Abstract
Src signaling was considered a potential mechanism of resistance to androgen-targeted therapy. In a randomized trial, we studied whether the addition of dasatinib would delay prostate cancer progression compared with abiraterone alone. Progression-free survival was not prolonged, although there were numerically more complete responses. Circulating tumor cell kinetics with a “flare” phenomenon were noted.
Background
Signaling via the Src pathway is thought to be a mediator of resistance to androgen targeted therapy in prostate cancer. We studied whether adding the Src inhibitor dasatinib to abiraterone would delay progression.
Patients and Methods
Eligible patients had metastatic castration-resistant prostate cancer (mCRPC), without prior chemotherapy. Abiraterone was prescribed at 1000 mg daily with prednisone 5 mg twice daily in both arms, and dasatinib 100 mg daily was added for Arm B. The primary endpoint was progression-free survival (PFS). The interim analysis was planned after 48 subjects, but the study was terminated early. PFS was evaluated using a 1-sided log rank test. The Fisher exact test was used for other categorical data analyses. Circulating tumor cells (CTCs) were identified with the Epic platform.
Results
With 26 men randomized and a median follow up of 41.8 months, the median PFS was 15.7 months (95% confidence interval, 8.2–49.0+ months) for Arm B and 9.0 months (95% confidence interval, 4.4–30.7 months) for Arm A (P = .15). Response Evaluation Criteria in Solid Tumors responses were seen in 5 (36%) of 14 patients, including 2 complete responses (CRs) on Arm B, and 2 (17%) of 12 responses without CR on Arm A (P = .39). Grade ≥ 3 toxicities more common in Arm B included hypertension, pleural effusion/dyspnea, and gastrointestinal effects. CTCs were detected at baseline in 10 of 19 evaluable patients (median, 2.7/mL blood [range 0.41–59.7]). At week 4, CTCs increased in 1 (10%) of 10 patients on Arm A and 4 (44%) of 9 patients on Arm B.
Conclusion
Dasatinib did not significantly prolong PFS in combination with abiraterone, although power was limited owing to the incomplete study cohort. Treatment with the combination was associated with robust objective responses, including Response Evaluation Criteria in Solid Tumors CRs.
Keywords: Androgen receptor, Castration resistant prostate cancer, Circulating tumor cells, Clinical trial, Src
Introduction
Suppression of testosterone production or signaling via the androgen receptor (AR) is the cornerstone of therapy for metastatic prostate cancer, even in the setting of castration resistance. Abiraterone acetate (AA) with prednisone was approved for the treatment of metastatic castration resistant prostate cancer (mCRPC) owing to prolongation of overall survival (OS) in patients who were pretreated with docetaxel1 and progression-free survival (PFS) in patients who were docetaxel-naive.2 However, progression inevitably occurs, with a median radiographic PFS of 5.6 months in the post-docetaxel population and 16.5 months in the docetaxel-naive population. Putative drivers of resistance to AA include AR mutations conferring independence, glucocorticoid signaling, and signaling through alternate pathways such as Src, reviewed by Galletti et al.3
Src is a member of a family of tyrosine kinases that have broad effects on cell proliferation, adhesion, migration, and survival.4 More specifically, Src interacts with multiple growth factor receptors, including the AR when signaling with testosterone.5 Although androgen-induced phosphorylation of AR is inhibited by Src inhi-bition,6,7 there is also higher level Src expression in cells with suppressed AR activity.8 In animal models of breast cancer, inhibition of Src was associated with a reduction in the development of bone metastases,9–11 making Src inhibition of considerable interest in prostate cancer, with its bone-predominant metastatic pattern.
Dasatinib (Das) is an oral multi-targeted tyrosine kinase inhibitor that induces strong inhibition of Src family kinases, as well as BCR-Abl, c-kit, and PDGFR.12–14 It has been approved by the United States Food and Drug Administration for treatment of chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. The phase II clinical trial of Das in men with mCRPC who had not received prior docetaxel treatment documented modest clinical activity. In this study, 43% of men had not progressed at week 12, and 17% had not progressed at week 24.15 Objective responses were seen, both by Response Evaluation Criteria in Solid Tumors (RECIST) criteria for 2 of 14 patients with measurable disease, and by prostate-specific antigen (PSA) declines, although only 1 patient had a confirmed 50% reduction in PSA. Importantly, the majority of patients experienced reductions in urine n-telopeptide and bone-specific alkaline phosphatase. With the hypothesis that adding Src inhibition would prolong PFS in men taking AA, a randomized phase II study of AA plus prednisone, with or without Das, was undertaken.
