Abstract
Objective:
The objectives were to investigate and compare the risks and incidences of venous thromboembolism (VTE) between the 2 groups of patients with coronavirus disease 2019 (COVID-19) pneumonia and community-acquired pneumonia (CAP).
Approach and Results:
Medical records of 616 pneumonia patients who were admitted to the Yichang Central People’s Hospital in Hubei, China, from January 1 to March 23, 2020, were retrospectively reviewed. The patients with COVID-19 pneumonia were treated in the dedicated COVID-19 units, and the patients with CAP were admitted to regular hospital campus. Risks of VTE were assessed using the Padua prediction score. All the patients received pharmaceutical or mechanical VTE prophylaxis. VTE was diagnosed using Duplex ultrasound or computed tomography pulmonary angiogram. Differences between COVID-19 and CAP groups were compared statistically. All statistical tests were 2 sided, and P<0.05 was considered as statistically significant. All data managements and analyses were performed by IBM SPSS, version 24, software (SPSS, Inc, Chicago, IL). Of the 616 patients, 256 had COVID-19 pneumonia and 360 patients had CAP. The overall rate of VTE was 2% in COVID-19 pneumonia group and 3.6% in CAP group, respectively (P=0.229). In these two groups, 15.6% of the COVID-19 pneumonia patients and 10% of the CAP patients were categorized as high risk for VTE (Padua score, >4), which were significantly different (P=0.036). In those high-risk patients, the incidence of VTE was 12.5% in COVID-19 pneumonia group and 16.7% in CAP group (P=0.606). Subgroup analysis of the critically ill patients showed that VTE rate was 6.7% in COVID-19 group versus 13% in CAP group (P=0.484). In-hospital mortality of COVID-19 and CAP was 6.3% and 3.9%, respectively (P=0.180).
Conclusions:
Our study suggested that COVID-19 pneumonia was associated with hypercoagulable state. However, the rate of VTE in COVID-19 pneumonia patients was not significantly higher than that in CAP patients.
Keywords: critical illness, hospital mortality, incidence, pneumonia, venous thromboembolism
Highlights.
This study investigated and compared the risks and incidences of venous thromboembolism between the 2 groups of patients with coronavirus disease 2019 (COVID-19) pneumonia and community-acquired pneumonia.
COVID-19 pneumonia was associated with hypercoagulable state.
The rate of venous thromboembolism in COVID-19 pneumonia patients was not significantly higher than that in community-acquired pneumonia patients.
See accompanying editorial on page 1958
Coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has spread around the planet. The clinical presentations of COVID-19 vary from asymptomatic to severe acute respiratory syndrome. It has been suggested that coronavirus, like some other viruses, may also have significant impact on the hematopoietic and hemostatic systems resulting in thrombotic and bleeding complications.1–5 Recent publications reported that SARS-CoV-2 infection might increase the risks of venous thromboembolism (VTE), especially in hospitalized patients with severe symptoms such as COVID-19 pneumonia.5–7 However, the real incidence of VTE in COVID-19 inpatients has not been well documented, and the VTE risks in COVID-19 patients have not been compared with community-acquired pneumonia (CAP) patients. This study compares the risks and incidences of VTE between the 2 groups of patients with COVID-19 pneumonia and CAP.
Approach
The authors declare that all supporting data are available within this article.
Total of 616 pneumonia patients were admitted to the Yichang Central People’s Hospital—a tertiary regional medical center in Hubei, China, from January 1 to March 23, 2020. All the patients presenting with fever or respiratory symptoms had COVID-19 screening tests, and the diagnosis of COVID-19 pneumonia was made according to the following published criteria: (1) symptomatic patients with bilateral pulmonary infiltrates and multifocal ground-glass opacities consistent with atypical pneumonia on chest computed tomography scan, (2) rhinopharyngeal specimen reverse transcription polymerase chain reaction (RT-PCR) test positive for SARS CoV-2, or (3) SARS CoV-2 gene assay positive.8
Our institution opened fever clinic for COVID-19 patients on January 1, 2020, but RT-PCR test was not started until the end of January. The suspected COVID-19 patients were initially diagnosed based on travel/contact history, clinical manifestations, and chest computed tomography findings. We excluded the patients who were discharged before RT-PCR test available. All the 616 patients in this study were positive from 1 or 2 rounds of RT-PCR tests during hospitalization. The patients who presented with diagnosed COVID-19 pneumonia were directly admitted to the dedicated units. The patients with clinical manifestations and imaging evidence of pneumonia, but negative for first COVID-19 test, were admitted to transitional isolated units until repeated tests were performed. Those patients who were confirmed to have COVID-19 infection by the repeated test were then transferred to the dedicated units. The patients who were negative per repeated COVID-19 test were then transferred to regular hospital wards from transitional units.
