Abstract
The cricetine rodent Peromyscus leucopus is an important reservoir for several human zoonoses, including Lyme disease, in North America. Akin to hamsters, the white-footed deermouse has been unevenly characterized in comparison to the murid Mus musculus. To further understanding of P. leucopus’ total genomic content, we investigated gut microbiomes of an outbred colony of P. leucopus, inbred M. musculus, and a natural population of P. leucopus. Metagenome and whole genome sequencing were combined with microbiology and microscopy approaches. A focus was the genus Lactobacillus, four diverse species of which were isolated from forestomach and feces of colony P. leucopus. Three of the species—L. animalis, L. reuteri, and provisionally-named species “L. peromysci”—were identified in fecal metagenomes of wild P. leucopus but not discernibly in samples from M. musculus. L. johnsonii, the fourth species, was common in M. musculus but absent or sparse in wild P. leucopus. Also identified in both colony and natural populations were a Helicobacter sp. in feces but not stomach, and a Tritrichomonas sp. protozoan in cecum or feces. The gut metagenomes of colony P. leucopus were similar to those of colony M. musculus at the family or higher level and for major subsystems. But there were multiple differences between species and sexes within each species in their gut metagenomes at orthologous gene level. These findings provide a foundation for hypothesis-testing of functions of individual microbial species and for interventions, such as bait vaccines based on an autochthonous bacterium and targeting P. leucopus for transmission-blocking.
Introduction
Epigraph: “I have always looked at problems from an ecological point of view, by placing most emphasis not on the living things themselves, but rather on their inter-relationships and on their interplay with surroundings and events.” René Dubos, 1981 [1, 2]
Peromyscus leucopus, the white-footed deermouse, is one of the most abundant wild mammals in central and eastern United States and adjacent regions of Canada and Mexico [3, 4]. The rodent is an omnivore, consuming a variety of seeds, such as oak acorns, as well as insects and other invertebrates. Its wide geographic range extends from rural areas to suburbs and even cities, and it is especially common in areas where humans and wildland areas interface [5]. Conditions permitting, P. leucopus is procreatively proliferant, with 20 or more litters during a female’s period of fecundity [6].
Although commonly called a “mouse”, this species and other members of the genus Peromyscus belong to the family Cricetidae, which includes hamsters and voles, and not the family Muridae, which includes Mus and Rattus. The pairwise divergence time for the genera Peromyscus and Mus is estimated to be ~27 million years ago [7, 8], approximately the time since divergence of the family Hominidae, the great apes and hominids, from Cercopithecidae, the Old World monkeys [7, 9]. While only a minority of a birth cohort of P. leucopus typically survive the predation and winter conditions of their first year in nature [10, 11], in captivity Peromyscus species can live twice as long as the laboratory mouse or rat [12]. P. leucopus differs in its social behavior and reproductive physiology from rodents that are traditional experimental models [13, 14].
P. leucopus also merits attention as a natural host and keystone reservoir for several tickborne zoonoses of humans (reviewed in [15]. These include Lyme disease, babesiosis, anaplasmosis, a form of relapsing ever, an ehrlichiosis, and a viral encephalitis. For humans these infections are commonly disabling and sometimes fatal, but P. leucopus is remarkably resilient in the face of persistent infections with these pathogens, singly or in combination. How this species tolerates infections to otherwise thrive as well as it does is poorly understood.
P. leucopus’ importance as a pathogen reservoir, its resilience in the face of infection, and its appealing features as an animal model [6, 16], prompted our genetic characterization of this species, beginning with sequencing and annotating its nuclear and mitochondrial genomes [17, 18]. The present study represents the third leg of this project, namely the microbial portion of the total animal “genome” for this species. Given the development of bait-delivered oral vaccines targeting P. leucopus [19] and plans to genetically modify and release this species [20, 21], pushing ahead on these interventional fronts without better understanding Peromyscus microbiota, the gastrointestinal (GI) tract’s in particular, seemed shortsighted.
Accordingly, we carried out a combined microbiologic and metagenomic study of the GI microbiome of P. leucopus. In our exploration of bacteria, protozoa, and DNA viruses in this species’ gut, we included whole genome sequences from cultured organisms as well as large contigs assembled from metagenome reads of uncultured organisms in our in-depth exploration of this. Attention initially focused on animals of a stock colony that has for many years been the major source of animals for different laboratories and spin-off breeding programs, including our own, in North America. The study extended to samples of P. leucopus deermice in their natural environments and, for a comparative animal, vivarium-reared Mus musculus under similar husbandry. While our investigations revealed similarities between the microbiota of the white-footed deermouse and the house mouse, there were also notable differences. These included a greater abundance and diversity of lactobacilli in P. leucopus. The investigation of four Lactobacillus species, particularly in their niches in the stomach of P. leucopus, was a special emphasis. A comparison of the GI microbiota of a natural population of P. leucopus and the stock colony animals revealed several species in common, albeit with larger variance among the wild animals.
Results and discussion
High coverage sequencing of fecal metagenome
At the start of our investigation there was only limited information in the literature on the GI microbiota of P. leucopus, and the studies were restricted to data on bacterial 16S ribosomal RNA genes sequences, as in the examples of references [22] and [23]. For a fuller description of the microbiota, including parasites and DNA viruses, as well as bacteria, of representatives of this species, we took an untargeted, metagenomic approach. This was followed by bioinformatic characterization of the breadth of organisms represented in the metagenome and then assembly and annotation of large chromosomal contigs of selected microorganisms in the metagenome.
We began with deep sequencing of a library of DNA extracted from a pooled sample of fecal pellets collected from two adult males and two adult females of the LL stock colony. This yielded 332,279,332 paired-end reads of average length 247 nt, or ~8 x 1010 bp, after quality control and trimming of adapters. The mean % GC content was 47; 90% of the trimmed reads had PHRED scores of ≥ 30. The reads were characterized as to families of bacteria, parasites, and DNA viruses at the metagenomic server MG-RAST (http://mgrast.org). Annotated proteins accounted for 65% of the reads, followed by unknown proteins at 34%, and then ribosomal RNA (rRNA) genes at 0.8%. The rarefaction curve became asymptotic at ~200,000 reads and a species count of 9000 (S1 Fig). The alpha diversity for this pooled sample was 250 species. By phylum 94% of the matched reads were either Bacteroidetes (60%) or Firmicutes (34%) (S2 Fig). Higher level functional categories included carbohydrates (16.4% of reads), clustering-based subsystems (14.8%), protein metabolism (8.9%), amino acids and derivatives (7.8%), RNA metabolism (6.6%), and DNA metabolism (5.7%) (S3 Fig).
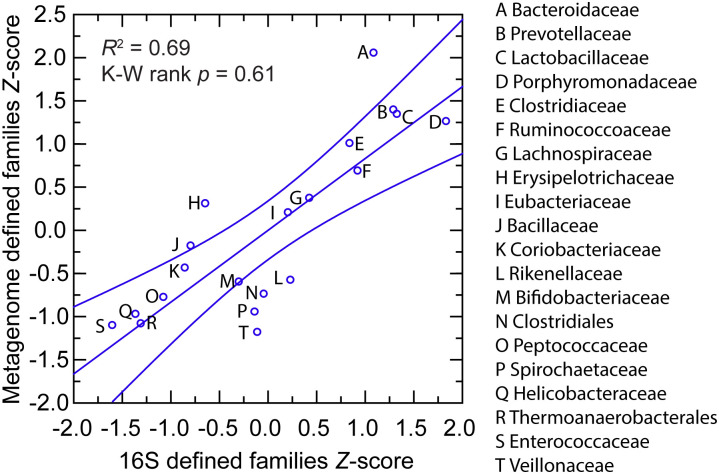
A portion of the DNA was also submitted to a reference laboratory for 16S rRNA determinations from metagenomic analysis of microbiota. As illustrated in Fig 1 and detailed in S1 and S2 Tables, for the 20 most abundant taxa at the family or higher, there was concordance between the methods in the rankings. The most common families by the metagenomic accounting were members of the gram-negative bacterial order Bacterioidales (Bacterioidaceae, Porphyromonadaceae, Prevotellaceae, and Rikenellaceae), the gram-positive phylum Actinobacteria (Bifidobacteriaceae and Coriobacteriaceae), or the gram-positive phylum Firmicutes (Bacillaceae, Enterococcaceae, Lactobacillaceae, unclassified Clostridiales, Eubacteriaceae, Lachnospiraceae, Peptococcaceae, Ruminococcoceae, Thermoanaerobacterales Family III, Erysipelotrichaceae, and Veillonellaceae). Two exceptions were organisms of the families Spirochaetaceae of the phylum Spirochaetes and Helicobacteraceae of the phylum Proteobacteria.
Fig 1. Scatter plot of relative abundances of commonly occurring bacterial families or orders in fecal metagenomes of Peromyscus leucopus LL stock by 16S ribosomal RNA gene criterion (x-axis) and by genome-wide metagenome criterion (y-axis).
The values of the two methods were normalized by Z-score. The different taxa are indicated in the graph by capital letters, which are defined in the box to the right. The linear regression curve and its 95% confidence interval is shown. The coefficient of determination (R2) value and the Kruskal-Wallis (K-W) test by ranks p value are given.
A de novo assembly yielded 16,945 ungapped contigs of ≥ 10 kb from 197,369,943 reads and totaling 385 Mb of sequence with an average coverage of 104X. Of the total, 219 contigs were ≥ 100 kb in length and with ≥ 30X coverage. These were used in searches of non-redundant nucleotide and protein databases for provisional classifications. The identified taxa included Bacteroidales, Clostridia, Clostridiaceae, Erysipelotrichales, Lactobacillaceae, Muribaculaceae, Firmicutes, and Spirochaetaceae. Three organisms represented among the high coverage contigs could be unambiguously classified as to species: Lactobacillus animalis, which is considered in detail below, and two Parabacteroides species: distasonis and johnsonii. Another Lactobacillus species represented among the highly ranked contigs could not be identified with a known species represented in the database [24].
Among the high coverage contigs were also representatives of Rhodospirillales of the class Alphaproteobacteria, Mycoplasmataceae of Mollicutes, and the little-characterized group of bacteria called Elusimicrobia [25]. Nearly as prevalent were organisms closely related to the phylum-level designation Candidatus Melainabacteria [26]. On the list of organisms identified by searches with metagenomic contigs of databases and cumulatively accounting for 95% of the matched reads were the unexpected finding of the protozoan taxon Trichomonadidae with 41,614 or 0.12% of the reads (S2 Table). Enterobacteriaceae at 0.3% accounted for a relatively small proportion of matched reads.
As in humans [27], Bacteroidaceae, Lachnospiraceae, Prevotellaceae, and Ruminococcaceae were abundant in the gut metagenome and cumulatively accounted for approximately half of the identified families in the P. leucopus sample. One difference between humans and this P. leucopus sample was the much higher prevalence in P. leucopus of the family Lactobacillaceae, which on average represented only ~0.2% of metagenomes in a European population [27] and by 16S sequencing ≤ 0.4% on the fecal microbiota in other studies [28]. A higher proportion of lactobacilli in the fecal microbiota was previously noted in other rodents [29] and specifically in Peromyscus species [22].
Selected taxa
Escherichia coli
Although Enterobacteriaceae were infrequently represented among the metagenomic sequences, their cultivability under routine laboratory conditions and the availability of a vast database for some species prompted our isolation of Enterobacteriaceae from LL stock P. leucopus fecal pellets on selective media. The predominant isolate on the plates was an Escherichia coli, which we designated LL2. The whole genome sequence of isolate LL2’s chromosome and plasmids was sequenced and assembled using a hybrid of long reads and short reads (Table 1) for an overall coverage of 90X. The chromosome in two contigs of 3.4 Mb and 1.6 Mb totaled 5.0 Mb with a GC content of 50%.
Table 1. Resources from this study.
| Organism and strain | Description | BioProject | BioSample | SRA or MG-RAST a | Accession No. |
|---|---|---|---|---|---|
| Cultured Lactobacillus johnsonii LL8 | 2,045,501 bp WGS chromosome; 40 contigs | PRJNA589091 | SAMN13266521 | SRX7128459 | WKKC00000000 |
| Cultured Lactobacillus johnsonii LL8 | 75,746 bp plasmid | PRJNA589091 | SAMN13266521 | SRX7128459 | CM019125 |
| Cultured Escherichia coli LL2 | Chromosome; 3,345,873 and 1,610,537 bp contigs | PRJNA533838 | SAMN11468944 | SRR9087223 SRR9087224 | VBVB01000001 VBVB01000002 |
| Cultured Escherichia coli LL2 | 121,192 bp plasmid | PRJNA533838 | SAMN11468944 | SRR9087223 SRR9087224 | CM017030 |
| Cultured Escherichia coli LL2 | 90,617 bp plasmid | PRJNA533838 | SAMN11468944 | SRR9087223 SRR9087224 | CM017032 |
| Cultured Escherichia coli LL2 | 56,474 bp plasmid | PRJNA533838 | SAMN11468944 | SRR9087223 SRR9087224 | CM017031 |
| Gut metagenome | 50–450 kb contigs of fecal metagenome | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | JAAGKN000000000 |
| Uncultured Helicobacter sp. LL4 | 290,716 bp 172,100 bp 203,294 bp chromosome fragments | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MN577567 MN577568 MN577569 |
| Uncultured Helicobacter sp. LL4 | 16S ribosomal RNA gene, partial | n.a. b | n.a. | n.a. | MT114577 |
| Uncultured Candidatus Melainabacteria bacterium isolate LL20 | 270,170 bp chromosome fragment | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MN577570 |
| Uncultured Elusimicrobia bacterium LL30 | 232,820 bp 215,518 bp chromosome fragments | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MN577571 MN577572 |
| Uncultured Clostridiales bacterium LL40 | 438,773 bp chromosome fragment | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MN577573 |
| Uncultured Spirochaetaceae bacterium LL50 | 277,828 bp chromosome fragment | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MN577574 |
| Uncultured Prevotella sp. LL70 | 456,702 bp 379,405 bp chromosome fragments | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MN990733 MN990734 |
| Uncultured Rhodospirillales bacterium LL75 | 145,048 bp 150,471 bp 130,339 bp 104,625 bp 177,737 bp chromosome fragments | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MN990728 MN990729 MN990730 MN990731 MN990732 |
| Uncultured Mycoplasmataceae bacterium LL85 | 126,601 bp 100,243 bp chromosome fragments | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MN991199 MN991200 |
| Uncultured Muribaculaceae bacterium LL71 | 125,326 bp 103,384 bp chromosome fragments | PRJNA540317 | SAMN11533720 | mgm4799371.3 mgm4799372.3 | MT002444 MT002445 |
| Uncultured Tritrichomonas sp. LL5 | 1,501 bp of small subunit ribosomal RNA | PRJNA540317 | SAMN13920683 | mgm4864879.3 | MN120899 |
| Uncultured Tritrichomonas sp. LL5 | 989 bp partial iron hydrogenase gene of hydrogenosome | PRJNA540317 | SAMN13920683 | mgm4864879.3 | MN985504 |
| Uncultured Tritrichomonas sp. LL5 | 5298 bp fragment with DNA polymerase type B, organellar and viral family protein | PRJNA540317 | SAMN13920683 | mgm4864879.3 | MT002461 |
| Uncultured Lactobacillus sp. (“peromysci”) BI7442 | rpsA, ftsK, ftsZ, dnaA, dnaN, recD, ileS, recA, topA | PRJNA593618 | SAMN13482862 | SRX7285441 | MN792760 -MN792768 |
| Uncultured Lactobacillus animalis 7442BI | 51 large and small ribosomal proteins | PRJNA593618 | SAMN13482862 | SRX7285441 | MN817867 -MN817918 |
E. coli LL2 had the following MLST schema types (http://pubmlst.org or http://enterobase.org): Achtman ST-278, Pasteur ST-357, and ribosomal protein ST-122394. The ribosomal protein profile was unique among thousands of isolates in the database. The 121 kb, 56.5 kb, and 91 kb plasmids of strain LL2 were similar to the following E coli plasmids, respectively: a 185 kb plasmid (NC_007675) found in an avian strain, a 58 kb plasmid (CP024858) of a multiply antibiotic-resistant human isolate, and an 89 kb plasmid (CM007643) in an organism isolated from sewage. E. coli LL2 was susceptible to ampicillin, ciprofloxacin, gentamicin, and sulfamethoxazole-trimethoprim by in vitro testing.
