Abstract
Background:
The SWORD trials showed that in participants who achieved virologic suppression taking 3-drug or 4-drug regimens, switching to the 2-drug regimen dolutegravir plus rilpivirine was noninferior in maintaining HIV-1 RNA <50 copies/mL at the week 48 primary endpoint. We present pooled week 148 analysis results from both studies.
Setting:
SWORD-1: 65 centers, 13 countries; SWORD-2: 60 centers, 11 countries.
Methods:
SWORD-1 and SWORD-2 are identical, open-label, phase III studies. Participants with screening HIV-1 RNA <50 copies/mL for ≥6 months; no prior virologic failure; and no documented resistance-associated major protease inhibitor, integrase inhibitor, nucleoside reverse transcriptase inhibitor (NRTI), or non-NRTI mutations or integrase resistance-associated substitution R263K were randomly assigned 1:1 to switch to once-daily dolutegravir 50 mg plus rilpivirine 25 mg on day 1 (early-switch group) or to continue their current antiretroviral regimen and, if virologically suppressed at week 48, switch to dolutegravir plus rilpivirine at week 52 (late-switch group) until week 148.
Results:
Using snapshot algorithm at week 148, 432 of 513 (84%) early-switch participants (148 weeks of exposure) and 428 of 477 (90%) late-switch participants (96 weeks of exposure) maintained HIV-1 RNA <50 copies/mL. Eleven participants (1%) on dolutegravir plus rilpivirine met the confirmed virologic withdrawal criterion through week 148 (early-switch group, n = 8; late-switch group, n = 3) with no integrase resistance identified. Non-NRTI resistance-associated mutations were identified in 6 participants (<1%). Drug-related adverse events (grades 2–4) were observed in 31 (6%) early-switch and 16 (3%) late-switch participants. Significant improvements in bone biomarkers were observed. Significant improvements were observed in renal biomarkers in participants taking tenofovir disoproxil fumarate pre‐switch.
Conclusion:
Switching to the 2-drug regimen dolutegravir plus rilpivirine maintained virologic suppression for a high proportion of participants through 3 years, with low rates of virologic failure and a well-tolerated safety profile.
Key Words: 2-drug regimen, integrase strand transfer inhibitor, non-nucleoside reverse transcriptase inhibitor, antiretroviral therapy
INTRODUCTION
The efficacy of 3-drug–containing antiretroviral therapy (ART) is well established for the treatment of HIV-1 infection and typically composed of 2 nucleoside reverse transcriptase inhibitors (NRTIs) and a third drug from another class: protease inhibitor, integrase strand transfer inhibitor (INSTI), or non-nucleoside reverse transcriptase inhibitor (NNRTI).1 As effective treatments are prolonging life expectancy of people living with HIV-1, strategies to manage lifelong exposure to pharmacotherapy and minimize ART-related comorbidities are needed. Switching from 3-drug regimens to 2-drug regimens (2DRs) can potentially reduce exposure to antiretroviral drugs, avoid adverse drug–drug interactions when switching from boosted regimens, and reduce treatment-associated toxicities.
Guidelines recommend the use of dolutegravir plus rilpivirine as a 2-drug switch strategy for virologically suppressed individuals without prior virologic failure or transmitted drug resistance.1–3 Primary analysis of pooled data from the SWORD-1 and SWORD-2 trials demonstrated that switching from standard ART to the 2DR of dolutegravir plus rilpivirine was noninferior in maintaining HIV-1 RNA <50 copies/mL in adults with virologic suppression on a 3-drug or 4-drug regimen (94.7% vs. 94.9%, respectively).4 Interim analyses of pooled SWORD-1 and SWORD-2 data at week 100 showed HIV-1 suppression in 89% (456/513) of participants continuing dolutegravir plus rilpivirine for 2 years (early-switch group) and in 93% (444/477) of those who switched to the 2DR after the week 48 primary endpoint (late-switch group).5 Importantly, over this 2-year period, confirmed virologic failure occurred in 8 of 990 (1%) participants (6 over 100 weeks in the early-switch group and 2 over 48 weeks in the late-switch group) with rilpivirine resistance-associated mutations (RAMs) detected in 4 of these participants.5 Here, we report findings from the pooled analysis of SWORD-1 and SWORD-2 at week 148 assessing long-term efficacy and safety of 2DR treatment (148 weeks for the early-switch group and 96 weeks for the late-switch group).
