Abstract
The coronavirus disease 2019 (COVID-19) pandemic presents an unprecedented challenge and opportunity for translational investigators to rapidly develop safe and effective therapeutic interventions. Greater risk of severe disease in COVID-19 patients with comorbid diabetes mellitus, obesity, and heart disease may be attributable to synergistic activation of vascular inflammation pathways associated with both COVID-19 and cardiometabolic disease. This mechanistic link provides a scientific framework for translational studies of drugs developed for treatment of cardiometabolic disease as novel therapeutic interventions to mitigate inflammation and improve outcomes in patients with COVID-19.
Keywords: inflammation, morbidity, mortality, pandemics, viruses
Highlights.
Patients with diabetes mellitus, obesity, and heart disease are at a greater risk for severe complications of coronavirus disease 2019 (COVID-19).
Vascular inflammation and trained immunity associated with cardiometabolic diseases may increase risk of hyperinflammatory response to COVID-19 infection.
Drugs with anti-inflammatory properties developed for the treatment of cardiometabolic disease are being evaluated in clinical trials of COVID-19 patients.
An outbreak of the novel severe acute respiratory syndrome (SARS) coronavirus-2 causing coronavirus disease 2019 (COVID-19) originally emerged from Wuhan, Hubei province in China, in December 2019.1 On March 11, 2020, COVID-19 was declared a pandemic by the World Health Organization, and by July 30, 2020, the virus had infected over 17 million people worldwide across 216 countries with over 668 000 fatalities.2
SARS coronavirus-2 is a single-stranded enveloped RNA virus similar in structure and pathogenicity to SARS coronavirus from the 2002 SARS and the 2012 Middle East respiratory syndrome coronavirus outbreaks.3 SARS-coronavirus-2 binds its S protein to ACE2 (angiotensin-converting enzyme 2) on the surface of cells and relies on the cellular serine protease TMPRSS2 to prime the S protein for host cell entry.4 ACE2 is expressed in type II alveolar cells of the lung and is highly expressed in cardiac myocytes, cardiac pericytes, and vascular endothelium.5,6 ACE2 converts angiotensin II to Ang (1–7; angiotensin 1–7) and exerts vasodilatory, natriuretic, anti-inflammatory, and antioxidant effects.7,8
Cardiovascular Comorbidities of COVID-19
In the original SARS outbreak, the presence of preexisting cardiovascular disease was independently associated with an increased risk of death.9,10 Reports from China noted similar risks for a more severe clinical course in COVID-19 patients with hypertension, diabetes mellitus, or cardiovascular disease at baseline.11–13 Data from 2 cohorts derived from academic medical centers in New York City identified age, obesity, and the presence of preexisting heart disease as strong predictors for hospitalization among COVID-19 patients.14,15 National data from the Centers for Disease Control reported diabetes mellitus and cardiovascular disease as the most common comorbid conditions in hospitalized or intensive care unit patients.16 Registry data from United Kingdom healthcare systems also identified advanced age, obesity, diabetes mellitus, and hypertension as risk factors for more severe COVID-19 morbidity and mortality.17,18 In contrast, higher body mass index was not associated with increased mortality risk in hospitalized COVID-19 patients in a single center in New York City.19 In this prepublication report, age and increased blood levels of proinflammatory cytokines were independently associated with decreased survival.
Cardiovascular Manifestations of COVID-19 Infection
Three distinct phases of COVID-19 are described beginning with mild upper respiratory syndrome, a parenchymal pulmonary phase characterized by marked hypoxemia, and progression to a hyperinflammatory prothrombotic phase with multiorgan dysfunction and thromboembolism in a subset of patients.13,20,21 Elevation in serum cardiac biomarkers (troponin, brain natriuretic peptide) is common in hospitalized patients. Patients may present with COVID-19 and electrocardiographic findings consistent with ST-segment–elevation myocardial infarction with or without obstructive coronary lesions.11,22 Isolated cases of suspected acute myocarditis have been reported in COVID-19 patients based on clinical findings of typical electrocardiographic changes, elevated biomarkers, echocardiographic wall motion abnormalities, cardiac magnetic resonance imaging, and hemodynamic instability.23–25 However, histological changes consistent with myocarditis have not been identified in autopsy specimens.26–28 A New York City autopsy series reported platelet-fibrin thrombi in the cardiac microvasculature and venules and cases of venous thrombosis associated with regional myocardial infarction.29
Vascular complications of COVID-19 have also been reported including stroke, cutaneous chilblains-like lesions on the toes, and case reports of systemic vasculitis resembling Kawasaki disease in children with severe COVID-19 (pediatric multisystem inflammatory syndrome).30–36 Other autopsy series of COVID-19 patients report evidence of viral particles within vascular endothelial cells and diffuse vascular endothelial cell injury in lung, heart, kidney, and intestinal tissues.37,38 The inflammatory response to viral infections upregulates expression of tissue factor, markers of thrombin generation and platelet activation, complement activation, and risk of intravascular thrombosis.22,39,40 Whether, and to what degree, the clinically recognized cardiovascular manifestations of COVID-19 are a result of direct viral injury, prolonged hypoxemia, vascular endothelial cell infection or inflammation, cardiac pericyte infection, or intravascular thrombosis remains unknown.