The chromosome was notable for the following: CRISPR-Cas1 and–Cas3 arrays; ISas1, ISNCY, IS3, IS110 and IS200 family transposases; restriction-modification systems; fimbria and curli biosynthesis and transport systems; type II toxin-antitoxin systems; and type II, type III and type VI secretion systems. The plasmids encoded fimbrial and pilin proteins, type I, type II, and type IV secretion systems, colicins, CdiA-type contact-dependent inhibition toxin, and three conjugative transfer systems, but no discernible coding sequences for antibiotic resistance.
Serial dilutions of feces of LL stock 20 animals (11 females and 9 males) in phosphate-buffered saline and plated on agar selective for gram-negative enteric bacteria yielded a mean (asymmetric 95% confidence interval) of 3,491 (677–18,010) colony-forming units (cfu) of E. coli per g of feces. This low density was consistent with the findings from metagenomic sequencing.
The origin of this E. coli strain in these animals is obscure. Its source may have been the vivarium for the breeding stock of the colony, which includes other Peromyscus species besides P. leucopus. But the strain appears to be stably maintained among the gut microbiota of this population of P. leucopus. This adaptation and its susceptibilities to commonly-used antibiotics may make it a candidate as a vector for delivering oral vaccines to this species [30].
Lactobacillus
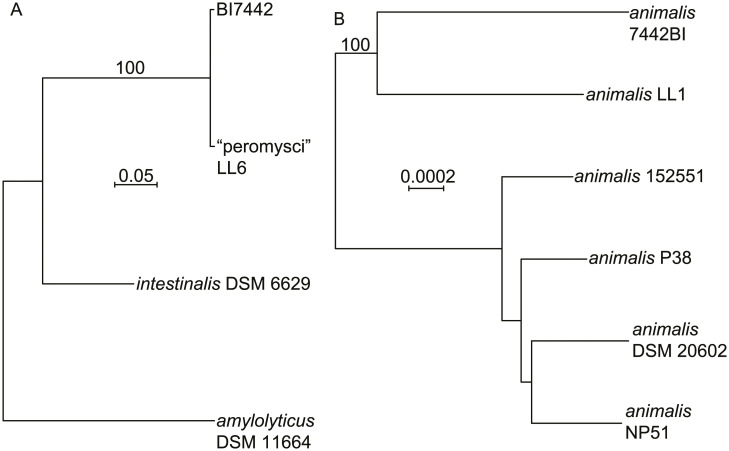
We isolated lactobacilli from fecal pellets of stock colony P. leucopus on plates of selective medium that were incubated under microaerophilic and hypercapnic conditions at 37 °C. Four different species were identified. The genomes of three of organisms, namely L. animalis strain LL1, L. reuteri strain LL7, and a new species, designated as Lactobacillus sp. LL6 and provisionally named as “L. peromysci”, have been reported [24]. The fourth genome, of the LL8 strain of L. johnsonii, is described first here (Table 1). L. johnsonii’s chromosome from cumulative contigs was 2,045,501 bp, about the same size as that of “L. peromysci” at 2,067,236 bp, but shorter than the 2,280,577 bp length for L. animalis and 2,205,740 bp for L. reuteri. The % GC content of “L. peromysci” at 33.5 was closer to L. johnsonii (34.4) than to either L. animalis (41.0) or L. reuteri (38.9).
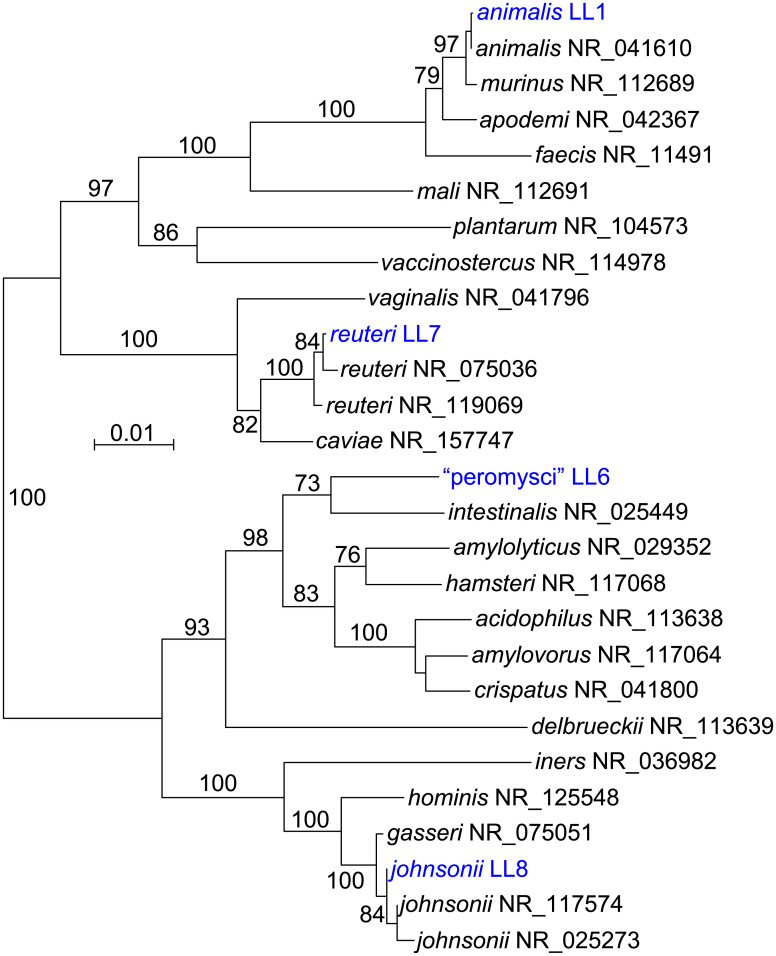
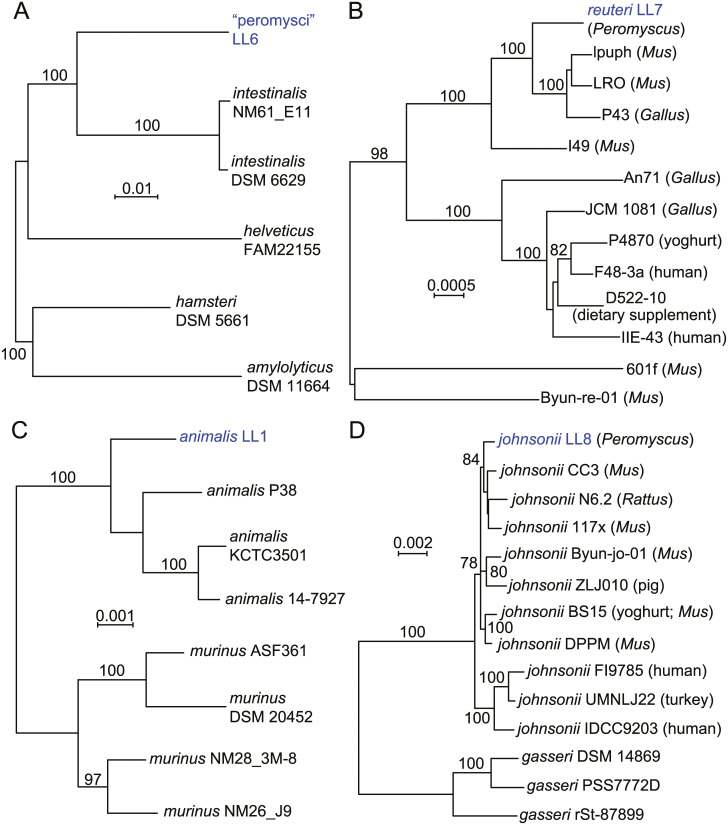
Fig 2 is a distance phylogram of 1385 aligned sites of 16S ribosomal RNA genes for the four different lactobacilli. These were distributed across four major groups of the genus Lactobacillus. The phylogenetic relationships were examined in more depth by multilocus sequence typing of the 53 genes for ribosomal proteins. These were identified in the genomes, compared with other deposited sequences in the ribosomal MLST database (https://pubmlst.org) [31], concatenated, and then aligned with analogously concatenated DNA sequences from related species (S3 Table). Bacteria with identical sequences for the 53 ribosomal protein genes were not found in the rMLST database of 133,460 profiles. The % GC contents of the concatenated coding sequences were 39.5, 40.8, 42.2, and 42.3 for L. johnsonii, “L. peromysci”, L reuteri, and L. animalis, respectively. Fig 3 shows the distance phylograms for ~20 kb of aligned positions for the four species, each grouped with other strains or species within their respective phylogenetic clusters.
Fig 2. Neighbor-joining distance phylogram of 1420 aligned positions of 16S ribosomal RNA genes of the culture isolates of four Lactobacillus species from Peromyscus leucopus and selected other Lactobacillus spp.
The sources for the accession numbers for the strains are given in Methods (L. animalis, L. reuteri, and “L. peromysci”) or in Table 1. The other organisms represented are from Reference RNA sequences database of the National Center for Biotechnology Information; the accession numbers are given after the species name. The scale for distance by criterion of observed differences is indicated. Percent bootstrap (100 iterations) support values of ≥ 90% at a node are shown.
Fig 3. Neighbor-joining distance phylograms of codon-aligned, concatenated nucleotide sequences for complete sets of ribosomal proteins of “L. peromysci” (panel A), L. reuteri (panel B), L. animalis (panel C), and L. johnsonii (panel D) of P. leucopus compared with Lactobacillus spp. (strain identifier) of other sources.
The scales for distance by Jukes-Cantor criterion are indicated in each panel. Percent bootstrap (100 iterations) support values of ≥ 75% at a node are shown. In panels B and D the host animal or other origin for a given isolate are given in parentheses.
“Lactobacillus peromysci” was distant from other sequenced lactobacilli by rMLST (panel A), as well as by its 16S ribosomal RNA gene (Fig 2). The nearest taxon in the sequence alignment was L. intestinalis, which was first isolated from the intestines of Mus musculus and other murids [32]. The unique ST for the rMLST for strain LL6 of this organism is 115326.
Draft and complete genomes of numerous L. reuteri strains have been sequenced, for example, strain Byun-re-01, which was isolated from M. musculus small intestine [33]. Many of these are utilized in the fermented foods industry, such as production of kimchi, or as dietary supplements, but others were isolated as constituents of the GI microbiota of several varieties of animals. L. reuteri strain LL7 was in a cluster that mainly comprised isolates from M. musculus (panel B).
L. animalis and L. murinus are closely related species that primarily have been associated with GI microbiota of rodents and some other mammals. Isolate LL1 grouped with representatives of L. animalis in the analysis (panel C) and not L. murinus [34]. LL1’s 16 ribosomal RNA sequence was identical to that of the type strain ATCC 35046 of L. animalis [35] at 1488 of 1489 positions (GCA_000183825) [36]. Another pair of closely-related species are L. johnsonii and L. gasseri, for which there are several sequenced genomes. The LL8 isolate from fecal pellets of P. leucopus clustered with L. johnsonii strains from mice and rats (panel D). More distant were strains of L. johnsonii isolated from humans and a bird; more distant still were representatives of L. gasseri.
Plasmids were identified in each of the four species on the basis of a circularly permuted sequence for a contig and presence of coding sequences that were homologous to known plasmid replication or partition proteins (Table 1). Large plasmids of 179 kb and 76 kb were present in L. reuteri and L. johnsonii, respectively. L. animalis and “L. peromysci” had small plasmids of 4 kb and 7 kb, respectively. Megaplasmids of greater than 100 kb have been observed in other Lactobacillus spp. [37]. In all genomes there was evidence of lysogenic bacteriophages or their remnants. All species except L. reuteri discernibly had coding sequences for Class I or Class III bacteriocins or their specific transport and immunity proteins (S4 Table).