METHODS
The identically designed, open-label, phase III SWORD-1 (65 centers, 13 countries) and SWORD-2 (60 centers, 11 countries) studies enrolled participants with virologic suppression on 3-drug or 4-drug regimens who had no observed instance of HIV-1 RNA ≥50 copies/mL for 6 months before screening and no instance of HIV-1 RNA ≥200 copies/mL in the previous 6–12-month period before screening (complete methods have been previously published).5 Participants were randomized (1:1; stratified by baseline third agent class, age, and planned participation in a bone substudy) to switch to once-daily oral dolutegravir 50 mg plus rilpivirine 25 mg (2DR) either on day 1 (early-switch group; 148 weeks of exposure) or at week 52 and continued 2DR until week 148 (late-switch group; 96 weeks of exposure).
Secondary endpoints included incidence of observed genotypic and phenotypic resistance to dolutegravir or rilpivirine for participants meeting the confirmed virologic withdrawal (CVW) criterion. CVW was defined as a participant having one measurement of HIV-1 RNA ≥50 copies/mL [suspected virologic withdrawal (SVW)] followed by a second confirmatory measurement of HIV-1 RNA ≥200 copies/mL, resulting in study withdrawal and resistance testing. For participants meeting CVW, integrase genotypic and phenotypic resistance assessments used GenoSeq Integrase and PhenoSense Integrase (Monogram Biosciences, South San Francisco, CA); genotypic and phenotypic resistance assessments for reverse transcriptase and protease used PhenoSense GT (Monogram Biosciences) on the sample collected at SVW. The PhenoSense GT plus Integrase assay (Monogram Biosciences) for reverse transcriptase, protease, and integrase was used in cases where other testing did not work. Baseline genotyping was performed on whole blood or peripheral blood mononuclear cells from participants meeting CVW using GenoSure Archive proviral DNA testing, but this platform does not provide phenotypic data. Predefined exploratory endpoints from this pooled 148-week analysis included proportion of participants maintaining virologic suppression (HIV-1 RNA <50 copies/mL per US Food and Drug Administration snapshot algorithm), change from baseline in CD4+ cell count, and incidence of disease progression (HIV‐1– associated conditions, AIDS, and death) for all randomly assigned participants receiving ≥1 dose of any study drug. Adverse events (AEs), as well as renal and bone biomarkers, and fasting lipids were recorded throughout the study as previously described.5
Snapshot algorithm was performed after switch for proportion of participants with HIV-1 RNA <50 copies/mL at week 148. Biomarkers and lipids were analyzed as change from baseline for the early-switch group or from late-switch baseline (ie, last assessment before switch, normally at week 48) for the late-switch group. Statistical significance of change from baseline/late-switch baseline within each group was assessed using a 1-sample 2-sided t test for bone biomarkers and a nonparametric sign test for percentage change in renal biomarkers of ratios of urine retinol-binding protein/creatinine and urine β2 microglobulin/creatinine by previous exposure to tenofovir disoproxil fumarate, at a significance level of α = 5%. Simple statistics [ie, mean (SD), median (interquartile range; IQR)] were used to summarize changes in estimated glomerular filtration rate. Missing data were not imputed. Participants reported adherence to their current regimen over the past several weeks by marking on a linear visual analog scale with response options ranging from 0 (no HIV medication) to 100 (every dose of HIV medication). Treatment satisfaction was self-reported using a 10-item scale by specific domains (eg, convenience, flexibility), and each item was scored on a 0–6-point scale. Higher scores indicated greater improvement.
SWORD-1 and SWORD-2 were performed in accordance with the 2008 Declaration of Helsinki under the approval of national, regional, and investigational site ethics committees. All participants provided written informed consent before screening.