Medical records of the 616 patients were reviewed retrospectively. This study was approved by the Institutional Review Board of the Yichang Central People’s Hospital. Informed written consent was not required for this deidentified retrospective study. Patients’ demographic data were collected and analyzed statistically. The risks of VTE were assessed using Padua prediction score.9 All patients received VTE prophylaxis following standard protocols with low-molecular-weight heparin or unfractionated heparin or mechanical intermittent pneumatic compression device if contraindicated to anticoagulants. High-risk (Padua score, >4) patients were screened using Duplex ultrasound or computed tomography pulmonary angiogram to rule out VTE.
Descriptive analyses were reported as relative frequencies for discrete variables. Continuous variables were reported as mean±SD or median and interquartile range for normal and non-normal distributed variables, respectively. To determine the differences of observational parameters between COVID-19 pneumonia and CAP groups, χ2 test, Fisher exact test, t test, or Mann-Whitney U test were performed. All statistical tests were 2 sided, and P<0.05 was considered as statistically significant. All data managements and analyses were performed using IBM SPSS, version 24, software (SPSS, Inc, Chicago, IL).
Results
Of the 616 patients, 256 (men:women, 131:125; age, 55.5 [0.5–87] years) were diagnosed to have COVID-19 pneumonia, and 360 patients (men:women, 211:149; age, 61 [15–95] years) had CAP. Demographics and characteristics of all patients were collected and summarized in Table 1. In general, COVID-19 pneumonia patients were younger with less underlying diseases, whereas more CAP patients had chronic comorbidities, including coronary artery disease, cerebrovascular disease, hypertension, diabetes mellitus, and history of malignancy. However, acute liver and renal dysfunction occurred more common in COVID-19 pneumonia patients, especially in critically ill patients (Table 1).
Table 1.
Demographics and Characteristics of COVID-19 Pneumonia and CAP Patients
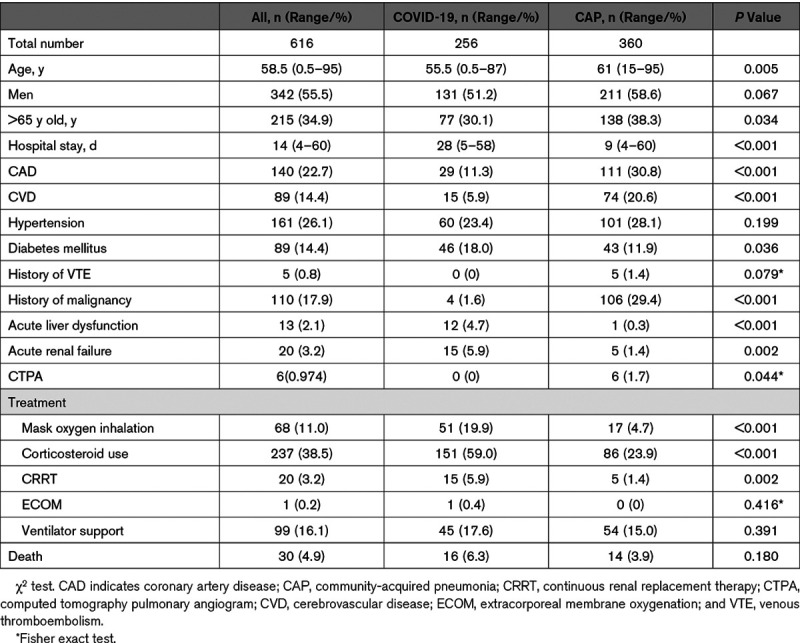
Overall rate of VTE was 2% in COVID-19 pneumonia group and 3.6% in CAP group, respectively, with no significant difference (P=0.229; Table 2). D-dimmer was elevated in both groups (Table 3). Average Padua score was significantly higher in COVID-19 group, compared with CAP group (2.36±1.51 versus 1.88±1.36; P<0.001). In these two groups, 15.6% (40 of 256) of the COVID-19 pneumonia patients and 10% (36 of 360) of the CAP patients were categorized as high risk for VTE development with a Padua score >4. The percentage of high-risk patients in COVID-19 group was significantly higher than that in CAP group (P=0.036).
Table 2.