Table 2 summarizes differentiating genetic profiles among the four species for 11 selected genes or pathways. Two species, L. reuteri and “L. peromysci”, had coding sequences for a urease, which could provide for tolerance of acidic conditions, such as in the stomach. A urease had previously been identified in a L. reuteri strain that was considered a gut symbiont in rodents [38]. The four species had secY1-secA1 transport and secretion systems. Accessory Sec systems (secY2-secA2) were identified in genomes of L. reuteri, L. johnsonii, and L. animalis but not in “L. peromysci”. The LL7 strain of L. reuteri on its megaplasmid also had coding sequences for a third SecY-SecA system. An accessory Sec system was involved with adhesion and biofilm formation in the Lactobacillales bacterium Streptococcus pneumoniae [39]. A coding sequence for an IgA protease was identified in L. johnsonii but not in the other three species. An IgA protease in another strain of L. johnsonii was associated with long-term persistence in the gut of mice [40]. The presence or absence of other genes or pathways that differentiated between the four species were an L-rhamnose biosynthesis pathway in one species, a luxS gene associated with a quorum sensing system in L. reuteri and L. johnsonii [41], a type 1 CRISPR-Cas3 array in “L. peromysci” [42], pathways for thiamine biosynthesis [43] and for reduction of nitrate [44] in three species, an arginine deiminase and its repressor in L. reuteri [45], and a type VII secretion system in L. animalis [46].
Table 2. Selected genes and pathways in four species of Lactobacillus of the gastrointestinal microbiota of Peromyscus leucopus.
| Species | Urease | secY2- secA2 | secY3- secA3 | IgA protease (pfam07580) | L-rhamnose pathway | luxS | Type 1 CRISPR Cas | Thiamine biosynthesis | Nitroreductase nfnB-nifU | Arginine deiminase/ repressor | Type VII secretion system |
|---|---|---|---|---|---|---|---|---|---|---|---|
| reuteri | + | + | + | - | - | + | - | - | - | + | - |
| johnsonii | - | + | - | + | - | + | - | + | + | - | - |
| animalis | - | + | - | - | - | - | - | + | + | - | + |
| “peromysci” | + | - | - | - | + | - | + | + | + | - | - |
Of the four species found in P. leucopus feces, only L. johnsonii and L. reuteri have been commonly isolated from human feces [28]. While various strains of L. reuteri, L. johnsonii, and either L. animalis or the closely related L. murinus have been observed among the GI microbiota of M. musculus represenstatives [47], an organism similar to “L. peromysci” has not. Whether this is an indication of a restricted host range or a specific adaptation for this bacterium is considered below. Lactobacilliaceae organisms were previously noted in the gut microbiota of Peromyscus species but were not further classified as to species or otherwise characterized [22, 23].
Helicobacter
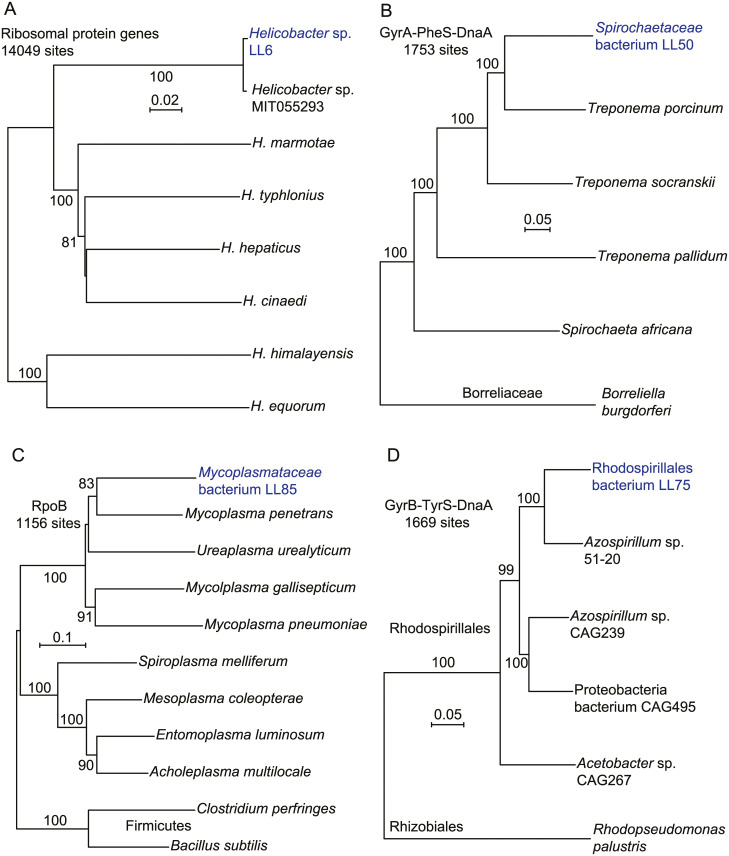
Among the assembled metagenomic contigs were three totaling 666,100 bp of a Helicobacter genome (Table 1). The contigs had non-overlapping in genetic content, and blast searches with translated genes from each of the 3 contigs yielded the identical rankings of taxa for homologous proteins. On these bases, we concluded that the contigs represented a single type of Helicobacter bacterium, and designated it strain LL4. Using the DNA sequences for 53 ribosomal proteins of this organism, we compared it with similar sets from other Helicobacter spp. (panel A of Fig 4). This analysis showed that the organism was near-identical to orthologous sequences of Helicobacter sp. MIT 05–5293 (accession JROZ02000000), which had been cultivated from a wild P. leucopus captured in Massachusetts ([48]; J.G. Fox, personal communication). This finding indicated that the organism was autochthonous for Peromyscus and had not been acquired from another rodent housed in the same vivarium. Baxter et al. reported “abundant representatives” of the genus Helicobacter in wild P. maniculatus and P. leucopus captured in Michigan [22].
Fig 4. Neighbor-joining distance phylograms of concatenated nucleotide (panel A) or amino sequences (panels B-C) of Helicobacter spp. (panel A), Spirochaetaceae bacteria (panel B), Mollicutes and Firmicutes bacteria (panel C) and Rhodospirillales bacteria (panel D) of gut metagenome of P. leucopus and from other sources.
The accession numbers for the other bacteria represented in the phylograms are given in S5 Table. The respective phylogenetic analyses used concatenated sequences of the following: ribosomal protein genes (panel A); the DNA gyrase A (GyrA), phenylalanyl t-RNA synthase, alpha subunit (PheS), and chromosomal replication iniator protein (DnaA) (panel B); DNA-directed RNA polymerase, beta-subunit (RpoB) (panel C); and DNA gyrase B (GyrB), tyrosyl t-RNA synthase (TyrS), and DnaA (panel D). The distance criteria were Jukes-Cantor for the codon-aligned nucleotide sequences and Poisson for amino acid sequences. The scales for distance are shown in each panel. Percent bootstrap (100 iterations) support values of ≥ 80% at a node are shown.
Strains LL4 and MIT 05–5293 are in a cluster of species known as “enterohepatic” Helicobacter for their primary residence in the intestine rather than the stomach and for their frequent presence in liver tissue [49]. These species may not be benign. H. hepaticus is associated with hepatitis, bowel inflammation, and carcinoma [50], and H. typhlonius is associated with reduced fecundity in mice [51].
Spirochaetaceae
The phylogenetic relationship of the uncultured Spirochaetaceae bacterium LL50 to selected other spirochetes (S5 Table) is shown in panel B of Fig 4. The organism was classified in the genus Treponema by the MG-RAST analysis program. Bacteria that have assigned to this genus are highly divergent and include free-living organisms in a variety of environments, symbionts of termites, the agent of syphilis, and gut residents, such as T. porcinum, which was isolated from the feces of pigs [52]. More distant still was the agent of Lyme disease, Borreliella burgdorferi, of the family Borreliaceae [53]. In our view naming the organism as a “treponeme” on the evidence to date would provide little insight about its role in the microbiome and may even be misleading.
Seven other bacterial taxa
The Mycoplasmataceae bacterium LL85 (panel C of Fig 4) was unlike any other mollicute represented in the database but was in a cluster with vertebrate-associated species, like M. pneumoniae. But there is also deep branching in this tree, as the tree including two Firmicutes as outgroups shows (S5 Table). Panel D is a phylogram of selected alphaproteobacteria and includes the organism LL75 (S5 Table). The algorithmic analysis identified this at the genus level as Azospirillum, which is a largely uncharacterized taxon with highly divergent members. While assignments as to genus or family are uncertain at this time, LL75 clustered within the order Rhodospirillales and not with rhizobacteria (S5 Table).
Table 1 lists five other types of novel bacteria that were identified in the P. leucopus gut metagenome and partially sequenced and annotated. These were a Candidatus Melainabacteria bacterium (isolate LL20), an Elusimicrobia bacteria (isolate LL30), a Clostridiales bacterium (isolate LL40), a Prevotella species (isolate LL70), and a Muribaculaceae bacterium (isolate LL71). Candidatus Melainabacteria is either a non-photosynthetic sister phylum of cyanobacteria or a class within the phylum Cyanobacteria [26]. Besides a variety of environmental sources, including hot springs and microbial mats, these poorly-characterized organisms have also been identified in the feces of humans and other animals. The phylum Elusimicrobia, formerly “Termite Group 1” [54], is a strictly-anaerobic, deeply-branched lineage of gram-negative bacteria, representatives of which were first observed in the hindgut of termites [25]. The family Muribaculaceae (formerly “family S24-7”) of the order Bacteroidales were first identified among gut microbiota of mice and subsequently in the intestines of other animals, including humans and ruminants [55].
DNA viruses
Of 112,677,080 reads of the metagenome high-coverage sequencing of the LL stock animals, 97,147 (0.09%) were assigned to one of 28 DNA virus families. Three classifications accounted 92% of the reads: Siphoviridae (50%), which are bacteriophages with long contractile tails; Myoviridae (21%), which are bacteriophages with contractile tails; and “unclassified viruses” (21%). At the species level, 31,812 (68%) of the 46,904 Siphoviridae-matching reads mapped specifically to bacteriophages of Lactobacillus spp.
Tritrichomonas protozoan
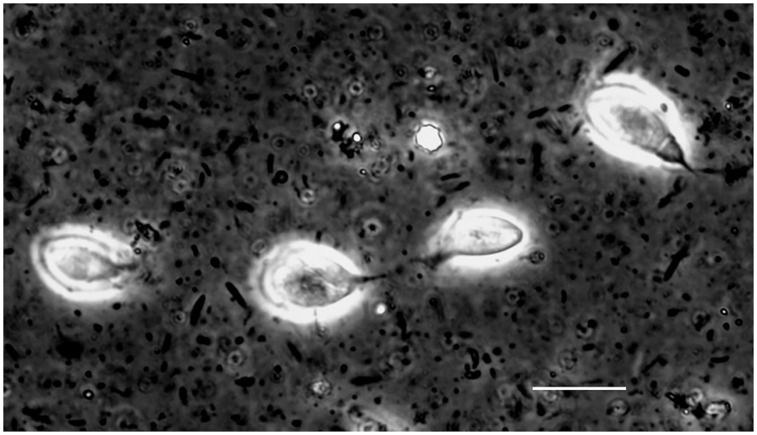
Intestinal flagellated protozoa named “Trichomonas muris” or “Tritrichomonas muris” had previously been identified in wild P. leucopus and P. maniculatus [56]. While laboratory mice are typically free of intestinal protozoa [57], the anaerobic Tritrichomonas muris has been reported in some populations of colony M. musculus [58]. To further investigate the protozoa that were provisionally identified as “Trichomondidae” at the family level in the metagenome analysis, we euthanized 14 healthy adult animals (6 females and 8 males) and examined fresh cecal contents by phase microscopy. Six of the LL stock animals had been born at the PGSC facility, and 8 had been born at U.C. Irvine.
In each of the 14 animals examined there were numerous motile flagellates consistent in morphology with T. muris in their ceca [59]. These were each at a cell density of ~106 per milliliter of unconcentrated cecal fluid (Fig 5; S1 Movie). Entire ceca and their contents from two adult females and two adult males were subjected to DNA extraction, library preparation from the DNA, sequencing, and de novo assembly of contigs.
Fig 5. Photomicrograph of live Tritrichomonas flagellated protozoan in the cecal fluid of P. leucopus LL stock.
Four organisms against the background of intestinal bacteria were visualized in the wet mount by differential interference microscopy. Bar, 10 μm.
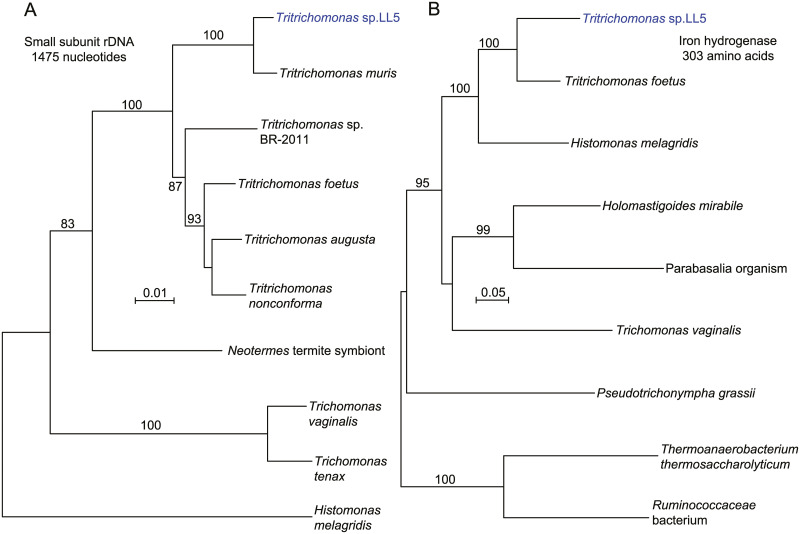
Fig 6 shows phylograms of nucleotide sequence of the small subunit (SSU) ribosomal RNA gene (panel A; S5 Table) and of the partial amino acid sequence of the iron hydrogenase of the hydrogenosome of anaerobic protozoa (panel B; S5 Table) [60]. The SSU of isolate LL5 indicates that it is probably synonymous with Tritrichomonas muris, for which only a SSU sequence was available. The sequence of the iron hydrogenase further supported placement in the genus Tritrichomonas. Histomonas melagridis, a sister taxon by this analysis, is recognized as a pathogen of poultry. Another sequence of the LL5 organism encodes a type B DNA polymerase (Table 1), which likewise matched closely with an ortholog in the Tritrichomonas foetus genome sequence (S4 Fig). T. foetus is a sexually-transmitted pathogen of cattle [61] and a cause of chronic diarrhea in domestic cats [62].
Fig 6. Neighbor-joining distance phylograms of nucleotide (panel A) or amino acid (panel B) partial sequences of small subunit ribosomal RNA gene (rDNA) (panel A) and iron hydrogenase protein (panel B) of Tritrichomonas sp. LL5 of P. leucopus and selected other parabasilids and other microbes.
The distance criteria were observed differences for nucleotide alignment and Poisson for amino acid alignment. The scales for distance are shown in each panel. Percent bootstrap (100 iterations) support values of ≥ 80% at a node are shown.