Patients and Methods
Institutional Review Board approval was obtained, and the trial was registered at clinicaltrials.gov (NCT01685125). Eligible subjects included patients with mCRPC (defined on the basis of either PSA or radiographic progression while receiving castration therapy) who had not received prior chemotherapy for CRPC with normal organ function. Prior immunotherapy and unlimited prior hormonal therapies were allowed; concurrent administration of bone support with zoledronic acid or denosumab was allowed. After signing written informed consent, eligible patients were randomly assigned 1:1 to Arm A (AA 1000 mg orally daily with prednisone 5 mg orally twice daily) or Arm B (same plus Das 100 mg orally daily).
Imaging was repeated after 12 and 24 weeks, then per clinical indication. PSA was measured every 4 weeks; however, changes in PSA were not used as criteria to discontinue study therapy.
The primary endpoint was PFS, which was calculated from cycle 1, day 1 until progression or death for any reason, whichever was observed first, or was censored at the latest follow-up. For purpose of calculating the PFS, progression was defined only by imaging (RECIST 1.1 for soft tissue, Prostate Cancer Working Group 2 for bone scan), and PSA progression was not included. Secondary endpoints included PSA changes, rate of objective response, and overall survival. A 1-sided, 0.05-level log-rank test was planned for comparing the 2 arms in terms of PFS. The study planned to have 96 patients (48 in each arm) enrolled over a period of 2 years, to ensure 85% power to detect an increase in PFS—with the increase reflected by an increase at 12 weeks from 40% to 60% with the addition of Das. An interim analysis for futility was planned after 24 subjects were evaluable at 12 weeks on each arm.
Correlative studies included serial assessment of high-definition circulating tumor cells (HD-CTCs) using the high-definition single cell assay platform.16,17 All P-values reported were 2-sided.
Results
The trial was active between September 2012 and April 2015, at which time the trial was closed owing to slow accrual. At the time of study closure, 26 men had been randomized, 12 to arm A (AA alone) and 14 to Arm B (AA + Das). A CONSORT diagram is available (see Supplemental Figure 1 in the online version). Baseline and demographic characteristics are summarized in Table 1. The population was typical for first-line mCRPC, as only 1 patient had received ketoconazole, and 1 had received enzalutamide. The median age was 66.5 years (range, 55–85 years), and the median baseline PSA was 19.8 ng/mL (range, 0.84–1387 ng/mL). The median follow-up for the study cohort is 41.8 months (range, 12.9–51.8 months).
Table 1.
Baseline and Demographic Characteristics of Study Participants
| Total Patients (N = 26), n (%) | Arm A: Abiraterone (n = 12), n (%) | Arm B: Abiraterone + Dasatinib (n = 14), n (%) | |
|---|---|---|---|
| Median age at study entry, y (range) | 66.5 (55.6–85.3) | 66.4 (55.6–75.9) | 66.5 (55.7–85.3) |
| Race | |||
| Caucasian | 11 (42) | 6 (50) | 5 (36) |
| Hispanic | 9 (35) | 4 (33) | 5 (36) |
| Black | 3 (12) | 1 (8) | 2 (14) |
| Asian | 3 (12) | 1 (8) | 2 (14) |
| Prior therapy | |||
| Bicalutamide | 21 (81) | 8 (67) | 13 (93) |
| Ketoconazole | 1 (4) | 0 (0) | 1 (7) |
| PSA level at study entry, ng/mL | |||
| Median (range) | 19.8 (0.84–1387) | 22.2 (0.9–1387) | 19.8 (0.8–184.1) |
| ECOG PS | |||
| 0 | 22 (85) | 9 (75) | 13 (93) |
| 1–2 | 4 (15) | 3 (25) | 1 (7) |
| Disease site involvement at baseline | |||
| Lymph node only | 6 (23) | 2 (17) | 4 (29) |
| Bone only | 12 (46) | 8 (67) | 4 (29) |
| Soft tissue ± bone | 8 (31) | 2 (17) | 6 (43) |
| Radiographic progressive disease prior to study entry | |||
| No | 3 (12) | 1 (8) | 2 (14) |
| Yes | 23 (88) | 11 (92) | 12 (86) |
Abbreviations: ECOG PS = Eastern Cooperative Oncology Group performance status; PSA = prostate-specific antigen.