RESULTS
Participant baseline characteristics were balanced across the early-switch and late-switch groups, with 28% aged ≥50 years, 22% female, and 80% White. Through week 148, 77 of 513 (15%) early-switch participants discontinued (AE, n = 38; withdrew consent, n = 14; lack of efficacy, n = 12; protocol deviation, n = 7; lost to follow-up, n = 4; and protocol-defined stopping criteria, n = 2), and 46 of 477 (10%) late-switch participants discontinued (AE, n = 18; withdrew consent, n = 11; lack of efficacy, n = 6; investigator decision, n = 5; lost to follow-up, n = 3; and protocol deviation, n = 3). At screening, the most commonly received NRTIs were tenofovir disoproxil fumarate (early-switch group, 73%; late-switch group, 70%) and emtricitabine (early-switch group, 69%; late-switch group, 67%). The most common third agent classes at baseline were NNRTIs (early-switch group, 54%; late-switch group, 56%), protease inhibitors (early-switch group, 26%; late-switch group, 25%), and INSTIs (early-switch group, 20%; late-switch group, 19%).4
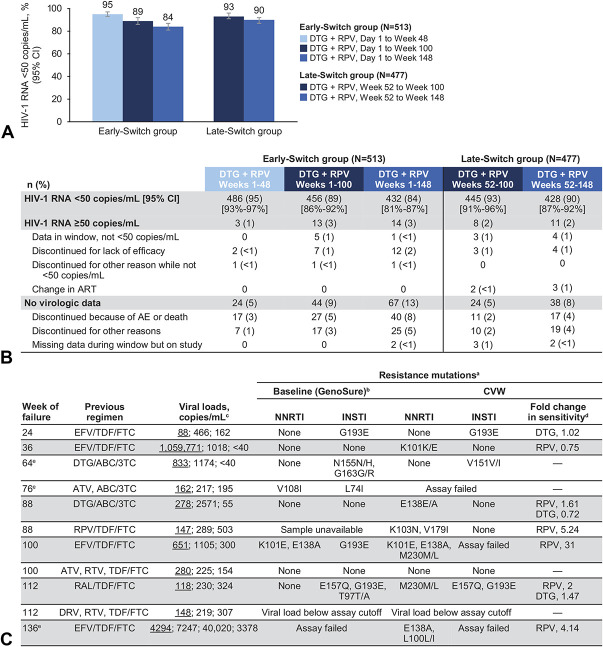
At week 148, 84% of early-switch participants {432/513 [95% confidence interval (CI): 81% to 87%]} and 90% [428/477 (95% CI: 87% to 92%)] of late-switch participants maintained virologic suppression (Fig. 1A). Data from the Snapshot analyses conducted at weeks 48, 100, and 148 are illustrated in Figure 1B. Median increase from baseline in CD4+ cell count was 46.0 cells/mm3 (IQR, −57.0 to 147.0) in the early-switch group and 5.5 cells/mm3 (IQR, −86.0 to 110.0) from late-switch baseline in the late-switch group. After switching to dolutegravir plus rilpivirine, 4 participants (2 per group; 3 with CDC category A and 1 with CDC category B at baseline) experienced disease progression to a new CDC category C event, 1 of whom progressed after week 100. Three deaths were reported in participants who received dolutegravir plus rilpivirine, all in the early-switch group, and none were considered related to study treatment (1 each of Kaposi's sarcoma, week 30; natural causes, week 110; and suicide, week 112).
FIGURE 1.
A, Proportion of participants with plasma HIV-1 RNA <50 copies/mL at weeks 48, 100, and 148, (B) snapshot analysis at weeks 48, 100, and 148, and (C) cases of CVW through week 148. ABC, abacavir; ATV, atazanavir; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; FTC, emtricitabine; ND, not determined; NR, not reported; RAL, raltegravir; RAM, resistance-associated mutation; RPV, rilpivirine; RTV, ritonavir; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate. aShading represents participants with NNRTI RAMs. bHIV-1 baseline resistance testing was performed on integrated HIV-1 proviral DNA using GenoSure Archive assay (Monogram Biosciences). On-study resistance testing used standard plasma-based genotypic and phenotypic resistance testing. cViral load values are shown; underlined value denotes viral load meeting SVW criterion followed by viral load at the confirmatory (CVW) and withdrawal visits. dFold change data were generated from assays performed at CVW in participants with observed RAMs. eParticipants in the late-switch group.
The frequency of participants who received dolutegravir plus rilpivirine meeting the CVW criterion remained low through week 148 (11/990; 1%). For participants with available resistance testing data, no integrase resistance was observed at the CVW time point whereas NNRTI-associated or rilpivirine-associated resistance mutations were identified in 6 participants (<1%), with baseline rilpivirine RAMs detected in 1 of these participants (Fig. 1C). Of the 6 participants, 2 showed a phenotypic fold change (FC) in sensitivity to rilpivirine of <2, 1 had an FC value of 2, and 3 had FC values >2 in the phenotypic assay, which has a biological cutoff for rilpivirine of 2 (Fig. 1C).