Whether the T. muris is a commensal shared across natural populations of Peromyscus or a parasite acquired from another rodent during the colony’s history in a vivarium remains to be determined. As related below, there is sequence evidence of the same or related organism in several wild animals. Whatever the case, these organisms may have an effect on immune responses of P. leucopus, as has been reported for T. muris in M. musculus [63–65], and their presence needs to be taken into account in interpreting experimental results in the laboratory and in applications for field interventions.
Comparative study of GI microbiota of P. leucopus and M. musculus
The preceding study revealed several microbes that were either undescribed species or genera, e.g. “L. peromysci” or the Candidatus Melainabacteria bacterium, or new strains of known microbial species, e.g. L. animalis LL1 and T. muris LL4. These novelties notwithstanding, to what extent did the gut microbiota of this deermouse resemble that of the typical laboratory animal, a house mouse that was maintained under similar husbandry conditions, including diet? That question motivated the following experiment.
Fecal pellet samples from 20 adult P. leucopus (10 females and 10 males) and 20 adult BALB/c M. musculus (10 females and 10 males) were obtained and stored frozen at -80 °C until processing. All animals were approximately 10 weeks old. The animals were housed in the same vivarium facility, though in different rooms. The pellets were subjected to total DNA extractions, and paired-end Illumina sequencing with 250 cycles of indexed libraries were carried out. There were means (95% CI) of 3.4 (3.1–3.7) x 106 post-quality control reads for P. leucopus samples and 3.4 (3.2–3.6) x 106 for M. musculus samples (S6 and S7 Tables).
The reproducibility between replicate library constructions from the same sample was assessed with quantitations of reads assigned by taxonomic family for specimens from seven P. leucopus among the 20 total. Pairwise coefficients of determination (R2) for the 91 possible combinations were calculated (S8 Table). The mean (95% CI) of R2 values were 0.999 (0.999–1.0) for the 7 pairs of replicates and 0.930 (0.915–0.944) for the 84 non-replicate pairs. We concluded that most of the variation between samples was attributable to inter-specimen differences in the microbiota and not to technical issues in library preparation or sequencing.
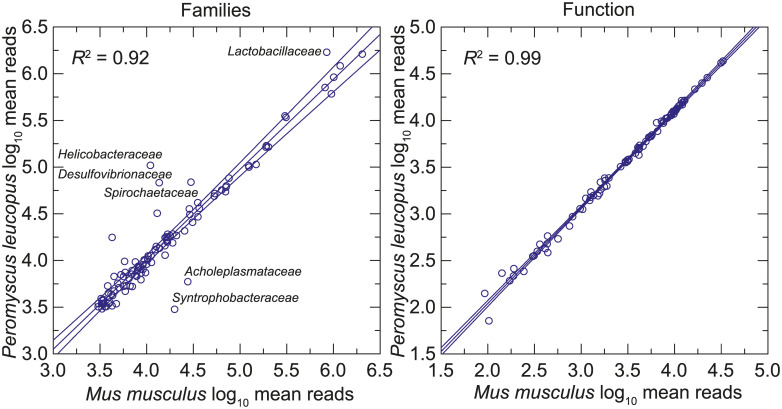
The prevalences of different taxonomic families in the P. leucopus and M. musculus gut metagenomes were similar (left panel of Fig 7 and S9 Table). But a few families stood out as either more or less common in the deermice. Notable among these were Lactobacillaceae, Helicobacteriaceae, and Spirochaetaceae, which were approximately 4x, 8x, and 2x, respectively, more prevalent on average among microbiota of P. leucopus than in M. musculus. There was no evidence of Tritrichomonas sp. in the BALB/c mice by this analysis, but direct examination of intestinal contents was not carried out.
Fig 7. Scatter plots of log-transformed normalized reads of the gut metagenomes of 20 P. leucopus on the gut metagenomes of 20 M. musculus by bacterial families (left panel) or by function at the pathway level (right panel).
The linear regression lines, their 95% confidence intervals, and coefficients of determination (R2) are shown. Selected families that are comparatively more or less prevalent in P. leucopus are indicated.
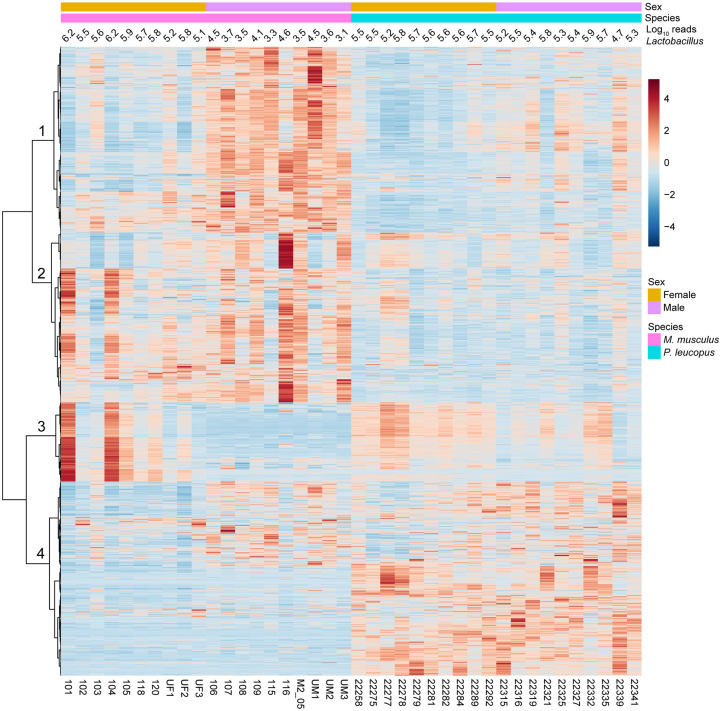
At the level of 86 operational KEGG pathways, the metagenomes of P. leucopus and M. musculus were nearly indistinguishable (right panel of Fig 7 and S10 Table). But, as shown in the heat map of Fig 8, at the homologous gene level there were many differences between these two species and also between females and males within each species (S12 and S13). Hierarchical clusters 2 and 4 of the analysis discriminated between mice and deermice regardless of sex, while clusters 1 and 3 signified marked differences by sex and less so by species.
Fig 8. Heat map-formatted shading matrix of KEGG orthology gene level annotations of gut metagenomes of P. leucopus and M. musculus.
The annotations were generated by MicrobiomeAnalyst (https://www.microbiomeanalyst.ca). Columns are grouped by species and by sex within each species. Individual animal identifications are given on the x-axis below the heat map. Above the heat map are the log-transformed reads mapping to the genus Lactobacillus for each animal’s fecal sample. Clustering of rows of genes were by Pearson correlation coefficient. Four major clusters are labeled 1–4 on the y-axis. Scaling is by relative abundances from low (blue) to high (red).
As one example of differences between species, there was higher representation of genes of the mevalonate pathway in the gut metagenomes of P. leucopus. Beginning with acetyl-CoA and ending with isopentenyl pyrophosphate, the central intermediate in the biosynthesis of isoprenoids in all organisms [66], the coding sequences for the following ordered enzymes (with Enzyme Commission [EC] number) in the pathway were comparatively higher in frequency: acetyl-CoA C-acetyltransferase (EC:2.3.1.9), hydroxymethylglutaryl-CoA synthase (EC:2.3.3.10), hydroxymethylglutaryl-CoA reductase (EC:1.1.1.88), mevalonate kinase (EC:2.7.1.36), phosphomevalonate kinase (EC:2.7.4.2), and diphosphomevalonate decarboxylase (EC:4.1.1.33).
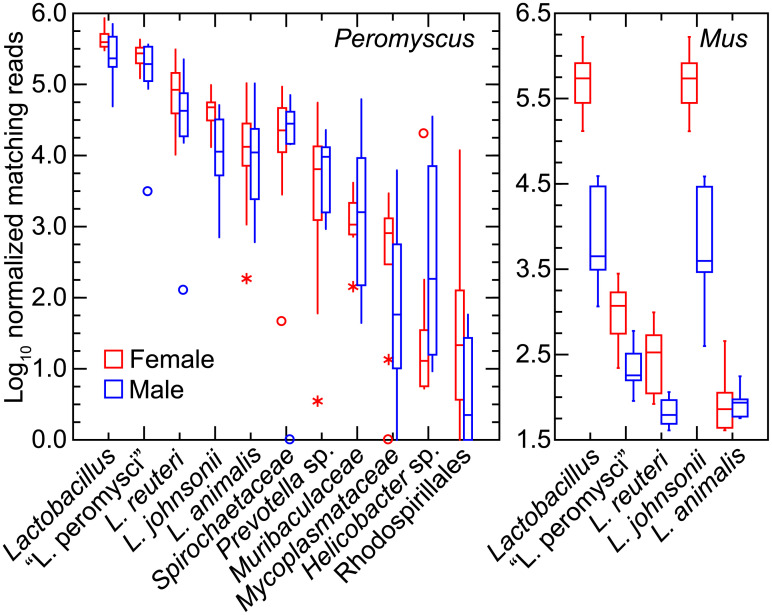
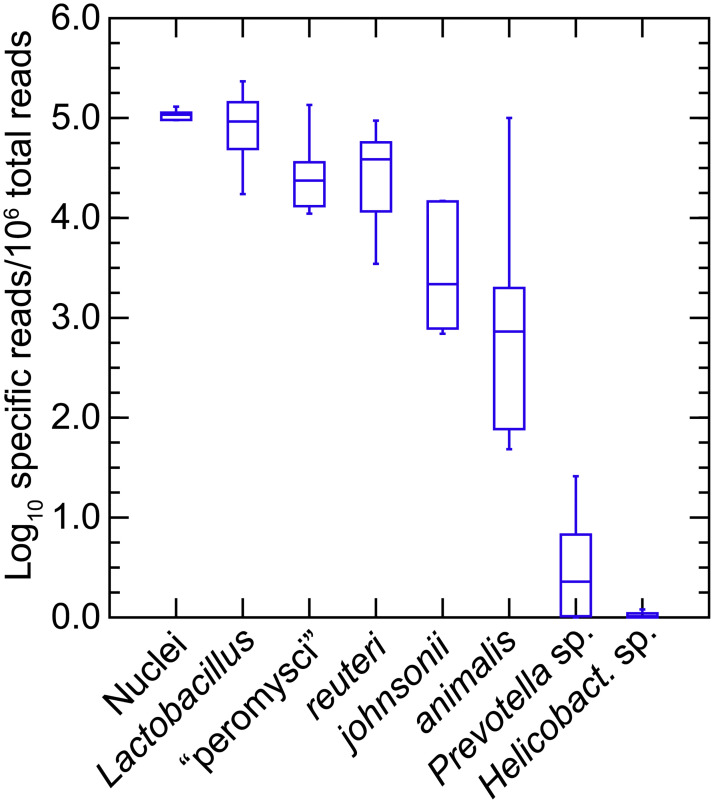
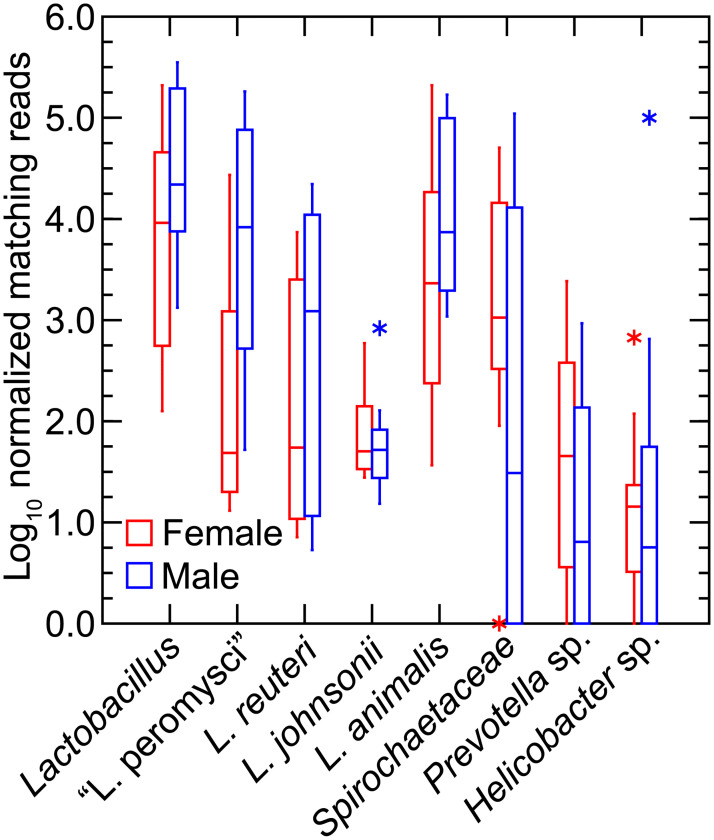
We further investigated specific differences between P. leucopus and M. musculus and between individual animals of each species in Lactobacillus spp. [67]. This was achieved by mapping reads to references of the chromosome sequences of the four species that had been isolated from the feces of LL stock P. leucopus. The caveat is that the lactobacilli in the mice would not be expected to be identical to the deermouse strains used as references. Fig 9 shows box plots for Peromyscus on the left and for Mus on the right for data given in S11 Table. Included in the analysis of P. leucopus gut metagenome reads were selected other bacteria that had been frequently identified among the metagenomic contigs and then further characterized by partial genome sequencing (see above).
Fig 9. Box-whisker plots of log-transformed normalized reads of gut metagenomes of P. leucopus (left panel) and M. musculus (right panel) that mapped to chromosomes of Lactobacillus spp. or other bacteria by host species and grouped by sex.
The references to which reads were mapped were complete chromosomes or partial chromosomes of organisms listed in Table 1. “Lactobacillus” in the first position of each panel were the cumulative reads for the four individual Lactobacillus species in this analysis.
All four species of the lactobacilli were represented in each of the 20 P. leucopus metagenomes. “L. peromysci” and L. reuteri tended to be the most common and consistently represented, while L. johnsonii and L. animalis varied more in prevalences between animals. Other bacteria were also identified in the samples of all or most of the individual animals. The Spirochaetaceae bacterium was ~10-fold less abundant than the cumulative Lactobacillus spp. in the P. leucopus samples.