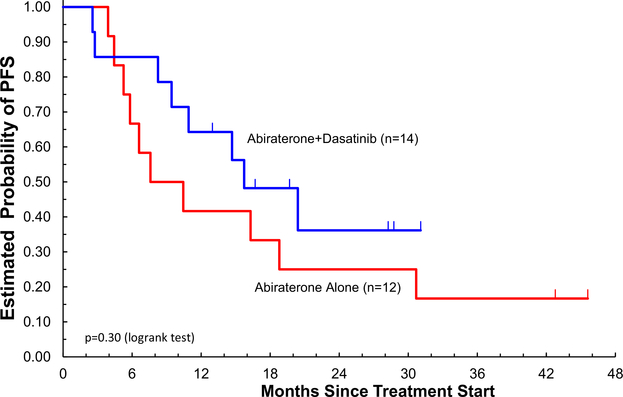
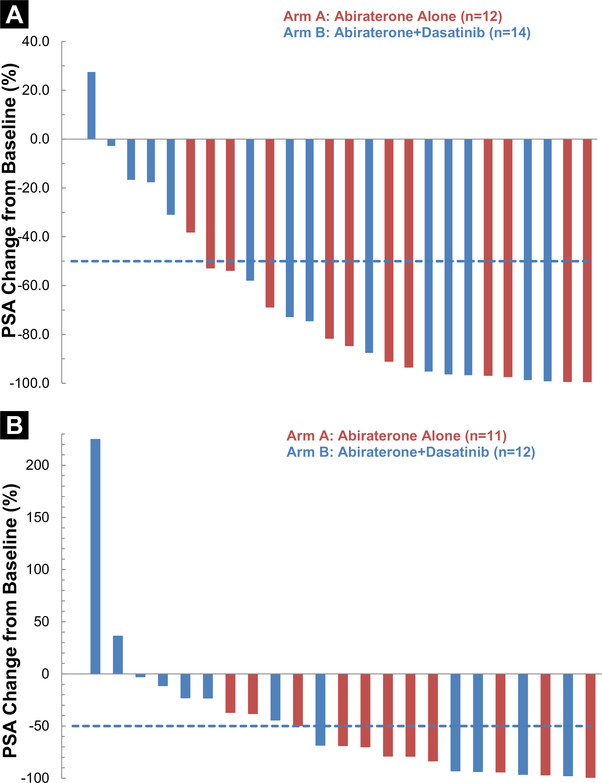
Outcomes for the study population are summarized in Table 2 and graphically represented in Figure 1. At 24 weeks, the probability of being free of radiographic progression was 75% (≥ 13%) for AA alone and 86% (≥ 9%) for AA + Das (P=.58). The median PFS was 9 months (range, 4.4–30.7 months) for AA alone and 15.7 months (range, 8.2–49+ months) for AA + Das (1-sided P = .15). The median overall survival is 26.9 months (95% confidence interval [CI], 12.5–51.8+ months) for AA alone and 41.2 months (95% CI, 39.7–49 months) for AA + Das. At the time of analysis, 14 subjects had died, 7 on each treatment arm, only 1 owing to prostate cancer, and no deaths were related to study treatment. From the intent-to treat analysis, RECIST objective responses were seen in 5 (36%; 95% CI, 15%-63%) of 14 patients with 2 complete responses (CRs) on AA + Das and 2 (17%; 95% CI, 3%-46%) of 12 patients without CR on AA (P = .34, comparing CR + partial response [PR] vs. stable disease [SD] + progressive disease [PD] + inevaluable). PSA changes are depicted in Figure 2.
Table 2.