Potential Pathophysiologic and Pharmacological Links Between Cardiometabolic Disease and COVID-19 Infection
Innate immunity is increasingly recognized to mediate vascular inflammation and atherosclerosis progression, in part, via upregulation of the NLRP3 (nucleotide-binding oligomerization domain, leucine-rich repeat–containing receptor family pyrin domain-containing 3) inflammasome pathway in settings of hypercholesterolemia, diabetes mellitus, obesity, and atherosclerosis development.41–46 This pathway regulates maturation and secretion of the proinflammatory cytokine IL (interleukin)-1β. In the LDL (low-density lipoprotein)-receptor knockout hypercholesterolemic mouse, activation of the NLRP3 inflammasome by exposure to the Western diet modulates long-term immune function by a transcriptomic and epigenetic reprogramming of myeloid precursors, so-called trained innate immunity.47–49 As a result, the myeloid precursors, and their derived cells, exhibit an enhanced inflammatory response upon secondary challenge with microbial ligands. Accordingly, NLRP3 inflammasome activation and trained immunity in association with cardiovascular risk factors and disease might confer increased risk of a hyperinflammatory response that augments the effects of COVID-19–induced inflammation or COVID-19–induced immune modulation of ACE2/Ang (1–7) signaling (Figure).50–53 This double-hit hypothesis is concordant with epidemiological observations linking cardiometabolic conditions to increased risk of severe complications of COVID-19 and provides a scientific framework for translational studies of drugs developed for treatment of cardiometabolic disease as novel therapeutic interventions in patients with COVID-19.
Figure.
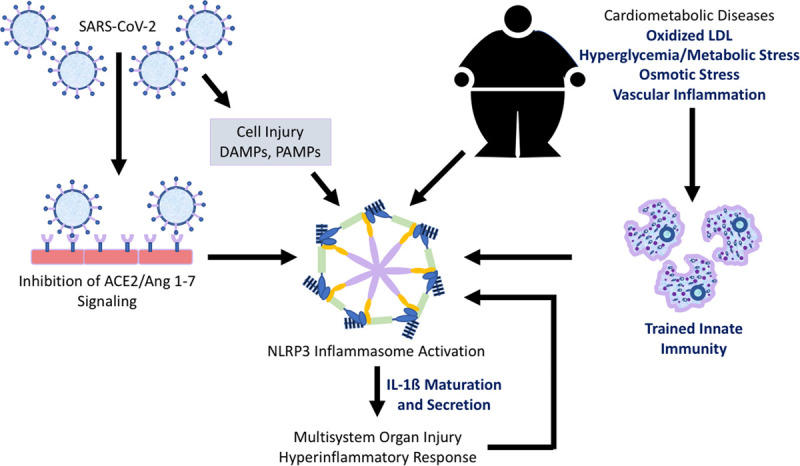
Possible mechanisms contributing to increased risk of severe complications in coronavirus disease 2019 (COVID-19) patients with comorbid cardiometabolic disease. NLRP3 (nucleotide-binding oligomerization domain, leucine-rich repeat–containing receptor family, pyrin domain-containing 3) inflammasome activation and trained immunity in association with cardiovascular disease and risk-enhancing conditions such as hypercholesterolemia, diabetes mellitus, and obesity might confer increased risk of a hyperinflammatory response that augments the effects of COVID-19–induced inflammation or COVID-19–induced immune modulation of ACE2 (angiotensin-converting enzyme 2)/Ang (1–7; angiotensin 1–7) signaling. This double-hit hypothesis is concordant with epidemiological observations linking cardiometabolic conditions to increased risk of severe complications of COVID-19 infection and provides a scientific framework for translational studies of drugs developed for the treatment of cardiometabolic disease as novel therapeutic interventions in patients with COVID-19 infection. Potential targets to reduce excessive COVID-19–induced inflammation in patients with cardiometabolic disease are listed in bolded text. DAMP indicates damage-associated molecular pattern; IL, interleukin; LDL, low-density lipoprotein; PAMP, pathogen-associated molecular pattern; and SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Aldose Reductase Inhibition
Aldose reductase—the first and rate-limiting step of the polyol pathway—channels excess glucose away from energy metabolism in cardiomyocytes and vascular cells during hyperglycemia and ischemia.54,55 Increased metabolic flux through the polyol pathway may mediate progression of diabetes mellitus–related end-organ complications due to increased osmotic stress, altered redox homeostasis, and augmented NF-κB (nuclear factor-kappa B) signaling and NLRP3 inflammasome activation.54–58 Transgenic mice expressing human aldose reductase exhibit increased expression of the transcription factor early growth response 1 and increased vascular proinflammatory and prothrombotic signaling.59 Aldose reductase inhibition protects both diabetic and nondiabetic hearts in experimental ischemia/reperfusion injury models, protects against lipopolysaccharide-induced cardiac dysfunction, reduces lung injury in experimental sepsis-induced inflammation, and reduces hyperglycemia-induced inflammasome activation in THP-1 monocytic cells and in the streptozotocin-induced diabetes mellitus mouse model.56,60–63 A double-blind, randomized placebo-controlled clinical trial of an aldose reductase inhibitor (zopolrestat) in diabetic patients demonstrated a significant increase in left ventricular ejection fraction during exercise when compared with placebo.64
AT-001 is a novel aldose reductase inhibitor in development to assess its safety and efficacy on functional capacity, biomarkers, and echocardiographic measures of cardiac structure and function in diabetic patients (NCT04083339). In a randomized proof-of-concept study conducted in diabetic patients, AT-001 therapy for 28 days reduced blood levels of sorbitol and NT-proBNP (N-terminal pro-B-type natriuretic peptide) levels when compared with placebo.65 A phase 2 open-label trial of 14 days of AT-001 therapy in COVID-19 diabetic patients with heart disease is ongoing to assess the safety and serial biomarkers of inflammation and cardiac injury (NCT04365699).