The mean number of lactobacilli in aggregate were ~2-fold more prevalent in P. leucopus females than males of the species (t-test p = 0.03). In M. musculus this sex difference for Lactobacillus was more pronounced; on average ~100-fold more reads from female mice mapped to Lactobacillus genomes than was found for male mice (t-test p < 0.001). The differences in amounts of fecal lactobacilli in the sample plausibly account for cluster 3 of the heatmap of Fig 8. L. johnsonii largely accounted for these differences between sexes in M. musculus; nearly all of the reads mapping to the Lactobacillus genus as a whole were mapping to the L. johnsonii genome. The three other species identified in P. leucopus were either not present or in much lower numbers in this sampling of M. musculus. Strains of L. johnsonii have been commonly detected in feces of laboratory mice [68].
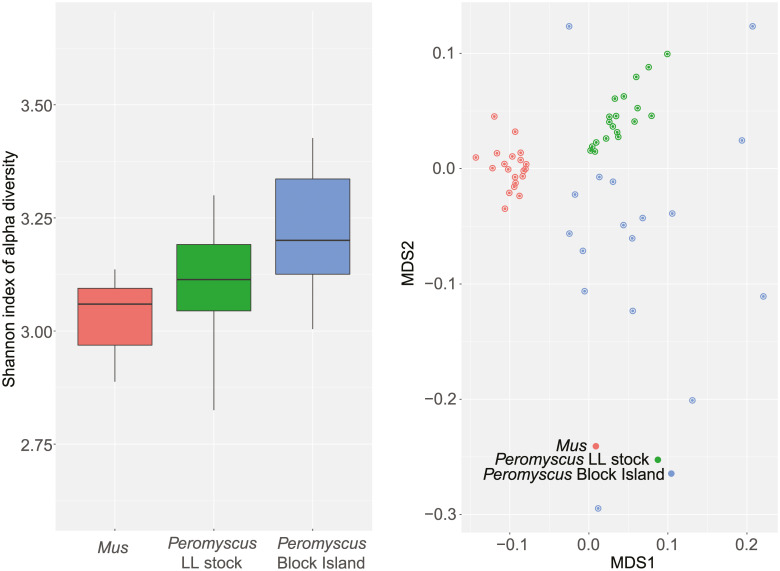
A limitation to the study was that the LL stock animals were outbred, and the BALB/c mice were inbred. An inbred lineage derived from the LL stock population was not available. On the other hand, this distinction provided a comparison of microbiome diversities between an outbred and inbred population. As expected, there was greater alpha diversity among the outbred samples than the inbred (Fig 10). Another limitation was the dependence on fecal pellets collected at one time point. The samples were from similar age P. leucopus and M. musculus and were obtained from the animals and then processed on the same day, but for this study we did not assess variation within individuals over time. The gut microbiomes of the mothers of these animals were uncharacterized, so we could not evaluate maternal effects in this study.
Fig 10. Alpha diversity (left) and beta diversity (right) of gut metagenomes of outbred Peromyscus leucopus (green), a natural population of P. leucopus (blue), and inbred Mus musculus (red).
Left panel, box-whisker plots of Shannon index of alpha diversity for 20 BALB/c M. musculus, 20 LL stock colony P. leucopus, and 18 P. leucopus trapped on Block Island, RI. The 3 pairwise, 2-tailed t-test p values between the groups were ≤ 0.02. Right panel, beta diversity by Bray-Curtis measure visualized by multi-dimensional scaling (MDS). A fuller description of the samples from the natural population from Block Island is provided below. The greater dispersion of values among these animals in panel B corresponded to the greater alpha diversity of this group (panel A).
Lactobacilli of the stomach of P. leucopus
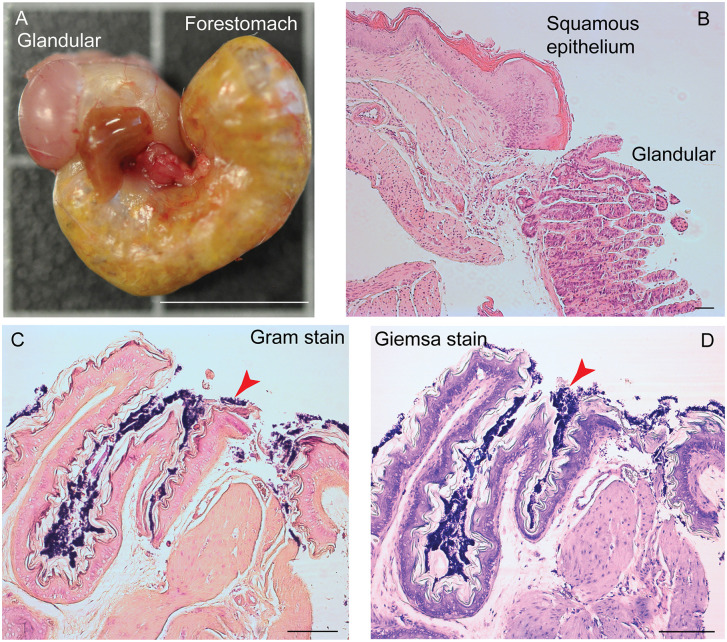
The differences between P. leucopus and M. musculus in the amount and species richness of the lactobacilli in their GI microbiota prompted further investigation of P. leucopus using histologic, microbiologic and genomic approaches. Fig 11 shows the gross morphology and histology of the stomach of representative LL stock P. leucopus animals [69]. The difference between forestomach with its stratified squamous epithelium and the discrete region lined with glandular mucosa are indicated in the dissecting scope and higher magnification light microscope views.
Fig 11. Gross morphology and histology of the stomach of Peromyscus leucopus LL stock.
The glandular mucosa portions of the stomach and the forestomach with stratified squamous epithelium are indicated. Panel A, whole stomach after dissection. Portions of the esophagus and small intestine are juxtaposed in the center in this view. Bar, 1 cm. Panel B, histology of hematoxylin and eosin-stained section of junction of glandular and squamous epithelium parts. Bar, 100 μm. Panels C and D, Gram stain (C) and Wright-Giemsa stain (D) of sections of squamous epithelium. Bar, 100 μm. Red arrowheads indicate gram-positive bacteria in a biofilm.
Staining of the sections of the fixed gastric tissue with Gram stain or Giemsa stain show a thick layer of gram-positive bacteria on the non-secretory epithelium portion of the stomach. This is similar to Savage et al. noted in the forestomachs of M. musculus [70]. The appearance is also consistent with the Lactobacillus biofilm that was described by Wesney et al. [71].
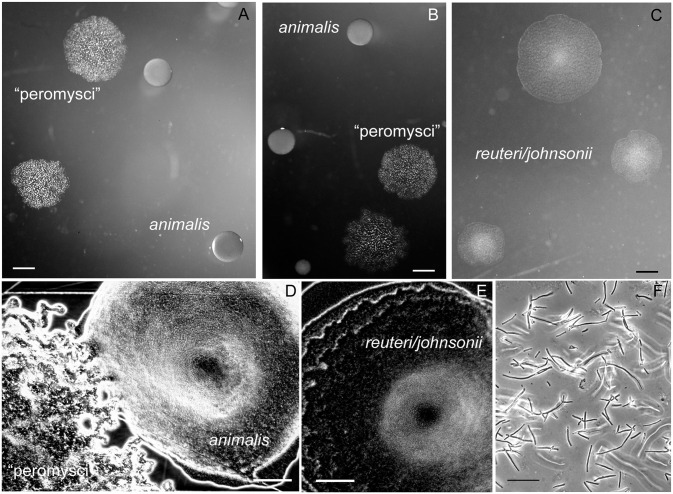
Two of the species, “L. peromysci” and L. animalis, could reliably be distinguished by their distinctive colony morphologies from the isolated strains of L. reuteri and L. johnsonii, which had colonies of similar appearance (Fig 12). The rough-surfaced, ropy colonies of “L. peromysci” and the compact smooth colonies of L. animalis were similar to what Dubos and colleagues described in their study of lactobacilli of the mouse stomach and gut [72].
Fig 12. Colonies and cells of lactobacilli of the P. leucopus stomach and gut.
Panels A-C show representative sizes and morphologies of colonies of “L. peromysci”, L. animalis, and the less distinguishable L. reuteri and L. johnsonii. Bars, 1 mm. Panels D and E show magnified view of colonies of “L. peromysci” and L. animalis (D) and that of L. reuteri and L. johnsonii (E). Bar, 100 μm. Panel F, phase microscopy of wet mount of unconcentrated broth culture of “L. peromysci”. Bar, 10 μm.
We next used a different set of 20 animals of the LL stock, 6 (2 females and 4 males) of which were born at the PGSC facility and 14 (7 females and 7 males) of which were born at U.C. Irvine. All animals were housed at U.C. Irvine for at least 26 weeks before euthanasia, dissection, and cultivation of the stomach tissue and contents.
Mean (95% CI) colony forming units of lactobacilli per gram of stomach tissue on selective medium plates were ten-fold higher in females at 7.4 (1.1–47.4) x 109 than in males at 0.76 (0.40–1.4) x 109 (t-test p = 0.02 for log-transformed values) (Table 3). There was no discernible association with place of birth, and there was no difference between females and males in the proportions of the lactobacilli were identified as “L. peromysci”, L. animalis, and L. reuteri/L. johnsonii. For five animals, whose lactobacilli were subjected to 16S ribosomal RNA gene PCR and sequencing for confirmation, the L. reuteri/L. johnsonii-type colonies were predominantly L. reuteri. But L. johnsonii was confirmed to be present as well and outnumbered L. reuteri in one animal. The results for 3 animals that had been on a 9% fat content diet, which was part of the breeding program, instead of 6% fat content were not distinguishable from those for the other 17.
Table 3. Colony forming units (cfu) of Lactobacillus spp. in Peromyscus leucopus stomach.
| Animal ID | Sex | Log10 total cfu/gm | % colony type | ||
|---|---|---|---|---|---|
| animalis | “peromysci” | reuteri/johnsonii | |||
| 22403 | F | 10.9 | 3 | 32 | 65 |
| 22404a | F | 11.3 | 61 | 4 | 35 |
| 25053 | F | 10.7 | 37 | 60 | 3 |
| 25054 | F | 11.0 | 96 | 4 | <1 b |
| 25055 | F | 10.4 | 94 | 6 | <1 |
| 25065 | F | 8.9 | 50 | 19 | 31 |
| 25062 | F | 8.5 | 60 | 14 | 26 (26/0) c |
| 25063 | F | 8.8 | 52 | 12 | 36 (33/3) |
| 25058 | F | 8.3 | 82 | 14 | 5 (5/0) |
| 22401a | M | 8.6 | 33 | 19 | 48 |
| 22375 | M | 8.8 | 58 | 3 | 40 |
| 22420a | M | 9.7 | 48 | 19 | 33 |
| 22377 | M | 9.0 | 81 | 10 | 9 |
| 26050 | M | 8.4 | 87 | 6 | 7 |
| 25056 | M | 9.6 | 60 | 15 | 26 |
| 25010 | M | 9.0 | 84 | 16 | <1 |
| 25060 | M | 8.8 | 55 | 23 | 23 |
| 25061 | M | 8.3 | 63 | 38 | <1 |
| 25059 | M | 8.5 | 50 | 9 | 41 (36/5) |
| 25011 | M | 9.0 | 61 | 24 | 14 (4/10) |
| Mean (95% CI) | n.a. | 9.3 (8.9–9.8) | 61 (51–70) | 17 (11–23) | ≤ 22 |
a 9% fat content diet instead of 6% fat content diet.
b <1, below limit of detection by serial dilution on plates
c (/), L. reuteri to L. johnsonii ratio by PCR and 16S ribosomal rDNA sequencing
A separate group of 9 adult LL stock P. leucopus (5 females and 4 males) were euthanized after withholding food overnight, and the freshly-excised stomachs were subjected to DNA extraction without prior washing of the stomach. A mean (95% CI) of 477,688 (408,988–546,388) PE250 Illumina reads were obtained for the 9 samples (S7 Table). These were mapped to the four Lactobacillus genomes as references, as described above, as well as to partial chromosomes for Prevotella sp. LL70 and Helicobacter sp. LL4 (Table 1). For an estimate of the number of mammalian nuclei represented in the stomach extract the P. leucopus genome (accession NMRJ00000000.1) served as the reference. Fig 13 shows the distributions of normalized reads mapping to the references as well as to the P. leucopus genome and cumulatively to all Lactobacillus spp. Females and males in this sample were indistinguishable by group by these measures. For this group of animals and this analysis, we confirmed the high prevalence of “L. peromysci” in the stomach as well as the comparatively greater representation of L. reuteri over L. johnsonii. In this sample L. animalis was more variable in numbers between animals. As further evidence that the Helicobacter sp. was of the enterohepatic type, it was near undetectable in the stomach extract, while a typically abundnant genus in the intestine, Prevotella, was present in small numbers in some samples. The lactobacilli in the stomach were about as numerous as the stomach tissue cells constituting the sample.
Fig 13. Box-whisker plots of log-transformed normalized reads of total metagenomes of the stomachs of 9 P. leucopus that mapped to chromosomes of Lactobacillus spp. or other bacteria by host species and by sex.
The references to which reads were mapped were complete chromosomes or partial chromosomes of organisms listed in Table 1. “Lactobacillus” in the first position of each panel were the cumulative reads for the four individual Lactobacillus species in this analysis. The number of cell “nuclei” present in the stomach tissue and DNA extract were estimated by mapping reads to the whole genome of P. leucopus as described in the text.
A strain of L. reuteri was shown to be the source of biofilm in the GI tract of mice in one study [73], and L. murinus, the sister taxa of L. animalis (Fig 3), accounted for the biofilm in another study of the upper GI tract of M. musculus [74]. L. johnsonii has also been demonstrated to produce an exopolysaccharide biofilm [75]. In a study of germ-free mice in which bacteria were experimentally introduced, L. taiwanensis, which is in the same cluster as L. johnsonii and L. gasseri [67], formed a mixed-species biofilm with L. reuteri [76].
One limitation of this experiment is that we may have overlooked species that were not identified because they were not cultivable by our method and conditions, which were microaerophilic, not strictly anaerobic. That said, if cells of such non-cultivable lactobacilli had been present in the feces or stomach, their numbers did not reach a threshold for assembly into contigs of the de novo assembly of the high coverage sequencing and then detection.
Gut metagenomes of a natural population
The foregoing studies were of animals born and reared under controlled conditions, including the same diet and environmental parameters for all individuals in the group. Infectious diseases and predators were not a variable. The LL stock P. leucopus were outbred but the effective population size was small compared to a wild population [18]. Which of our findings would hold for animals sampled in their native habitats?