Outcome Measures Summarizing Response to Treatment
| Total Patients (n = 26) | Arm A: Abiraterone Alone (n = 12) | Arm B Abiraterone + Dasatinib (n = 14) | P Value | |
|---|---|---|---|---|
| Best PSA changes from baseline during treatment | .26a | |||
| Median, % (95% CI) | −83 (−99 to 28) | −88 (−99 to 38) | −76 (−99 to 28) | |
| PSA changes from baseline at 12 weeks | .13a | |||
| Median, % (95% CI) | −69 (−99 to 225) | −79 (−99 to 37) | −34 (−98 to 225) | |
| Best PSA response, n (%) | .23b | |||
| PR | 20 (77) | 11 (91) | 9 (64) | |
| SD | 5 (19) | 1 (8) | 4 (29) | |
| PD | 1 (4) | 0 (0) | 1 (7) | |
| Best clinical response (RECIST), n (%) | .34b | |||
| CR/CRU | 2 (8) | 0 (0) | 2 (14) | |
| PR/PRU | 5 (15) | 2 (17) | 3 (21) | |
| SD | 14 (54) | 9 (64) | 5 (36) | |
| PD | 2 (8) | 0 (0) | 2 (14) | |
| Inevaluable | 3 (12) | 1 (8) | 2 (14) | |
| Overall survival | .44c | |||
| Median, mos (95% CI) | 39.7 (25.0–51.8+) | 26.9 (12.5–51.8+) | 41.2 (25.0–49.0+) | |
| PFS | .29c | |||
| Probability of PFS at 12 weeks | 0.92 ± 0.05 | 1.00 ± 0.00 | 0.86 ± 0.09 | .18c |
| Probability of PFS at 24 weeks | 0.81 ± 0.08 | 0.75 ± 0.13 | 0.86 ± 0.09 | .58c |
| Median, mos (95% CI) | 14.7 (7.5–25.0) | 9.0 (4.4–30.7) | 15.7 (8.2–49.0+) |
Abbreviations: CI = confidence interval; CR = complete response; CRU = unconfirmed complete response; PD = progressive disease; PFS = progression-free survival; PR = partial response; PRU = unconfirmed partial response; PSA = prostate-specific antigen; RECIST = Response Evaluation Criteria In Solid Tumors; SD = stabile disease.
P value based on Wilcoxon test.
P value based on Fisher’s exact test.
P value based on logrank test.
Figure 1.
Kaplan-Meier Curves for PFS for the Study Population, Separated by Treatment Arm
Figure 2.
Waterfall Plots Depicting PSA Changes on Study. A, “Best” or Maximal PSA Decline. B, 12-week PSA Change
Toxicity is summarized in Table 3; discontinuation owing to adverse events occurred in 1 subject on AA alone and 2 subjects on AA + Das. Eleven patients experienced grade 3+ toxicities, 3 on AA alone and 8 on AA + Das. Grade ≥ 3 toxicities more common with AA + Das included hypertension (43% vs. 8% for AA alone), pleural effusion/dyspnea (7% vs. 0% for AA alone), and gastrointestinal adverse effects (14% vs. 8% for AA alone).
Table 3.
Toxicities Experienced by Patients on Study
| Arm A: Abiraterone (n = 12) |
Arm B: Abiraterone + Dasatinib (n = 14) |
|||
|---|---|---|---|---|
| Toxicity | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 |
| Anemia | 3 | 0 | 7 | 1 |
| Constipation | 1 | 0 | 3 | 1 |
| Diarrhea | 0 | 1 | 6 | 0 |
| Nausea/dyspepsia | 2 | 0 | 2 | 1 |
| Edema | 1 | 0 | 4 | 0 |
| Fatigue | 7 | 0 | 10 | 0 |
| Elevated AST/ALT | 1 | 1 | 2 | 0 |
| Hyperglycemia | 2 | 0 | 3 | 0 |
| Hypokalemia | 1 | 0 | 2 | 2 |
| Dizziness | 1 | 0 | 2 | 0 |
| Headache | 1 | 0 | 3 | 0 |
| Insomnia | 1 | 0 | 1 | 0 |
| Dyspnea | 1 | 0 | 2 | 1a |
| Hypertension | 1 | 1 | 2 | 6 |
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Related to pleural effusion.