The Overall VTE Incidences in COVID-19 and CAP Groups
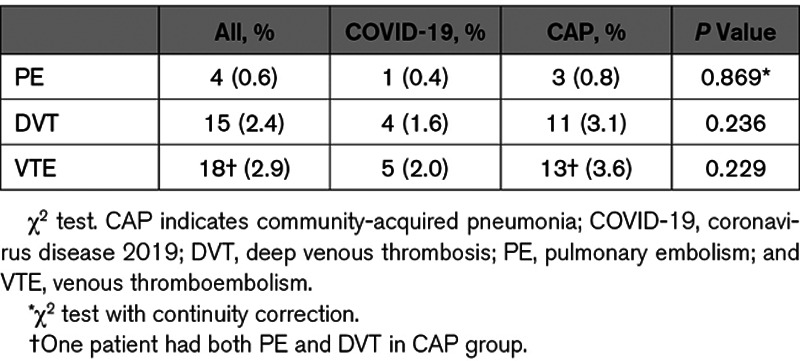
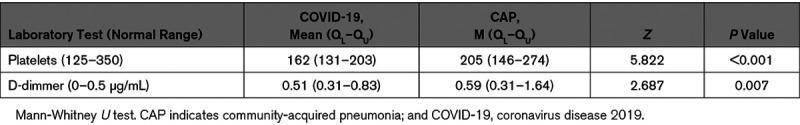
Table 3.
Platelet Counts and D-Dimmer in COVID-19 Pneumonia and CAP Patients
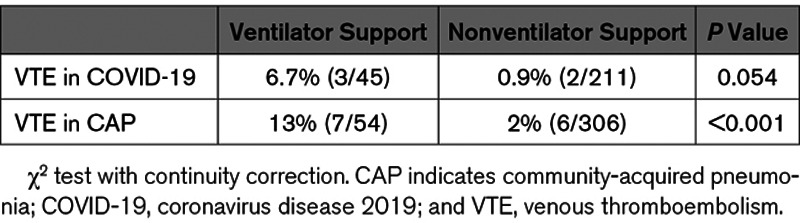
Subgroup analysis showed that in the high-risk (Padua score, >4) patients, the incidences of VTE were 12.5% (5 of 40) in COVID-19 pneumonia group and 16.7% (6 of 36) in CAP group, with P=0.606 (Table 4). The incidence of VTE in the critically ill patients who required ventilator support was 6.7% (3 of 45) in COVID-19 group and 13% (7 of 54) in CAP group, respectively (Table 4). Vertical comparison within each group showed the incidence of VTE in the patients who required ventilator support was higher than that in the patients without ventilator support (Table 5). However, cross-comparison demonstrated no statistical difference between the COVID-19 pneumonia and CAP groups (P=0.484).
Table 4.
Incidences of VTE in High-Risk Patients and Critically Ill Patients
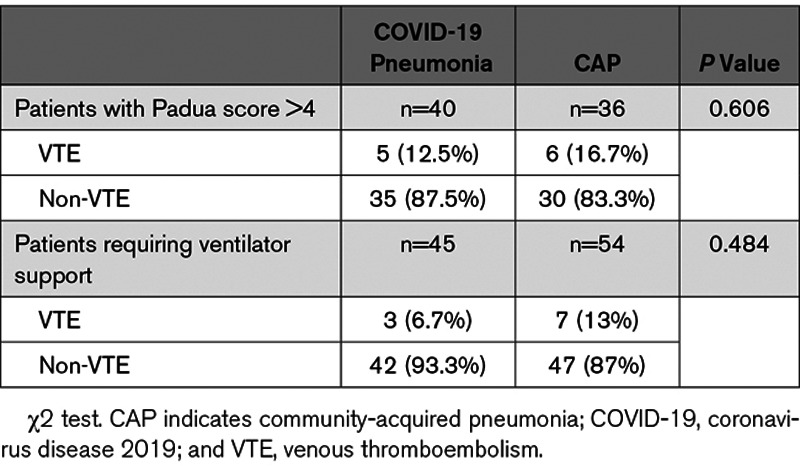
Table 5.
The Incidences of VTE in Critically Ill and Noncritically Ill Patients
The length of hospital stay of the COVID-19 pneumonia patients was significantly longer than CAP patients (28 versus 9 days; P<0.001). In-hospital mortality rate was 6.3% in COVID-19 group versus 3.9% in CAP group, but statistical difference was not reached (P=0.138; Table 1). In COVID-19 pneumonia group, 1 patient on ventilator support developed acute arterial ischemia with gangrene toes and fingers.
Discussion
Previous publications documented that coronaviruses may have strong influences on hematopoietic and hemostatic systems and are associated with thrombotic complications.1–6,10–12 The mechanisms are potentially due to endothelial cell dysfunction secondary to infection,1,13 venous stasis because of immobilization during hospitalization especially in critically ill patients, and hypoxia stimulation.14 Antiphospholipid, anticardiolipin, and anti–β2-glycoprotein I antibodies were detected in 3 patients with complicated severe COVID-19 infection,15 but data in large series of patients were not available. The mechanisms of this novel SARS-CoV-2 on thrombotic system have not been clearly understood.