SGLT2 Inhibitors
SGLT2 (sodium-glucose cotransporter 2) inhibitors inhibit glucose reabsorption in the proximal convoluted tubule of the kidney.66 This class of agents reduces risk of cardiovascular morbidity and mortality and progression of nephropathy in diabetic patients.67–71 SGLT2 inhibitors induce transcriptomic reprogramming mimicking a fasting state with increased fatty acid utilization and ketogenesis.72 This metabolic shift in response to SGLT2 inhibition is hypothesized to be associated with activation of SIRT-1 (Sirtuin 1) and HIF-1α (hypoxia-inducible factor-1 alpha) signaling, enhanced autophagy, decreased oxidative stress, and decreased NLRP3 inflammasome activation.73,74
The observed improvement in cardiorenal outcomes with SGLT2 inhibition is greater than that expected from the modest improvement in glycemic control reported in clinical trials.73 Dapagliflozin reduces hospitalizations and death in heart failure patients with or without diabetes mellitus.75 In light of these putative cytoprotective mechanisms not related directly to glycemic control, and its association with reduced cardiovascular risk in both diabetic and nondiabetic populations, dapagliflozin might reduce the inflammatory response in viral infections and sepsis and, therefore, decrease the risk of morbidity and mortality in COVID-19. This hypothesis will be tested in the DARE-19 trial (Dapagliflozin in Respiratory Failure in Patients With COVID-19; NCT04350593)—an international double-blind, placebo-controlled study of 900 COVID-19 patients.
Incretins
The DPP4 (dipeptidyl peptidase-4) inhibitors and GLP-1-RAs (GLP-1 [glucagon-like peptide-1] receptor antagonists) are pharmacological agents used to modulate the incretin pathway of gut hormones. DPP4 inhibitors improve glycemic control by inhibiting the degradation of GLP-1—a gut hormone secreted by intestinal neuroendocrine cells that stimulates postprandial insulin secretion.76 The GLP-1-RAs are either endogenous or exogenous analogues of GLP-1. Prospective randomized placebo-controlled cardiovascular outcome trials of several GLP-1-RAs have demonstrated reduction in risk of major adverse cardiac events and reduction of cardiovascular death with liraglutide.71,77,78 Pharmacological augmentation of incretin pathway signaling may improve cardiac outcomes, in part, by immunomodulatory pathways. DPP4 is a transmembrane glycoprotein expressed in cardiac and vascular tissues, kidneys, adipocytes, and inflammatory cells.79 DPP4 upregulates T-cell CD86 (cluster of differentiation 86) expression and nuclear signaling via the NF-κB pathway and increases inflammasome expression and activity.80 Inhibition of DPP4 increases incretin signaling, which in turn reduces proinflammatory and prothrombotic signaling in response to endotoxin in experimental models of sepsis.81,82 Linagliptin was shown to attenuate cardiac dysfunction in diabetic mice with sepsis,83 but there are no available data demonstrating a protective effect for DPP4 inhibitors in patients with sepsis. A meta-analysis of 74 studies showed no increased risk for respiratory infections associated with DPP4 inhibitors when compared with placebo or other antidiabetic agents.84
Immunomodulation by incretin signaling might provide therapeutic benefit for diabetic patients with COVID-19 illness. Two open-label randomized studies in diabetic patients with COVID-19 are planned to determine the effects of linagliptin and insulin versus insulin alone on glycemic control, COVID-19 disease progression, and hospital outcomes (NCT04341935 and NCT04371978).
Colchicine
Colchicine is an anti-inflammatory medication to treat gout, familial Mediterranean fever, and pericarditis. Colchicine decreases neutrophil-endothelial adhesion, neutrophil-platelet interaction, and neutrophil and NLRP3 inflammasome activation.85,86 In observational studies of gout patients, colchicine treatment is associated with reduced high-sensitivity C-reactive protein and reduced risk of cardiovascular events.87–89 In a double-blind randomized study, a short-term course of colchicine 1.8 mg administered at the time of percutaneous coronary intervention did not reduce postprocedure biomarkers of myocardial injury when compared with placebo but reduced postprocedure rises in IL-6 and C-reactive protein 24 hours after dosing.90 In patients with coronary artery disease and stable symptoms or recent myocardial infarction, colchicine 0.5 mg daily decreased the risk of adverse cardiovascular outcomes end point when compared with placebo.91,92
Given this profile of putative anti-inflammatory mechanisms and cardiovascular risk reduction, the COLCORONA trial (Colchicine Coronavirus SARS-CoV2 Trial) is currently recruiting ≈6000 subjects in a multinational, randomized, double-blind, placebo-controlled trial to evaluate the safety and efficacy of colchicine in outpatients diagnosed with COVID-19 (NCT04322682). There are 9 additional international trials of colchicine in COVID-19 listed on https://www.clinicaltrials.gov.