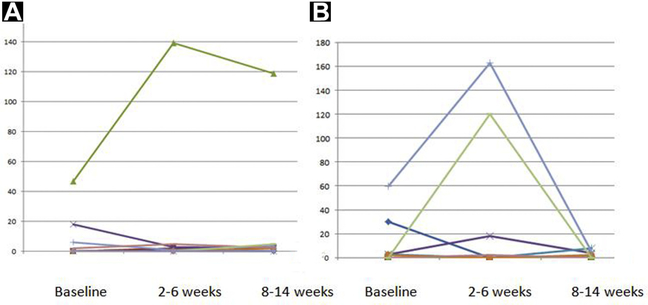
HD-CTCs were assessed serially in 19 subjects (10 subjects from AA alone and 9 subjects in AA + Das), with trends summarized in Figure 3. For the AA alone group, the median HD-CTCs cells/mL at baseline, week 4, and week 12 were: 0.2 (range, 0–18.1), 0 (range, 0–5.0), and 1.3 (range, 0–5.0), respectively. Similarly, for AA + Das, the median HD-CTCs cells/mL at baseline, week 4, and week 12 were: 1.9 (range, 1–60), 0 (range, 0–163), and 1.2 (range, 0–7.8), respectively. There was no correlation between changes in PSA and changes in CTC at any time point (see Supplemental Table 1 in the online version). The HD-CTC assay further allowed for assessment of CTC with an apoptotic phenotype,18 which was also found to vary across data points (Table 4). In 3 subjects, no HD-CTCs were detected at any time point. Baseline HD-CTC and post-treatment HD-CTC were not statistically associated with response to treatment (P = .24 and P = .2, respectively), but there was a trend toward longer PFS with lower HD-CTC at both time points (data not shown).
Figure 3.
Circulating Tumor Cell Changes in Patients During the Study: Abiraterone (A); Abiraterone + Dasatinib (B)
Table 4.
Baseline and Post-treatment CTC Levels for High-definition and Apoptotic CTC Levels by Treatment Arm
| Baseline, Median (Range) (Q1, Q3) |
Week 4, Median (Range) (Q1, Q3) |
Week 12, Median (Range) (Q1, Q3) |
||||
|---|---|---|---|---|---|---|
| Treatment Arm | HD-CTC | Apo-CTC | HD-CTC | Apo-CTC | HD-CTC | Apo-CTC |
| Abi alone (n = 10) | 0.2 (0–18.1) (0, 1.9) |
1.6 (0–17.0) (0, 2.1) |
0 (0–5.0) (0, 0.9) |
0.3 (0–6.7) (0, 5,4) |
1.3 (0–5.0) (0, 2.6) |
0.7 (0–2.6) (0, 1.8) |
| Abi + Das (n = 9) | 1.9 (0–60) (0, 2.9) |
1.4 (0–36) (0, 2.1) |
0 (0–163) (0, 18.0) |
1.3 (0–29.4) (0.9, 2.7) |
1.2 (0–7.8)a (0, 4.4) |
0.6 (0–64.5)a (0, 3.8) |
| P valueb | .53 | .97 | .36 | .20 | .71 | .82 |
Abbreviations: Abi = abiraterone; Apo = apoptotic; CTC = circulating tumor cell; Das = dasatinib; HD = high-definition.
Only 8 patients in this set.
P value based on Wilcoxon test.
Discussion
The Src family kinases have a proven role in tumorigenesis and in progression of various solid tumors, reviewed by Summy et al.19 Interactions of Src with AR and promotion of castration resistance, along with preclinical evidence of involvement by Src in the development of bone metastases, make it a target of great interest in mCRPC.5,9–11,20 Unfortunately, the addition of the Src inhibitor Das to AA in this study did not improve PFS, with the important caveat that an incomplete study cohort limited statistical power and the ability to draw conclusions. Nevertheless, these results are consistent with those of another study, a multi-arm protocol in which Das was added upon progression on AA and no benefit was identified.21 Furthermore, Das failed to prolong PFS when added to docetaxel in CRPC.22 Taken together, these data do not support the use of Das in an unselected population of patients with mCRPC.