Recent cohort studies and case reports suggested that the VTE risks were increased in COVID-19 patients.2–7,16,17 Current interim guidelines recommend to assess VTE risk in all COVID-19 patients admitted to hospital and provide pharmacological prophylaxis to all high-risk patients.18,19 It was also suggested that anticoagulant treatment was associated with decreased mortality in severe COVID-19 patients.6 However, no previous study has compared the VTE risks between the 2 groups of COVID-19 pneumonia patients and CAP patients. We conducted a retrospective chart review study to investigate the difference of VTE risks between the 2 groups of COVID-19 pneumonia and CAP patients, who were treated during the same time period at the same hospital with 2600 regular beds and 500 dedicated COVID-19 beds located in the Hubei province—the epidemic center in China.
Patients in the COVID-19 group were younger and healthier, whereas CAP patients had more chronic underlying diseases, such as coronary artery disease, cerebrovascular disease, hypertension, and diabetes mellitus. This reflected the characteristics of COVID-19 spreading among the populations who were socially more active (Table 1). Notably, rates of acute kidney and liver dysfunction were significantly higher in COVID-19 pneumonia patients than in CAP patients (5.9% versus 1.4%, P=0.002, and 4.7% versus 0.3%, P<0.001, respectively). Cytokine storm might be one of the mechanisms causing multisystem organ dysfunction.1,3,5 In-hospital mortality was as higher as 6.3% in COVID-19 pneumonia group versus 3.9% in CAP group but not statistically different (P=0.18). The length of hospital stay of the COVID-19 pneumonia patients was significantly longer than CAP patients. The main reason was COVID-19 patients had to wait until they became asymptomatic and were negative from 2 separate SARS-CoV-2 tests.
It has been reported that SARS-CoV-2 increases the risk for VTE in COVID-19 patients.2–7 Our data showed similar findings as the average Padua score was significantly higher in COVID-19 group than that in CAP group. However, the rates of VTE were not statistically different between the 2 groups, 2% versus 3.6% (P=0.229). These numbers of VTE in our groups were similar to previous report as 2.9% in COVID-19 patients.5
In this study, the percentage of high-VTE-risk patients with Padua score >4 was 15.6% in COVID-19 pneumonia group, which was significantly higher than the rate of 10% in CAP group (P=0.036). Longitudinal subgroup analysis demonstrated that the incidence of VTE increased from 2% in the entire COVID-19 group as a whole to 12.5% in the high-VTE-risk COVID-19 pneumonia patients with Padua score >4. In CAP group, the incidence of VTE increased from overall 3.6% to 16.7% in high-VTE-risk patients (Padua score, >4). Cross group comparison demonstrated that the differences of VTE between COVID-19 and CAP groups were not statistically significant (P=0.606). These findings suggested that SARS-CoV-2 infection was associated with hypercoagulable state especially in high-risk patients. However, the actual VTE incidence in COVID-19 pneumonia group was not significantly higher than that in CAP patients (2% versus 3.6%; P=0.229).
It was also reported that severity of the SARS-CoV-2 infection was associated with increased VTE risk.5–7 However, direct comparison of the VTE rates in critically ill patients has not been performed. Our analysis compared the incidences of VTE in the patients who required ventilator support with the patients with mild-to-moderate symptoms. Longitudinal comparison in COVID-19 group showed that the rates of VTE increased to 6.7% in ventilator support patients from 0.9% in the patients without respiratory distress, although statistical significance was not reached (P=0.054). In CAP group, the incidence of VTE was significantly higher in the patients who required ventilator support than that in the patients with no ventilator support, 13% versus 2% (P<0.001).
Our study revealed that elevated Padua prediction scores and increased VTE risks, which reflected hypercoagulable state, were associated with the severity of COVID-19 pneumonia and CAP. However, the incidences of VTE were not statistically different between these 2 groups based on cross group analyses. These real-world data suggested that SARS-CoV-2 infection might, similar to CAP, increase the VTE risk but did not further result in higher actual VTE events when routine deep venous thrombosis prophylaxis was given. We could imagine that complex mechanisms are involved in the pathophysiological processes of VTE development in the patients with SARS-CoV-2 infection. Attempting to interpret our findings that VTE incidence was not higher in COVID-19 pneumonia group than in CAP group, one possible explanation might be that COVID-19 patients were younger with fewer underlying diseases. Additionally, higher rates of acute liver and renal dysfunction in COVID-19 patients might cause some degree of coagulopathy.20 In contrast, chronic comorbidities and history of malignancy were more common in CAP group (Table 1), which could result in higher VTE risks.