IL-1 Inhibitors
The Toll-like receptor family plays a critical role in inducing innate immune signaling in response to microbial components (pathogen-associated molecular patterns), or damage-associated molecular patterns that occur with sterile inflammation and cell injury, including atherosclerosis and ischemic myocardial injury.93–95 Activation of these transmembrane receptors initiates signaling that ultimately leads to activation of the transcription factors NF-κB, IRF (inferon regulator factor)-3, and IRF7 and induction of antibacterial and antiviral gene expression. Among the genes upregulated are pro–IL-1β and components of the NLRP3 inflammasome, which upon assembly activates caspase-1–mediated IL-1β and IL-18 secretion, and a form of cell death called pyroptosis. IL-1β is a potent proinflammatory cytokine that acts via the IL-1R1 (IL-1 receptor, type 1) to induce fever, activation of innate and adaptive immune cell responses, the acute-phase response, and leukocyte-endothelial cell interactions.96 This proinflammatory signaling cascade is counterbalanced by IL-1Ra (IL-1 receptor antagonist), which binds to IL-1R1 without causing the conformational change required for IL-1R3 to bind, thereby abrogating transmembrane signaling. Anakinra is a recombinant form of IL-1Ra that was first approved for rheumatoid arthritis and is used to treat a variety of rheumatic and cardiovascular conditions. It is commonly used as a second-line agent for refractory pericarditis and has shown promising results in phase 2 studies of acute myocardial infarction and chronic heart failure.97–99 There are 2 ongoing trials of anakinra to prevent disease progression and cytokine storm severity in COVID-19 (NCT04362111 and NCT04341584).
Canakinumab is a human monoclonal antibody that targets IL-1β and neutralizes its downstream inflammatory effects (including generation of IL-6) implicated in the pathogenesis of atherothrombosis.100 The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study trial randomized over 10 000 patients with prior myocardial infarction and demonstrated a reduction in major adverse cardiovascular events (and cancer-related mortality) with canakinumab versus placebo.101 However, this benefit was offset by increased risk of fatal infection and sepsis. A phase 2 single-center study of canakinumab is currently recruiting COVID-19 patients with evidence of myocardial injury (NCT04365153). Patients will be randomized to the intervention drug or placebo with a primary outcome of time to clinical improvement or hospital discharge.
HMG-CoA Reductase Inhibitors (Statins)
Immunomodulatory effects of statins contribute to their reduction of cardiovascular disease risk beyond LDL cholesterol-lowering effects and thereby might also attenuate the inflammatory response in COVID-19. Inhibition of HMG-coenzyme A reductase exerts downstream effects on the mevalonic acid pathway leading to a reduction in geranylgeranylation and farnesylation of GTPases responsible for immune cell migration, cytokine production, and T-cell signaling.102,103 Statins reduce IL-6–induced expression of C-reactive protein at the transcriptional level and repress major histocompatibility complex class II molecule expression on antigen-presenting cells thereby decreasing the activation of T lymphocytes.104,105 The effects of various statins on NLRP3 inflammasome activation differ according to dose and pharmacokinetic properties.106,107 Simvastatin and mevastatin have been reported to inhibit oxidized LDL–mediated inflammasome activation in human endothelial cells by activation of nuclear pregnane X receptors.108,109 Statin use is associated with reduced risk of influenza-related hospitalization and death in observational studies.110–112 Conversely, a prospective randomized clinical trial of rosuvastatin for treatment of sepsis-associated adult respiratory distress syndrome was stopped early due to futility.113 In light of these data derived from non–COVID-19 populations, a randomized trial of preemptive administration of standard medications used in acute coronary syndrome (including atorvastatin, antiplatelets, and anticoagulants) in patients hospitalized with COVID-19 illness is currently recruiting participants (NCT04333407). A smaller randomized study in statin-naive patients with COVID-19 aims to assess the efficacy of atorvastatin to mitigate disease progression (NCT04380402).
Conclusions
The COVID-19 pandemic presents an unprecedented challenge and opportunity for translational investigators to rapidly develop safe and effective therapeutic interventions based on limited preclinical data. As of May 10, 2020, >1000 clinical trials in COVID-19 patients are registered on the World Health Organization database (https://clinicaltrials.gov/ct2/who_table). This brief review describes a small representative sample of clinical trials targeting cardiometabolic inflammatory pathways as a novel strategy to improve outcomes in COVID-19 patients. Numerous trials testing other classes of drugs that target angiotensin II signaling, IL-6 signaling, or other vascular inflammation signaling pathways are omitted due to limited space. The results of these clinical trials and ongoing observational biospecimen studies may provide clues to elucidate potential mechanistic links between cardiometabolic disease and host response to COVID-19 and identify novel targets for intervention in COVID-19 patients with comorbid cardiometabolic disease.
Sources of Funding
B. Shah is supported, in part, by the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development (iK2CX001074) and National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI; R01HL146206). S.D. Katz and M. Pillinger are supported, in part, by grant number NIH/NCATS (National Center for Advancing Translational Sciences) UL1 TR000038 from the National Center for Research Resources, National Institutes of Health. M.S. Garshick is supported by the American Heart Association Career Development Grant (Dallas, TX) 18CDA34080540. R. Ramasamy is supported, in part, by grants from NIH/NHLBI (P01HL143697-01 and R01 HL132516-01). J.D. Newman is supported, in part, by the NIH/NHLBI HL125991. K.J. Moore is supported by grants from the NIH/NHLBI P01HL131481 and R35HL135799.