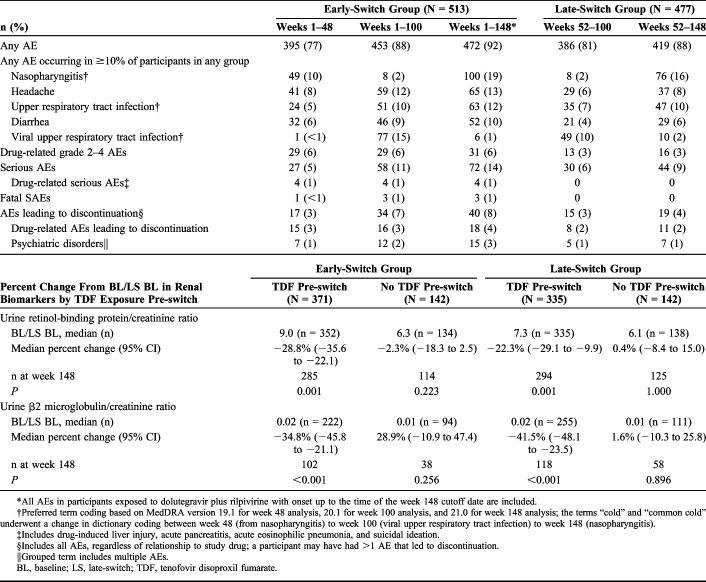
Low rates of drug-related grade 2–4 AEs were reported in the early-switch (31/513; 6%) and late-switch groups (16/477; 3%) after 148 and 96 weeks of exposure to dolutegravir plus rilpivirine, respectively. Discontinuations due to AEs occurred in 40 (8%) early-switch participants through 148 weeks of exposure and 19 (4%) late-switch participants through 96 weeks of exposure, with low rates of discontinuation due to psychiatric AEs during these exposure periods [early-switch group: 15/513 (3%); late-switch group: 7/477 (1%)]. Serious AEs occurred in 72 (14%) early-switch participants after 148 weeks of exposure [4 (<1%) were drug related] and 44 (9%) late-switch participants after 96 weeks of exposure (none were drug related; Table 1).
TABLE 1.
AEs Through Week 148 and Change From Baseline/Late-Switch Baseline in Renal Biomarkers at Week 148
| n (%) | Early-Switch Group (N = 513) | Late-Switch Group (N = 477) | |||
| Weeks 1–48 | Weeks 1–100 | Weeks 1–148* | Weeks 52–100 | Weeks 52–148 | |
| Any AE | 395 (77) | 453 (88) | 472 (92) | 386 (81) | 419 (88) |
| Any AE occurring in ≥10% of participants in any group | |||||
| Nasopharyngitis† | 49 (10) | 8 (2) | 100 (19) | 8 (2) | 76 (16) |
| Headache | 41 (8) | 59 (12) | 65 (13) | 29 (6) | 37 (8) |
| Upper respiratory tract infection† | 24 (5) | 51 (10) | 63 (12) | 35 (7) | 47 (10) |
| Diarrhea | 32 (6) | 46 (9) | 52 (10) | 21 (4) | 29 (6) |
| Viral upper respiratory tract infection† | 1 (<1) | 77 (15) | 6 (1) | 49 (10) | 10 (2) |
| Drug-related grade 2–4 AEs | 29 (6) | 29 (6) | 31 (6) | 13 (3) | 16 (3) |
| Serious AEs | 27 (5) | 58 (11) | 72 (14) | 30 (6) | 44 (9) |
| Drug-related serious AEs‡ | 4 (1) | 4 (1) | 4 (1) | 0 | 0 |
| Fatal SAEs | 1 (<1) | 3 (1) | 3 (1) | 0 | 0 |
| AEs leading to discontinuation§ | 17 (3) | 34 (7) | 40 (8) | 15 (3) | 19 (4) |
| Drug-related AEs leading to discontinuation | 15 (3) | 16 (3) | 18 (4) | 8 (2) | 11 (2) |
| Psychiatric disorders‖ | 7 (1) | 12 (2) | 15 (3) | 5 (1) | 7 (1) |
| Percent Change From BL/LS BL in Renal Biomarkers by TDF Exposure Pre-switch | Early-Switch Group | Late-Switch Group | ||
| TDF Pre-switch (N = 371) | No TDF Pre-switch (N = 142) | TDF Pre-switch (N = 335) | No TDF Pre-switch (N = 142) | |
| Urine retinol-binding protein/creatinine ratio | ||||
| BL/LS BL, median (n) | 9.0 (n = 352) | 6.3 (n = 134) | 7.3 (n = 335) | 6.1 (n = 138) |