There were several limitations in this report. First, this was a retrospective chart review study with patient selection bias. Second, the small numbers of VTE rates in subgroup analysis might generate type II statistical errors. Third, the underlying chronic comorbidities and different treatment regimens might also play an important role in VTE development. Finally, It has been reported that the sensitivity of RT-PCR test was 83.3% for the first round and 91.7% after repeated.21 Theoretically, the false negative tests could result in some crossover diagnoses between the COVID-19 and CAP groups. Fortunately, we did not have any later diagnosed COVID-19 pneumonia from the CAP group.
Disclosures
None.
Footnotes
Nonstandard Abbreviations and Acronyms
- CAP
- community-acquired pneumonia
- COVID-19
- coronavirus disease 2019
- RT-PCR
- reverse transcription polymerase chain reaction
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus-2
- VTE
- venous thromboembolism
For Disclosures, see page 2336.
F. Mei and J. Fan are joint first authors.
References
- 1.Goeijenbier M, van Wissen M, van de Weg C, Jong E, Gerdes VE, Meijers JC, Brandjes DP, van Gorp EC. Review: Viral infections and mechanisms of thrombosis and bleeding. J Med Virol 2012841680–1696doi: 10.1002/jmv.23354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020191145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, Psaltopoulou T, Gerotziafas G, Dimopoulos MA. Hematological findings and complications of COVID-19. Am J Hematol 202095834–847doi: 10.1002/ajh.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, Zhong N, Li S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol 20207e362–e363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu JF, Wang L, Zhao L, Li F, Liu L, Zhang L, Li Q, Gu J, Liang S, Zhao Q, et al. Risk assessment of venous thromboembolism and bleeding in COVID 19 [published online March 24, 2020]. Res Square Pulmonol. doi: 10.21203/rs-18340/v1. [Google Scholar]
- 6.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020181094–1099doi: 10.1111/jth.14817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui S., Chen S., Li X., Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020181421–1424doi: 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Commission of the People’s Republic of China. Diagnosis and management of COVID-19 pneumonia (3rd edition). doi: 10.46234/ccdcw2020.082. http://www.gov.cn/zhengce/zhengceku/2020-01/23/content_5471832.htm. Accessed January 23, 2020. [DOI] [PMC free article] [PubMed]
- 9.Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, De Bon E, Tormene D, Pagnan A, Prandoni P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost 201082450–2457doi: 10.1111/j.1538-7836.2010.04044.x [DOI] [PubMed] [Google Scholar]
- 10.Ng KH, Wu AK, Cheng VC, Tang BS, Chan CY, Yung CY, Luk SH, Lee TW, Chow L, Yuen KY. Pulmonary artery thrombosis in a patient with severe acute respiratory syndrome. Postgrad Med J. 2005;81:e3. doi: 10.1136/pgmj.2004.030049. doi: 10.1136/pgmj.2004.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 20033481986–1994doi: 10.1056/NEJMoa030685 [DOI] [PubMed] [Google Scholar]
- 12.Wong RS, Wu A, To KF, Lee N, Lam CW, Wong CK, Chan PK, Ng MH, Yu LM, Hui DS, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 20033261358–1362doi: 10.1136/bmj.326.7403.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res 201714938–44doi: 10.1016/j.thromres.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res 201918177–83doi: 10.1016/j.thromres.2019.07.013 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;383:e38. doi: 10.1056/NEJMc2007575. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J. 2020;41:1858. doi: 10.1093/eurheartj/ehaa254. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Wang X, Yang P, Zhang S. COVID-19 complicated by acute pulmonary embolism. Radiology. 2020;2:e200067. doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Surgeons. Clinical issues and guidance. https://www.facs.org/covid-19/clinical-guidance. Accessed April 16, 2020.
- 19.Hunt B, Retter A, McClintock C. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. ISTH Academy. & Claire McClintock B. 03/25/20; 290533. https://academy.isth.org/isth/2020/covid-19/290533/beverley.hunt.andrew.retter.26.claire.mcclintock.practical.guidance.for.the.html?f=menu%3D8%2Abrowseby%3D8%2Asortby%3D2%2Alabel%3D19794. Accessed April 16, 2020.
- 20.Escolar G, Díaz-Ricart M, Cases A. Uremic platelet dysfunction: past and present. Curr Hematol Rep 20054359–367 [PubMed] [Google Scholar]
- 21.Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, Zeng B, Li Z, Li X, Li H. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]