Disclosures
S.D. Katz has received an investigator-initiated research grant from Applied Therapeutics, Inc. R. Ramasamy is a consultant for Applied Therapeutics, Inc, and has received a research grant from them. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- ACE2
- angiotensin-converting enzyme 2
- Ang 1–7
- angiotensin 1–7
- COVID-19
- coronavirus disease 2019
- DPP4
- dipeptidyl peptidase-4
- GLP-1
- glucagon-like peptide-1
- GLP-1-RA
- glucagon-like peptide-1 receptor antagonists
- IL
- interleukin
- IL-1R1
- interleukin-1 receptor, type 1
- IL-1Ra
- interleukin-1 receptor antagonist
- IRF
- inferon regulator factor
- LDL
- low-density lipoprotein
- NF-κB
- nuclear factor-kappa B
- NLRP3
- nucleotide-binding oligomerization domain, leucine-rich repeat–containing receptor family pyrin domain-containing 3
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- SARS
- severe acute respiratory syndrome
- SGLT2
- sodium-glucose cotransporter 2
For Sources of Funding and Disclosures, see page 2050.
References
- 1.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020579270–273doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization World Health. World Health Organization. Coronavirus disease (COVID-19) pandemic. 2020 https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed July 31, 2020.
- 3.Lecis R, Mucedda M, Pidinchedda E, Pittau M, Alberti A. Molecular identification of Betacoronavirus in bats from Sardinia (Italy): first detection and phylogeny. Virus Genes 20195560–67doi: 10.1007/s11262-018-1614-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-Cov-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020181271–280.e8doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-COV-2. Cardiovasc Res 20201161097–1100doi: 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 200087E1–E9doi: 10.1161/01.res.87.5.e1 [DOI] [PubMed] [Google Scholar]
- 7.Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 2004383pt 145–51doi: 10.1042/BJ20040634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1-7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 2005289H2281–H2290doi: 10.1152/ajpheart.00618.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JW, Ng CK, Chan YH, Mok TY, Lee S, Chu SY, Law WL, Lee MP, Li PC. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax 200358686–689doi: 10.1136/thorax.58.8.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 20032892801–2809doi: 10.1001/jama.289.21.JOC30885 [DOI] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 20203951054–1062doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 20203231061–1069doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 20203821708–1720doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 20203951763–1770doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, Felix SEB, Tie Y, Fullerton KE. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 202069759–765doi: 10.15585/mmwr.mm6924e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, Andrews N, Byford R, Dabrera G, Elliot A, et al. Risk factors for SARS-CoV-2 among patients in the oxford royal college of general practitioners research and surveillance centre primary care network: a cross-sectional study [published online May 15, 2020 ]. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30371-6. doi: 10.1016/S1473-3099(20)30371-6 https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30371-6/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan RE, Adab P. Who is most likely to be infected with SARS-COV-2? [published online May 15, 2020]. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30395-9. doi: 10.1016/S1473-3099(20)30395-9. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30395-9/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Valle DM, Kim-Schulze S, Hsin-Hui H, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz T, Madduri D, Stock A, et al. An inflammatory cytokine signature helps predict COVID-19 severity and death [published online May 30, 2020]. medRxiv. 2020 doi: 10.1101/2020.05.28.20115758. [Google Scholar]
- 20.Akhmerov A, Marbán E. COVID-19 and the heart. Circ Res 20201261443–1455doi: 10.1161/CIRCRESAHA.120.317055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 20203951033–1034doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, et al. ST-segment elevation in patients with COVID-19 - a case series. N Engl J Med 20203822478–2480doi: 10.1056/NEJMc2009020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin [published online March 16, 2020]. Eur Heart J. doi: 10.1093/eurheartj/ehaa190. [Google Scholar]
- 24.Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, Wang LF, Gao H, Wang Y, Dong CF, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights [published online April 10, 2020]. Infection. doi: 10.1007/s15010-020-01424-5. doi: 10.1007/s15010-020-01424-5. https://link.springer.com/article/10.1007%2Fs15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol 2020153725–733doi: 10.1093/ajcp/aqaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in COVID-19: the first autopsy series from New Orleans [published online May 27, 2020]. medRxiv. doi: 10.1101/2020.04.06.20050575. [Google Scholar]
- 28.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 20205819–824doi: 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-firbin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series Megakaryocytes and platelet-firbin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. doi: 10.1016/j.eclinm.2020.100434. https://www.thelancet.com/action/showPdf?pii=S2589-5370%2820%2930178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol 202059739–743doi: 10.1111/ijd.14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royal College of Paediatrics and Child Health. Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19. doi: 10.1111/jpc.16408. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf. [DOI] [PubMed]