This particular study of a natural population had two specific purposes. The first was to assess the species richness or alpha diversity of microbiota within a given animal and differences in species composition or beta diversity between animals. The second objective’s question of whether any of the Lactobacillus species we identified in the stock colony were present in natural populations. For this survey we used fecal pellets from P. leucopus that were individually captured and then released on Block Island, several miles off-shore from the North American mainland. At time of capture the animals were identified as to species, sex, and stage of maturity.
We analyzed the data from fecal pellets of 18 different animals (10 females and 8 males), the majority of which were adults, collected from P. leucopus captured at different locations on Block Island (S14 Table). As expected, there was greater variation between individual animals than was observed with the stock colony animals maintained under same conditions. Fig 10 compares the alpha diversity by Shannon index and beta diversity by Bray-Curtis dissimilarity of the inbred BALB/c M. musculus, outbred LL stock P. leucopus, and the natural population of P. leucopus of Block Island.
By algorithmic assignment of reads to taxonomic family, Lactobacillaceae was one of the most prevalent bacteria with a mean of ~5% of reads, but this was over a range of 0.3% to 20%. As was the case for the stock colony P. leucopus, the frequency of Helicobacteraceae varied more widely between sampled animals than for comparably-prevalent taxa: a mean of ~1% but over a range of 0.03% to 12%. The frequency of a parabasalid protist in the metagenomes, by the criterion of reads matching to Trichomonadidae, was similar to what we observed in the metagenome of the stock colony P. leucopus: the mean was 0.11% with a range of 0.02 to 0.62%. This was an indication that the T. muris was autochthonous in P. leucopus, but we did not have direct observation of the protozoa to confirm that.
In descending order the two most prevalent DNA viruses in the samples, with the exception of one animal, were bacteriophages of the families Siphoviridae and Myoviridae (data available under MG-RAST accession numbers of S14 Table). These were the two virus families that were also most prevalent in the feces of the stock colony animals. In the sample from the exceptional animal (number 7464 of S14 Table), the two most prevalent virus types were the genus Dependovirus, a group of DNA viruses dependent on another virus in the cell for replication, and the genus Mastadenovirus, an adenovirus that can provide that function. Other than the adenovirus in the one sample, there were no discernible representatives of herpesviruses, poxviruses, or parvoviruses, groups of DNA viruses which include pathogens for mammals. The methods used would not have identified RNA viruses other than retroviruses.
Using the chromosome sequences of the four Lactobacillus species and partial chromosome sequences of Spirochaetaceae bacterium LL50, Prevotella sp. LL70, and Helicobacter sp. LL4 as references, we mapped and counted reads, as described for the LL stock and M. musculus study above. Fig 14 summarizes results for the 18 animals grouped by sex. Lactobacilli were common but, as seen with family level matching, there was greater variation between samples of the different animals than was observed for colony animals. There was also substantial variation in prevalences of the Spirochaetaceae bacterium and the Prevotella species. In most of the samples there was scant evidence of the Helicobacter species but in two animals, there were higher numbers of this organism, reaching 7% of the total reads in one fecal sample.
Fig 14. Box-whisker plots of log-transformed normalized reads of fecal metagenomes of 5 female (red) and 4 male (blue) P. leucopus of a natural population of Block Island, Rhode Island.
The reference genomes and other sequences were those described for Fig 13 and in addition the partial chromosome sequence of Spirochaetaceae sp. LL50. As an estimate of the number of mammalian cells in the extract, “nuclei” corresponded with normalized reads mapped to P. leucopus genome. Points at a greater distance from the median than 1.5 times the interquartile range are plotted as asterisks.
Among the lactobacilli used as references for this analysis, the two most prevalent species were “L. peromysci” and L. animalis. L. reuteri overall was about 10-fold lower in frequency, and L. johnsonii was about a hundred-fold lower in frequency. It is likely that reads called as L. johnsonii were the result of complete or partial matching to chromosomal loci that were highly conserved across the genus. Unlike the stock colony P. leucopus and the M. musculus, in this sampling of the Block Island population the samples from female animals had marginally lower representation of lactobacilli in the fecal samples than males.
To better characterize the two predominant Lactobacillus species in this set, we assembled contigs of reads mapping to “L. peromysci” or L. animalis from a higher coverage sequencing of the DNA of one of the Block Island samples. This yielded 51 of the 53 genes for ribosomal protein genes for a strain of L animalis, which was designated 7442BI, and several core or housekeeping genes for the “L. peromysci”-like organism, which was designated BI7442 (Table 1). Fig 15 shows phylograms of DNA sequences for these and related Lactobacillus species or strains. The concatenated sequence of the BI7442 isolate was 99.2% identical over the 11,252 nt aligned with the corresponding sequences of the LL6 isolate of “L. peromysci”. Isolate 7442BI was comparatively more distant from the LL1 isolate of L. animalis in the stock colony but still clustered with it rather than with other examples of L. animalis.
Fig 15. Distance phylograms of concatenated codon-aligned nucleotide sequences of two Lactobacillus spp. of P. leucopus of Block Island, Rhode Island.
Panel A, 10,152 positions of ftsK, ftsZ, dnaA, dnaN, ileS, and topA of “L. peromysci” LL6 and BI7442 and two other Lactobacillus species. Panel B, 18,552 positions of 51 of 53 ribosomal protein genes of six L. animalis strains, including LL1 and 7442BI. The distance criteria were Jukes-Cantor. The scales for distance are shown in each panel. Percent bootstrap (100 iterations) support values of ≥ 80% at a node are shown.
The sample size was limited, and we did not attempt culture isolations from the pellets. But the source of samples from P. leucopus was notable for its location on an island where B. burgdorferi is enzootic [77] and the risk of infection for residents and visitors is high [78]. If there were to be future interventions targeting P. leucopus to interrupt disease transmission, Block Island would likely be a candidate site for this application.
This survey also documented that a strain or strains of “L. peromysci” and L. animalis are present in the native deermice. The high degree of sequence identity between two “L. peromysci” examples, whose origins were North Carolina and a New England island, long separated from the mainland, suggest that this newly-discovered species is autochthonous and plausibly a narrowly host-restricted symbiont of P. leucopus. Host-range restrictions of lactobacilli for the stomachs of mice were demonstrated by Wesney and Tannock [71]. Supporting an assignment of a symbiont lifestyle was its smaller genome size and lower % GC content of this species in comparison with L. reuteri and L. johnsonii with their broader host ranges [79].
Conclusions
Six decades ago René Dubos (of the epigraph), Russell Schaedler, and their colleagues at what is now Rockefeller University reported in a series of ground-breaking papers on the “fecal flora” of mice and variations in that microflora between mouse strains [80–82]. They associated differences in gastrointestinal flora with growth rates of the mice and the mouse’s susceptibility to infection and endotoxin. A featured group of bacteria in their studies were lactobacilli. They showed that this group of bacteria were present in large numbers in the feces and that they predominated (up to 109 cfu per g of homogenate) in the stomachs of the mice [70], similarly to what we observed in P. leucopus. As their studies first intimated, the rodent may plausibly owe as much to the genomes of their microbiota as to the nuclei and mitochondria of their somatic cells for either ameliorating or exasperating disease [83].
There are also implications of our findings for development of oral vaccines that target P. leucopus to block transmission of pathogens either from tick to the reservoir or from the reservoir to the tick. Two of the candidate vehicles for the bait delivery of recombinant vaccine antigens to rodents have been E. coli and a Lactobacillus species [30, 84]. In neither case were the strains known to be adapted for life in P. leucopus. The success rate for achieving a protective response may be enhanced by use as the bacterial vehicle microbes that are adapted to P. leucopus. Such organisms presumably would more likely than an allochthonous bacterium to stably colonize and then proliferate to numbers large enough for the recombinant protein to elicit the sought-after immune responses.
Finally, this exploration and curation of microbes in the gut of the white-footed deermouse concludes the third leg of our project on the total genome of representative animals of the species: the nuclear genome [18], the mitochondrial genome [17], and now the GI microbiome [85]. This provides a foundation for testing of hypotheses by selective manipulation of the microbiota, for instance, by specifically targeting a certain species with a lytic phage or bacteriocin, to which it is not immune, and then evaluating the phenotype of the animal after this “knock-out”. Now that there is an annotated P. leucopus genome with millions of SNPs identified (UC Santa Cruz genome browser; http://googl/LwHDr5) it also feasible to investigate through forward genetics the host determinants of particular bacterial associations and for which there is evidence of variation within a population. An example would be the Helicobacter species that was highly variable in prevalence in both the wild animals and the stock colony animals.
Materials and methods
Colony animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. At the University of California Irvine protocol AUP-18-020 was approved by the Institutional Animal Care and Use Committee (IACUC)-approved protocol. Adult outbred P. leucopus of the LL stock were purchased from the Peromyscus Genetic Stock Center (PGSC) of the University of South Carolina [86]. The closed colony of the LL stock was founded with 38 animals captured near Linville, NC in the mid-1980’s. Some of the LL stock animals in the study were bred at the University of California Irvine’s animal care facility from pairs originating at the PGSC. Adult BALB/cAnNCrl (BALB/c) M. musculus were purchased from Charles River. For the species comparison experiment both the PGSC-bred P. leucopus and M. musculus animals were housed in Techniplast individual ventilated cages in vivarium rooms with a 12 hour-12 hours light-dark cycle, an ambient temperature of 22 ± 1 °C, stable humidity, and on an ad libitum diet of 2020X Teklad global soy protein-free extruded rodent chow with 6% fat content (Envigo, Placentia, CA). Other animals of PGSC origin, including for the high-coverage gut metagenome study, were also housed under the same conditions and on the same diet. Twenty U.C. Irvine-bred animals were under the housing conditions and on the diet except for three (1 female and 2 males) that were on 2019 Teklad global protein extruded rodent chow with 9% fat content. Fecal samples were 2–3 pellets (100–150 mg) collected from an individual animal in temporary (< 1 h) isolation in a clean cage. The pellets were collected with sterile forceps and pooled in a single sterile 1.5 ml microfuge tube on ice. The pellets for DNA extractions were stored frozen at -80° C. Pellets intended for cultivation, as described below, were processed as fresh specimens. Before euthanasia with carbon dioxide asphyxiation and cervical dislocation and then dissection of the stomach, food but not water was withheld for 12 h for selected animals. P. leucopus studied at the PGSC were under IACUC-approved protocol 2349-101211-041917 of the University of South Carolina and were euthanized by isoflurane inhalation.
Field site and animal trapping
The study was performed under IACUC-approved protocol AC-AAAS6470 of Columbia University [77]. Block Island, located 23 km from mainland Rhode Island, is part of the Outer Lands archipelagic region, which extends from Cape Cod, MA through to Staten Island, NY. Block Island is 25.2 sq. km, about 40% of which is maintained under natural conditions. The agent of Lyme disease Borreliella burgdorferi is enzootic on the island [87] Animals were trapped at three locations: 1, a nature conservation area (41.15694, -71.58972); 2, private land with woodlots and fields (41.16333, -71.56611); and 3, Block Island National Wildlife Refuge (41.21055, -71.57222). Trapping was carried out during the May-August period with Sherman live traps (H.B. Sherman Traps, Inc. Tallahassee, FL) that were baited with peanut butter, oats, and sunflower seeds. Traps were arranged in nine 200 m transects with one trap placed every 10 m for a total of 180 traps at each location. Animals were removed from traps, weighed, sexed, and assessed as to age (adult, subadult, or juvenile) by pelage. Fecal pellets were collected and kept at -20 °C on site, during shipment and until DNA extraction. The species identification of the source of the fecal pellets as P. leucopus was confirmed by sequencing of the D-loop of the mitochondrion as described [17].
Cultivation and enumeration of bacteria
Lactobacilli were initially isolated and then propagated on Rogosa SL agar plates (Sigma-Aldrich) in candle jars at 37° C. Gram-negative bacteria and specifically Escherichia coli were isolated and propagated on MacConkey Agar plates (Remel) incubated in ambient air at 37° C. For determinations of colony forming units (cfu) homogenates of stomach, cecum, or fecal pellets were suspended and the serially diluted in phosphate-buffer saline, pH 7.4, before plating in 100 μl volumes on solid media in 150 mm x 15 mm polystyrene Petri dishes. Colonies were counted manually. Liquid cultures of Lactobacillus spp. isolates or E. coli were in Difco Lactobacilli MRS Broth (Becton-Dickinson) or LB broth, respectively, and incubated at 37° C on a shaker. Bacteria were harvested by centrifugation at 8000 x g for 10 min. Antibiotic susceptibilities were determined by standard disk testing on Mueller-Hinton Agar (Sigma-Aldrich) plates and ciprofloxacin 5 μg, gentamicin 10 μg, ampicillin 10 μg, and sulfamethoxazole 23.75 –trimethoprim 1.25 μg BBL Sensi-Disc antibiotic disks (Becton-Dickinson) according the manufacturer instructions.
Histology
After the stomachs were removed from two euthanized P. leucopus LL stock adult females, they were opened longitudinally, gently flushed with PBS, and fixed in 10% buffered formalin (Thermo Fisher Scientific). Histological and histochemical analysis was performed on paraffin block sections of the stomach with Hematoxylin and Eosin, Wright-Giemsa and Gram stains (Abcam, Cambridge, UK).
Microscopy, photography, and video
Photographs of colonies on plates were taken with a Nikon Df DSLR camera and 60 mm Nikkor AF-S Micro lens with illumination by incident light above and reflected light below the plates on an Olympus SZ40 dissecting scope. An Olympus BX60 microscope with attached QIClick CCD camera and Q-Capture Pro7 software (Teledyne Photometrics, Tucson, AZ) was used for low-magnification images of colonies under bright light microscopy and 400X images under phase and differential interference microscopy. Histology slides were examined on a Leica DM 2500 microscope equipped with a MC120 HD digital camera (Leica Microsystems, Buffalo Grove, IL).
DNA extractions
DNA from fresh and frozen fecal pellets, from tissue of stomach or cecum, and from bacteria harvested from broth cultures were extracted with ZymoBIOMICS DNA Miniprep or Microprep kits (Zymo Research). Freshly-dissected, unwashed gastric or cecal tissues were first cut into small pieces with sterile instruments before homogenization in the lysis buffer in an Omni Bead Ruptor 4 bead beater (Omni International). DNA concentrations were determined by both NanoDrop spectrophotometer and Qubit fluorometer (Thermo Fisher Scientific).