| Median percent change (95% CI) | −28.8% (−35.6 to −22.1) | −2.3% (−18.3 to 2.5) | −22.3% (−29.1 to −9.9) | 0.4% (−8.4 to 15.0) |
| n at week 148 | 285 | 114 | 294 | 125 |
| P | 0.001 | 0.223 | 0.001 | 1.000 |
| Urine β2 microglobulin/creatinine ratio | ||||
| BL/LS BL, median (n) | 0.02 (n = 222) | 0.01 (n = 94) | 0.02 (n = 255) | 0.01 (n = 111) |
| Median percent change (95% CI) | −34.8% (−45.8 to −21.1) | 28.9% (−10.9 to 47.4) | −41.5% (−48.1 to −23.5) | 1.6% (−10.3 to 25.8) |
| n at week 148 | 102 | 38 | 118 | 58 |
| P | <0.001 | 0.256 | <0.001 | 0.896 |
All AEs in participants exposed to dolutegravir plus rilpivirine with onset up to the time of the week 148 cutoff date are included.
Preferred term coding based on MedDRA version 19.1 for week 48 analysis, 20.1 for week 100 analysis, and 21.0 for week 148 analysis; the terms “cold” and “common cold” underwent a change in dictionary coding between week 48 (from nasopharyngitis) to week 100 (viral upper respiratory tract infection) to week 148 (nasopharyngitis).
Includes drug-induced liver injury, acute pancreatitis, acute eosinophilic pneumonia, and suicidal ideation.
Includes all AEs, regardless of relationship to study drug; a participant may have had >1 AE that led to discontinuation.
Grouped term includes multiple AEs.
BL, baseline; LS, late-switch; TDF, tenofovir disoproxil fumarate.
The median urine retinol-binding protein/creatinine ratio decreased statistically significantly from baseline/late-switch baseline through week 148 in participants taking tenofovir disoproxil fumarate pre-switch in the early-switch and late-switch groups (Table 1). In participants not taking tenofovir disoproxil fumarate pre-switch, there was no statistically significant change in either the early-switch or late-switch group. Similar results were observed for the median urine β2 microglobulin/creatinine ratio (Table 1). For median estimated glomerular filtration rate by serum cystatin C,6 regardless of previous exposure to tenofovir disoproxil fumarate, there was no change from baseline through week 148 in the early-switch group [previous exposure: 0.00 (IQR, −8.92 to 8.22), n = 307; no previous exposure: 0.00 (IQR, −9.92 to 0.00), n = 125]. The late-switch group showed a transient 7% reduction at week 100 followed by a return to late-switch baseline values at week 148 [previous exposure: 0.00 (IQR, −8.45 to 0.00), n = 304; no previous exposure: 0.00 (IQR, −12.40 to 0.00), n = 129].