- 32.New York State Department of Health, Bureau of Communicable Disease Control Health advisory: pediatric multi-system inflammatory syndrome potentially associated with coronavirus disease (COVID-19) in children. May 6, 2020http://dmna.ny.gov/covid19/docs/all/DOH_COVID19_PediatricInflammatorySyndrome_050620.pdf
- 33.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, Milner JD. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 2020324294–296doi: 10.1001/jama.2020.10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020324259–269doi: 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D. COVID-19 presenting as stroke. Brain Behav Immun 202087115–119doi: 10.1016/j.bbi.2020.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, Bilaoglu S, HOchman K, Raz E, Galetta S, et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menter T, Haslbauer JD, Nienhold R, Savic S, Deigendesch H, Frank S, Turek D, Willi N, Pargger H, Bassetti S, et al. Post-mortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction [published online May 4, 2020]. Histopathology. 2020 doi: 10.1111/his.14134. doi: 10.1111/his.14134. https://onlinelibrary.wiley.com/doi/full/10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 20203951417–1418doi: 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goeijenbier M, van Wissen M, van de Weg C, Jong E, Gerdes VE, Meijers JC, Brandjes DP, van Gorp EC. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol 2012841680–1696doi: 10.1002/jmv.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cugno M, Meroni PL, Gualtierotti R, Griffini S, Grovetti E, Torri A, Panigada M, Aliberti S, Blasi F, Tedesco F, et al. Complement activation in patients with COVID-19: a novel therapeutic target. J Allergy Clin Immunol 2020146215–217doi: 10.1016/j.jaci.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity 2013381092–1104doi: 10.1016/j.immuni.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011145341–355doi: 10.1016/j.cell.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 20104641357–1361doi: 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin Y, Fu J. Novel insights into the NLRP 3 inflammasome in atherosclerosis. J Am Heart Assoc. 2019;8:e012219. doi: 10.1161/JAHA.119.012219. doi: 10.1161/JAHA.119.012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhoads JP, Lukens JR, Wilhelm AJ, Moore JL, Mendez-Fernandez Y, Kanneganti TD, Major AS. Oxidized low-density lipoprotein immuneomplex priming of the Nlrp3 inflammasome involves TLR and FcγR cooperation and is dependent on CARD9. J Immunol 20171982105–2114doi: 10.4049/jimmunol.1601563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res 2016118145–156doi: 10.1161/CIRCRESAHA.115.306656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christ A, Günther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Baßler K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 2018172162–175.e14doi: 10.1016/j.cell.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore KJ. Targeting inflammation in CVD: advances and challenges. Nat Rev Cardiol 20191674–75doi: 10.1038/s41569-018-0144-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, de Graaf J, Joosten LA, Netea MG, Gomes ME, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis 2016254228–236doi: 10.1016/j.atherosclerosis.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 50.Rheinheimer J, de Souza BM, Cardoso NS, Bauer AC, Crispim D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review. Metabolism 2017741–9doi: 10.1016/j.metabol.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 51.Passos-Silva DG, Verano-Braga T, Santos RA. Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci (Lond) 2013124443–456doi: 10.1042/CS20120461 [DOI] [PubMed] [Google Scholar]
- 52.Nieto-Torres JL, DeDiego ML, Verdiá-Báguena C, Jimenez-Guardeño JM, Regla-Nava JA, Fernandez-Delgado R, Castaño-Rodriguez C, Alcaraz A, Torres J, Aguilella VM, et al. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10:e1004077. doi: 10.1371/journal.ppat.1004077. doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farag NS, Breitinger U, Breitinger HG, El Azizi MA. Viroporins and inflammasomes: a key to understand virus-induced inflammation. Int J Biochem Cell Biol. 2020;122:105738. doi: 10.1016/j.biocel.2020.105738. doi: 10.1016/j.biocel.2020.105738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vedantham S, Ananthakrishnan R, Schmidt AM, Ramasamy R. Aldose reductase, oxidative stress and diabetic cardiovascular complications. Cardiovasc Hematol Agents Med Chem 201210234–240doi: 10.2174/187152512802651097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramasamy R, Goldberg IJ. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ Res 20101061449–1458doi: 10.1161/CIRCRESAHA.109.213447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase mediates NLRP3 inflammasome-initiated innate immune response in hyperglycemia-induced Thp1 monocytes and male mice. Endocrinology 20171583661–3675doi: 10.1210/en.2017-00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramana KV, Srivastava SK. Aldose reductase: a novel therapeutic target for inflammatory pathologies. Int J Biochem Cell Biol 20104217–20doi: 10.1016/j.biocel.2009.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation 20061141838–1846doi: 10.1161/CIRCULATIONAHA.106.630830 [DOI] [PubMed] [Google Scholar]
- 59.Vedantham S, Thiagarajan D, Ananthakrishnan R, Wang L, Rosario R, Zou YS, Goldberg I, Yan SF, Schmidt AM, Ramasamy R. Aldose reductase drives hyperacetylation of Egr-1 in hyperglycemia and consequent upregulation of proinflammatory and prothrombotic signals. Diabetes 201463761–774doi: 10.2337/db13-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramasamy R, Oates PJ, Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes 199746292–300doi: 10.2337/diab.46.2.292 [DOI] [PubMed] [Google Scholar]