PCR
The near-complete 16S ribosomal RNA gene for Lactobacillus spp. was amplified using PCR using custom primers specific for the genus Lactobacillus: forward 5’-CCTAATACATGCAAGTCG and reverse 5’-GGTTCTCCTACGGCTA. The Platinum Taq polymerase and master mix (ThermoFisher Scientific) contained uracil-DNA glycosylase. On a T100 thermal cycler (BioRad) PCR conditions (°C for temperature) The conditions were 37° for 10 min, 94° for min, 40 cycles of 94° for 10 s, 55° for 30 s, and 72° for 45 s. The 1.5 kb PCR product was isolated and purified from agarose gel using the NucleoSpin Gel and PCR Clean-up kit (Takara). The product was subjected to Sanger dideoxy sequencing at GENEWIZ (San Diego, CA).
Whole genome sequencing, assembly, and annotation
Long reads were obtained using an Oxford Nanopore Technology MinION Mk1B instrument with Ligation Sequencing Kit, R9.4.1 flow cell, MinKnow v. 19.6.8 for primary data acquisition, and Guppy v. 3.2.4 for base calling with default settings. Paired-end short reads were obtained on a MiSeq sequencer with paired-end v2 Micro chemistry and 150 cycles (Illumina, San Diego, CA). The library was constructed using the NEXTflex Rapid DNA-Seq kit (Bioo Scientific, Austin, TX), the quality of sequencing reads was analyzed using FastQC (Babraham Bioinformatics), and reads were trimmed of Phred scores <15 and corrected for poor-quality bases using Trimmomatic [88]. A hybrid assembly was carried out with Unicycler v.0.4.7 [89] with default settings and 16 threads on the High Performance Computing cluster of the University of California Irvine. Assembly of short reads alone were performed with the Assembly Cell program of CLC Genomics Workbench v. 11 (Qiagen). Annotation was provided by the NCBI Prokaryotic Genome Annotation Pipeline [90]. Putative bacteriocins and their associated transport and immunity functions were identified by BAGEL4 [91, 92]. For other analyses paired-end reads were mapped with a length fraction of 0.7 and similarity fraction of 0.9 to whole genomes sequences or concatenated large contigs representing partial genomes (Table 1). Mapped reads were normalized for length of reference sequence and for total reads after quality control and removal of adapters.
Metagenome sequencing
The library was constructed using NEXTflex Rapid DNA-Seq kit v2 (Bioo Scientific) and the NEXTflex Illumina DNA barcodes after shearing the DNA with a Covaris S220 instrument, end repair and adenylation, and clean-up of the reaction mixture with NEXTFLEX Clean Up magnetic beads (Beckman Coulter, Brea, CA). The library was quantified by qPCR with the Kapa Sybr Fast Universal kit (Kapa Biosystems, Woburn, MA), and the library size was determined by analysis using the Bioanalyzer 2100 DNA High Sensitivity Chip (Agilent Technologies). Multiplexed libraries were loaded on either an Illumina HiSeq 2500 sequencer (Illumina, San Diego, CA) with paired-end chemistry for 250 cycles or a MiSeq Sequencer (Illumina, San Diego, CA) with paired-end v2 Micro chemistry and 150 cycles. The Illumina real time analysis software RTA 1.18.54 converted the images into intensities and base calls. De novo assemblies were performed with De Novo Assembly v. 1.4 of CLC Genomics Workbench v. 11 with the following settings: mismatch, insertion, and deletion costs of 3 each; length fraction of 0.3, and similarity fraction of 0.93.
16S ribosomal RNA gene analysis
The same DNA extract used for the metagenome sequencing at University of California Irvine was submitted to the reference laboratory ID Genomics, Inc. (Seattle, WA) and subjected the company’s 16S rRNA Metagenomics service (http://idgenomics.com/our-services), which used the 16S Metagenomics v. 1.01 program (Illumina). Of the 333,358 reads 82%, were classified as to taxonomic family.
Metagenome analysis
Fastq files were uploaded to the metagenomic analysis server MG-RAST (https://www.mg-rast.org) [93]. Reads were joined using join paired reads function on the browser and filtered for Mus musculus v37 genome. Artificial replicate sequences produced by sequencing artifacts were removed by the method of Gomez-Alvarez et al. [94]. Low quality reads (Phred score <15 for no more than 5 bases) were removed using SolexQA, a modified DynamicTrim protocol [95]. When the term "alpha diversity" was reported in the results and tables for MG-RAST analyses, this stood for species richness or count of the species as calculated by MG-RAST. The output of the MG-RAST protocol was also analyzed in R using the vegan package (https://cran.r-project.org, https://github.com/vegandevs/vegan). By this means we also represented alpha diversity by the Shannon index, which accounts for evenness as well as richness [96]. Beta diversity as expressed as the Bray-Curtis Dissimilarity statistic [97] was calculated using the avgdist function with 1000 sample depth, the median as the function, and 100 iterations (https://github.com/vegandevs/vegan/blob/master/man/avgdist.Rd). Data was visualized using Nonmetric multidimensional scaling in two dimensions [98]. MicrobiomeAnalyst (https://www.microbiomeanalyst.ca) [99] was used for hierarchical clustering by distance criterion and by means of Pearson correlations. The DFAST prokaryotic genome annotation pipeline (https://dfast.nig.ac.jp) was used for annotation of incomplete chromosomes and large contigs [100]. For Lactobacillus spp. and the Helicobacter sp. the lactic acid bacteria database and Helicobacter database, respectively, options were chosen. Alignments and phylogenetic analysis were carried out with the SeaView v. 4 suite [101].
Statistical analysis
Normalized reads and other values whose distributions spanned more than one order of magnitude were log-transformed before parametric analysis by 2-tailed t-test. Inverse transformation was carried out to provide nonparametric means and corresponding asymmetric 95% confidence intervals. Nonparametric analysis by rank order was by Kruskal-Wallis test. The Z-score was the number of standard deviations below or above the population mean a give raw value was. The False Discovery Rate (FDR) with corrected p value was estimated by the method of Benjamini and Hochberg [102]. The box-whisker plot graphs were made with SYSTAT v. 13.1 software (Systat Software, Inc.).
Supporting information
(EPS)
(EPS)
(EPS)
The GenBank accession numbers for the sequences are given next to organism name. The bootstrapped tree was generated with PhyML with the setstings of the LG model, 4 rate classes, and 100 replicates.
(EPS)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(MOV)
Acknowledgments
We thank Emma Keay and Vanessa Cook at the University of California Irvine and Asieh Naderi and Vimala Kaza at the University of South Carolina for their contributions to the study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The research was supported by National Institutes of Health (nih.gov) grant R21 AI136523 to A.G.B., National Science Foundation (nsf.gov) grants 1736150 and 1755670 to I.C. and H. Kiaris (University of South Carolina), and National Science Foundation (Division of Integrative Organismal Systems) grant IOS1755286 to D.M.T. and M.A.D. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dubos R. Autobiographical Manuscript. New York: Rockefeller University; 1981. p. Folder 14. [Google Scholar]
- 2.Sangodeyi F. Rene Dubos and the Emerging Science of Human Microbial Ecology New York: Rockefeller University; 2012. [https://www.issuelab.org/resources/27965/27965.pdf] [Google Scholar]
- 3.Hall ER. The Mammals of North America. Second ed New York: John Wiley and Sons; 1981. [Google Scholar]
- 4.Lackey JA, Huckaby DG, Ormiston BG. Peromyscus leucopus. Mammalian Species. 1985;247:1–10. [Google Scholar]
- 5.Munshi-South J, Kharchenko K. Rapid, pervasive genetic differentiation of urban white-footed mouse (Peromyscus leucopus) populations in New York City. Mol Ecol. 2010;19(19):4242–54. 10.1111/j.1365-294X.2010.04816.x [DOI] [PubMed] [Google Scholar]
- 6.Crossland JP, Dewey MJ, Barlow SC, Vrana PB, Felder MR, Szalai GJ. Caring for Peromyscus spp. in research environments. Lab Animal. 2014;43(5):162–6. 10.1038/laban.504 [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Stecher G, Suleski M, Hedges SB. TimeTree: a resource for timelines, timetrees, and divergence times. Mol Biol Evol. 2017;34:1812–9. 10.1093/molbev/msx116 [DOI] [PubMed] [Google Scholar]
- 8.Steppan SJ, Schenk JJ. Muroid rodent phylogenetics: 900-species tree reveals increasing diversification rates. PLoS One. 2017;12(8):e0183070 10.1371/journal.pone.0183070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrago CG, Voloch CM. The precision of the hominid timescale estimated by relaxed clock methods. J Evol Biol. 2013;26(4):746–55. 10.1111/jeb.12076 [DOI] [PubMed] [Google Scholar]
- 10.Schug MD, Vessey SH, Korytko AI. Longevity and survival in a population of white-footed mice (Peromyscus leucopus). J Mammal. 1991;72:360–6. [Google Scholar]
- 11.Bunikis J, Tsao J, Luke CJ, Luna MG, Fish D, Barbour AG. Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: a longitudinal study in an area where Lyme Borreliosis is highly endemic. J Infect Dis. 2004;189(8):1515–23. 10.1086/382594 [DOI] [PubMed] [Google Scholar]
- 12.Sacher G, Hart R. Longevity, aging, and comparative cellular and molecular biology of the house mouse, Mus musculus, and the white-footed mouse, Peromyscus leucopus. Birth Defects, Orig Artic Ser;(United States). 1978;14(1). [PubMed] [Google Scholar]
- 13.Shorter KR, Owen A, Anderson V, Hall-South AC, Hayford S, Cakora P, et al. Natural genetic variation underlying differences in Peromyscus repetitive and social/aggressive behaviors. Behavior Genet. 2014;44(2):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veres M, Duselis AR, Graft A, Pryor W, Crossland J, Vrana PB, et al. The biology and methodology of assisted reproduction in deer mice (Peromyscus maniculatus). Theriogenology. 2012;77(2):311–9. 10.1016/j.theriogenology.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbour AG. Infection resistance and tolerance in Peromyscus spp., natural reservoirs of microbes that are virulent for humans. Semin Cell Devel Biol. 2017;61(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedford NL, Hoekstra HE. Peromyscus mice as a model for studying natural variation. eLife. 2015;4:e06813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbour AG, Shao H, Cook VJ, Baldwin-Brown J, Tsao JI, Long AD. Genomes, expression profiles, and diversity of mitochondria of the White-footed Deermouse Peromyscus leucopus, reservoir of Lyme disease and other zoonoses. Sci Rep. 2019;9(1):17618 10.1038/s41598-019-54389-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long A, Baldwin-Brown J, Tao Y, Cook V, Balderrama-Gutierrez G, Corbett-Detig R, et al. The genome of Peromyscus leucopus, natural host for Lyme disease and other emerging infections. Sci Advances. 2019;5(7):eaaw6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes-Solecki MJ, Brisson DR, Dattwyler RJ. Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine. 2006;24(20):4440–9. 10.1016/j.vaccine.2005.08.089 [DOI] [PubMed] [Google Scholar]
- 20.Najjar DA, Normandin AM, Strait EA, Esvelt KM. Driving towards ecotechnologies. Pathogens Global Health. 2017;111(8):448–58. 10.1080/20477724.2018.1452844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Specter M. Annals of Science: Rewriting the Code of Life. The New Yorker. 2017;93(1); January 2. [Google Scholar]
- 22.Baxter NT, Wan JJ, Schubert AM, Jenior ML, Myers P, Schloss PD. Intra- and interindividual variations mask interspecies variation in the microbiota of sympatric Peromyscus populations. Appl Environ Microbiol. 2015;81(1):396–404. 10.1128/AEM.02303-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohl KD, Dearing MD, Bordenstein SR. Microbial communities exhibit host species distinguishability and phylosymbiosis along the length of the gastrointestinal tract. Mol Ecol. 2018;27(8):1874–83. 10.1111/mec.14460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassam K, Milovic A, Barbour AG. Genome sequences of three Lactobacillus species strains of the stomach of the White-footed Deermouse (Peromyscus leucopus). Microbiol Res Announc. 2019;8(40). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herlemann DP, Geissinger O, Ikeda-Ohtsubo W, Kunin V, Sun H, Lapidus A, et al. Genomic analysis of "Elusimicrobium minutum," the first cultivated representative of the phylum "Elusimicrobia" (formerly termite group 1). Appl Environ Microbiol. 2009;75(9):2841–9. 10.1128/AEM.02698-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soo RM, Skennerton CT, Sekiguchi Y, Imelfort M, Paech SJ, Dennis PG, et al. An expanded genomic representation of the phylum Cyanobacteria. Genome Biol Evol. 2014;6(5):1031–45. 10.1093/gbe/evu073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–4. 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- 28.Walter J. Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol. 2008;74(16):4985–96. 10.1128/AEM.00753-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tannock G. The microecology of lactobacilli inhabiting the gastrointestinal tract In: Marshall KC, editor. Advances in Microbial Ecology. 11 Boston, MA: Springer; 1990. p. 147–71. [Google Scholar]
- 30.Gomes-Solecki M, Richer L. Recombinant E. coli dualistic role as an antigen-adjuvant delivery vehicle for oral immunization In: Pal U, Buyuktanir O, editors. Borrelia burgdorferi Methods in Molecular Biology. New York: Humana Press; 2018. p. 347–57. 10.1007/978-1-4939-7383-5_27 [DOI] [PubMed] [Google Scholar]
- 31.Jolley KA, Bliss CM, Bennett JS, Bratcher HB, Brehony C, Colles FM, et al. Ribosomal multilocus sequence typing: universal characterization of bacteria from domain to strain. Microbiology. 2012;158(Pt 4):1005–15. 10.1099/mic.0.055459-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujisawa T, Itoh K, Benno Y, Mitsuoka T. Lactobacillus intestinalis (ex Hemme 1974) sp. nov., nom. rev., isolated from the intestines of mice and rats. Int J Syst Bacteriol. 1990;40(3):302–4. 10.1099/00207713-40-3-302 [DOI] [PubMed] [Google Scholar]
- 33.Kim D, Cho MJ, Cho S, Lee Y, Byun SJ, Lee S. Complete Genome Sequence of Lactobacillus reuteri Byun-re-01, isolated from mouse small intestine. Microbiol Res Announc. 2018;7(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Z, Harris HM, McCann A, Guo C, Argimon S, Zhang W, et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat Commun. 2015;6:8322 10.1038/ncomms9322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dent V, Williams R. Lactobacillus animalis sp. nov., a new species of Lactobacillus from the alimentary canal of animals. Zentralblatt für Bakteriologie Mikrobiologie und Hygiene: I Abt Originale C: Allgemeine, angewandte und ökologische Mikrobiologie. 1982;3(3):377–86. [Google Scholar]