Although 2 CRs were seen on the combination arm of this trial, the study did not clearly demonstrate that Src is a driver in CRPC, and a biomarker for Src-driven disease remains absent. Future investigation of Src inhibitor therapy might best be supported by elucidation of which patient populations have cancer that is at least partially driven by Src signaling. Alternatively, novel pharmacologic strategies to interrupt Src signaling may be required. For instance, inhibition of post-translational modifications may represent an effective strategy. Early preclinical data found reduced prostate cancer growth stimulated by Src and AR when N-myristoyl-transferase was inhibited.23 Co-activators of Src-AR signaling may also need to be targeted, such as the aryl hydrocarbon receptor.24 Based on emerging data, targeted use of Src inhibition in patients expressing ARv7 as their main driver of resistance could be of interest.25 MicroRNA are also being investigated as potential means to interfere with Src-AR signaling.26,27
We identified an interesting “flare” phenomenon, with certain patients exhibiting a marked increase in CTCs at 2 to 6 weeks, followed by a decrease at weeks 8 to 14. This has not previously been described in the literature. Owing to small numbers, it is not possible to determine whether this has clinical implications. Although enumeration has fallen out of favor, changes in CTC numbers has proven to yield strong, independent prognostic information in the setting of mCRPC using the Veridex CellSearch system.28,29 The EPIC platform allows for sophisticated interrogation of CTC, and has yielded insights regarding the importance of nuclear localization of ARv7 and phenotypic heterogeneity in predicting response to therapy.30,31 We did find apoptotic CTC, which have been associated with poor PFS in small-cell lung cancer.32 In patients with mCRPC, apoptotic CTC numbers are highly correlated with overall CTC numbers, making their specific prognostic implication challenging to isolate.33 As0 the landscape for treatment of advanced prostate cancer becomes more complicated and greater subcategorization of prostate cancer occurs, CTCs are likely to play a valuable role in illuminating mechanisms of therapeutic resistance and delineating individualized treatment paradigms. Importance of sub-populations, including apoptotic CTC, is an area of active investigation.
Conclusion
In conclusion, Das did not improve the primary outcome when added to AA in mCRPC in this small cohort; however, there was a strong objective response rate. Given the lack of proven benefit, further studies of Src inhibition in CRPC should only be conducted if new biologic evidence emerges linking Src to disease progression.
Supplementary Material
Clinical Practice Points.
Src signaling is thought to mediate resistance to androgen-targeted therapy. We studied the addition of dasatinib (Src inhibitor) to abiraterone but found no delay in disease progression for the combination. The study was underpowered, as 26 patients were randomized rather than the 96 planned for 85% power.
A “flare” pattern in circulating tumor cells was identified at 2 to 6 weeks into treatment; further work would be needed to define the clinical significance of this phenomenon.
Acknowledgments
This study was funded by Bristol-Myer Squibb. This work was partially supported by Award Numbers U54CA143906 P30CA014089 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health, who had no influence on the design, analysis, or conclusions of the study.
Footnotes
Disclosure
T. Dorff reports consulting for Astra Zeneca, Bayer, Intas, and Janssen, and promotional speaker for Exelixis and Prometheus; P. Kuhn is a shareholder in and advisor to Epic Sciences, which holds the exclusive commercialization license to the HD-CTC technology from The Scripps Research Institute; J. Pinski reports promotional speaker for Janssen; D. Quinn reports consulting for Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Clovis Dendreon, Pfizer, and Sanofi. All other authors state that they have no conflicts of interest.
Supplemental Data
Supplemental figure and table accompanying this article can be found in the online version at https://doi.org/10.1016/j.clgc.2019.02.010.