- 61.Ramasamy R, Trueblood N, Schaefer S. Metabolic effects of aldose reductase inhibition during low-flow ischemia and reperfusion. Am J Physiol 1998275H195–H203doi: 10.1152/ajpheart.1998.275.1.H195 [DOI] [PubMed] [Google Scholar]
- 62.Ravindranath TM, Mong PY, Ananthakrishnan R, Li Q, Quadri N, Schmidt AM, Ramasamy R, Wang Q. Novel role for aldose reductase in mediating acute inflammatory responses in the lung. J Immunol 20091838128–8137doi: 10.4049/jimmunol.0900720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, Yan S, Ii S, Itakura M, Rui L, et al. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J 2004181192–1199doi: 10.1096/fj.03-1400com [DOI] [PubMed] [Google Scholar]
- 64.Johnson BF, Nesto RW, Pfeifer MA, Slater WR, Vinik AI, Chyun DA, Law G, Wackers FJ, Young LH. Cardiac abnormalities in diabetic patients with neuropathy: effects of aldose reductase inhibitor administration. Diabetes Care 200427448–454doi: 10.2337/diacare.27.2.448 [DOI] [PubMed] [Google Scholar]
- 65.Perfetti R, Shendelman S. Clinical assessment of at-001, an aldose reductase inhibitor in development for diabetic cardiomyopathy: a 28-day proof of concept study. Circulation. 2019;140:A13475. [Google Scholar]
- 66.Kalra S. Sodium glucoseo-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther 20145355–366doi: 10.1007/s13300-014-0089-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. ; EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 20153732117–2128doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 68.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017377644–657doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 69.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. ; DECLARE–TIMI 58 Investigators Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019380347–357doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 70.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. ; CREDENCE Trial Investigators Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 20193802295–2306doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 71.Wilcox T, De Block C, Schwartzbard AZ, Newman JD. Diabetic agents, from metformin to SGLT2 inhibitors and GLP1 receptor agonists: JACC focus seminar. J Am Coll Cardiol 2020751956–1974doi: 10.1016/j.jacc.2020.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osataphan S, Macchi C, Singhal G, Chimene-Weiss J, Sales V, Kozuka C, Dreyfuss JM, Pan H, Tangcharoenpaisan Y, Morningstar J, et al. SGLT2 inhibition reprograms systemic metabolism via FGF21-dependent and -independent mechanisms. JCI Insight. 2019;4:e123130. doi: 10.1172/jci.insight.123130. doi: 10.1172/jci.insight.123130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail 202022618–628doi: 10.1002/ejhf.1732 [DOI] [PubMed] [Google Scholar]
- 74.Yaribeygi H, Katsiki N, Butler AE, Sahebkar A. Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys. Drug Discov Today 201924256–262doi: 10.1016/j.drudis.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 75.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. ; DAPA-HF Trial Committees and Investigators Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 20193811995–2008doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 76.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 198663492–498doi: 10.1210/jcem-63-2-492 [DOI] [PubMed] [Google Scholar]
- 77.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. ; SUSTAIN-6 Investigators Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 20163751834–1844doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 78.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. ; LEADER Steering Committee; LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016375311–322doi: 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andersen ES, Deacon CF, Holst JJ. Do we know the true mechanism of action of the DPP-4 inhibitors? Diabetes Obes Metab 20182034–41doi: 10.1111/dom.13018 [DOI] [PubMed] [Google Scholar]
- 80.Liu T, Zhang L, Joo D, Sun SC. Nf-kappab signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ku HC, Chen WP, Su MJ. GLP-1 signaling preserves cardiac function in endotoxemic Fischer 344 and DPP4-deficient rats. Naunyn Schmiedebergs Arch Pharmacol 2010382463–474doi: 10.1007/s00210-010-0559-9 [DOI] [PubMed] [Google Scholar]
- 82.Steven S, Jurk K, Kopp M, Kröller-Schön S, Mikhed Y, Schwierczek K, Roohani S, Kashani F, Oelze M, Klein T, et al. Glucagon-like peptide-1 receptor signalling reduces microvascular thrombosis, nitro-oxidative stress and platelet activation in endotoxaemic mice. Br J Pharmacol 20171741620–1632doi: 10.1111/bph.13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al Zoubi S, Chen J, Murphy C, Martin L, Chiazza F, Collotta D, Yaqoob MM, Collino M, Thiemermann C. Linagliptin attenuates the cardiac dysfunction associated with experimental sepsis in mice with pre-existing type 2 diabetes by inhibiting NF-κB. Front Immunol. 2018;9:2996. doi: 10.3389/fimmu.2018.02996. doi: 10.3389/fimmu.2018.02996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang W, Cai X, Han X, Ji L. DPP-4 inhibitors and risk of infections: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev 201632391–404doi: 10.1002/dmrr.2723 [DOI] [PubMed] [Google Scholar]