- 36.Nam SH, Choi SH, Kang A, Kim DW, Kim RN, Kim A, et al. Genome sequence of Lactobacillus animalis KCTC 3501. J Bacteriol. 2011;193(5):1280–1. 10.1128/JB.01505-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claesson MJ, Li Y, Leahy S, Canchaya C, van Pijkeren JP, Cerdeno-Tarraga AM, et al. Multireplicon genome architecture of Lactobacillus salivarius. Proc Natl Acad Sci U S A. 2006;103(17):6718–23. 10.1073/pnas.0511060103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frese SA, Benson AK, Tannock GW, Loach DM, Kim J, Zhang M, et al. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 2011;7(2):e1001314 10.1371/journal.pgen.1001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandara M, Skehel JM, Kadioglu A, Collinson I, Nobbs AH, Blocker AJ, et al. The accessory Sec system (SecY2A2) in Streptococcus pneumoniae is involved in export of pneumolysin toxin, adhesion and biofilm formation. Microbes Infect. 2017;19(7–8):402–12. 10.1016/j.micinf.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denou E, Pridmore RD, Berger B, Panoff J-M, Arigoni F, Brüssow H. Identification of genes associated with the long-gut-persistence phenotype of the probiotic Lactobacillus johnsonii strain NCC533 using a combination of genomics and transcriptome analysis. J Bacteriol. 2008;190(9):3161–8. 10.1128/JB.01637-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson CM, Aggio RB, O'Toole PW, Villas-Boas S, Tannock GW. Transcriptional and metabolomic consequences of LuxS inactivation reveal a metabolic rather than quorum-sensing role for LuxS in Lactobacillus reuteri 100–23. J Bacteriol. 2012;194(7):1743–6. 10.1128/JB.06318-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hidalgo-Cantabrana C, Goh YJ, Pan M, Sanozky-Dawes R, Barrangou R. Genome editing using the endogenous type I CRISPR-Cas system in Lactobacillus crispatus. Proc Natl Acad Sci U S A. 2019;116(32):15774–83. 10.1073/pnas.1905421116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saulnier DM, Santos F, Roos S, Mistretta TA, Spinler JK, Molenaar D, et al. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS One. 2011;6(4):e18783 10.1371/journal.pone.0018783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreno-Vivian C, Cabello P, Martinez-Luque M, Blasco R, Castillo F. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J Bacteriol. 1999;181(21):6573–84. 10.1128/JB.181.21.6573-6584.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hwang H, Lee JH. Characterization of arginine catabolism by lactic acid bacteria isolated from kimchi. Molecules (Basel, Switzerland). 2018;23(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer JR, Blosser EG, Zindl CL, Silberger DJ, Conlan S, Laufer VA, et al. Preventing dysbiosis of the neonatal mouse intestinal microbiome protects against late-onset sepsis. Nat Med. 2019;25(11):1772–82. 10.1038/s41591-019-0640-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peña JA, Li SY, Wilson PH, Thibodeau SA, Szary AJ, Versalovic J. Genotypic and phenotypic studies of murine intestinal lactobacilli: species differences in mice with and without colitis. Appl Environ Microbiol. 2004;70(1):558–68. 10.1128/aem.70.1.558-568.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheh A, Shen Z, Fox JG. Draft genome sequences of eight enterohepatic Helicobacter species isolated from both laboratory and wild rodents. Genome Announc. 2014;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suerbaum S, Josenhans C, Sterzenbach T, Drescher B, Brandt P, Bell M, et al. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc Natl Acad Sci U S A. 2003;100(13):7901–6. 10.1073/pnas.1332093100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4(1):22–30. 10.1038/mi.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bracken TC, Cooper CA, Ali Z, Truong H, Moore JM. Helicobacter infection significantly alters pregnancy success in laboratory mice. J Am Assoc Lab Animal Sci. 2017;56(3):322–9. [PMC free article] [PubMed] [Google Scholar]
- 52.Nordhoff M, Taras D, Macha M, Tedin K, Busse HJ, Wieler LH. Treponema berlinense sp. nov. and Treponema porcinum sp. nov., novel spirochaetes isolated from porcine faeces. Int J Syst Evol Microbiol. 2005;55(Pt 4):1675–80. 10.1099/ijs.0.63388-0 [DOI] [PubMed] [Google Scholar]
- 53.Barbour AG. Borreliaceae In: Whitman WB, Rainey R, Kämpfe P, Trujillo M, Chun J, DeVos P, et al. , editors. Bergey's Manual of Systematics of Archaea and Bacteria. 2018. p. 1–9. [Google Scholar]
- 54.Geissinger O, Herlemann DP, Morschel E, Maier UG, Brune A. The ultramicrobacterium "Elusimicrobium minutum" gen. nov., sp. nov., the first cultivated representative of the termite group 1 phylum. Appl Environ Microbiol. 2009;75(9):2831–40. 10.1128/AEM.02697-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagkouvardos I, Lesker TR, Hitch TCA, Galvez EJC, Smit N, Neuhaus K, et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome. 2019;7(1):28 10.1186/s40168-019-0637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doran DJ. A catalogue of the protozoa and helminths of North American rodents. I. Protozoa and Acanthocephala. Am Midland Naturalist. 1954;52(1):118–28. [Google Scholar]
- 57.Ericsson AC, Gagliardi J, Bouhan D, Spollen WG, Givan SA, Franklin CL. The influence of caging, bedding, and diet on the composition of the microbiota in different regions of the mouse gut. Sci Rep. 2018;8(1):4065 10.1038/s41598-018-21986-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steiner JM, Schwamberger S, Pantchev N, Balzer HJ, Vrhovec MG, Lesina M, et al. Use of ronidazole and limited culling to eliminate Tritrichomonas muris from laboratory mice. J Am Assoc Lab Animal Sci. 2016;55(4):480–3. [PMC free article] [PubMed] [Google Scholar]
- 59.Koyama T, Endo T, Asahi H, Kuroki T. Life cycle of Tritrichomonas muris. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene Series A: Med Microbiol, Infect Dis, Virol, Parasitol. 1987;264(3–4):478–86. [DOI] [PubMed] [Google Scholar]
- 60.Hackstein JH, Akhmanova A, Boxma B, Harhangi HR, Voncken FG. Hydrogenosomes: eukaryotic adaptations to anaerobic environments. Trends Microbiol. 1999;7(11):441–7. 10.1016/s0966-842x(99)01613-3 [DOI] [PubMed] [Google Scholar]
- 61.Rae DO, Crews JE. Tritrichomonas foetus. Vet Clin North Am Food Animal Practice. 2006;22(3):595–611. 10.1016/j.cvfa.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 62.Yao C, Koster LS. Tritrichomonas foetus infection, a cause of chronic diarrhea in the domestic cat. Vet Res. 2015;46:35 10.1186/s13567-015-0169-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Escalante NK, Lemire P, Cruz Tleugabulova M, Prescott D, Mortha A, Streutker CJ, et al. The common mouse protozoa Tritrichomonas muris alters mucosal T cell homeostasis and colitis susceptibility. J Exp Med. 2016;213(13):2841–50. 10.1084/jem.20161776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351(6279):1329–33. 10.1126/science.aaf1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chudnovskiy A, Mortha A, Kana V, Kennard A, Ramirez JD, Rahman A, et al. Host-rotozoan interactions protect from mucosal infections through activation of the inflammasome. Cell. 2016;167(2):444–56.e14. 10.1016/j.cell.2016.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lange BM, Croteau R. Isopentenyl diphosphate biosynthesis via a mevalonate-independent pathway: Isopentenyl monophosphate kinase catalyzes the terminal enzymatic step. Proc Natl Acad Sci USA. 1999;96(24):13714–9. 10.1073/pnas.96.24.13714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hammes WP, Vogel RF. The genus Lactobacillus In: Wood B, Holzapfel W, editors. The Genera of Lactic Acid Bacteria: Springer; 1995. p. 19–54. [Google Scholar]
- 68.Salzman NH, de Jong H, Paterson Y, Harmsen HJM, Welling GW, Bos NA. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiol. 2002;148(Pt 11):3651–60. [DOI] [PubMed] [Google Scholar]
- 69.Carleton MD. A survey of gross stomach morphology in New World Cricetinae (Rodentia, Muroidea), with comments on functional interpretations. Miscellaneous Publications, Museum of Zoology, University of Michigan No 146. June 14, 1973. pp. 1–43.
- 70.Savage DC, Dubos R, Schaedler RW. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968;127(1):67–76. 10.1084/jem.127.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wesney E, Tannock GW. Association of rat, pig, and fowl biotypes of lactobacilli with the stomach of gnotobiotic mice. Microbial Ecol. 1979;5(1):35–42. [DOI] [PubMed] [Google Scholar]
- 72.Dubos R, Schaedler RW, Costello R, Hoet P. Indigenous, normal, and autochthonous flora of the gastrointestinal tract J Exp Med. 1965;122:67–76. 10.1084/jem.122.1.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salas-Jara MJ, Ilabaca A, Vega M, Garcia A. Biofilm forming Lactobacillus: new challenges for the development of probiotics. Microorganisms. 2016;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Almiron M, Traglia G, Rubio A, Sanjuan N. Colonization of the mouse upper gastrointestinal tract by Lactobacillus murinus: a histological, immunocytochemical, and ultrastructural study. Curr Microbiol. 2013;67(4):395–8. 10.1007/s00284-013-0367-9 [DOI] [PubMed] [Google Scholar]
- 75.Dertli E, Mayer MJ, Narbad A. Impact of the exopolysaccharide layer on biofilms, adhesion and resistance to stress in Lactobacillus johnsonii FI9785. BMC Microbiol. 2015;15:8 10.1186/s12866-015-0347-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin XB, Wang T, Stothard P, Corander J, Wang J, Baines JF, et al. The evolution of ecological facilitation within mixed-species biofilms in the mouse gastrointestinal tract. ISME J. 2018;12(11):2770–84. 10.1038/s41396-018-0211-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang CI, Kay SC, Davis S, Tufts DM, Gaffett K, Tefft B, et al. High burdens of Ixodes scapularis larval ticks on white-tailed deer may limit Lyme disease risk in a low biodiversity setting. Ticks Tick-Borne Dis. 2019;10(2):258–68. 10.1016/j.ttbdis.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finch C, Al-Damluji MS, Krause PJ, Niccolai L, Steeves T, O'Keefe CF, et al. Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS One. 2014;9(1):e84758 10.1371/journal.pone.0084758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–90. 10.1146/annurev.genet.41.110306.130119 [DOI] [PubMed] [Google Scholar]
- 80.Dubos R, Schaedler RW. Some biological effects of the digestive flora. Am J Med Sci. 1962;244:265–71. 10.1097/00000441-196209000-00001 [DOI] [PubMed] [Google Scholar]
- 81.Dubos RJ, Schaedler RW. The effect of diet on the fecal bacterial flora of mice and on their resistance to infection. J Exp Med. 1962;115:1161–72. 10.1084/jem.115.6.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schaedler RW, Dubos RJ. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962;115:1149–60. 10.1084/jem.115.6.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell. 2017;171(5):1015–28.e13. 10.1016/j.cell.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Del Rio B, Seegers JF, Gomes-Solecki M. Immune response to Lactobacillus plantarum expressing Borrelia burgdorferi OspA is modulated by the lipid modification of the antigen. PLoS One. 2010;5(6):e11199 10.1371/journal.pone.0011199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carroll IM, Threadgill DW, Threadgill DS. The gastrointestinal microbiome: a malleable, third genome of mammals. Mamm Genome. 2009;20(7):395–403. 10.1007/s00335-009-9204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peromyscus Genetic Stock Center. Peromyscus Genetic Stock Center, Columbia, SC: University of South Carolina; 2017. [http://stkctr.biol.sc.edu]. [Google Scholar]
- 87.Tufts DM, Diuk-Wasser MA. Transplacental transmission of tick-borne Babesia microti in its natural host Peromyscus leucopus. Parasit Vectors. 2018;11(1):286 10.1186/s13071-018-2875-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haft DH, DiCuccio M, Badretdin A, Brover V, Chetvernin V, O'Neill K, et al. RefSeq: an update on prokaryotic genome annotation and curation. Nucl Acids Res. 2018;46(D1):D851–d60. 10.1093/nar/gkx1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Jong A, van Heel AJ. BAGEL4 Groningen, the Netherlands: University of Groningen; 2019 [http://bagel4.molgenrug.nl].
- 92.van Heel AJ, de Jong A, Song C, Viel JH, Kok J, Kuipers OP. BAGEL4: a user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucl Acids Res. 2018;46(W1):W278–W81. 10.1093/nar/gky383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, et al. The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gomez-Alvarez V, Teal TK, Schmidt TM. Systematic artifacts in metagenomes from complex microbial communities. ISME J. 2009;3(11):1314–7. 10.1038/ismej.2009.72 [DOI] [PubMed] [Google Scholar]
- 95.Cox MP, Peterson DA, Biggs PJ. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics. 2010;11(1):485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hill MO. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54(2):427–32. [Google Scholar]
- 97.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs. 1957;27(4):325–49. [Google Scholar]
- 98.Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58(301):236–44. [Google Scholar]
- 99.Chong J, Liu P, Zhou G, Xia J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nature Protocols. 2020;15(3):799–821. 10.1038/s41596-019-0264-1 [DOI] [PubMed] [Google Scholar]
- 100.Tanizawa Y, Fujisawa T, Nakamura Y. DFAST: a flexible prokaryotic genome annotation pipeline for faster genome publication. Bioinformatics. 2017;34(6):1037–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gouy M, Guindon S, Gascuel O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27(2):221–4. 10.1093/molbev/msp259 [DOI] [PubMed] [Google Scholar]
- 102.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological). 1995:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(EPS)
(EPS)
(EPS)
The GenBank accession numbers for the sequences are given next to organism name. The bootstrapped tree was generated with PhyML with the setstings of the LG model, 4 rate classes, and 100 replicates.
(EPS)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(MOV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.