In both groups, statistically significant decreases from baseline/late-switch baseline, evaluated for the SWORD studies population as a whole, were observed in bone-specific alkaline phosphatase [baseline/late-switch baseline mean (SD): early-switch group, 15.9 (6.7), n = 511; late-switch group, 17.1 (7.0), n = 477; mean (SD) change at week 148: early-switch group, −3.4 (4.9), P < 0.001, n = 432; late-switch group, −3.5 (25.1), P = 0.004, n = 433], osteocalcin [baseline/late-switch baseline mean (SD): early-switch group, 23.8 (9.8), n = 511; late-switch group, 22.9 (8.1), n = 477; mean (SD) change at week 148: early-switch group, −4.9 (7.7), P < 0.001, n = 431; late-switch group, −4.8 (7.4), P < 0.001, n = 433], procollagen 1 N-terminal propeptide [baseline/late-switch baseline mean (SD): early-switch group, 53.0 (23.2), n = 512; late-switch group, 54.5 (20.7), n = 477; mean (SD) change at week 148: early-switch group, −4.2 (22.8), P < 0.001, n = 431; late-switch group, −7.3 (23.7), P < 0.001, n = 431], and type 1 collagen C-telopeptide [baseline/late-switch baseline mean (SD): early-switch group, 0.7 (0.3), n = 512; late-switch group, 0.6 (0.9), n = 477; mean (SD) change at week 148: early-switch group, −0.1 (0.3), P < 0.001, n = 433; late-switch group, −0.1 (0.9), P = 0.008, n = 431]. For the SWORD studies population as a whole, no clinically meaningful differences were detected in mean change from baseline/late-switch baseline at week 148 for cholesterol (early-switch group, 0.13 mmol/L, n = 360; late-switch group, 0.08 mmol/L, n = 356), high-density lipoprotein cholesterol (early-switch group, 0.05 mmol/L, n = 360; late-switch group, 0.01 mmol/L, n = 356), low-density lipoprotein cholesterol (early-switch group, 0.11 mmol/L, n = 349; late-switch group, 0.12 mmol/L, n = 352), triglycerides (early-switch group, −0.02 mmol/L, n = 360; late-switch group, −0.12 mmol/L, n = 356), or ratio of total cholesterol to high-density lipoprotein cholesterol (early-switch group, −0.04, n = 360; late-switch group, 0.03, n = 356).
Self-reported regimen adherence was high (>98%) at baseline and remained stable at each time point in both the early-switch and late-switch groups up to week 148. High levels of treatment satisfaction at baseline or late-switch baseline were maintained up to week 148 after switching to dolutegravir plus rilpivirine.
DISCUSSION
Pooled 148-week results from SWORD-1 and SWORD-2 demonstrate long-term efficacy of the 2DR in maintaining virologic suppression for up to 3 years. Of note, the late-switch group at week 148 achieved a level of efficacy comparable with that of the early-switch group at week 100,5 highlighting and reproducing the 2-year clinical efficacy of this 2DR. Similar to the 100-week analysis,5 frequency of CVW from day 1 through week 148 remained low (11/990; 1%). Interestingly, in 1 participant with baseline integrase substitutions N155N/H and G163G/R by proviral DNA genotype assay, only the integrase polymorphism mixture (V151V/I), which is not associated with dolutegravir resistance, was detected at virologic failure. NNRTI RAMs were observed in 6 participants (early-switch group, 5/513; 1%; late-switch group, 1/477; <1%). There were no notable safety concerns with up to 148 weeks of treatment, and safety profiles were comparable between the early-switch group at week 100 and the late-switch group at week 148 (100 vs. 96 weeks of 2DR exposure, respectively).5 Drug-related AEs were reported less frequently in the late-switch group, possibly reflecting increased investigator experience with this 2DR.
In addition to our data demonstrating long-term efficacy of switching to dolutegravir plus rilpivirine, recent reports in clinical settings also support switching to this 2DR. In a cohort of 102 patients, switching to dolutegravir plus rilpivirine maintained virologic suppression in 93% of patients, with only 1 case of virologic failure at 48 weeks.7 In a separate multicenter cohort of 187 virologically suppressed patients with 371 person-years of follow-up, switching to dolutegravir plus rilpivirine was efficacious in maintaining suppression, with 5 (2.7%) cases of virologic failure, which was defined as 2 consecutive measurements of HIV-1 RNA ≥50 copies/mL in 3 months or a single measurement of HIV-1 RNA ≥1000 copies/mL.8 Although we observed a lower frequency of virologic failure in the SWORD studies at week 48 (3/513; <1%), direct comparison between trials may be confounded by differences in patient populations, particularly previous ART experience, and analysis methods.4 Recent reports in clinical settings also support the long-term efficacy of switching to other 2DRs. In the open-label phase 3 TANGO study9 assessing the efficacy and safety of switching to a fixed-dose 2DR of dolutegravir plus lamivudine, 344 of 369 (93.2%) participants who switched to dolutegravir plus lamivudine had HIV-1 RNA <50 copies/mL at week 48. The low rates of virologic failure observed in SWORD (<1%) and TANGO (0%) at 48 weeks support the robustness of a dolutegravir-based 2DR.4,9
This analysis detected NNRTI RAMs at low frequency (6/990; 0.6%) over up to 148 weeks of exposure and was comparable with other NNRTI-based switch studies. In a 48-week switch study (immediate switch or delayed switch at week 24) in virologically suppressed participants with HIV-1 switching to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from a protease inhibitor–based regimen,10 4 of 469 (0.9%) participants in the rilpivirine group analyzed for resistance at week 48 had evidence of NNRTI and/or NRTI resistance mutations.
