T cell depletion from pooled MLNs from each vaccination group of mice 24 hours after the mice were infected with S. aureus (day 1). Cells from (A) and (B) were gated on CD3+ cells.
(TIF)
(A-B) Two sets of wild-type female mice were immunized once a week for 3 weeks with PBS (n = 5), Empty-GPs (n = 5), or 4X-SA-GP (n = 5). Serum was collected from one set of mice (PBS, Empty-GP, 4X-SA-GP; n = 5 mice/group) 2 weeks after the final vaccination and 8 weeks after the final immunization for the other set of mice (PBS, Empty-GP, 4X-SA-GP; n = 5 mice/group). The serum was diluted 3 times at 1:1000, 1:10,000 and 1;100,000 and was then tested for antibodies specific for each of the 4 S. aureus proteins encapsulated in 4X-SA-GP by ELISA. The subclasses IgG1(A) and IgG2c (B) were analyzed for specificity towards rClfA, rIsdA, rMntC, and rSdrE. The read-out of the assay is the optical density (OD) at 450 nm for each serum sample. Each data point represents an individual mouse. Data analysis was performed using ANOVA. *p<0.05, **p<0.005. Data are representative of at least two experiments for serum at two weeks and a single experiment for serum at 8 weeks.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.