References
- 1.DeBono JS, Logothetis CJ, Molina A, et al. , COU-AA-301 Investigators. Abir-aterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan CJ, Smith MR, deBono JS, et al. , COU-AA-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galletti G, Leach BI, Lam L, Tagawa ST. Mechanisms of resistance to systemic therapy in metastatic castration-resistant prostate cancer. Cancer Treat Rev 2017; 57:16–27. [DOI] [PubMed] [Google Scholar]
- 4.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 1997; 13:513–609. [DOI] [PubMed] [Google Scholar]
- 5.Migliaccio A, Castoria C, DiDomenico M, et al. Steroid-induced androgen receptor-oestradiol receptor b-Src complex triggers prostate cancer cell proliferation. EMBO J 2000; 19:5406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kousteni S, Bellido T, Plotkin LI, et al. Non-genotropic, sex non-specific signaling through the androgen or estrogen receptors: dissociation from transcriptional activity. Cell 2001; 104:719–30. [PubMed] [Google Scholar]
- 7.Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res 2006; 66:11047–54. [DOI] [PubMed] [Google Scholar]
- 8.Mendiratta P, Mostaghel E, Guinney J, et al. Genomic strategy for targeting therapy in castration-resistant prostate cancer. J Clin Oncol 2009; 27:2022–9. [DOI] [PubMed] [Google Scholar]
- 9.Myoui A, Nishimura R, Williams PJ, et al. C-SRC tyrosine kinase activity is associated with tumor colonization in bone and lung in an animal model of human breast cancer metastasis. Cancer Res 2003; 63:5028–33. [PubMed] [Google Scholar]
- 10.Rucci N, Recchia I, Angelucci A, et al. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther 2006; 318:161–72. [DOI] [PubMed] [Google Scholar]
- 11.Saad F. Src as a therapeutic target in men with prostate cancer and bone metastases. BJU Int 2009; 103:434–40. [DOI] [PubMed] [Google Scholar]
- 12.Lombardo LJ, Lee FY, Chen P, et al. Discovery of n-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 2004; 47:6658–61. [DOI] [PubMed] [Google Scholar]
- 13.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistnat Abl kinase domain mutants. Cancer Res 2005; 65:4500–5. [DOI] [PubMed] [Google Scholar]
- 14.Nam S, Kim D, Cheng JQ, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res 2005; 65: 9185–9. [DOI] [PubMed] [Google Scholar]
- 15.Yu EY, Massard C, Gross ME, et al. Once-daily dasatininb: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology 2011; 77:1166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazar DC, Cho EH, Luttgen MS, et al. Cytometric comparisons between circulating tumor cells from prostate cancer patients and the prostate-tumor-derived LNCaP cell line. Phys Biol 2012; 9:016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrinucci D, Bethel K, Kolatkar A, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 2012; 9:016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wendel M, Bazhenova L, Boshuizen R, et al. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys Biol 2012; 9:016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res 2006; 12:1398–401. [DOI] [PubMed] [Google Scholar]
- 20.Su B, Gillard B, Gao L, Eng KH, Gelman IH. Src controls castration recurrence of CWR22 prostate cancer xenografts. Cancer Med 2013; 2:784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efstathiou E, Tsikkinis A, Wen S, et al. Abiraterone acetate followed by randomization to dasatinib or sunitinib malate in metastatic castration resistant prostate cancer. Ann Oncol 2016; 27(Suppl 6), abstract 727PD. [Google Scholar]
- 22.Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (REAADY): a randomized double-blind phase 3 trial. Lancet Oncol 2013; 14:1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Alsaidan OA, Goodwin O, et al. Blocking myristoylation of Src inhibits its kinase activity adn suppresses prostate cancer progression. Cancer Res 2017; 77: 6950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghotbaddini M, Cisse K, Carey A, Powell JB. Simultaneous inhibition of aryl hydrocarbon receptor (AhR) and Src abolishes androgen receptor signaling. PLoS One 2017; 12:e0179844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szafran AT, Stephan C, Bolt M, Mancini MG, Marcelli M, Mancini MA. High-content screenign identifies Src family kinases as potential regulators of AR-v7 expression and androgen-independent cell growth. Prostate 2017; 77: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu YN, Yin J, Barrett B, et al. Loss of androgen-regulated microRNA1 activates Src and promotes prostate cancer bone metastasis. Mol Cell Biol 2015; 35:1940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi XB, Ma AH, Xue L, et al. miR-124 and andrgoen receptor signaling inhibitors repress protate cancer growth by downregulating androgen receptor splice variants, EZH2, and Src. Cancer Res 2015; 75:5309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.deBono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival bneefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14:6302–9. [DOI] [PubMed] [Google Scholar]
- 29.Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol 2014; 32:1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scher HI, Graf RP, Schreiber NA, et al. Nuclear-specific AR-v7 protein localization is necessary to guide treatment selection in metastatic castration-resistant prostate cancer. Eur Urol 2017; 71:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scher HI, Graf RP, Schreiber NA, et al. Phenotypic heterogeneity of circulating tumor cells informs clinical decisions between AR signaling inhibitors and taxanes in metastatic prostate cancer. Cancer Res 2017; 77:5687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou J-M, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012; 30:525–32. [DOI] [PubMed] [Google Scholar]
- 33.McDaniel AS, Ferraldeschi R, Krupa R, et al. Phenotypic diversity of circulating tumour cells in patients with metastatic castration-resistant prostate cancer. BJU Int 2017; 120:E30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.