Data from the pooled SWORD studies demonstrate high rates of virologic suppression, a low rate of virologic failure with limited observed NNRTI RAMs, no integrase inhibitor resistance, and no notable safety concerns in participants with up to 3 years of exposure to dolutegravir plus rilpivirine. These data support this 2DR as an effective treatment alternative with a good safety profile for individuals who are already suppressed on standard 3-drug or 4-drug regimens without previous virologic failure and concurrent hepatitis B infection for long-term HIV-1 management.
ACKNOWLEDGMENTS
The authors thank the study participants; their families and caregivers; the ViiV Healthcare, GlaxoSmithKline, and Janssen study team members; and all investigators and site staff who participated in the study. Editorial assistance was provided under the direction of the authors by Aarthi Gobinath, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and funded by ViiV Healthcare.
Footnotes
Presented in part at the 25th Annual Conference of the British HIV Association; April 2–5, 2019; Bournemouth, United Kingdom; Poster P008.
J.v.W., L.P.K., J.M., R.W., M.U., B.W., M.‐C.N., M.G., and K.Y.S. are employees of ViiV Healthcare and own stock in GlaxoSmithKline. M.U. has a patent pending (US20170079982). C.O. has received grants from ViiV Healthcare and grants, personal fees, nonfinancial support, and travel and speaking fees from ViiV Healthcare, Gilead, Janssen, AbbVie, Bristol-Myers Squibb, and Merck Sharpe and Dohme. R.R. has received speaking fees and support for conference attendance from ViiV Healthcare, speaking fees and grants from Gilead and Janssen, and speaking fees from Merck Sharpe and Dohme. J.R.B. has received personal fees from ViiV Healthcare, Gilead, Merck Sharpe and Dohme, Janssen, and Hexal. D.B. has received grants from ViiV Healthcare and grants, pe rsonal fees, nonfinancial support, and travel and speaking fees from Gilead, AbbVie, Bristol-Myers Squibb, ViiV Healthcare, and Merck Sharpe and Dohme. D.P. has received consulting fees from ViiV Healthcare, Gilead, Theratechnologies, and speaking fees from Gilead. K.A. is an employee of and owns stock in GlaxoSmithKline. K.V. is an employee of Janssen Pharmaceutica NV. This study was funded by ViiV Healthcare and Janssen Pharmaceutica NV. The remaining author has no funding or conflicts of interest to disclose.
REFERENCES
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV: Department of Health and Human Services; 2019. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed June 3, 2020. [Google Scholar]
- 2.European AIDS Clinical Society. EACS Guidelines Version 10.0: European AIDS Clinical Society; 2019. Available at: https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf. Accessed June 3, 2020. [Google Scholar]
- 3.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society–USA Panel. JAMA. 2018;320:379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391:839–849. [DOI] [PubMed] [Google Scholar]
- 5.Aboud M, Orkin C, Podzamczer D, et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV. 2019;6:e576–e587. [DOI] [PubMed] [Google Scholar]
- 6.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casado JL, Monsalvo M, Fontecha M, et al. Dolutegravir plus rilpivirine as dual regimen in virologically suppressed HIV-1 infected patients in a clinical setting. HIV Res Clin Pract. 2019;20:64–72. [DOI] [PubMed] [Google Scholar]
- 8.Ciccullo A, Baldin G, Capetti A, et al. A comparison between two dolutegravir-based two-drug regimens as switch strategies in a multicentre cohort of HIV-1-infected patients. Antivir Ther. 2019;24:63–67. [DOI] [PubMed] [Google Scholar]
- 9.van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose two-drug regimen versus continuing a tenofovir alafenamide-based three- or four-drug regimen for maintenance of virologic suppression in adults with HIV-1: phase 3, randomized, non-inferiority TANGO study. Clin Infect Dis. 2020; ciz1243. doi: 10.1093/cid/ciz1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palella FJ, Jr, Fisher M, Tebas P, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS. 2014;28:335–344. [DOI] [PubMed] [Google Scholar]