- 85.Leung YY, Yao Hui LL, Kraus VB. Colchicine– update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 201545341–350doi: 10.1016/j.semarthrit.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006440237–241doi: 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 87.Crittenden DB, Lehmann RA, Schneck L, Keenan RT, Shah B, Greenberg JD, Cronstein BN, Sedlis SP, Pillinger MH. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol 2012391458–1464doi: 10.3899/jrheum.111533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krasnokutsky S, Romero AG, Bang D, Pike VC, Shah B, Igel TF, Dektiarev I, Guo Y, Zhong J, Katz SD, et al. Impaired arterial responsiveness in untreated gout patients compared with healthy non-gout controls: association with serum urate and C-reactive protein. Clin Rheumatol 2018371903–1911doi: 10.1007/s10067-018-4029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis 2016751674–1679doi: 10.1136/annrheumdis-2015-207984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah B, Pillinger M, Zhong H, Cronstein B, Xia Y, Lorin JD, Smilowitz NR, Feit F, Ratnapala N, Keller NM, et al. Effects of acuteolchicine administration prior to percutaneousoronary intervention: COLCHICINE-PCI randomized trial. Circ Cardiovasc Interv. 2020;13:e008717. doi: 10.1161/CIRCINTERVENTIONS.119.008717. doi: 10.1161/CIRCINTERVENTIONS.119.008717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low-doseolchicine after myocardial infarction. N Engl J Med 20193812497–2505doi: 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 92.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 201361404–410doi: 10.1016/j.jacc.2012.10.027 [DOI] [PubMed] [Google Scholar]
- 93.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 20111173720–3732doi: 10.1182/blood-2010-07-273417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res 20201261260–1280doi: 10.1161/CIRCRESAHA.120.315937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martin SJ. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J 20162832599–2615doi: 10.1111/febs.13775 [DOI] [PubMed] [Google Scholar]
- 96.Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 20182818–27doi: 10.1111/imr.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brucato A, Imazio M, Gattorno M, Lazaros G, Maestroni S, Carraro M, Finetti M, Cumetti D, Carobbio A, Ruperto N, et al. Effect of anakinra on recurrent pericarditis among patients witholchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 20163161906–1912doi: 10.1001/jama.2016.15826 [DOI] [PubMed] [Google Scholar]
- 98.Abbate A, Trankle CR, Buckley LF, Lipinski MJ, Appleton D, Kadariya D, Canada JM, Carbone S, Roberts CS, Abouzaki N, et al. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2020;9:e014941. doi: 10.1161/JAHA.119.014941. doi: 10.1161/JAHA.119.014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, Abouzaki NA, Dixon D, Kadariya D, Christopher S, et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail. 2017;10:e004373. doi: 10.1161/CIRCHEARTFAILURE.117.004373. doi: 10.1161/CIRCHEARTFAILURE.117.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, Gao P, Saleheen D, Rendon A, Nelson CP, et al. ; IL6R Genetics Consortium Emerging Risk Factors Collaboration Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 20123791205–1213doi: 10.1016/S0140-6736(11)61931-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. ; CANTOS Trial Group Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 20173771119–1131doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 102.Zeiser R. Immune modulatory effects of statins. Immunology 201815469–75doi: 10.1111/imm.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hechinger AK, Maas K, Dürr C, Leonhardt F, Prinz G, Marks R, Gerlach U, Hofmann M, Fisch P, Finke J, et al. Inhibition of protein geranylgeranylation and farnesylation protects against graft-versus-host disease via effects on CD4 effector T cells. Haematologica 20139831–40doi: 10.3324/haematol.2012.065789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, Mach F. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol 2005251231–1236doi: 10.1161/01.ATV.0000163840.63685.0c [DOI] [PubMed] [Google Scholar]
- 105.Mach F. Statins as immunomodulatory agents. Circulation 200410921 suppl 1II15–II17doi: 10.1161/01.CIR.0000129502.10459.fe [DOI] [PubMed] [Google Scholar]
- 106.Koushki K, Shahbaz SK, Mashayekhi K, Sadeghi M, Zayeri ZD, Taba MY, Banach M, Al-Rasadi K, Johnston TP, Sahebkar A. Anti-inflammatory action of statins in cardiovascular disease: the role of inflammasome and toll-like receptor pathways [published online May 6, 2020]. Clin Rev Allergy Immunol. doi: 10.1007/s12016-020-08791-9. doi: 10.1007/s12016-020-08791-9. https://link.springer.com/article/10.1007/s12016-020-08791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Satoh M, Tabuchi T, Itoh T, Nakamura M. NLRP3 inflammasome activation in coronary artery disease: results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin Sci (Lond) 2014126233–241doi: 10.1042/CS20130043 [DOI] [PubMed] [Google Scholar]
- 108.Howe K, Sanat F, Thumser AE, Coleman T, Plant N. The statin class of HMG-CoA reductase inhibitors demonstrate differential activation of the nuclear receptors PXR, CAR and FXR, as well as their downstream target genes. Xenobiotica 201141519–529doi: 10.3109/00498254.2011.569773 [DOI] [PubMed] [Google Scholar]
- 109.Wang S, Xie X, Lei T, Zhang K, Lai B, Zhang Z, Guan Y, Mao G, Xiao L, Wang N. Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFα in vascular endothelialells through a PXR-dependent mechanism. Mol Pharmacol 201792256–264doi: 10.1124/mol.116.108100 [DOI] [PubMed] [Google Scholar]
- 110.Vandermeer ML, Thomas AR, Kamimoto L, Reingold A, Gershman K, Meek J, Farley MM, Ryan P, Lynfield R, Baumbach J, et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis 201220513–19doi: 10.1093/infdis/jir695 [DOI] [PubMed] [Google Scholar]
- 111.Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest 20071311006–1012doi: 10.1378/chest.06-1997 [DOI] [PubMed] [Google Scholar]
- 112.Kwong JC, Li P, Redelmeier DA. Influenza morbidity and mortality in elderly patients receiving statins: a cohort study. PLoS One. 2009;4:e8087. doi: 10.1371/journal.pone.0008087. doi: 10.1371/journal.pone.0008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, Brower RG, Shanholtz C, Rock P, Douglas IS, et al. ; National Heart, Lung, and Blood Institute ACTN Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 20143702191–2200doi: 10.1056/NEJMoa1401520 [DOI] [PMC free article] [PubMed] [Google Scholar]


